Abstract
Background—
The prevalence of pre–diabetes mellitus and its consequences in patients with heart failure and reduced ejection fraction are not known. We investigated these in the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial.
Methods and Results—
We examined clinical outcomes in 8399 patients with heart failure and reduced ejection fraction according to history of diabetes mellitus and glycemic status (baseline hemoglobin A1c [HbA1c]: <6.0% [<42 mmol/mol], 6.0%–6.4% [42–47 mmol/mol; pre–diabetes mellitus], and ≥6.5% [≥48 mmol/mol; diabetes mellitus]), in Cox regression models adjusted for known predictors of poor outcome. Patients with a history of diabetes mellitus (n=2907 [35%]) had a higher risk of the primary composite outcome of heart failure hospitalization or cardiovascular mortality compared with those without a history of diabetes mellitus: adjusted hazard ratio, 1.38; 95% confidence interval, 1.25 to 1.52; P<0.001. HbA1c measurement showed that an additional 1106 (13% of total) patients had undiagnosed diabetes mellitus and 2103 (25%) had pre–diabetes mellitus. The hazard ratio for patients with undiagnosed diabetes mellitus (HbA1c, >6.5%) and known diabetes mellitus compared with those with HbA1c<6.0% was 1.39 (1.17–1.64); P<0.001 and 1.64 (1.43–1.87); P<0.001, respectively. Patients with pre–diabetes mellitus were also at higher risk (hazard ratio, 1.27 [1.10–1.47]; P<0.001) compared with those with HbA1c<6.0%. The benefit of LCZ696 (sacubitril/valsartan) compared with enalapril was consistent across the range of HbA1c in the trial.
Conclusions—
In patients with heart failure and reduced ejection fraction, dysglycemia is common and pre–diabetes mellitus is associated with a higher risk of adverse cardiovascular outcomes (compared with patients with no diabetes mellitus and HbA1c <6.0%). LCZ696 was beneficial compared with enalapril, irrespective of glycemic status.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01035255.
Keywords: clinical trial, diabetes mellitus, heart failure, prognosis, treatment outcome
Heart failure and type 2 diabetes mellitus are 2 of the great epidemics of modern times.1,2 Although each begets the other, the links between the 2 conditions are not fully elucidated.3 Although it is widely acknowledged that diabetes mellitus is a risk marker for the development of heart failure and greatly heightens the risk of worse outcomes once heart failure develops,4–6 the relationship between heart failure and the development of diabetes mellitus is less well understood. Although heart failure seems to be a state of insulin resistance, the mechanisms underlying this are not clear.7 Few studies have investigated the prevalence of pre–diabetic dysglycemia in patients with heart failure and even fewer its clinical consequences (and with conflicting findings).8,9 Identification of an association, if any, between pre–diabetes mellitus and adverse clinical outcomes is of clinical importance from 2 contrasting perspectives. There has been recent concern that hypoglycemic agents might contribute to the poor cardiovascular outcomes, including heart failure, in patients with diabetes mellitus.3 Demonstration that patients with pre–diabetes mellitus, untreated with hypoglycemic agents, have worse outcomes than normoglycemic patients would support the view that dysglycemia per se is harmful in heart failure. If so, treatment of such patients with hypoglycemic agents might prevent the development of diabetes mellitus and improve heart failure outcomes. We, therefore, investigated the prevalence of diabetes mellitus and pre–diabetes mellitus in patients with heart failure and reduced ejection fraction (HF-REF) who participated in the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial10 and examined the relationship between glycemic status and clinical outcomes in this trial. We also compared the effect of sacubitril/valsartan (LCZ696) with enalapril in patients in the PARADIGM-HF trial according to glycemic status.
Methods
The design and primary results of the PARADIGM-HF trial have been described in detail.10–12
The trial was approved by the ethics committee at each study center. All the patients provided written informed consent.
Study Patients
The inclusion criteria for the PARADIGM-HF trial included New York Heart Association class II–IV symptoms, EF ≤40% (changed to ≤35% by amendment), and a plasma B-type natriuretic peptide (BNP) ≥150 pg/mL (or N-terminal pro-BNP, ≥600 pg/mL). Patients who had been hospitalized for heart failure within the preceding 12 months could be enrolled with a lower natriuretic peptide concentration (BNP, ≥100 pg/mL or N-terminal pro-BNP, ≥400 pg/mL). Patients were required to be taking an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker at a dose equivalent to enalapril 10 mg daily for at least 4 weeks before screening, along with a stable dose of a β-blocker (unless contraindicated or not tolerated) and a mineralocorticoid receptor antagonist, if indicated. The exclusion criteria included history of intolerance of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, symptomatic hypotension (or a systolic blood pressure, <100 mm Hg at screening/<95 mm Hg at randomization), an estimated glomerular filtration rate (eGFR) <30 mL/min per 1.73 m2, a serum potassium concentration >5.2 mmol/L at screening (>5.4 mmol/L at randomization), or a history of angioedema.
Study Procedures
On trial entry, existing treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker was stopped, but other treatments for heart failure were continued. Patients first received enalapril 10 mg twice daily for 2 weeks (single-blind) and then LCZ696 (single-blind) for an additional 4 to 6 weeks, initially at 100 mg twice daily and then 200 mg twice daily. Patients tolerating both drugs at target doses were randomly assigned in a 1:1 ratio to double-blind treatment with either enalapril 10 mg twice daily or LCZ696 200 mg twice daily. The dose of enalapril was selected based on its effect to reduce the risk of death compared with placebo in the Studies of Left Ventricular Dysfunction (SOLVD) treatment trial.13 LCZ696 200 mg twice daily delivers the equivalent of valsartan 160 mg twice daily and significant and sustained neprilysin inhibition.
Definition of Pre–Diabetes Mellitus, Undiagnosed Diabetes Mellitus, and Diabetes Mellitus
For the purposes of this study, patients without a previous diagnosis of diabetes mellitus were divided into 3 categories according to the hemoglobin A1c (HbA1c) level using the International Diabetes Expert Committee criteria14,15: (1) normal, <6.0% (<42 mmol/mol); (2) pre–diabetes mellitus, 6.0% to 6.4% (42–47 mmol/mol); and (3) undiagnosed diabetes mellitus, ≥6.5% (≥48 mmol/mol). Patients with a previous diagnosis of diabetes mellitus (irrespective of HbA1c level) were considered to have diabetes mellitus.
Study Outcomes
The PARADIGM-HF trial was designed to recruit ≈8400 patients and continue until 1229 patients experienced cardiovascular deaths and 2410 patients experienced either a first hospitalization for heart failure or cardiovascular death (primary outcome). However, an independent data and safety monitoring board recommended early termination of the study when the prespecified boundary for overwhelming benefit for both cardiovascular mortality and the primary outcome had been crossed. The primary outcome of this analysis was a composite of death from cardiovascular causes or a first hospitalization for heart failure. The secondary outcomes of the PARADIGM-HF trial were the time to death from any cause, the change from baseline to 8 months in the clinical summary score on the Kansas City Cardiomyopathy Questionnaire (KCCQ; on a scale from 0 to 100, with higher scores indicating fewer symptoms and physical limitations associated with heart failure), the time to a new onset of atrial fibrillation, and the time to the first occurrence of a decline in renal function (which was defined as end-stage renal disease or as a decrease in the eGFR of at least 50% or a decrease of >30 mL/min per 1.73 m2 from randomization to <60 mL/min per 1.73 m2); there were too few patients with new onset atrial fibrillation and decline in renal function for meaningful analysis in the current study of HbA1c subgroups. Adjudication of these outcomes was carried out in a blinded fashion by a clinical end point committee according to pre–specified criteria. Safety outcomes included hypotension, elevation of serum creatinine, hyperkalemia, cough, and angioedema, as previously reported.11
Statistical Analysis
Baseline characteristics are presented as mean with SDs for continuous variables and frequencies and percentages for categorical variables. Unadjusted event rates are reported per 100 patient-years of follow-up according to diabetic status. Cox proportional hazard models were applied to calculate hazard ratios (HRs) for the outcomes in patients with pre–diabetes mellitus, undiagnosed diabetes mellitus, and diabetes mellitus with normoglycemic patients as reference, as well as treatment effect of LCZ696 for the outcomes according to glycemic status. Event-free survival curves (Figure 1) were calculated using Kaplan–Meier estimates. In additional Cox models, we examined the relationship between diabetic status and outcomes stratified by EF and kidney function (Figures 2 and 3). The adjusted Cox regression models included information on age, sex, race (white versus all other), geographical region, heart failure duration, New York Heart Association class, EF, heart rate, KCCQ score, body mass index, eGFR, N-terminal pro-BNP, ischemic cause, and history of myocardial infarction, stroke, and atrial fibrillation. We compared the frequency of a ≥5-point decline in the KCCQ score at 8-month follow-up according to diabetes mellitus status and used logistic regression to calculate odds ratios for this reduction in patients with diabetes mellitus and pre–diabetes mellitus compared with normoglycemic patients. We applied a cubic spline model to assess the relationship between HbA1c and the primary composite outcome in patients not treated with glucose-lowering drugs. All P values are 2-sided, and a P value of <0.05 was considered significant. Analyses were performed using Stata version 13 (Stata Corp, College Station, TX), and SAS version 9.4 (SAS Institute, Cary, NC).
