Abstract
Many species of small desert mammals are known to have expanded auditory bullae. The ears of gerbils and heteromyids have been well described, but much less is known about the middle ear anatomy of other desert mammals. In this study, the middle ears of three gerbils (Meriones, Desmodillus and Gerbillurus), two jerboas (Jaculus) and two sengis (elephant‐shrews: Macroscelides and Elephantulus) were examined and compared, using micro‐computed tomography and light microscopy. Middle ear cavity expansion has occurred in members of all three groups, apparently in association with an essentially ‘freely mobile’ ossicular morphology and the development of bony tubes for the middle ear arteries. Cavity expansion can occur in different ways, resulting in different subcavity patterns even between different species of gerbils. Having enlarged middle ear cavities aids low‐frequency audition, and several adaptive advantages of low‐frequency hearing to small desert mammals have been proposed. However, while Macroscelides was found here to have middle ear cavities so large that together they exceed brain volume, the bullae of Elephantulus are considerably smaller. Why middle ear cavities are enlarged in some desert species but not others remains unclear, but it may relate to microhabitat.
Keywords: communication, desert, gerbil, jerboa, low‐frequency, malleus, middle ear, sengi
Introduction
The tympanic membrane covers the external entrance to the air‐filled middle ear cavity. In many mammals, this cavity is enclosed within a bony auditory bulla, visible as a swelling at the base of the skull (exceptions include Old World primates such as humans, in which the middle ear cavity is enclosed within the temporal bone but not within a bulla). Airborne sound causes the membrane to vibrate, which in turn sets into vibration the three auditory ossicles: the malleus, incus and stapes. The footplate of the stapes is enclosed within the oval window (fenestra vestibuli), the entrance to the fluid‐filled inner ear within which sound vibrations are transduced by hair cells into electrical signals. Possession of three middle ear ossicles is characteristic of all mammals, but middle ear morphology otherwise varies considerably between different groups (Fleischer, 1978; Mason, 2013).
A smaller head means a smaller interaural time‐of‐arrival difference for sound presented at any given angle, and a smaller interaural intensity difference due to reduced sound shadowing, while a smaller pinna reduces availability of monaural directional cues (Heffner & Heffner, 1992a). In order to achieve accurate sound localization, a smaller mammal needs to detect higher frequencies, which are affected more by the head and pinna. Smaller vocal organs should lead to higher‐pitched vocalizations, used in intraspecific communication. For these and other reasons, small mammals are generally expected to benefit from high‐frequency hearing. Indeed, the high‐frequency hearing limit of rodents (at 60 dB SPL) shows a strong, negative correlation with functional interaural distance (Heffner & Heffner, 1992a; Heffner et al. 2001).
Some small desert mammals, however, are known to have relatively acute hearing within the low‐frequency range, below about 3 kHz. The results of both electrophysiological and behavioural studies show that this ability is well developed in gerbils (Finck & Sofouglu, 1966; Ryan, 1976) and kangaroo‐rats (Moushegian & Rupert, 1970; Vernon et al. 1971; Webster & Webster, 1972; Heffner & Masterton, 1980). Unlike in some subterranean mammals, in which high‐frequency hearing has been lost and localization abilities are compromised (Heffner & Heffner, 1990, 1992b, 1993), gerbils and kangaroo‐rats can also hear well into the ultrasonic range: the behavioural audiogram of Meriones unguiculatus extends up to nearly 60 kHz at 60 dB SPL (Ryan, 1976), while that of Dipodomys merriami extends to 52 kHz (Heffner & Masterton, 1980). The ability of these species to localize sound is similar to that of other small rodents (Heffner & Masterton, 1980; Heffner & Heffner, 1988).
For reasons discussed in the companion paper (Mason, 2015b), one of the key adaptations towards improving low‐frequency hearing is a voluminous middle ear cavity, and this is a feature of both gerbils and kangaroo‐rats. A large cavity should be easy to accommodate within a large skull, but a small mammal would require a disproportionately large auditory bulla (Fleischer, 1978). Lataste (1882) may have been the first author to note that desert rodents often have enlarged bullae, writing ‘Je crois en effet pouvoir énoncer cette règle, que les espèces d'un même genre et les genres d'une même famille ont les bulles d'autant plus développées qu'ils sont plus désertiques’, which translates as ‘I believe in fact to be able to formulate this rule, that the species of the same genus and the genera of the same family which are more of the desert have better‐developed bullae’. Since then, many others have commented on the presence of larger bullae in desert mammals, including Heim de Balsac (1936), Zavattari (1938), Petter (1953), Prakash (1959) and Oaks (1967).
Among desert rodents with enlarged bullae, the auditory structures of kangaroo‐rats and ‐mice in the family Heteromyidae, subfamily Dipodomyinae (Webster, 1961; Webster & Webster, 1975, 1977), and those of gerbils in the family Muridae, subfamily Gerbillinae (Oaks, 1967; Lay, 1972; Buytaert et al. 2011; von Unge et al. 2011; Salih et al. 2012), have been particularly well described. The most familiar of these is the Mongolian gerbil or jird (Meriones unguiculatus), a domestic species that, in the wild, inhabits the steppes of central Asia (Gulotta, 1971). Laboratory colonies are said to have originated from 20 pairs of animals captured in 1935 in the Amur river basin of eastern Mongolia (Norris, 1987), and this gerbil has since become a key model species in hearing research. Acute low‐frequency hearing in Meriones has been confirmed in a large number of experimental studies, and a great deal is now known about the function of its middle ear (see Mason 2015b).
This study compares the middle ear morphology of Meriones with that of less familiar desert mammals. What features of the middle ear have evolved convergently within arid‐region species of different families, and to what extent does morphology vary among members of the same family? Jerboas (Rodentia; Dipodidae) are native to the desert and semi‐desert regions of North Africa and the Middle East (Nowak, 1999). Limited information about their middle ears can be found in Howell (1932), Ognev (1948) and the comprehensive but unpublished thesis of Oaks (1967). Most papers on the middle ears of sengis, also known as elephant‐shrews (Macroscelidea; Macroscelididae), have concentrated on details of bullar structure (Van der Klaauw, 1931; Evans, 1942; Saban, 1956; MacPhee, 1981; Benoit et al. 2013, 2014), although very brief descriptions of the ear ossicles also exist (Doran, 1878; Segall, 1970). In the present study, the ears of two sengi and two gerbil species, from specimens all captured in the same part of Namibia, were examined and compared with those of two jerboas and the Mongolian gerbil. Middle ear structures were examined using micro‐computed tomography (micro‐CT) followed by dissection under light microscopy. Preliminary notes on the ear anatomy of the jerboas have been reported elsewhere (Mason, 2015a).
Most of the recent work on the Mongolian gerbil's auditory system has been published in specialized journals, and the interpretation of these papers requires a fairly advanced knowledge of acoustics. The companion paper (Mason, 2015b) introduces middle ear function to readers who lack this background, using the Mongolian gerbil as a case‐study, and explains what can and what cannot reasonably be inferred about hearing based on middle ear anatomy.
Materials and methods
One head each of Desmodillus auricularis (Cape short‐tailed gerbil; CAS MAM 30155), Gerbillurus setzeri (Setzer's hairy‐footed gerbil; CAS MAM 30154), Elephantulus rupestris (Western rock sengi; CAS MAM 30153) and Macroscelides flavicaudatus (Namib round‐eared sengi; CAS MAM 30152) were obtained on loan from the collection of the Department of Ornithology & Mammalogy, California Academy of Sciences, San Francisco, California, USA. Macroscelides flavicaudatus, previously regarded as a subspecies of Macroscelides proboscideus, has recently been elevated to a full species (Dumbacher et al. 2012). The heads were skinned, preserved in alcohol and subsequently kept in a freezer. These animals had originally been collected in the Kunene Region, Namibia, in 2013 (Rathbun et al. 2015). The heads of four M. unguiculatus (Mongolian gerbil) were obtained as corpses from another research project at the University of Cambridge. They originated from a laboratory breeding colony and were frozen prior to examination. Two Jaculus orientalis (greater Egyptian jerboa) and one Jaculus jaculus (lesser Egyptian jerboa) were obtained as frozen corpses from a commercial rodent breeder.
Micro‐CT scans were made of the skinned heads of the four Namibian specimens, one Meriones and one J. orientalis, wrapped in cellophane to reduce the rate of drying. One auditory bulla was then dissected out from each of these specimens, and either this isolated bulla or the remaining bulla within the basicranium was scanned again, at higher magnification. In the case of Meriones, a further bulla was scanned, from a different specimen. The only scan made of J. jaculus was of a partial bulla preparation.
The head scans of the Jaculus and Namibian specimens were made using a Metris X‐Tek HMX 160 micro‐CT scanner. Settings of 45–55 kV and 85–100 μA were used. The images were constructed from 720 projections, with 32 frames averaged per projection. The software used in the processing of the data included ixs Integrated X‐ray System Control version 4.1.29 (X‐Tek Systems, 2002), ngi ct Control version 1.5.4 (X‐Tek Systems, 2005) and ct‐pro 2.0 (Metris, 2008). Cubic voxel side‐lengths were 35–46 μm. The other CT‐scans were made at the Cambridge Biotomography Centre using a Nikon XT H 225 scanner; the settings were 110–132 kV and 120–180 μA. The images were constructed from 1080 projections, each with 1000 ms exposure and two frames averaged per projection. The software used in the processing of the scan data included ct agent xt 3.1.9 and ct pro 3d xt 3.1.9 (Nikon Metrology, 2004–2013). Cubic voxel side‐lengths were 21 μm for the Meriones head scan, and 8–14 μm for the bullar scans.