Figure 1.
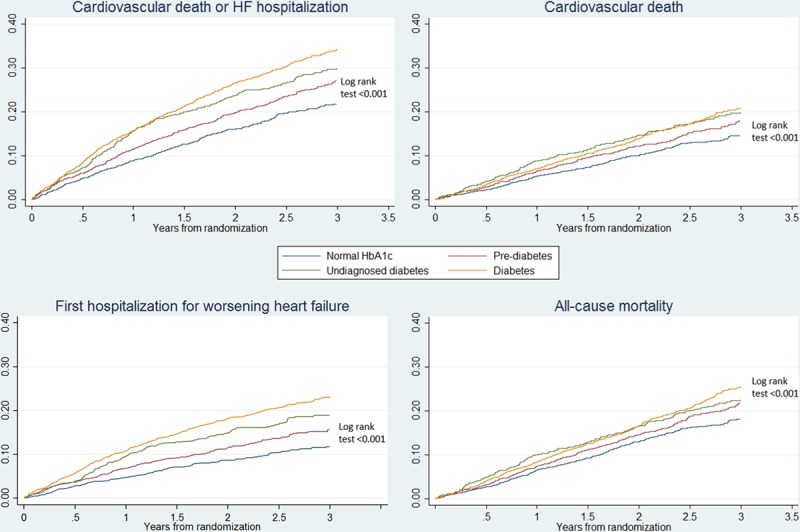
Kaplan–Meier curves for the primary composite end point of cardiovascular death or heart failure (HF) hospitalization, each of the components separately, and all-cause mortality according to history of diabetes mellitus and glycemic status. HbA1c indicates hemoglobin A1c.
Figure 2.
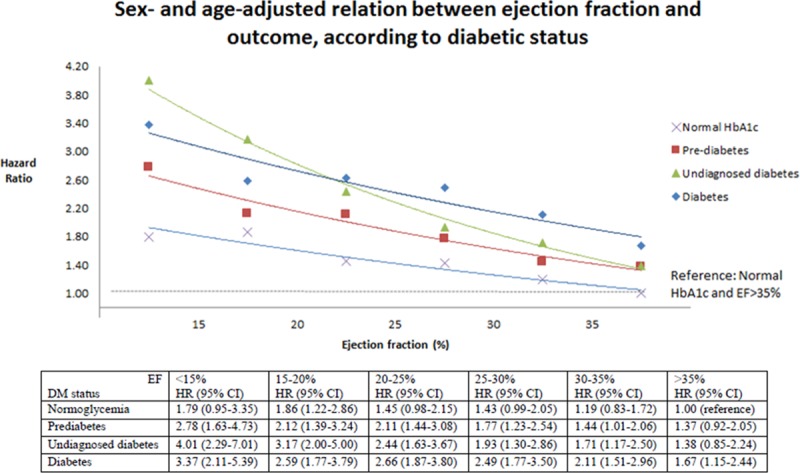
Relationship between ejection fraction (EF) and the primary outcome stratified by history of diabetes mellitus (DM) and glycemic status. CI indicates confidence interval; HbA1c, hemoglobin A1c; and HR, hazard ratio.
Results
Overall, 8274 patients had known diabetes mellitus or a measurement of HbA1c at baseline. Of these, 2907 (35%) had a history of diabetes mellitus. Of the 5367 (65%) patients with no history of diabetes mellitus, 2160 (40% [26% of total]) had HbA1c <6.0%, 2103 (39% [25% of total]) had HbA1c 6.0% to <6.5%, and 1106 (21% [13% of total]) had HbA1c ≥6.5% (“undiagnosed diabetes mellitus”). A total of 4013 (49%) patients were, therefore, defined as having diabetes mellitus based on history (n=2907) or HbA1c ≥6.5% (n=1106). The median follow-up in patients with normal HbA1c was 26 months, and it was 27 months in both patients with pre–diabetes mellitus and diabetes mellitus.
Baseline Characteristics
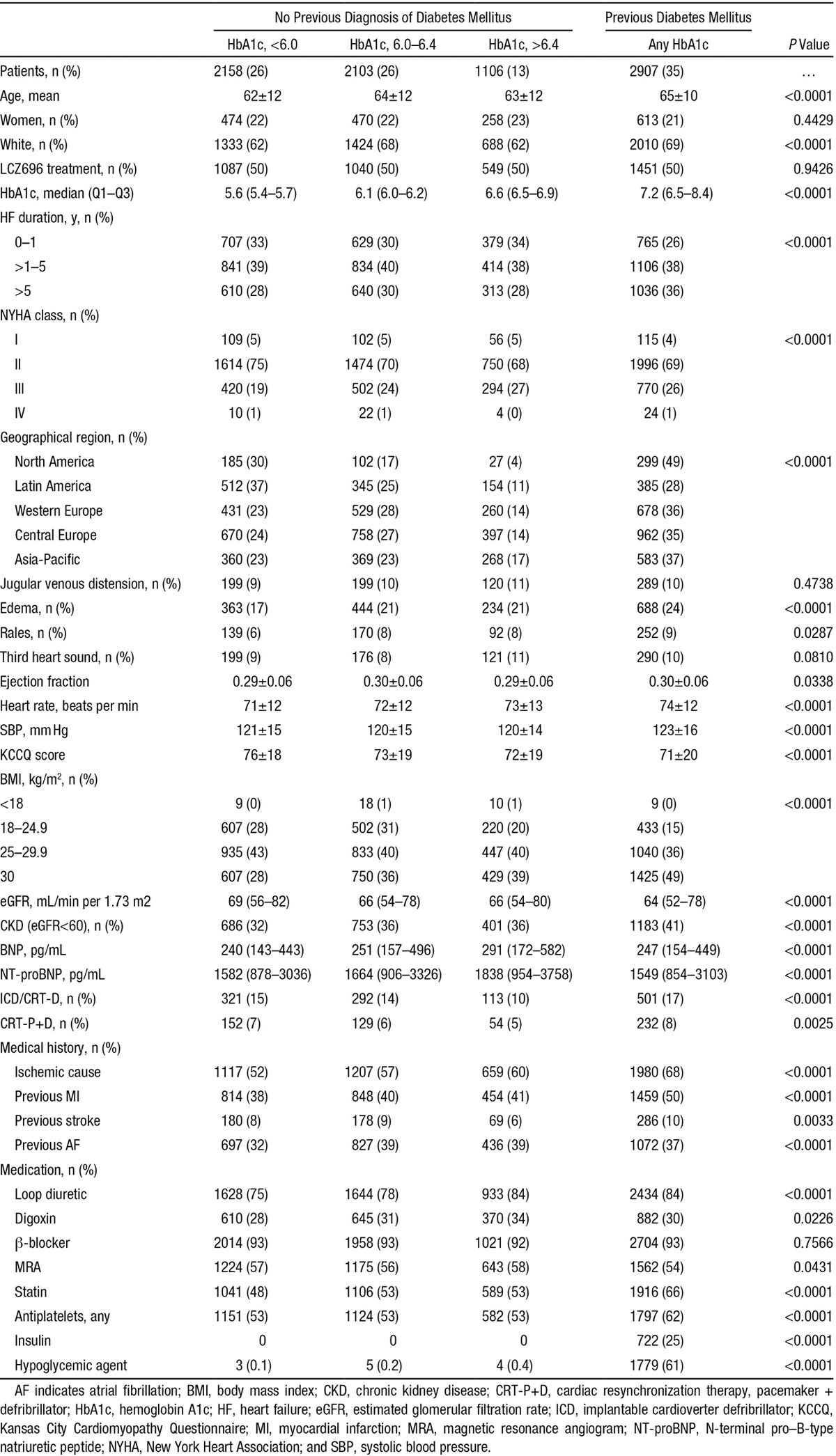
Patients with pre–diabetes mellitus and diabetes mellitus were older, more often whites, had longer heart failure duration, a higher body mass index (and more obesity), and evidence of overall worse heart failure status (Table 1). Manifestations of worse heart failure status included higher New York Heart Association class and BNP levels, lower KCCQ score and eGFR, more edema, and greater use of diuretics (Table 1). The exception to this was EF, which was marginally although insignificantly higher in patients with pre–diabetes mellitus and diabetes mellitus compared with those with normal HbA1c. Patients with pre–diabetes mellitus and diabetes mellitus also more commonly had a history of myocardial infarction and atrial fibrillation. Generally, the trends identified were most marked in patients with diabetes mellitus and intermediate between diabetes mellitus and normoglycemia in individuals with pre–diabetes mellitus. Patients in Latin America had the lowest prevalence of pre–diabetes mellitus/diabetes mellitus and the highest proportion of normoglycemia. The prevalence of diabetes mellitus was most prevalent in North America and the Asia-Pacific region. However, when both diabetes mellitus and pre–diabetes mellitus were taken into account, the rate of dysglycemia was similar in Western/Central Europe and the Asia-Pacific region and less in North America, compared with these other regions.