Exported tiff stacks were converted to jpg files using irfanview 4.37 (Irfan Skiljan, 2014) or adobe photoshop cs 8.0 (Adobe Systems, 2003). Some 3D reconstructions were made using microview 2.1.2 (GE Healthcare, 2000–2006). winsurf 4.0 (Moody & Lozanoff, 1998) was used to construct other three‐dimensional images, following visual identification and manual tracing of the borders of relevant ear structures. The inner walls of the middle ear cavities and bony labyrinth were traced in this way. Ossicular volumes were subtracted from middle ear cavity volumes: for simplicity, the epitympanic recess was taken to contain half the volume of the malleus plus incus combined, while the rest of the ossicular volume was taken to lie within the tympanic cavity. Images were laterally inverted, where required, to facilitate comparisons.
Following CT‐scanning, the isolated bullae of these animals were then dissected further. The middle ear structures were exposed and examined under light microscopy.
The nomenclature relating to middle ear subcavities and ossicular types varies according to author: Mason (2015aa) discusses some of the synonyms. The term ‘tympanic cavity’ is used in this paper to mean that part of the middle ear cavity that includes the pars tensa of the tympanic membrane and the cochlear promontory. It fills much of the tympanic bulla, as seen from a ventral view; the Eustachian tube enters the rostromedial tympanic cavity. The epitympanic recess is defined as a dorsal diverticulum of the tympanic cavity within which are found the heads of the malleus and incus; the pars flaccida of the tympanic membrane forms part of its lateral wall. A dorsal mastoid cavity (DMC) is a postero‐dorsal diverticulum of the middle ear cavity, contained within the petrosal bone. When large, the DMC may result in a visible swelling on the skull, caudally and dorsally. A ventral mastoid cavity (VMC) is a postero‐ventral diverticulum of the middle ear cavity, contained within the petrosal bone. The division between two communicating subcavities is sometimes marked by no more than a slight constriction, but in other cases there may be a dividing septum, penetrated by a discrete foramen.
Anatomical results
This paper focuses on the morphological features of the middle ear that are most likely to have an effect on hearing: the cavities, the auditory ossicles, the middle ear muscles and arteries.
Middle ear cavity structure
The auditory bullae are conspicuous from ventral views of the skulls (Fig. 1). They are relatively largest in Macroscelides, and in both this sengi and in the gerbils the right and left bullae closely converge in the midline (see later). In all but Elephantulus, the mastoid region is significantly inflated by extensions of the middle ear cavity: this mastoid inflation extends upwards around the back of the skull and is visible from a dorsal view. The extent of this inflation is greatest in Macroscelides, in which the DMC forms large swellings on top of the skull.
Figure 1.
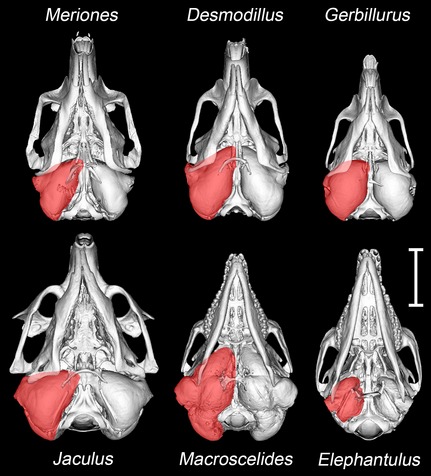
Microview reconstructions of the skulls of Meriones unguiculatus, Desmodillus auricularis, Gerbillurus setzeri, Jaculus orientalis, Macroscelides flavicaudatus and Elephantulus rupestris, seen to scale in the ventral view. In each case, the approximate extent of the right auditory bulla (including middle ear cavities and bony external meatus) is shaded in red. Scale bar: 10 mm.
In all the rodents, the tympanic cavity and epitympanic recess are each formed from tympanic and petrosal components (Fig. 2A). The tympanic bone forms the walls of these cavities ventrally, rostrally and laterally, supporting the tympanic membrane. The petrosal contribution is dorsal, medial and caudal: further inflation caudally results in the development of the mastoid cavities. No other bones were found to contribute to the middle ear cavity walls.
Figure 2.
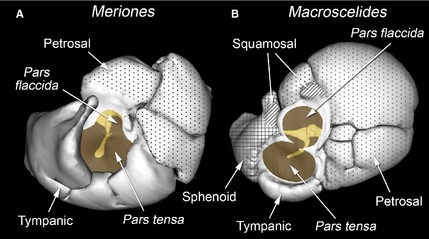
Winsurf reconstructions of the inner walls of the left middle ear cavities of (A) Meriones unguiculatus and (B) Macroscelides flavicaudatus, seen from approximately lateral views. The walls are represented as opaque and contributing bony elements are indicated. Unpatterned = tympanic bone (certainly including the ectotympanic: any possible entotympanic contribution could not be discerned); dotted = petrosal; cross‐hatched = sphenoid (probably basisphenoid and alisphenoid, fused); diagonal hatching = squamosal. In some places, these bony elements are overlapped externally by other bones, so the composition of the exterior of the auditory bulla differs somewhat. The openings into the middle ear cavity, covered by the pars tensa and pars flaccida of the tympanic membrane, are shaded brown. The malleus and incus, visible through these openings, are shaded yellow. Not to scale.
In the sengis, the tympanic cavity and epitympanic recess also have major tympanic and petrosal components, but other bones contribute too (Fig. 2B). A very small, dorsolateral diverticulum of the tympanic cavity forms within the squamosal, and there is a separate, small diverticulum of the epitympanic recess also walled by this bone. A much more capacious rostral extension of the tympanic cavity is formed from other bony elements, which appear to include fused basisphenoid and alisphenoid components, with a possible pterygoid contribution. In Macroscelides only, this portion of the bulla meets its contralateral counterpart in the midline. Right and left tympanic cavities converge here to the point that they are divided by a common bony septum (Fig. 3A). This septum, although very thin, is intact, so there is no intercommunication between right and left cavities. The right and left tympanic cavities closely converge in the midline in the gerbils too, but the bony walls of each bulla remain separated and the area of near‐contact is smaller (Fig. 3B). In both Macroscelides and Elephantulus, there was an osseous discontinuity in the ventromedial bullar wall where the tympanic, sphenoid and petrosal elements failed to unite: the Eustachian tube emerges from this region.
Figure 3.
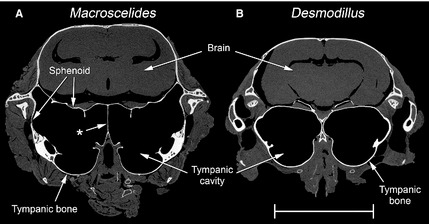
Micro‐CT transverse sections of the skinned heads of (A) Macroscelides flavicaudatus and (B) Desmodillus auricularis, at the level of the rostral tympanic cavities. In Macroscelides, the left and right cavities converge to the point where they share a common, midline septum (marked with an asterisk), probably formed from the basisphenoid bone. The bulla in this region has both tympanic and sphenoid components. In Desmodillus, the two bullae closely approximate each other in the midline but remain clearly separate; the bulla in this region is composed of the tympanic bone only. The other species studied had bullae less closely convergent than Desmodillus. Scale bar: 10 mm.
Middle ear subcavities
In Elephantulus, which has by far the smallest middle ear cavity (Table 1), the tympanic cavity and epitympanic recess have not expanded caudally beyond the facial nerve (Figs 4 and 5). The semicircular canals lie posterior to the middle ear cavity. In all other species, the middle ear cavity has expanded into the mastoid region of the skull, wrapping around the facial nerve and encroaching upon the semi‐circular canals (Figs 4 and 5). In Desmodillus and Meriones, diverticula of the cavities pass through the arcs of the canals and occupy the space between them, while in Jaculus the middle ear cavity does not pass through the arcs and the space between the canals is occupied by the parafloccular lobe of the cerebellum. The other species show intermediate conditions. In gerbils, the tympanic cavity has expanded laterally around the pars tensa of the tympanic membrane, into the walls of the bony external auditory meatus. While occupying under 10% of the total middle ear cavity volume in most species, the epitympanic recess is much more voluminous in Jaculus (Table 1).
Table 1.
Measurements of middle ear cavity and subcavity volumes, based on micro‐CT reconstructions
| Species | Body mass (g) | Total middle ear cavity volume (mm3) | Tympanic cavity volume (mm3) | Epitympanic recess volume (mm3) | DMC volume (mm3) | VMC volume (mm3) |
|---|---|---|---|---|---|---|
| Meriones unguiculatus (specimen 1) | 101 | 264 | 189 | 20 | 40 | 15 |
| Meriones unguiculatus (specimen 2) | 112 | 254 | 182 | 17 | 39 | 16 |
| Desmodillus auricularis | 40 | 383 | 251 | 25 | 107 | – |
| Gerbillurus setzeri | 29 | 283 | 232 | 10 | 41 | – |
| Jaculus orientalis | 85 | 543 | 250 | 155 | 138 | – |
| Macroscelides flavicaudatus | 34 | 748 | 275 | 61 | 300 | 112 |
| Elephantulus rupestris | 51 | 81 | 71 | 7 | – | 3 |
Volumetric measurements were obtained from one specimen of each species except for Meriones, where two bullae were scanned and measured.
DMC, dorsal mastoid cavity; VMC, ventral mastoid cavity.
Figure 4.
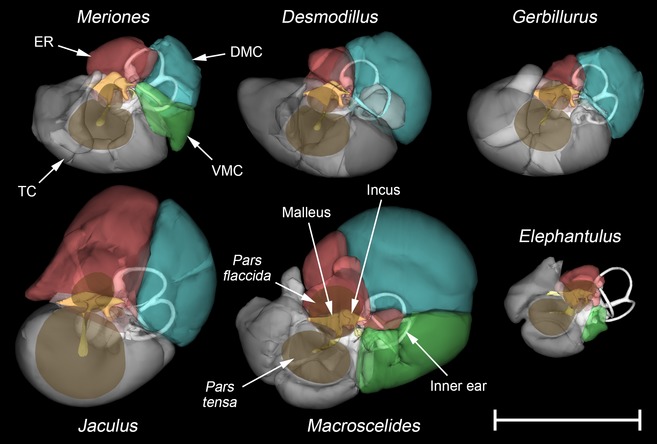
Winsurf reconstructions of the left middle ear cavities of Meriones unguiculatus, Desmodillus auricularis, Gerbillurus setzeri, Jaculus orientalis, Macroscelides flavicaudatus and Elephantulus rupestris, seen from approximately lateral views. The tympanic cavity (TC) is shaded in grey, the epitympanic recess (ER) in red, the dorsal mastoid cavity (DMC) in blue and the ventral mastoid cavity (VMC) in green. Only Meriones and Macroscelides have both mastoid cavities. Middle ear cavities are shown semitranslucent to reveal the inner ear (white) and middle ear ossicles (yellow). The tympanic membrane's pars tensa and pars flaccida are also shown, in translucent brown. Scale bar: 10 mm.
Figure 5.
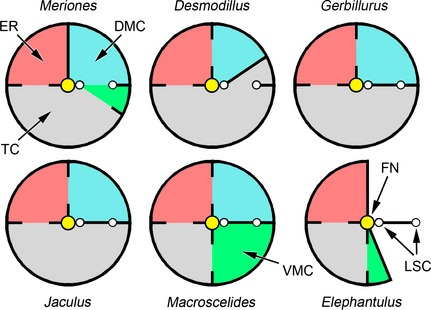
Diagrammatic representations of the left middle ear subcavities of Meriones unguiculatus, Desmodillus auricularis, Gerbillurus setzeri, Jaculus orientalis, Macroscelides flavicaudatus and Elephantulus rupestris, seen from approximately lateral views. The central, yellow circle represents the facial nerve (FN) and posterior to this are two sections through the lateral semicircular canal (LSC). The tympanic cavity (TC) is shaded in grey, the epitympanic recess (ER) in red, the dorsal mastoid cavity (DMC) in blue and the ventral mastoid cavity (VMC) in green. The septa or partial septa between the various subcavities are indicated as black lines. Cavities and subcavities are not drawn to scale. Compare these diagrams with the reconstructions of Fig. 4.
In some rodents (Desmodillus, Gerbillurus, Jaculus) and in Macroscelides, the DMC is a posterior diverticulum of the epitympanic recess. In the rodents, these two subcavities communicate via a discrete foramen within a dividing septum; the division between cavities is less distinct in the sengi. In Meriones, uniquely among the species considered, the DMC is divided from the epitympanic recess by a complete septum. It communicates instead with the region where the VMC and posterior tympanic cavity converge, extending from here through the arc of the lateral semicircular canal. A DMC is lacking in Elephantulus.
A VMC is found in Meriones and sengis only, as a posterior diverticulum of the tympanic cavity. The VMC is very small in Meriones and Elephantulus, but more capacious in Macroscelides (Table 1). There is no communication between VMC and DMC in Macroscelides.
Auditory ossicles
In the rodents, the anterior process of the malleus is a tapering lamina (Fig. 6). CT scans showed that the narrow tip of the process is synostosed to the bone of the tympanic cavity wall, but the bone here is so thin that the connection appeared to be quite flexible. The ossicles of Meriones, Desmodillus and Jaculus fall more‐or‐less into Fleischer's (1978) ‘freely mobile’ category, characterized by this flexible articulation, a relatively large head of the malleus, the absence of an orbicular apophysis and a manubrium roughly perpendicular to the anatomical axis of rotation (taken to extend from the anterior process of the malleus to the short process of the incus). A muscular process of the malleus was visible in all the rodents. In addition, in the gerbils only, a small, spinous process of the malleus projects caudally from the base of the manubrium. Nothing appeared to insert on this process, but the chorda tympani nerve passes over its base.
Figure 6.
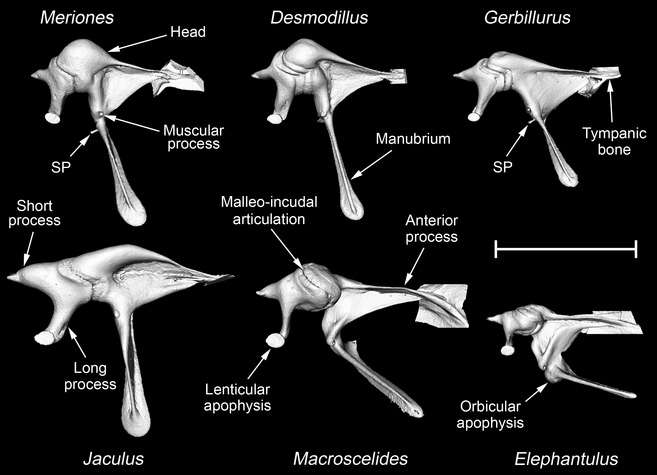
MicroView reconstructions of the left malleus and incus of Meriones unguiculatus, Desmodillus auricularis, Gerbillurus setzeri, Jaculus orientalis, Macroscelides flavicaudatus and Elephantulus rupestris, seen from within the middle ear cavity. In each case, the anterior process of the malleus is fused to the tympanic bone: a small part of the tympanic bone is shown where the fusion occurs. The head, manubrium, anterior process, muscular process and orbicular apophysis are all parts of the malleus, as is what is here termed the spinous process (SP). The short process, long process and lenticular apophysis are parts of the incus. An orbicular apophysis is only found in Elephantulus; a spinous process is found in Meriones and Gerbillurus and, less prominently, in Desmodillus. The other labelled structures are common to all six species. Scale bar: 3 mm.
The jerboas are characterized by an unusually wide manubrial blade, as seen from rostrally or caudally. The ossicles of Jaculus jaculus are very similar to those of J. orientalis, except that the pedicle connecting the lenticular apophysis to the long process of the incus is relatively longer in the former species (Fig. 7).
Figure 7.
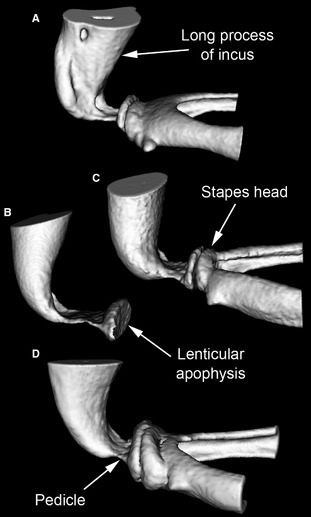
MicroView reconstructions of the region of articulation between left incus and stapes in (A) Gerbillurus setzeri, (B) Jaculus jaculus, (C) Jaculus orientalis and (D) Macroscelides flavicaudatus, seen from a caudal and slightly dorsal position. Only the distal long process of the incus and (in all but B) the head and crura of the stapes are shown. The lenticular apophysis of the incus is attached to its long process by means of a narrow, bony pedicle, which is particularly long in Jaculus jaculus. The head of the stapes articulates with the lenticular apophysis. Not to scale.
The malleus of Elephantulus (Fig. 6) falls into Fleischer's (1978) ‘microtype’ morphological category. It has a less tapering anterior process, which is more extensively fused to the skull than in the ‘freely mobile’ species, and the manubrium forms a more acute angle with the anatomical axis. The bony swelling near the base of the manubrium represents an orbicular apophysis. The mallei of Gerbillurus and especially Macroscelides are intermediate between freely mobile and microtype morphologies in terms of the angle of the manubrium and the relatively small head (Fig. 6), but these ossicles lack an orbicular apophysis and the region of synostosis between the anterior process and the tympanic bone is in both cases very narrow.