Table 1.
Baseline Characteristics According to the Presence of Diabetes Mellitus, Defined by Previous Diagnosis, Undiagnosed Diabetes Mellitus (HbA1c, ≥6.5), Pre–Diabetes Mellitus (HbA1c, 6.0–6.4), or Normoglycemia (HbA1c, <6.0)
Clinical Outcomes According to HbA1c Category and Diabetes Mellitus Status
The clinical outcomes of interest according to the predefined glycemia categories are summarized in Table 2 and illustrated in Figure 1. The rates of both the primary composite outcome and all-cause death were the lowest in the normal HbA1c group, significantly higher in the pre–diabetes mellitus category, and the highest in individuals with undiagnosed and known diabetes mellitus (Table 2; Figure 1). Patients with a history of diabetes mellitus were at higher risk of the primary composite outcome of heart failure hospitalization and cardiovascular mortality compared with those with normal HbA1c: adjusted HR, 1.64; 95% confidence interval, 1.44 to 1.88; P<0.001. The HR for patients with undiagnosed diabetes mellitus (HbA1c, >6.5%) compared with those with HbA1c <6.0% was 1.39 (1.18–1.64); P<0.001. The elevation in risk related to dysglycemia seemed more marked for heart failure hospitalization than for cardiovascular death or all-cause death. These differences in risk persisted after adjusting for other prognostic variables. In particular, the elevated risk related to pre–diabetes mellitus and diabetes mellitus was apparent across the spectrum of EF, although nonsignificantly so in patients with EF >35% and tended to be accentuated at lower EF (Figure 2). A similar pattern was observed when we assessed the risk related to diabetes mellitus and pre–diabetes mellitus according to kidney function (Figure 3). In a cubic spline analysis restricted to patients not on glucose-lowering drugs (n=6069), we found a correlation between increasing HbA1c and elevated risk of the primary outcome (Figure 4).
Table 2.
Event Rates and Risks of the Primary End Point (CV Death or Heart Failure Hospitalization), CV Death, Heart Failure Hospitalization, All-Cause Mortality, and Worsening KCCQ Score, According to History of DM and Glycemic Status
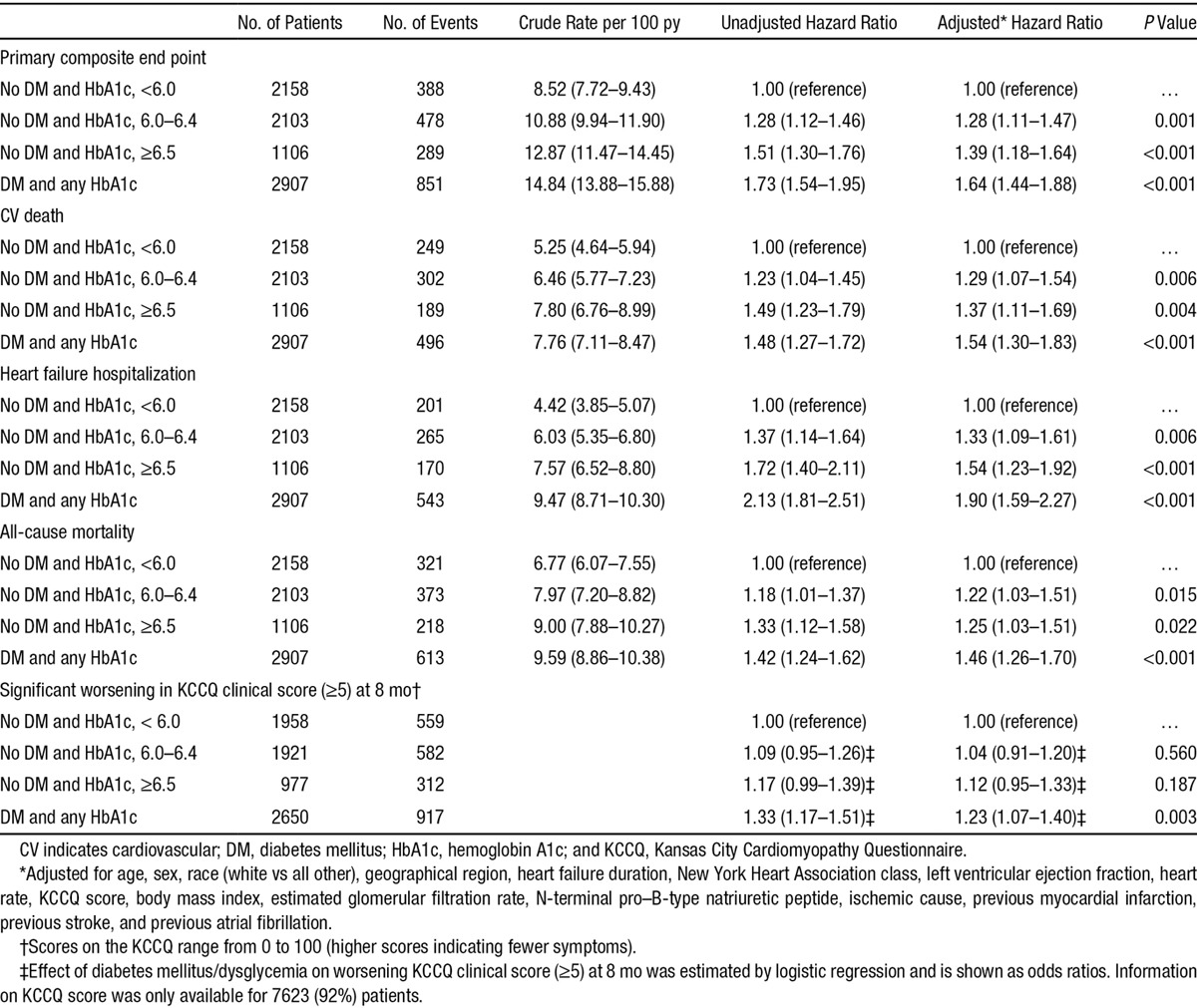
Figure 3.
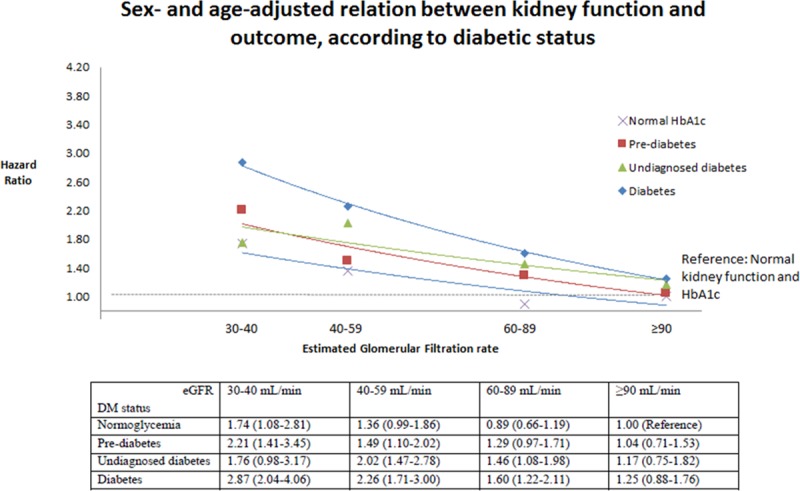
Relationship between diabetic status and the primary outcome stratified by kidney function. DM indicates diabetes mellitus; eGFR, estimated glomerular filtration rate; and HbA1c, hemoglobin A1c.