The stapedes of all species studied were similar in structure, featuring relatively long, internally excavated crura and oval footplates. CT reconstructions suggest that the stapes footplate in Gerbillurus fits less snugly into the oval window than in the other species (Fig. 8). The wider gap that was observed between footplate and oval window rim, which was close to being symmetrical all the way around, was presumably occupied by a broader annular ligament. The width of the ligament in Gerbillurus, estimated from gap width, varied between 27 and 35 μm around the perimeter of the footplate, whereas in Desmodillus, Meriones, J. orientalis and Macroscelides it was between about 9 and 17 μm. These measurements should be considered very rough estimates owing to the limited resolution of the CT scans. The resolution was too poor to make an estimate from the Elephantulus scan, while the J. jaculus scan did not include this region of the ear.
Figure 8.
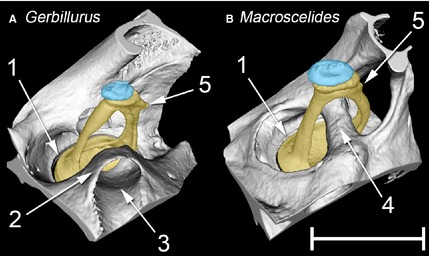
MicroView reconstructions of the left stapes and associated structures of (A) Gerbillurus setzeri and (B) Macroscelides flavicaudatus, seen from a rostral, ventral and lateral position. The stapes is in each case shaded in yellow, the lenticular apophysis (part of the incus) in blue. Key: 1 = rim of oval window, containing the stapes footplate; 2 = bony collar surrounding course of stapedial artery; 3 = canal for stapedial artery; 4 = bony tube for stapedial artery; 5 = muscular process for the insertion of the m. stapedius on the stapes. Note that the enclosure of the stapedial artery within a bony tube is nearly complete in Macroscelides, but far less so in Gerbillurus. In Macroscelides, the stapes footplate fits the oval window more snugly than in Gerbillurus. Scale bar: 1 mm.
Other structures of the middle ear
A stapedius muscle was found in all species, inserting on the muscular process of the stapes (Fig. 8) by means of a thin tendon. The tensor tympani muscle was present in all gerbils and sengis. A very small tensor tympani, inserting on the malleus by means of an extremely delicate, thread‐like tendon, was identified in both ears of one specimen of J. orientalis; although a muscular process was found on the malleus of the other specimen and on that of J. jaculus, no trace of the muscle could be found in these two individuals.
In all the rodents, the stapedial artery enters the middle ear from ventrally, crosses the promontory, passes through the intercrural foramen of the stapes and then enters a tube on the other side. From here it leaves the middle ear, without having branched. The artery is enclosed within a bony tube for much of its passage through the middle ear, but the tube becomes an open canal as it crosses the promontory and is missing where it passes through the stapes; there is a bony collar between these two open segments in the gerbils (Fig. 8A). In the sengis the pattern is different: the internal carotid artery enters the middle ear cavity and divides into promontorial and stapedial branches near the oval window. After the stapedial branch passes through the stapes it bends rostrally, running parallel to the promontorial branch before dividing into two at the roof of the middle ear cavity. All three branches ultimately enter the cranial cavity. In Macroscelides, the arteries of the middle ear are almost completely surrounded within bony tubes, although a small part of the tube passing through the stapes remained unossified in the specimen examined (not visible in Fig. 8B). In Elephantulus, however, the internal carotid and promontorial arteries are not enclosed within bony tubes, or even in canals: only the stapedial artery enters a bony tube, after it has passed through the stapes.
Discussion
The present comparison of the ear morphology of gerbils, jerboas and sengis has shown that middle ear cavity expansion has occurred in several different ways. Other features of the middle ear, including ossicular structure, arterial pattern and the nature of the middle ear muscles, also differ between the groups. Clearly, the small number of specimens of each species that could be obtained for destructive sampling represents a limitation of this study. No significant differences were found between the ears of the four M. unguiculatus specimens examined here. Although it should be borne in mind that these animals were raised together and are likely to have been related, wider experience suggests that variations between middle ears of individuals of similar age tend to be slight (Decraemer et al. 2014; pers.obs.). Degenerate middle ear muscles represent a possible exception to this, considered later. The results of other anatomical studies of these animals (listed in the Introduction) are largely consistent with the current findings, so we can be confident that the substantial interspecies differences highlighted here are real.
Comparative anatomy of the middle ear
The middle ear cavity volume in an adult rat (Rattus norvegicus), a much larger animal than any of the species studied here, is reported to be 61 mm3 (Zimmer et al. 1994). Like rats, ancestral members of both the Muridae (the rodent family including gerbils) and Dipodidae (the family including jerboas) almost certainly had small middle ear cavities and ‘microtype’ ossicles (Mason, 2015aa). The fact that all the rodents examined in the present study had cavity volumes at least four times larger than those of Rattus (Table 1) suggests that significant bullar hypertrophy has convergently evolved among both the gerbils and jerboas.
During prenatal development, the tympanic cavity forms as a diverticulum of the nasopharynx, to which it remains connected via the Eustachian tube. While its epithelial lining is derived from endodermal cells, the dorsal part of the middle ear cavity forms by cavitation of neural‐crest‐derived mesenchyme, at least in mice (Thompson & Tucker, 2013). Comparing the diagrams of Thompson & Tucker with the bullar structure of the rodents examined here, the endoderm‐derived cells would appear to line the ectotympanic component of the mouse middle ear cavity walls, whereas the neural crest‐derived cells line the petrosal component. Unlike mice, the desert rodents described here have voluminous mastoid subcavities (Figs 2, 4 and 5). The embryological origin of these subcavities remains unknown, but given that they are housed within the petrosal it would be interesting to establish whether their lining epithelium is neural crest‐derived.
As has been remarked on previously, differences in subcavity structure exist even among gerbils (Oaks, 1967; Lay, 1972; Pavlinov, 1988). A recent rodent phylogeny produced by Fabre et al. (2012) places Desmodillus and Gerbillurus within one major division of the Gerbillinae, and Meriones in the other. The communication of the DMC with the epitympanic recess (Desmodillus and Gerbillurus), or with the VMC and tympanic cavity (Meriones), suggests that the DMC evolved separately in the two gerbil lineages. Lay (1972) found a very small communication between DMC and posterior tympanic cavity in one specimen of Desmodillus, but this was not present in the present study or mentioned by Oaks (1967).
The cavity volume of Desmodillus was substantially larger than that of either Gerbillurus or Meriones; its tympanic membrane was also the largest of the three gerbils (see Table 1 and Mason 2015b). Ear cavity structure in Desmodillus is more like that of Gerbillurus, its nearer relative, but its ear ossicles more closely resemble those of Meriones in size and shape (Fig. 6).
Although the very tips of the anterior processes of the mallei remain fused to the tympanic bones, the rodents considered here have essentially ‘freely mobile’ malleus morphologies, another example of convergent evolution within the Muridae and Dipodidae. It is interesting to speculate that the tiny, spinous process extending caudally from the base of the manubrium in the gerbils might represent a vestigial remnant of the microtype orbicular apophysis.
The m. tensor tympani was found here in just one J. orientalis specimen but not the other; it was absent in J. jaculus, although a muscular process was present on the malleus. Oaks (1967) described a small muscle in J. orientalis, and found a vestigial muscle belly but no inserting tendon in J. jaculus. Perhaps the muscle degenerates postnatally in these jerboas, to the point where it is lacking in some individuals. Similarly, Begall & Burda (2006) found that the m. stapedius was present, but very weakly developed, in only some specimens of the subterranean rodent Spalacopus cyanus. Other curious anatomical features of the rodents studied here include the long pedicle supporting the lenticular apophysis of the incus in Jaculus and the wide annular ligament of the stapes in Gerbillurus, discussed later.
The sengis, belonging to the order Macroscelidea within the Afrotheria clade, are only very distantly related to rodents. Accordingly, they have a very different bullar structure and middle ear arterial pattern. It is well known that their auditory bullae are made from an unusually large number of bony elements, although there has been some disagreement about the relative contributions (van Kampen, 1905; Van der Klaauw, 1931; Saban, 1956; Novacek, 1977; MacPhee, 1981). As well as the usual tympanic and petrosal components, which together make up the entirety of the bullae in the rodents, the sengi middle ear cavity walls include two small contributions from the squamosal and a substantial rostral component apparently composed of fused basisphenoid/alisphenoid elements (Fig. 2B). An entotympanic component could not be distinguished in the bullae of any of the mammals examined here, but might be visible in younger specimens. Interestingly, the bony composition of the middle ear cavity walls proved to be very similar in Macroscelides and Elephantulus despite the bullae being nearly 10 times larger in the former. Much of this enlargement is due to a considerable expansion of the petrosal component. The right and left tympanic cavities of Macroscelides are separated in the midline only by a shared, thin bony lamina (Fig. 3A): this is probably formed from the basisphenoid, given that this is the only unpaired, midline bony element contributing to the bulla.
Macroscelides flavicaudatus has relatively enormous middle ear cavities: right and left cavity volumes considered together represent 130% of brain cavity volume. Together with the kangaroo‐mouse Microdipodops, which also has outsized bullae (Pye, 1965; Webster & Webster, 1975), it must be a contender for the largest middle ear cavities relative to head size of any mammal. The malleus of Macroscelides shows ‘freely mobile’ characteristics in its reduced connection to the tympanic and the absence of an orbicular apophysis – although the acute angle between manubrium and rotatory axis looks like a retained microtype feature. Contrary to the statement by Benoit et al. (2013), the ossicles of neither Macroscelides nor Elephantulus are obviously inflated. The enclosure of middle ear arteries within bony tubes was more complete in Macroscelides than in any other species studied here.