Figure 4.
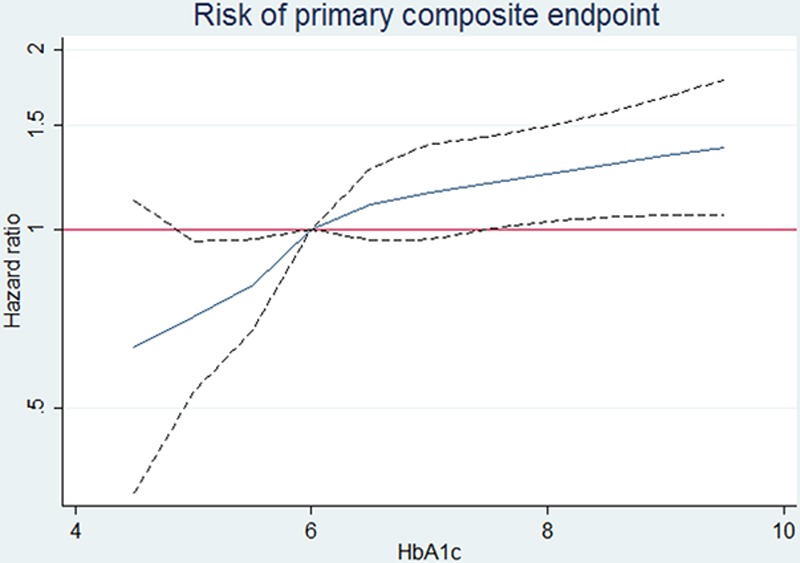
Risk of the primary composite outcome according to hemoglobin A1c (HbA1c) in patients not receiving glucose-lowering drugs.
At 8 months after randomization, more patients with known diabetes mellitus (35%) and undiagnosed diabetes mellitus (32%) had a decline of ≥5 points in KCCQ score, compared with patients with pre–diabetes mellitus (30%) and those with normal HbA1c (29%); P value for difference is 0.0002. Compared with the group with normal HbA1c, the adjusted odds ratios for a 5-point reduction were 1.23 (1.07–1.40) for patients with known diabetes mellitus, 1.12 (0.95–1.33) for those with undiagnosed diabetes mellitus, and 1.04 (0.91–1.20) for patients with pre–diabetes mellitus (Table 2).
Effect of LCZ696 (Sacubitril/Valsartan) According to Diabetes Mellitus Status
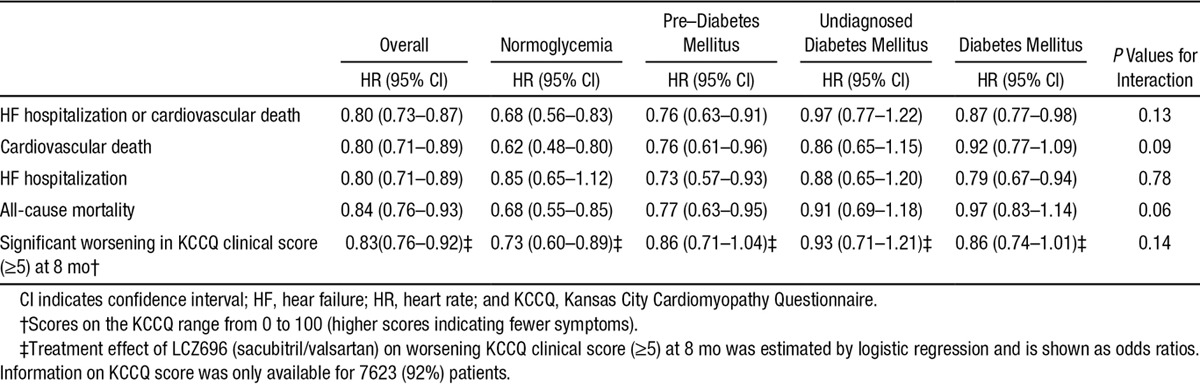
The effect of LCZ696 on the different outcomes is shown in Table 3. In each of the 3 predefined glycemia categories, LCZ696 reduced the occurrence of the primary composite outcome compared with enalapril. Fewer patients treated with LCZ696 considered themselves worse 8 months into the study (defined by a reduction in KCCQ score of ≥5 points) in all 4 predefined glycemia categories, with no significant interaction between glycemia category and treatment (P=0.14).
Table 3.
Treatment Effects of LCZ696 (Sacubitril/Valsartan) According to History of Diabetes Mellitus and Glycemic Status
Prespecified Safety Assessments
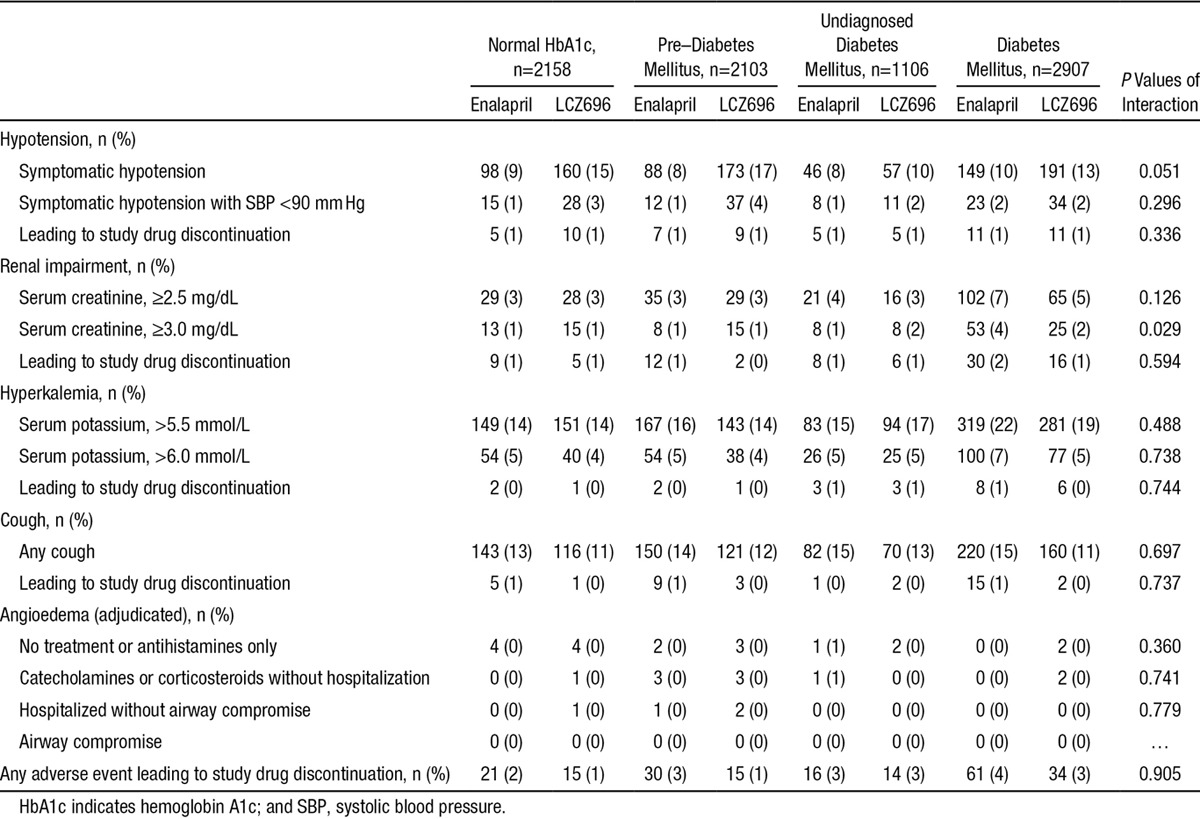
Adverse events causing drug discontinuation were overall rare, although more prevalent in patients with diabetes mellitus, compared with patients with normal HbA1c, and intermediate in the pre–diabetes mellitus group (Table 4). Renal impairment and hyperkalemia were more prevalent adverse events in patients with diabetes mellitus. We found no interaction with LCZ696 treatment, except for a higher likelihood of increase in serum creatinine ≥3.0 mg/dL, but importantly, this did not lead to more study drug discontinuation. Angioedema was very rare, regardless of diabetic status and assigned treatment.