The large size of the bullae of Elephantulus species has been commented on in the literature (Evans, 1942; Benoit et al. 2013). Although they are indeed relatively large in comparison with those of a rat, E. rupestris actually has by far the smallest middle ear cavities of the desert species considered here (Table 1). Elephantulus has microtype ossicles and middle ear arteries that are mostly free of bony tubes. The microtype morphology is similar in many respects to what is regarded as the primitive morphology for therian mammals; it is found in many small mammals known to be high‐frequency specialists, such as mice, shrews and bats, and among afrotherians it is found in the tenrecs (Fleischer, 1978; Mason, 2013). It is very probable that Elephantulus retains something approaching the primitive middle ear morphology for sengis. Further comparisons with appropriate afrotherian outgroups are required to assess whether its middle ear structures show significant modification beyond the primitive condition for its group.
‘Low‐frequency’ middle ear specializations in desert mammals
Experiments where parts of the middle ear cavities of kangaroo‐rats were filled with plasticene showed no obvious effect on equilibrium or locomotion, providing evidence against some early ideas of what bullar hypertrophy might be for (Webster, 1962). Attention focused on the likely effects of cavity expansion on hearing.
Based on the model of a simple resonator, Legouix et al. (1954) and Legouix & Wisner (1955) concluded that low‐frequency hearing in gerbils should be augmented by their enlarged bullae: this has been confirmed in more recent and more detailed experimental studies (Ravicz et al. 1992; Ravicz & Rosowski, 1997). Consistent with expectation, sensitivity at low frequencies tends to be greater in species with larger bullae than in those with smaller bullae (Lay, 1972; Webster & Webster, 1980; Plassmann et al. 1987; Shaffer & Long, 2004). Partially filling the bullae of Meriones (Legouix & Wisner, 1955) and Dipodomys species (Webster, 1962; Webster & Webster, 1972) was found to have a negative impact on low‐frequency hearing in particular. In the companion paper (Mason, 2015b), it is calculated that the difference in middle ear cavity volumes in Elephantulus and Macroscelides, the species with the smallest and largest middle ear cavities, respectively, could result in a tympanic membrane velocity that is four times greater in Macroscelides, at low frequencies. Hearing has not been directly tested in sengis (or in jerboas) to the knowledge of the author, but this prediction suggests that Macroscelides should have considerably more acute low‐frequency hearing than Elephantulus.
Across mammals in general, there appears to be a correlation between ossicular morphology and the frequencies that an animal can hear: species with ‘microtype’ ossicles, which feature a very stiff connection between malleus and tympanic bone, tend to be high‐frequency specialists, while species with good low‐frequency hearing tend to have ‘freely mobile’ ossicles (Fleischer, 1978; Heffner et al. 2001; Mason, 2013). As discussed in the companion paper, enlarged middle ear cavities and low ossicular stiffness are both required in order to transmit low‐frequency sound effectively, which explains why these two characteristics have evolved in parallel in gerbils, jerboas and sengis. The fact that middle ear cavity stiffness still represents about 75% of the total impedance at low frequencies in Meriones (Ravicz et al. 1992) is initially surprising, given that the cavities are so enlarged in gerbils. Presumably, it is easier to loosen ossicular connections than it is to expand the middle ear cavities, owing to the constraint of head size in these small mammals.
The widths of the annular ligaments of the stapes footplate estimated here for Desmodillus, Meriones, Jaculus and Macroscelides are very close to the 8–18 μm cited for Meriones by Buytaert et al. (2011), whereas the ligament in Gerbillurus was found to be about twice this width. All else being equal, a wider ligament would be expected to reduce the overall stiffness of the ossicular chain, and this could therefore represent another adaptation to improve low‐frequency sound transmission. It might also allow the stapes to vibrate in different modes. Why Gerbillurus should differ in this respect from the other species studied, however, remains mysterious.
The enclosure of middle ear arteries within bony tubes is believed to reduce low‐frequency noise, which would otherwise interfere with hearing (Fleischer, 1978; Packer, 1987), so this is also seen as a ‘low‐frequency’ characteristic.
Middle ear features of less clear adaptive function
The loss of middle ear muscles, which is especially common in subterranean species, is discussed by Mason (2013). The proposed functional link between the loss of the tensor tympani and a flattened, compliant malleo‐incudal articulation would appear not to hold for jerboas, in which the saddle‐shaped joint between the two ossicles does not appear to be unusual.
The pedicle supporting the lenticular apophysis on the long process of the incus represents a point of flexibility within the ossicular chain of mammals: models suggest that more movement may be possible here than at the nearby synovial joint between lenticular apophysis and stapes, at least in cats (Funnell et al. 2005). A relatively long incudal pedicle, which would be expected to confer increased flexibility, was found here in Jaculus species (Fig. 7), and has previously been observed in the mole‐rat Spalax (Mason et al. 2010). Spalax communicates with neighbours by head‐thumping on its burrow walls (Heth et al. 1987; Rado et al. 1987). A long and flexible pedicle may help to protect the inner ear of this mole‐rat from the impacts made by head‐thumping by decoupling the stapes from vibrations of the malleus and incus. Jaculus species are saltatorial, and it is conceivable that their long pedicles might similarly confer protection, in this case from the impacts of jumping. Further discussion of the role of flexibility within the ossicular chain may be found in Mason & Farr (2013), and in the companion paper (Mason, 2015b). It is interesting to note that Spalax also resembles Jaculus in lacking a tensor tympani muscle, but shares with Gerbillurus an unusually wide annular ligament (Mason et al. 2010).
The tympanic membrane includes a significant pars flaccida in all species studied here. This structure is absent in some gerbils and jerboas with relatively unspecialized middle ears (Lay, 1972), suggesting that the pars flaccida may have expanded in rodent species with hypertrophied bullae. This is surprising, given that other small rodents known or suspected to emphasize low‐frequency sound transmission, including caviomorphs, members of the squirrel‐related rodent clade and subterranean species, lack this structure (Mason, 2015a). As discussed in the companion paper, the adaptive advantage of the pars flaccida to gerbils and other desert species remains very much uncertain.
Advantages of low‐frequency hearing to desert mammals
Although some of the anatomical features of the middle ear described here remain of unknown functional significance, the hypertrophied cavities, freely‐mobile ossicular structure, and partial or complete enclosure of middle ear arteries within bony tubes are all consistent with a hypothesis that, with the exception of Elephantulus, the middle ears of the species studied here are adapted towards the transmission of low‐frequency sound. Several possible adaptive explanations for evolution of low‐frequency hearing in desert mammals have been proposed over the years, of which three are discussed below.
Communication over long distances
In regions of low relative humidity, high‐frequency airborne sound attenuates faster than lower‐frequency sound (Kinsler et al. 1982; Huang et al. 2002). Given the low population densities supported by deserts, acoustic communication between individual animals might have to occur across relatively long distances (Petter, 1953, 1961), and low frequencies would be favoured for this. It has also been suggested that low‐frequency hearing might be of use to gerbils in a form of ‘acoustic homing’ over long distances (Petter, 1968).
Among four species of Algerian gerbils, those with lower population densities were found to have relatively larger bullae (Petter, 1953), but the evidence that this is specifically associated with low‐frequency communication calls or ‘acoustic homing’ appears to be very limited. Recorded calls of Dipodomys and Jaculus contain greatest energy at frequencies from 800 Hz to 3 kHz, while those of gerbils have greatest energy between 1.7 and 6 kHz (Eisenberg, 1975). Although the kangaroo‐rat Dipodomys spectabilis has been observed calling between neighbours, from its mounds (Gibbs, 1955), such behaviour has apparently not been noted in other species of desert rodents, which do not appear to be particularly vocal animals. Shorter‐distance vocalizations tend to be at higher frequencies: several gerbil species including M. unguiculatus and G. setzeri are known to make ultrasonic vocalizations during encounters between individuals (Holman, 1980; Dempster & Perrin, 1991; Dempster et al. 1991). Sengis also vocalize (Nowak, 1999), but less seems to be known about their calls.
Detection of seismic signals
Seismic signals of low frequencies tend to propagate well through sand: several desert animals are believed to make use of such vibrations in detecting prey (Brownell, 1977; Hetherington, 1992; Narins et al. 1997; Young & Morain, 2002). Ground vibrations may be detected directly, either through the somatosensory system or via bone conduction to the inner ear, but some of the transmitted energy will also radiate into the air. This component can, at least in principle, be detected by the auditory system as low‐frequency airborne sound.
Species that eat insects would presumably benefit from being able to detect prey vibrations, but at least some of the animals studied here make more obvious use of ground vibrations in their foot‐drumming or ‐thumping. While drumming in the presence of predators such as snakes may be aimed at the predators, small mammals often foot‐drum during reproductive interactions between conspecifics or, in the case of some kangaroo‐rats, in territorial defence (Randall, 2010). Dipodomys spectabilis foot‐drums on top of its mounds and appears to be able to hear the drumming of a neighbour from at least 16–27 m away (Randall, 1984). Most of the energy in the airborne part of the signal is between 200 Hz and 2 kHz (Randall, 1984). The foot‐drums made by a signalling animal can be transmitted between neighbouring burrow systems, whereupon they radiate out into the burrow chamber (Randall & Lewis, 1997). Foot‐drumming is also characteristic of gerbils, including Meriones and Gerbillurus species (Lay, 1974; Swanson, 1974; Daly & Daly, 1975; Bridelance & Paillette, 1985; Bridelance, 1986; Dempster & Perrin, 1989), although G. setzeri only appears to ‘shiver’ its hindquarters and does not produce an audible sound (Dempster & Perrin, 1989). Sengis are known to foot‐drum and this has been described in detail in Elephantulus species (Faurie et al. 1996); Elephantulus seems to engage in this activity much more readily than Macroscelides (G. Rathbun, pers. comm.). Foot‐drumming was not observed in jerboas by Petter (1961), but Eisenberg (1975) mentions that Jaculus thumps its hind feet when confronted by unfamiliar animals.
The ‘predator avoidance’ hypothesis
The attack approaches of owls (Otus asio) and sidewinder rattlesnakes (Crotalus cerastes) were found to generate acoustic signals at frequencies under 2 kHz (Webster, 1962). Kangaroo‐rats (Dipodomys) appear to use these sounds to trigger avoidance behaviours (Webster, 1962; Webster & Webster, 1971). The response of a kangaroo‐rat to a predator strike is to leap at the last minute, in which case accurate localization of the predator itself may not be necessary (Webster & Webster, 1972). Among heteromyids in a cage experiment, larger bullar volumes were found to correlate with a decreased chance of a successful owl strike (Longland & Price, 1991), and the tendency of these rodents to forage in the open has been found to correlate positively with bulla volume (Kotler, 1984). There is indirect evidence that success of adult gerbils (Meriones) in avoiding owl strikes may also relate to their hearing (Lay, 1974). The results of these studies are consistent with the hypothesis that low‐frequency hearing, facilitated by larger bullae, might improve the chances of detecting predators, allowing these desert mammals to undertake more risky foraging strategies. It should be borne in mind, however, that locomotion method (e.g. bipedality or quadrupedality) is also likely to affect the success of a predator strike (Longland & Price, 1991).
Although this ‘predator avoidance’ hypothesis has been widely accepted, not all data are consistent. Although Dipodomys merriami proved to be very good at avoiding rattlesnake attacks in an experimental setting, the kangaroo‐mouse Microdipodops megacephalus, which also has large bullae, was surprisingly vulnerable to such attacks (Pierce et al. 1992). Pierce et al. suggested that this may be because Microdipodops has less exposure to these snakes in the wild. The strongest challenge to the ‘predator avoidance’ hypothesis, however, has come from Hafner (1993). Some of his key arguments include the following: (i) the audiograms of kangaroo‐rats as published by Webster & Webster (1980) show a broad range of high sensitivity, rather than a peak at frequencies coinciding with predator strike noises; (ii) kangaroo‐rats with experimentally reduced middle ear volumes were still effective at avoiding rattlesnake strikes, as long as they remained sighted (Webster & Webster, 1971); (iii) ‘natural selection’ experiments looking at the survival chances of animals with reduced middle ear volumes that were released back into the wild (Webster & Webster, 1971) did not yield statistically compelling results; and (iv) there is no documented relationship between habitat and bullar volume within the Heteromyidae. Counterarguments to these points include the following: (i) hearing is likely used for many different purposes in these animals, and there is no reason to expect it to be tuned very specifically to the sounds made by predators; (ii) the fact that experimentally blinded kangaroo‐rats were able to avoid snakes in the Webster & Webster study, as long as their ears were intact, suggests that the auditory system can be used for predator detection in the absence of sight; (iii) the results from the Websters’ natural selection experiment are at least suggestive, even if not statistically significant; (iv) looking over a broader range of rodents, enlarged bullae do seem to be associated with arid environments (Mason, 2015a). Although in the opinion of the present author none of Hafner's challenges are fatal to the ‘predator avoidance’ hypothesis, they do raise important questions about the quality of the evidence available to support it, as well as the broader point about the tendency of many to accept adaptationist hypotheses even in the absence of rigorous proof.
The smaller ears of Elephantulus
Although most of the species studied here show middle ear hypertrophy and cognate adaptations associated with augmenting low‐frequency hearing, E. rupestris does not. This animal has a much smaller middle ear cavity than any of the others (Table 1), a microtype malleus that appears to be stiffly articulated with the skull and the least development of bony tubes for its middle ear arteries. These characteristics, which are likely to be primitive for sengis, suggest that the low‐frequency hearing of Elephantulus must be considerably inferior to that of Macroscelides or the gerbils.
The fact that Elephantulus species appear to foot‐drum more than Macroscelides species shows that this behaviour alone is not sufficient to drive middle ear hypertrophy in desert mammals. However, there are at least three things distinguishing Elephantulus from the other species studied here, any or all of which might be relevant to its hearing.
Although the Elephantulus specimen described here was captured in the same, small region of Namibia as Macroscelides, Desmodillus and Gerbillurus, its preferred microhabitat differs. Elephantulus rupestris tends to live among rocks, scree and boulders while M. flavicaudatus lives on gravel plains (Rathbun, 2009); D. auricularis prefers ‘calcareous ground, fine soils or consolidated sand (sometimes covered in pebbles) with a sparse cover of grass or low shrub’, while G. setzeri inhabits ‘hot, dry gravel plains with shallow, semi‐compacted soil lacking vegetation’ (Happold, 2013). Elephantulus, then, prefers rockier ground than the other species.
The desert rodents considered here are largely vegetarian, although gerbils will also take insects (Nowak, 1999). Sengis are usually considered to be insectivorous but M. proboscideus seems to eat more vegetable matter than members of other genera (Kerley, 1995). If this is also true of its close relative M. flavicaudatus in Namibia, Elephantulus would be the species in this study with the highest proportion of insects in its diet.
Elephantulus species are sometimes known as ‘long‐eared elephant‐shrews’ and Macroscelides species as ‘short‐eared elephant‐shrews’ (Nowak, 1999), reflecting differences in pinna size, although the difference is not very pronounced. It has been noted that larger pinnae in other desert mammals are often associated with smaller bullae (Howell, 1932; Heim de Balsac, 1936).
Pavlinov & Rogovin (2000) looked for correlations between microhabitat, diet, pinna size and other traits, in rodents. They proposed that the relative sizes of pinnae and middle ear structures might relate to mechanism of escape from predators, which in turn might relate to foraging strategy. Both are likely to be influenced by microhabitat. Further study of sengis, both in terms of their natural history and the examination of the middle ears of more species, is clearly required to test the hypothesis that microhabitat ultimately underlies the dramatic differences in ear structure found in these animals.
Concluding remarks
All available experimental evidence is consistent with the notion that enlarged bullae in small desert mammals augment low‐frequency hearing; reasons why this should be so are well understood theoretically (see companion paper). The present study has highlighted the fact that bullar enlargement has occurred convergently and in different anatomical patterns among different species. Other features of hypertrophied middle ears include a ‘freely mobile’ ossicular structure and the enclosure of middle ear arteries within bony tubes, and these also appear to be associated with improved low‐frequency hearing. Although several plausible hypotheses relating to the adaptive advantages of low‐frequency sensitivity in these animals have been proposed, the evidence supporting each of them remains limited. Not all species living in arid regions have hypertrophied middle ears, and the main selective pressure driving this adaptation remains elusive.
Acknowledgements
The California Academy of Sciences was instrumental to this study: the author extends his heartfelt thanks to Galen Rathbun for collecting the specimens and for the discussions, insights and help, while Jack Dumbacher and Maureen Flannery made possible the loan of the Namibian specimens. Thanks are also due to Simon's Rodents of Abbotsley, Cambridgeshire, Frank Jiggins and Maggie Dinsdale for the provision of the other specimens. Andrei Serov kindly provided Russian translations. Most of the CT scans were made courtesy of the Cambridge Biotomography Centre. The author also thanks Alan Heaver of the Department of Engineering for making the other CT scans, and Prof. Norman Fleck for the use of his scanner. Finally, the author wishes to thank Abigail Tucker and the Anatomical Society for the invitation to present the lecture upon which this work is based, and the two reviewers of this paper for their helpful comments and suggestions.