Table 4.
Prespecified Safety Assessments According to History of Diabetes Mellitus and Glycemic Status
Patients With Previously Known Diabetes Mellitus Versus Undiagnosed Diabetes Mellitus
Notable differences between these 2 groups included older age, longer duration of heart failure, lower eGFR, and more frequent ischemic cause (and previous myocardial infarction), in patients with known diabetes mellitus (Table 1). In terms of medication, patients with known diabetes mellitus were more likely to be treated with antiplatelet agents and statins. The risk of the primary outcome was higher in patients with known diabetes mellitus (P=0.025), primarily because of a higher risk of heart failure hospitalization (P=0.032), whereas the risk of cardiovascular death was similar in those with known and undiagnosed diabetes mellitus (P=0.205). Finally, the risk of all-cause mortality seemed higher in patients with known diabetes mellitus, compared with patients with HbA1c ≥6.5%, and more so in adjusted analyses (HR, 1.46 [1.26–1.70] versus HR, 1.25 [1.03–1.51]; P=0.07).
Discussion
This study has 3 key findings. First, although it is known that the prevalence of diabetes mellitus is high in patients with HF-REF, it seems that both pre–diabetes mellitus and undiagnosed diabetes mellitus are also common in these patients. Second, non–diabetic dysglycemia (pre–diabetes mellitus) is associated with a substantially increased risk of adverse outcomes in HF-REF. Finally, LCZ696 (sacubitril/valsartan) is superior to enalapril, irrespective of glycemic status.
The first of our findings shows that a patient with HF-REF without a history of diabetes mellitus has approximately a 1-in-5 chance of actually having the condition (but not yet diagnosed) and a >1-in-3 chance of having pre–diabetes mellitus, based on HbA1c testing. Few previous studies have reported the prevalence of non–diabetic dysglycemia in HF-REF. In 1 seminal report, describing a substudy of 663 patients in the Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) pilot study,16 27% had known diabetes mellitus. Among the remaining patients, 11% had undiagnosed diabetes mellitus (fasting plasma glucose, ≥7.1 mmol/L) and 12% a fasting glucose between 6.1 and 7.1 mmol/L diagnostic of the pre–diabetic condition impaired fasting glycemia. Egstrup et al17 used the more sensitive approach of oral glucose tolerance testing to explore the same question in 227 ambulatory patients with HF-REF without known diabetes mellitus attending a heart failure clinic in Denmark. Of these, 60% had normal glucose tolerance, 22% impaired glucose tolerance, and 18% undiagnosed diabetes mellitus (an additional 20% of the study cohort had known diabetes mellitus). Among patients without diabetes mellitus in our much larger and geographically diverse population, the proportions of patients with pre–diabetes mellitus (38%) and undiagnosed diabetes mellitus (20%) were both higher. The overall prevalence of diabetes mellitus and pre–diabetes mellitus was, therefore, a remarkable 74%. We found some geographic variation in prevalence, with patients from Latin America having the lowest prevalence of dysglycemia and patients in the Asia-Pacific region and Europe the highest. This contrasts strikingly with the prevalence of diabetes mellitus in the general population. For example, using similar HbA1c diagnostic thresholds, the prevalence of diagnosed diabetes mellitus, undiagnosed diabetes mellitus, and pre–diabetes mellitus in US residents aged ≥65 years was 17.7% (95% confidence interval, 15.6–19.8), 3.5% (2.6–4.4), and 8.1% (6.6–9.6), respectively, giving a total of 29.3% individuals with diabetes mellitus or pre–diabetes mellitus, an overall prevalence considerably less than half of that observed in our patients with HF-REF.18
The significance of this finding is related to the worse clinical status and substantially elevated risk of adverse clinical outcomes conferred by both pre–diabetes mellitus and diabetes mellitus. In 1 study, pre–diabetes mellitus and insulin resistance were correlated with worse symptom status, reduced exercise tolerance, and neurohumoral activation, and another study showed that elevated HbA1c was associated with increased mortality in nondiabetic patients referred for suspected heart failure.19,20 Our findings confirm and extend these previous observations from RESOLVD pilot study, particularly with the demonstration of a worse KCCQ score, more edema and higher natriuretic peptide levels in patients with pre–diabetes mellitus compared with those with normal HbA1c.16 The finding that lower HbA1c in patients without known diabetes mellitus corresponded to a better prognosis is in contrast with the observed U-shaped relationship between HbA1c and adverse outcomes in patients with known and treated diabetes mellitus.21 We also found, as previously, that these manifestations of worse clinical status were apparent despite a similar or even higher EF than in the group with normal HbA1c, which is an unexplained and perhaps paradoxical finding.
Although the heightened risk related to diabetes mellitus is well known, the risk associated with pre–diabetes mellitus is not. This finding is important for many reasons. Most significantly, it shows that dysglycemia itself, rather than the use of hypoglycemic drugs, is a risk factor for adverse outcomes. Recently, there has been concern that the agents used to lower blood glucose may be harmful in patients with heart failure.3,22,23 As our patients with pre–diabetes mellitus were not receiving these treatments, hypoglycemic agents cannot account for the worse outcomes in this group compared with subjects with normal HbA1c. However, patients with diabetes mellitus also did worse than patients with pre–diabetes mellitus (and those with known diabetes mellitus did worse than those with undiagnosed diabetes mellitus), still leaving open the possibility of harm related to hypoglycemic drugs (although there are other reasons why the more severe and probably longer duration of hyperglycemia in diabetes mellitus might be associated with worse outcomes than pre–diabetes mellitus).
Second, these findings are important as they emphasize the need to better understand the effect of treatments for dysglycemia on outcomes in patients with heart failure. If hypoglycemic treatments were shown to improve outcomes across the range of dysglycemia, including both pre–diabetes mellitus and diabetes mellitus, potentially a large proportion of patients would be eligible for such treatment. Although the relationship between dysglycemia and adverse events in heart failure is clear and strong, it is only an association and a clear cause-and-effect mechanistic pathway has not been confirmed. Moreover, as alluded to above, there has been concern that at least some hypoglycemic agents may increase rather than decrease the risk of heart failure–related events.23
As anticipated, renal dysfunction and hyperkalemia were more common among patients with diabetes mellitus (compared with those with normoglycemia) in the enalapril group; however, both these adverse effects were numerically (but statistically insignificantly) less common in the LCZ696 group, compared with the enalapril group, across all glycemia categories. Renal dysfunction was also more frequent in angiotensin-converting enzyme inhibitor–treated patients with diabetes mellitus than in those without diabetes mellitus.24 Marked renal dysfunction (serum creatinine, ≥3.0 mg/dL) was less frequent with LCZ696 than with enalapril, irrespective of glycemia status. Hypotension was more common overall with LCZ696 compared with enalapril; the increment in hypotension with LCZ696 was smaller in patients with diabetes mellitus than in the other glycemia groups.
This study has many limitations. It is a retrospective analysis. Our dysglycemia categorization is based on 1 set of criteria, and other slightly different criteria exist.25,26 Our patients had only 1 measurement of HbA1c and not at least 2 measurements or supplementary analyses of fasting glucose and oral glucose tolerance, as recommended in guidelines.14,15
In summary, we have shown that in patients with chronic HF-REF, dysglycemia is common and pre–diabetes mellitus, as well as diabetes mellitus, is associated with worse clinical status and a significantly increased risk of adverse cardiovascular outcomes compared with normoglycemic patients. LCZ696 was beneficial, irrespective of HbA1c concentration and diabetes mellitus status.
Sources of Funding
This study was funded by Novartis. Dr Kristensen was supported by a Research Fellowship from the Heart Failure Association of the European Society of Cardiology.
Disclosures
Drs Jhund, Squire, Cardoso, Merkely, Martinez, Starling, Desai, Lefkowitz, Rouleau, Shi, Solomon, Swedberg, Zile, McMurray, and Packer and A.R. Rizkala have consulted for or received research support from Novartis, sponsor of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial. Dr Packer has consulted for Novartis, Pfizer, Sanofi, Cytokinetics, Cardiokinetix, BioControl, Janssen, Amgen, CardioMEMS, and Cardiorentis. Dr McMurray’s employer, University of Glasgow, was paid by Novartis for Dr McMurray’s time spent as cochairman of the PARADIGM-HF trial. Dr Desai consulted for Novartis, Relypsa and St. Jude Medical. Drs Lefkowitz and Shi are employees of Novartis. Drs Swedberg, and Zile have received honoraria from Novartis for sponsored lectures. Dr Martinez are on the speaker’s bureau of Novartis. Dr Squire received honoraria from Novartis for participating in various activities. Drs Kristensen and Preiss report no conflicts.
Appendix
PARADIGM-HF Collaborators: Vanhaecke J, Ramires F, Katova T, Arnold M, Huang J, Gomez E, Belohlavek J, Refsgaard J, Lederballe O, Gonzalez A, Duarte YC, Rosenthal A, Peuhkurinen K, Hagege AA, Böhm M, Arango JL, Andersen K, Senni M, Erglis A, Petrulioniene Z, Llamas EB, Mosterd A, Cabrera W, Sibulo AS, Jr, Negrusz-Kawecka M, Silva-Cardoso J, Vinereanu D, Boytsov S, Wong R, Dukat A, Burgess L, Kim KS, Calvo C, Fu M, Chen CH, Kiatchoosakun S, Kozan O, Starling R, Teerlink J, Dargie H, Foley R, Francis GS, Komajda M, Pocock S, Barkoudah E, Bello N, Brahimi A, Charytan D, Duong C, Finn P, Hartley H, Vazir A, Weinrauch L, Kaplan AP, Brown N, Zuraw B, Greenlaw N, Albisu J, Alvarisqueta A, Amuchastegui M, Astesiano A, Avila E, Beloscar J, Berli M, Borrego C, Bustos B, Calella P, Carbajales J, Caruso G, Caruso O, Casabé H, Cimbaro Canella J, Colombo Berra F, Colque R, Costello R, Dran R, Ferre Pacora F, Gabito A, Glenny J, Guzman P, Ibañez J, Ingaramo R, Kotliar C, Leon de la Fuente R, Liberman A, Liniado G, Llanos J, Lobo Marquez L, Luquez H, Mackinnon I, Mallagray M, Martingano R, Mercado D, Moises Azize G, Parody L, Perez Rey R, Petenian E, Rodríguez M, Rojas C, Romano A, Salvatierra Ruiz A, Sanchez A, Sarjanovich R, Sarries A, Schiavi L, Sessa H, Soler J, Vico M, Zaidman C, Vanhaecke J, Decroly P, Dendale P, Ector B, Friart A, Heyse A, Missault L, Mulleners T, Smolders W, Vandekerckhove H, Vincent M, Weytjens C, Wollaert B, Ramires F, Andrade C, Bassan R, Borelli F, Botelho R, Braga J, Braile M, Costa C, Costa F, Duda N, Garcia M, Greco O, Hernandes M, Jaeger C, Koehler I, Luiz Rech R, Mesquita E, Moraes J, Jr, Neto J, Neuenschwander F, Paiva M, Rassi S, Reis G, Rossi P, Rabelo A, Reis H, Saporito W, Simoes M, Souza W, Vilas Boas F, Katova T, Benov H, Boeva-Chompalova B, Chobanska P, Denchev S, Dimov B, Donova T, Georgiev P, Gotchev D, Goudev A, Gruev T, Hergeldjieva V, Lazov P, Manukov I, Marchev S, Mihov L, Milanova M, Mileva-Manolova A, Mincheva-Kabakchieva V, Parvanova Z, Pencheva G, Petranov S, Raev D, Ramshev K, Sirakova V, Staneva A, Tisheva-Gospodinova S, Todorov G, Tokmakova M, Tzekova M, Valchanova P, Yotov Y, Zhelev V, Arnold M, Bergeron S, Bourgeois R, Bourgeois S, Cha J, DeGrâce M, Delgado D, Deslongchamps F, Dion D, Giannetti N, Huynh T, Johnston J, Klinke P, Kornder J, Labonte R, Lauzon C, Lepage S, Mak S, Moe G, Murthy D, Pandey S, Parker J, Rajda M, Robinson S, Rupka D, Sabe-Affaki G, Sestier F, Sheppard R, Yao L, Albornoz F, Avendaño P, Cobos L, Escobar E, Fernandez M, Jalil J, Lanas F, Sepulveda P, Stockins B, Yovaniniz P, Huang J, Gui M, Hu HD, Ke YN, Li LG, Li XD, Li XL, Li WM, Liu SW, Liu XH, Ma GS, Sun NL, Xu G, Yang K, Yuan ZY, Zhang J, Zhao RP, Gomez E, Accini J, Almanzar A, Coronel J, Cotes C, Echeverria L, Manzur F, María E, Reynales H, Rodriguez M, Sotomayor A, Urina M, Velasquez J, Vélez S, Vesga B, Belohlavek J, Burianova H, Carda J, Cech V, Cepelak M, Hanustiakova A, Horny I, Kolar K, Krupicka J, Kvasnicka J, Lindovsky P, Lorenc Z, Malek F, Malik J, Mandovec A, Petrova I, Podzemska B, Povolny P, Radvan M, Richter M, Riha V, Sabl P, Slaby J, Svejda J, Telekes P, Ulman J, Vomacka Z, Zemek S, Lederballe O, Refsgaard J, Andersen H, Egstrup K, Elming H, Eske N, Gøtzsche O, Jensen J, Køber L, May O, Pedersen O, Rickers H, Steffensen F, Gonzalez A, Paulino A, Martinez P, Duarte YC, Delgado C, Duarte Y, Hidalgo FL, Mariscal C, Marmol R, Rosenthal A, Kaasik A, Kaik J, Laane E, Peuhkurinen K, Jaaskelainen T, Kiilavuori K, Taurio J, Hagege AA, Alexeeva-Kovalchuk A, Bauer F, Berdague P, Berneau JB, Bouvier JM, Damien L, Damy T, Davy JM, Decoulx DE, El-Mansour N, Etchecopar C, Galinier M, Gibelin P, Gosse P, Guetlin A, Labeque JN, Livarek B, Logeart D, Martelet M, Nazeyrollas P, Neuder Y, Nourredine M, Poulard JE, RaidRihani R, Sabatier R, Tran NT, Yannick N, Zannad F, Bohm M, Adelberger V, Al-Zoebi A, Bastian A, Behrens S, Bessler H, Braun R, Brehm B, Buhr M, Cieslinski G, vom Dahl J, Daut W, Demmig HJ, Denny S, Ebert HH, Fechtrup C, Fischer S, Frick HM, Genth-Zotz S, Gerbaulet U, Germann H, Geßner S, Gola G, Grönefeld G, Hagenow A, Hampf J, Hartmann A, Hauf G, Hegeler-Molkewehrum C, Hegemann P, Hermes S, Himpel-Bönninghoff A, Hoeltz S, Jerwan-Keim R, Kadel C, Karle C, Kindermann I, Knapp M, Krause KH, Krosse B, Kühne U, Kuhrs M, Leicht M, Löbe M, Loos H, Mehling H, Melchior K, Menzel F, Münzel T, Natour M, Naudts I, Naumann R, Nischik R, Olbrich HG, Pohl W, Prohaska M, Proskynitopoulus N, Regner S, Reimer D, Roeder S, Rummel R, Salbach P, Schäfer T, Schaum T, Schenkenberger I, Schindler A, Schmidt A, Schmidt E, Schnabel A, Schneider R, Schneider W, Schreckenberg A, Schreibmüller F, Schreiner M, Schröder T, Schumacher M, Segner A, Seibert H, Siao G, Siao K, Sohn HY, Stöhring R, Tangerding G, Taubert G, Terhorst A, Tesch C, Toursarkissian N, Tyler K, Uebel P, Weyland K, Wilke A, Yilmaz A, Zemmrich C, Zeh W, Zotz R, Arango JL, Arriola J, Corona V, Leal S, López E, Muñoz R, Ovando A, Paniagua A, Rodríguez D, Velasquez L, Wyss F, Tse HF, Li SK, Yan B, Yip G, Merkely B, Andrassy G, Andreka P, Bakai J, Csapo K, Cziraki A, Édes I, Forster T, Hajko E, Illes A, Jánoskuti L, Kalina A, Kovacs Z, László Z, Lupkovics G, Matoltsy A, Müller G, Nagy A, Noori E, Nyolczas N, Papp A, Salamon C, Szántai G, Szocs A, Tomcsányi J, Toth D, Varjú I, Veress G, Vertes A, Zámolyi K, Zilahi Z, Andersen K, Gudnason T, Sigurdsson A, Thorgeirsson G, Abhyankar A, Agarwal D, Aggarwal R, Bagirath R, Banker D, Bisne V, Bohra P, Chopra V, Dani S, Dharmadhikari A, Fulwani M, Gadkari M, Ghaisas N, Basavanagowdappa H, Gupta S, Hiremath S, Jagtap P, Jain A, Jain V, Jindal R, Joseph S, Kerkar P, Kumbla M, Malipeddi B, Mathan G, Mehta A, Mohan M, Murthy L, Nair A, Pai V, Pandey A, Prakash V, Rao M, Rao NS, Reddy N, Sarma P, Shah P, Shamsudden K, Sharma K, Sinha S, Thakkar B, Thanvi S, Trivedi P, Vijan V, Yugandhar B, Aronson D, Ben Gal T, Goland S, Katz A, Keren A, Lewis B, Marmor A, Mayler S, Shochat M, Senni M, Anastasio L, Baldin M, Brunelli C, Casolo G, Coppolino C, Cosmi F, Danzi G, Destro M, Di Napoli T, D’Ospina A, Fucili A, Gigantino A, Liberato NL, Lombardi F, Lembo G, Magrini F, Mannarino E, Marchese D, Minneci C, Modena M, Mos L, Napoli T, Opasich C, Pajes G, Perticone F, Pileri P, Poddighe G, Ronchi E, Saba