References
- Begall S, Burda H (2006) Acoustic communication and burrow acoustics are reflected in the ear morphology of the coruro (Spalacopus cyanus, Octodontidae), a social fossorial rodent. J Morphol 267, 382–390. [DOI] [PubMed] [Google Scholar]
- Benoit J, Orliac M, Tabuce R (2013) The petrosal of the earliest elephant‐shrew Chambius (Macroscelidea: Afrotheria) from the Eocene of Djebel Chambi (Tunisia) and the evolution of middle and inner ear of elephant‐shrews. J Syst Paleontol 11, 907–923. [Google Scholar]
- Benoit J, Crumpton N, Merigeaud S, et al. (2014) Petrosal and bony labyrinth morphology supports paraphyly of Elephantulus within Macroscelididae (Mammalia, Afrotheria). J Mammal Evol 21, 173–193. [Google Scholar]
- Bridelance P (1986) Les podophones de Psammomys obesus en milieu naturel: comparaison avec les podophones de Meriones (Gerbillidae, Rodentia). Mammalia 50, 145–152. [Google Scholar]
- Bridelance P, Paillette M (1985) Un système original de communication sonore chez des rongeurs désertiques: la podophonie chez quatre espèces de Meriones (Rongeurs, Gerbillidés). Mammalia 49, 161–172. [Google Scholar]
- Brownell PH (1977) Compressional and surface waves in sand: used by desert scorpions to locate prey. Science 197, 479–482. [DOI] [PubMed] [Google Scholar]
- Buytaert JA, Salih WH, Dierick M, et al. (2011) Realistic 3D computer model of the gerbil middle ear, featuring accurate morphology of bone and soft tissue structures. J Assoc Res Otolaryngol 12, 681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M, Daly S (1975) Behavior of Psammomys obesus (Rodentia: Gerbillinae) in the Algerian Sahara. Zeitschrift für Tierpsychologie 37, 298–321. [Google Scholar]
- Decraemer WF, de La Rochefoucauld O, Funnell WRJ, et al. (2014) Three‐dimensional vibration of the malleus and incus in the living gerbil. J Assoc Res Otolaryngol 15, 483–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster ER, Perrin MR (1989) A comparative study of agonistic behaviour in hairy‐footed gerbils (genus Gerbillurus). Ethology 83, 43–59. [Google Scholar]
- Dempster ER, Perrin MR (1991) Ultrasonic vocalizations of six taxa of Southern African gerbils (Rodentia: Gerbillinae). Ethology 88, 1–10. [Google Scholar]
- Dempster ER, Dempster R, Perrin MR (1991) Behaviour associated with ultrasonic vocalizations in six gerbilline rodent species. Ethology 88, 11–19. [Google Scholar]
- Doran AHG (1878) Morphology of the mammalian ossicula auditûs . Trans Linn Soc Lond, 2nd series, 1, 371–497. [Google Scholar]
- Dumbacher JP, Rathbun GB, Smit HA, et al. (2012) Phylogeny and taxonomy of the round‐eared sengis or elephant‐shrews, genus Macroscelides (Mammalia, Afrotheria, Macroscelidea). PLoS One 7, e32410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg JF (1975) The behavior patterns of desert rodents In: Rodents in Desert Environments. (eds Prakash I, Ghosh PK.), pp. 189–224. The Hague: Dr. W. Junk bv Publishers. [Google Scholar]
- Evans FG (1942) The osteology and relationships of the elephant shrews (Macroscelididae). Bull Am Mus Nat Hist 80, 85–125. [Google Scholar]
- Fabre P‐H, Hautier L, Dimitrov D, et al. (2012) A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol Biol 12, 88. doi:10.1186/1471‐2148‐12‐88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurie AS, Dempster ER, Perrin MR (1996) Footdrumming patterns of southern African elephant‐shrews. Mammalia 60, 567–576. [Google Scholar]
- Finck A, Sofouglu M (1966) Auditory sensitivity of the Mongolian gerbil (Meriones unguiculatus). J Aud Res 6, 313–319. [Google Scholar]
- Fleischer G (1978) Evolutionary principles of the mammalian middle ear. Adv Anat Embryol Cell Biol 55, 1–70. [DOI] [PubMed] [Google Scholar]
- Funnell WR, Heng Siah T, McKee MD, et al. (2005) On the coupling between the incus and the stapes in the cat. J Assoc Res Otolaryngol 6, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RH (1955) Vocal sound produced by the kangaroo rat, Dipodomys spectabilis . J Mammal 36, 463. [Google Scholar]
- Gulotta EF (1971) Meriones unguiculatus . Mammal Species 3, 1–5. [Google Scholar]
- Hafner JC (1993) Macroevolutionary diversification in heteromyid rodents: heterochrony and adaptation in phylogeny In Biology of the Heteromyidae. (eds Genoways HH, Brown JH.), pp. 291–318. Provo, Utah: American Society of Mammalogists. [Google Scholar]
- Happold D (2013) Mammals of Africa, Volume III: Rodents, Hares and Rabbits. London: Bloomsbury. [Google Scholar]
- Heffner RS, Heffner HE (1988) Sound localization and use of binaural cues by the gerbil (Meriones unguiculatus). Behav Neurosci 102, 422–428. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE (1990) Vestigial hearing in a fossorial mammal, the pocket gopher (Geomys bursarius). Hear Res 46, 239–252. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE (1992a) Evolution of sound localization in mammals In: The Evolutionary Biology of Hearing. (eds Webster DB, Fay RR, Popper AN.), pp. 691–715. New York: Springer. [Google Scholar]
- Heffner RS, Heffner HE (1992b) Hearing and sound localization in blind mole rats (Spalax ehrenbergi). Hear Res 62, 206–216. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE (1993) Degenerate hearing and sound localization in naked mole rats (Heterocephalus glaber), with an overview of central auditory structures. J Comp Neurol 331, 418–433. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Masterton B (1980) Hearing in Glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am 68, 1584–1599. [Google Scholar]
- Heffner RS, Koay G, Heffner HE (2001) Audiograms of five species of rodents: implications for the evolution of hearing and the perception of pitch. Hear Res 157, 138–152. [DOI] [PubMed] [Google Scholar]
- Heim de Balsac H (1936) Biogéographie des mammifères et des oiseaux de l'Afrique du Nord. Suppléments au Bulletin Biologique de France et de Belgique 21, 1–446. [Google Scholar]
- Heth G, Frankenberg E, Raz A, et al. (1987) Vibrational communication in subterranean mole rats (Spalax ehrenbergi). Behav Ecol Sociobiol 21, 31–33. [Google Scholar]
- Hetherington TE (1992) Behavioural use of seismic cues by the sandswimming lizard Scincus scincus . Ethol Ecol Evol 4, 5–14. [Google Scholar]
- Holman SD (1980) Sexually dimorphic, ultrasonic vocalizations of Mongolian gerbils. Behav Neural Biol 28, 183–192. [Google Scholar]
- Howell AB (1932) The saltatorial rodent Dipodomys: the functional and comparative anatomy of its muscular and osseous systems. Proc Am Acad Arts Sci 67, 377–536. [Google Scholar]
- Huang GT, Rosowski JJ, Ravicz ME, et al. (2002) Mammalian ear specializations in arid habitats: structural and functional evidence from sand cat (Felis margarita). J Comp Physiol A 188, 663–681. [DOI] [PubMed] [Google Scholar]
- van Kampen PN (1905) Die Tympanalgegend des Säugetierschädels. Gegenbaurs Morphologisches Jahrbuch 34, 321–722. [Google Scholar]
- Kerley GIH (1995) The round‐eared elephant‐shrew Macroscelides proboscideus (Macroscelidea) as an omnivore. Mammal Rev 25, 39–44. [Google Scholar]
- Kinsler LE, Frey AR, Coppens AB, et al. (1982) Fundamentals of Acoustics. New York: John Wiley. [Google Scholar]
- Kotler BP (1984) Risk of predation and the structure of desert rodent communities. Ecology 65, 689–701. [Google Scholar]
- Lataste F (1882) Mammifères nouveaux d'Algérie. Le Naturaliste 14, 107–109. [Google Scholar]
- Lay DM (1972) The anatomy, physiology, functional significance and evolution of specialised hearing organs of gerbilline rodents. J Morphol 138, 41–120. [DOI] [PubMed] [Google Scholar]
- Lay DM (1974) Differential predation on gerbils (Meriones) by the little owl, Athene brahma . J Mammal 55, 608–614. [Google Scholar]
- Legouix JP, Wisner A (1955) Rôle fonctionnel des bulles tympaniques géantes de certains rongeurs (Meriones). Acustica 5, 208–216. [Google Scholar]
- Legouix J‐P, Petter F, Wisner A (1954) Étude de l'audition chez des mammifères à bulles tympaniques hypertrophiées. Mammalia 18, 262–271. [Google Scholar]
- Longland WS, Price MV (1991) Direct observations of owls and heteromyid rodents: can predation risk explain microhabitat use? Ecology 72, 2261–2273. [Google Scholar]
- MacPhee RDE (1981) Auditory Regions of Primates and Eutherian Insectivores. Basel: S. Karger. [Google Scholar]
- Mason MJ (2013) Of mice, moles and guinea‐pigs: functional morphology of the middle ear in living mammals. Hear Res 301, 4–18. [DOI] [PubMed] [Google Scholar]