P, Sicuro M, Silvestri F, Salerno Uriarte J, Spagnuolo V, Sprovieri M, Taddei S, Terrosu P, Tespili M, Uriarte J, Vergoni W, Volterrani M, Erglis A, Dormidontova G, Eglite R, Lvova T, Rancane G, Sime I, Petrulioniene Z, Luksiene D, Maleckas T, Mazutavicius R, Miliuniene R, Petrulioniene Z, Slapikas R, Ahmad W, Chew D, Ismail O, Ong T, Llamas EB, Aguilera M, Arenas J, Carrillo J, González J, Leon S, Llamas G, Macias A, Meaney A, Orihuela O, Pavía A, Rodriguez I, Rodriguez T, Salcido E, Solache G, Velasco R, Mosterd A, Basart D, Bellersen L, Derks A, Dijkgraaf R, Dunselman P, van Eck J, Gurlek C, den Hartog F, Hoedemaker G, Kaplan R, Koolen J, Liem L, Milhous J, de Nooijer C, Pronk A, Brunner-la Rocca H, Ronner E, Swart H, Tjeerdsma G, Willems F, Avilés E, Frago G, González B, Nieto R, Cabrera W, Alegre R, Azañero R, García JH, Godoy A, Heredia J, Lu L, Orihuela B, Rodriguez A, Roldan Y, Torres P, Urquiaga J, Sibulo AS, Jr, Anonuevo J, Atilano A, Borromeo A, Castillo R, Chua P, Ferrolino A, Guerrero A, Locnen S, Manlutac B, Rogelio G, Rosita R, Ruales A, Vilela G, Negrusz-Kawecka M, Bebenek W, Sobkowicz B, Cymerman K, Dabrowska M, Foczpaniak M, Jazwinska-Tarnawska E, Kabara A, Kania G, Kolaczyk P, Kucharski W, Landa K, Mirek-Bryniarska E, Piepiorka M, Pijanowski Z, Sciborski R, Szpajer M, Tyminski M, Weglarz P, Wojciechowska C, Wronska D, Almeida F, Andrade A, Braganca N, Carvalho S, Fonseca C, Oliveira L, Padua F, Silvestre I, Soares R, Vinereanu D, Andor M, Bartos D, Basarab G, Coman I, Copaci I, Cristea M, Dragulescu S, Enache D, Fruntelata A, Iliescu L, Istratoaie O, Lighezan D, Militaru C, Nanea T, Nechita C, Puschita M, Tomescu M, Tudoran M, Boitsov S, Ageev F, Averkov O, Akimov A, Ballyuzek M, Baranov E, Baranova E, Barbarash O, Berkovich O, Berns S, Bessonova N, Boyarkin M, Bulashova O, Chernetsov V, Chukaeva I, Kamensky I, Dovgalevsky Y, Dovgolis S, Duplyakov D, Ermoshkina L, Fitilev S, Galyavich A, Gendlin G, Gofman A, Goloschekin B, Gomova T, Gordienko A, Karpov Y, Kastanayan A, Khromtsova O, Kisliak O, Kobalava Z, Konradi A, Korolev M, Kosmacheva E, Kostenko V, Koziolova N, Kuimov A, Kulibaba E, Lebedev P, Lesnov V, Libis R, Lopatin Y, Makukhin V, Masterov I, Moiseeva Y, Morozova T, Motylev I, Murashkina S, Nosov V, Oleynikov V, Palatkina T, Parmon E, Pimenov L, Privalov D, Rafalsky V, Rebrov A, Reznik I, Ruda M, Saifutdinov R, Sayganov S, Shvarts Y, Shpagina L, Shustov S, Shutemova E, Sitnikova M, Sizova J, Smolenskaya O, Solovieva A, Staroverov I, Struk R, Svistov A, Tarasov N, Tarlovskaya E, Tereschenko S, Trofimov N, Uspensky Y, Vasilieva E, Vezikova N, Vishnevsky A, Volkov D, Yakhontov D, Yakovlev A, Yavdosyuk A, Zateyshchikova A, Zharkov O, Zhilyaev E, Zotov D, Zrazhevsky K, Wong R, Lee C, Ong H, Yeo D, Dukát A, Antalík L, Baníková A, Demešová D, Dvoržák M, Fazekaš F, Foldiová D, Fülöp P, Kabaivanov P, Kovács J, Maček V, Majerčák I, Mazúr J, Mihalíková A, Olexa P, Pacherová J, Palinský M, Pálka J, Pella D, Remišová S, Schichorová J, Smik R, Sokolová B, Šuch S, Viňanská D, Burgess L, Ahmed F, Baben L, Badat A, Basson D, Bester F, Bruning A, Delport E, Dindar F, Foccart J, Gani M, Gerntholtz T, Hellstrom E, Horak A, Ismail S, Jamjam L, Kapp C, Latiff G, Lerumo T, Lombaard J, Manga P, van der Merwe N, Mkhwanazi M, Mohamed Z, Mpe M, Naidoo D, Padayachee T, Ranjith N, van Rensburg DJ, Saaiman J, Sebopa B, Tayob M, Theron H, Thomas M, Vally T, Venter T, Wellmann H, van Zyl L, Kim KS, Baek SH, Zo JH, Hong GR, Kang DH, Kang SM, Kim DS, Kim BJ, Kim U, Park DG, Shin JH, Yoo BS, Calvo C, Luis-Arias J, Arias-Castaño JC, Comín J, de Teresa L, Fernandez-Aviles F, Gomez-Huelgas R, González-Bueno M, Cosín J, Cremer D, Crespo M, Deben F, Freixa R, Galve E, Garcia M, Gomez R, Jiménez M, Mainar L, Marin I, Martinez F, Martínez-Sellés M, Marzal D, Muñoz B, Núñez J, Pascual D, Peña G, Reyes A, Sanmartín M, Torres F, Vida M, Fu M, Ahlström P, Hagerman I, Hajimirsadeghi A, Hansson A, Kempe A, Thorsén C, Zethson-Halldén M, Chen CH, Chen CP, Chen PS, Hsu KL, Lin LY, Pai PY, Tsao HM, Tzeng BH, Kiatchoosakun S, Hengrussamee K, Piyayotai D, Sanguanwong S, Thongsri T, Kozan O, Aktoz M, Barcin C, Birdane A, Camsari A, Ermis C, Guray Y, Kudat H, Ural D, Yavuzgil O, Yenigun M, Yigit Z, Yilmaz MB, Yokusoglu M, Squire I, Apostolakis S, Banerjee P, Barr C, Bhatia V, Bogle R, Boos C, Brigden G, Brown N, Bulugahapitiya S, Dayer M, Dutka D, El-Harari M, Fisher M, Gaballa M, Ghandi N, Glover J, James R, Kadr H, Kalra P, Kardos A, Lang C, Leslie S, Levy T, Lynch M, MacFadyen R, Mahmood S, Mamas M, Martin W, Megarry S, Mohindra R, More R, Moriarty A, Murphy J, Muthusamy R, Neyses L, Nightingale A, O’Toole L, Price D, Purvis J, Ryding A, Smith D, Sobolewska J, Soo L, Strain D, Trelawny J, Trevelyan J, Watkin R, Witherow F, Woldman S, Yousef Z, Teerlink J, Adamson P, Akinboboye O, Akyea-Djamson A, Amin A, Amkieh A, Amos A, Anand I, Awasty V, Banish D, Bank A, Bargout R, Barnard D, Beacom M, Berg I, Berk M, Best J, Bilazarian S, Bouchard A, Bozkurt B, Breisblatt W, Brookfield L, Brown C, Browne K, Canadas-Zizzias R, Carr K, Chapman D, Chu A, Chung E, Colan D, Davis B, Denning S, Desai V, Dexter J, Dharma C, Edwards J, Efstratiadis S, Eisen H, Fattal P, Fenster B, Fernandez J, Flores A, Flores E, Floro J, Frivold G, Fuhs B, Goldscher D, Gould R, Grazette L, Laufer N, Lieber IH, Haas G, Habet K, Hack T, Haidar A, Halpern S, Hargrove J, Harris J, Hart T, Hass G, Hattler B, Hazelrigg M, Heilman K, Heiman M, Heroux A, Herzog W, Hoffman M, Hotchkiss D, Hunter C, Hunter J, Iteld B, Jackson D, Jaffrani N, Janik M, Jardula M, Joseph J, Kaneshige A, Khan M, Klapholz M, Koren M, Kostis J, Larrain G, Lasala G, Laufer N, Lee K, Leonen M, Lieber I, Liu M, Magno J, Maher J, Maisel A, Maislos F, Malkowski M, Mallis G, Mandviwala M, Mani C, Markham D, Marple R, Maurer M, McKenzie W, Mehrle A, Mendez J, Miller A, Miller R, Miller V, Mishkin J, Mitchell J, Mody F, Montgomery B, Murray D, Murray A, Naidu J, Neutel J, Nguyen D, O’Brien T, Olsen S, Ooi H, Orchard R, Parrott C, Petersen J 2nd, Poling T, Prodafikas J, Ptacin M, Quinlan E 3rd, Quinn T, Rama B, Ramanathan K, Rawitscher D, Rosado J, Rosenthal S, Oberoi MS, Samal A, Schmalfuss C, Schwartz S, Seals A, Selektor Y, Schaefer S, Seto T, Shah S, Shanes J, Sims J, Singh S, Sooudi S, Sotolongo R, Suiter D, Sunderam S, Thadani U, Thrasher J, Trichon B, Vicuna R, Vranian R, Jackson R, Wallach S, Ward N, Weinstein D, Wells T, Wickemeyer W, Wight J, Williams C, Wu L, Xu G, Zebrack J, Mendoza I, Avendaño A, Alvarez M, Silva E, Vergara G
Footnotes
A list of all PARADIGM-HF Investigators and Committees is given in the Appendix.
Guest Editor for this article was Gregg C. Fonarow, MD.
CLINICAL PERSPECTIVE
In this study, we examined the prevalence of pre–diabetes mellitus and diabetes mellitus in patients with heart failure and reduced ejection fraction in the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial and their relationship with clinical outcomes. We also examined whether dysglycemia modified the benefit of sacubitril/valsartan compared with enalapril. Pre–diabetes mellitus (25% of patients), undiagnosed diabetes mellitus (13%), and known diabetes mellitus (35%) were common and associated with worse symptoms, more edema, and higher natriuretic peptide levels than normoglycemia. Patients with dysglycemia had a higher risk of cardiovascular death and heart failure hospitalization. The benefit of sacubitril/valsartan was consistent irrespective of glycemic status. These findings confirm and extend previous observations that patients with pre–diabetes mellitus and diabetes mellitus have worse clinical status and outcomes than normoglycemic patients, despite similar or higher ejection fraction. In particular, the observation that untreated dysglycemia is associated with adverse outcomes is notable. The potential mechanistic pathway(s) linking dysglycemia to adverse outcomes in heart failure and reduced ejection fraction remain to be elucidated, as do the effects of hypoglycemic agents in the large segment of these patients with pre–diabetic dysglycemia.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure) National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–851. doi: 10.1016/S2213-8587(14)70031-2. doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 6.Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, Sleight P, Teo K ONTARGET/TRANSCEND Investigators. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation. 2007;115:1371–1375. doi: 10.1161/CIRCULATIONAHA.106.661405. doi: 10.1161/CIRCULATIONAHA.106.661405. [DOI] [PubMed] [Google Scholar]
- 7.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–532. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 8.Tomova GS, Nimbal V, Horwich TB. Relation between hemoglobin a(1c) and outcomes in heart failure patients with and without diabetes mellitus. Am J Cardiol. 2012;109:1767–1773. doi: 10.1016/j.amjcard.2012.02.022. doi: 10.1016/j.amjcard.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerstein HC, Swedberg K, Carlsson J, McMurray JJ, Michelson EL, Olofsson B, Pfeffer MA, Yusuf S CHARM Program Investigators. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168:1699–1704. doi: 10.1001/archinte.168.15.1699. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15:1062–1073. doi: 10.1093/eurjhf/hft052. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Committees Investigators. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail. 2014;16:817–825. doi: 10.1002/ejhf.115. doi: 10.1002/ejhf.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 14.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, Guidelines ESCCfP, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG Task Force Members. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 16.Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, Pinkett T, Ghali JK, Wilson AC. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol. 1996;77:1017–1020. doi: 10.1016/s0002-9149(97)89163-1. [DOI] [PubMed] [Google Scholar]
- 17.Egstrup M, Schou M, Gustafsson I, Kistorp CN, Hildebrandt PR, Tuxen CD. Oral glucose tolerance testing in an outpatient heart failure clinic reveals a high proportion of undiagnosed diabetic patients with an adverse prognosis. Eur J Heart Fail. 2011;13:319–326. doi: 10.1093/eurjhf/hfq216. doi: 10.1093/eurjhf/hfq216. [DOI] [PubMed] [Google Scholar]
- 18.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AlZadjali MA, Godfrey V, Khan F, Choy A, Doney AS, Wong AK, Petrie JR, Struthers AD, Lang CC. Insulin resistance is highly prevalent and is associated with reduced exercise tolerance in nondiabetic patients with heart failure. J Am Coll Cardiol. 2009;53:747–753. doi: 10.1016/j.jacc.2008.08.081. doi: 10.1016/j.jacc.2008.08.081. [DOI] [PubMed] [Google Scholar]
- 20.Goode KM, John J, Rigby AS, Kilpatrick ES, Atkin SL, Bragadeesh T, Clark AL, Cleland JG. Elevated glycated haemoglobin is a strong predictor of mortality in patients with left ventricular systolic dysfunction who are not receiving treatment for diabetes mellitus. Heart. 2009;95:917–923. doi: 10.1136/hrt.2008.156646. doi: 10.1136/hrt.2008.156646. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar D, Bozkurt B, Ramasubbu K, Deswal A. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol. 2009;54:422–428. doi: 10.1016/j.jacc.2009.04.049. doi: 10.1016/j.jacc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komajda M, McMurray JJ, Beck-Nielsen H, Gomis R, Hanefeld M, Pocock SJ, Curtis PS, Jones NP, Home PD. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J. 2010;31:824–831. doi: 10.1093/eurheartj/ehp604. doi: 10.1093/eurheartj/ehp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL SAVOR-TIMI 53 Steering Committee and Investigators*. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 24.Packer M, Lee WH, Medina N, Yushak M, Kessler PD, Gottlieb SS. Influence of diabetes mellitus on changes in left ventricular performance and renal function produced by converting enzyme inhibition in patients with severe chronic heart failure. Am J Med. 1987;82:1119–1126. doi: 10.1016/0002-9343(87)90213-0. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippatos TD, Rizos EC, Gazi IF, Lagos K, Agouridis D, Mikhailidis DP, Elisaf MS. Differences in metabolic parameters and cardiovascular risk between American Diabetes Association and World Health Organization definition of impaired fasting glucose in European Caucasian subjects: a cross-sectional study. Arch Med Sci. 2013;9:788–795. doi: 10.5114/aoms.2013.38671. doi: 10.5114/aoms.2013.38671. [DOI] [PMC free article] [PubMed] [Google Scholar]