- Mason MJ (2015a) Functional morphology of rodent middle ears In: Evolution of the Rodents: Advances in Phylogeny, Functional Morphology and Development. (eds Cox PG, Hautier L.). Cambridge: Cambridge University Press; 373–404. [Google Scholar]
- Mason MJ (2015b) Structure and function of the mammalian middle ear. II: Inferring function from structure. J Anat 228, 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Farr MRB (2013) Flexibility within the middle ears of vertebrates. J Laryngol Otol 127, 2–14. [DOI] [PubMed] [Google Scholar]
- Mason MJ, Lai FWS, Li J‐G, et al. (2010) Middle ear structure and bone conduction in Spalax, Eospalax and Tachyoryctes mole‐rats (Rodentia: Spalacidae). J Morphol 271, 462–472. [DOI] [PubMed] [Google Scholar]
- Moody D, Lozanoff S (1998) SURFdriver: a practical computer program for generating three‐dimensional models of anatomical structures using a PowerMac. Clin Anat 11, 132. [Google Scholar]
- Moushegian G, Rupert A (1970) Response diversity of neurons in ventral cochlear nucleus of kangaroo rat to low‐frequency tones. J Neurophysiol 33, 351–364. [DOI] [PubMed] [Google Scholar]
- Narins PM, Lewis ER, Jarvis JUM, et al. (1997) The use of seismic signals by fossorial southern African mammals: a neuroethological gold mine. Brain Res Bull 44, 641–646. [DOI] [PubMed] [Google Scholar]
- Norris ML (1987) Gerbils In: The UFAW Handbook on the Care and Management of Laboratory Animals. (ed. Poole TB.), pp. 360–376. Harlow: Longman Scientific & Technical. [Google Scholar]
- Novacek MJ (1977) Aspects of the problem of variation, origin and evolution of the eutherian auditory bulla. Mammal Rev 7, 131–149. [Google Scholar]
- Nowak RM (1999) Walker's Mammals of the World. Baltimore: The Johns Hopkins University Press. [Google Scholar]
- Oaks E.C.J. (1967) Structure and function of inflated middle ears of rodents PhD thesis, Yale University. [Google Scholar]
- Ognev SI (1948) Zveri vostochnoy Yevropy i severnoy Azii. Tom 6. Gryzuny (prodolzheniye). Moscow: Izdatel'stvo Akademii Nauk SSSR. [Google Scholar]
- Packer DJ (1987) The influence of carotid arterial sounds on hearing sensitivity in mammals. J Zool 211, 547–560. [Google Scholar]
- Pavlinov IY (1988) Evolution of mastoid part of the bulla tympani in specialized arid rodents [in Russian]. Zoologicheskii Zhurnal 67, 739–750. [Google Scholar]
- Pavlinov IY, Rogovin KA (2000) Relation between size of pinna and of auditory bulla in specialized desert rodents [in Russian]. J Gen Biol 61, 87–101. [PubMed] [Google Scholar]
- Petter F (1953) Remarques sur la signification des bulles tympaniques chez les mammifères. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, Paris 237, 848–849. [Google Scholar]
- Petter F (1961) Répartition géographique et écologie des rongeurs désertiques (du Sahara occidental à l'Iran oriental). Mammalia 25, 1–219. [Google Scholar]
- Petter F (1968) Retour au gîte et nomadisme chez un rongeur à bulles tympaniques hypertrophiées. Mammalia 32, 537–549. [Google Scholar]
- Pierce BM, Longland WS, Jenkins SH (1992) Rattlesnake predation on desert rodents: microhabitat and species‐specific effects on risk. J Mammal 73, 859–865. [Google Scholar]
- Plassmann W, Peetz W, Schmidt M (1987) The cochlea in gerbilline rodents. Brain Behav Evol 30, 82–101. [DOI] [PubMed] [Google Scholar]
- Prakash I (1959) Hypertrophy of the bullae tympanicae in the desert mammals. Sci Cult 24, 580–582. [Google Scholar]
- Pye A (1965) The auditory apparatus of the Heteromyidae (Rodentia, Sciuromorpha). J Anat 99, 161–174. [PMC free article] [PubMed] [Google Scholar]
- Rado R, Levi N, Hauser H, et al. (1987) Seismic signalling as a means of communication in a subterranean mammal. Anim Behav 35, 1249–1251. [Google Scholar]
- Randall JA (1984) Territorial defense and advertisement by footdrumming in bannertail kangaroo rats (Dipodomys spectabilis) at high and low population densities. Behav Ecol Sociobiol 16, 11–20. [Google Scholar]
- Randall JA (2010) Drummers and stompers: vibrational communication in mammals In: The Use of Vibrations in Communication: Properties, Mechanisms and Function across Taxa. (ed. O'Connell‐Rodwell CE.), pp. 99–120. Kerala: Research Signpost. [Google Scholar]
- Randall JA, Lewis ER (1997) Seismic communication between burrows of kangaroo rats, Dipodomys spectabilis . J Comp Physiol A 181, 525–531. [DOI] [PubMed] [Google Scholar]
- Rathbun GB (2009) Why is there discordant diversity in sengi (Mammalia: Afrotheria: Macroscelidea) taxonomy and ecology? Afr J Ecol 47, 1–13. [Google Scholar]
- Rathbun GB, Osborne TO, Coals PGR (2015) Distribution of the Etendeka round‐eared sengi (Macroscelides micus), a Namibian endemic mammal. Journal (Namibia Scientific Society), 63, in press. [Google Scholar]
- Ravicz ME, Rosowski JJ (1997) Sound‐power collection by the auditory periphery of the Mongolian gerbil Meriones unguiculatus: III. Effect of variations in middle‐ear volume. J Acoust Soc Am 101, 2135–2147. [DOI] [PubMed] [Google Scholar]
- Ravicz ME, Rosowski JJ, Voigt HF (1992) Sound‐power collection by the auditory periphery of the Mongolian gerbil Meriones unguiculatus. I: middle‐ear input impedance. J Acoust Soc Am 92, 157–177. [DOI] [PubMed] [Google Scholar]
- Ryan A (1976) Hearing sensitivity of the mongolian gerbil, Meriones unguiculatis . J Acoust Soc Am 59, 1222–1226. [DOI] [PubMed] [Google Scholar]
- Saban R (1956. –1957) Les affinités du genre Tupaia Raffles 1821, d'après les caractères morphologiques de la tête osseuse. Annales de Paléontologie, 42, 43, 169–224, 1–44. [Google Scholar]
- Salih WHM, Buytaert JAN, Aerts JRM, et al. (2012) Open access high‐resolution 3D morphology models of cat, gerbil, rabbit, rat and human ossicular chains. Hearing Research, 284, 1–5. [DOI] [PubMed] [Google Scholar]
- Segall W (1970) Morphological parallelisms of bulla and auditory ossicles in some insectivores and marsupials. Fieldiana Zool 51, 169–205. [Google Scholar]
- Shaffer LA, Long GR (2004) Low‐frequency distortion product otoacoustic emissions in two species of kangaroo rats: implications for auditory sensitivity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190, 55–60. [DOI] [PubMed] [Google Scholar]
- Swanson HH (1974) Sex differences in behaviour of the Mongolian gerbil (Meriones unguiculatus) in encounters between pairs of same or opposite sex. Anim Behav 22, 638–644. [Google Scholar]
- Thompson H, Tucker AS (2013) Dual origin of the epithelium of the mammalian middle ear. Science 339, 1453–1456. [DOI] [PubMed] [Google Scholar]
- von Unge M, Buytaert JA, Dirckx JJ (2011) Anatomical boundary of the tympanic membrane pars flaccida of the Meriones unguiculatus (Mongolian gerbil). Anat Rec 294, 987–995. [DOI] [PubMed] [Google Scholar]
- Van der Klaauw CJ (1931) The auditory bulla in some fossil mammals, with a general introduction to this region of the skull. Bull Am Mus Nat Hist 62, 1–352. [Google Scholar]
- Vernon J, Herman P, Peterson E (1971) Cochlear potentials in the kangaroo rat, Dipodomys merriami . Physiol Zool 44, 112–118. [Google Scholar]
- Webster DB (1961) The ear apparatus of the kangaroo rat, Dipodomys . Am J Anat 108, 123–148. [DOI] [PubMed] [Google Scholar]
- Webster DB (1962) A function of the enlarged middle‐ear cavities of the kangaroo rat, Dipodomys . Physiol Zool 35, 248–255. [Google Scholar]
- Webster DB, Webster M (1971) Adaptive value of hearing and vision in kangaroo rat predator avoidance. Brain Behav Evol 4, 310–322. [DOI] [PubMed] [Google Scholar]
- Webster DB, Webster M (1972) Kangaroo rat auditory thresholds before and after middle ear reduction. Brain Behav Evol 5, 41–53. [DOI] [PubMed] [Google Scholar]
- Webster DB, Webster M (1975) Auditory systems of Heteromyidae: functional morphology and evolution of the middle ear. J Morphol 146, 343–376. [DOI] [PubMed] [Google Scholar]
- Webster DB, Webster M (1977) Auditory systems of Heteromyidae: cochlear diversity. J Morphol 152, 153–170. [DOI] [PubMed] [Google Scholar]
- Webster DB, Webster M (1980) Morphological adaptations of the ear in the rodent family Heteromyidae. Am Zool 20, 247–254. [Google Scholar]
- Young BA, Morain M (2002) The use of ground‐borne vibrations for prey localization in the Saharan sand vipers (Cerastes). J Exp Biol 205, 661–665. [DOI] [PubMed] [Google Scholar]
- Zavattari E (1938) Significato e funzione delle bulle timpaniche ipertrofiche dei mammiferi Sahariani. Riv Biol Coloniale 1, 249–259. [Google Scholar]
- Zimmer WM, Rosin DF, Saunders JC (1994) Middle‐ear development VI: structural maturation of the rat conducting apparatus. Anat Rec 239, 475–484. [DOI] [PubMed] [Google Scholar]


