Abstract
Background—
Cardiac sarcoidosis is associated with an increased risk of heart failure and sudden death, but its risk in patients with preserved left ventricular ejection fraction is unknown. Using cardiovascular magnetic resonance in patients with extracardiac sarcoidosis and preserved left ventricular ejection fraction, we sought to (1) determine the prevalence of cardiac sarcoidosis or associated myocardial damage, defined by the presence of late gadolinium enhancement (LGE), (2) quantify their risk of death/ventricular tachycardia (VT), and (3) identify imaging-based covariates that predict who is at greatest risk of death/VT.
Methods and Results—
Parameters of left and right ventricular function and LGE burden were measured in 205 patients with left ventricular ejection fraction >50% and extracardiac sarcoidosis who underwent cardiovascular magnetic resonance for LGE evaluation. The association between covariates and death/VT in the entire group and within the LGE+ group was determined using Cox proportional hazard models and time-dependent receiver–operator curves analysis. Forty-one of 205 patients (20%) had LGE; 12 of 205 (6%) died or had VT during follow-up; of these, 10 (83%) were in the LGE+ group. In the LGE+ group (1) the rate of death/VT per year was >20× higher than LGE− (4.9 versus 0.2%, P<0.01); (2) death/VT were associated with a greater burden of LGE (14±11 versus 5±5%, P<0.01) and right ventricular dysfunction (right ventricular EF 45±12 versus 53±28%, P=0.04). LGE burden was the best predictor of death/VT (area under the receiver-operating characteristics curve, 0.80); for every 1% increase of LGE burden, the hazard of death/VT increased by 8%.
Conclusions—
Sarcoidosis patients with LGE are at significant risk for death/VT, even with preserved left ventricular ejection fraction. Increased LGE burden and right ventricular dysfunction can identify LGE+ patients at highest risk of death/VT.
Keywords: cardiac arrhythmias, cardiac magnetic resonance, cardiomyopathy, defibrillator, gadolinium, heart failure, sarcoidosis
Sarcoidosis is a systemic disorder characterized by noncaseating granulomatous infiltration that most commonly affects the lungs and lymph nodes. However, autopsy data suggest that the major cause of death is cardiac arrhythmia and heart failure due to myocardial infiltration.1 Manifestations of cardiac involvement can range from no symptoms to advanced heart failure requiring transplantation and to sudden cardiac death. Regions of granulomatous infiltration are thought to evolve into scar tissue that serves as a substrate for re-entry ventricular tachycardia (VT)2 and atrial arrhythmias.3,4 Current guidelines from the American Heart Association consider cardiac sarcoidosis (CS) a class IIA indication for implantable cardioverter defibrillator (ICD) insertion. However, the level of evidence to support this recommendation is limited5 and no specific guidelines are provided accounting for the associated changes in left ventricular ejection fraction (LVEF) and it is unclear if this recommendation should also apply to patients with CS or other forms of associated myocardial damage who have a preserved LVEF of >50%. It has previously been shown that the primary and secondary prevention annualized ICD therapy rates in patients with CS are 10% and 20%.6,7
See Editorial by Greulich and Mahrholdt
Unfortunately, because CS is a patchy disorder that often involves only small amounts of myocardium without causing obvious abnormalities in LV function, commonly used tests, such as the ECG, echocardiogram, and stress testing do not reliably detect CS.8 It has recently been shown that cardiovascular magnetic resonance (CMR) with late gadolinium enhancement (LGE) imaging can readily identify individuals with CS or associated myocardial damage that are not otherwise clinically recognized because of its ability to accurately detect even small areas of abnormality.9 In fact, >15% of patients with sarcoidosis have cardiac involvement based on CMR, despite the absence of significant LV dysfunction.10 Although it is well known that the presence of CS in individuals with reduced LV systolic function portends a poor prognosis,11,12 the prognosis for those with CS or associated myocardial damage and preserved LV function is unclear. In fact, due to insufficient data, a recent expert consensus document from the Heart Rhythm Society did not identify these individuals as being at sufficient risk to warrant ICD implantation without further risk stratification.13
The aim of this study was to establish whether CMR with LGE imaging (LGE–CMR) can be used to risk stratify patients with known extracardiac sarcoidosis and preserved LVEF (>50%). The specific objectives were to determine (1) the prevalence of CS or associated myocardial damage in this patient population; (2) the rates of major adverse cardiac events in sarcoidosis patients with and without cardiac involvement, and (3) imaging-based covariates that may help to identify individuals with CS or other forms of associated myocardial damage who are at highest risk for adverse cardiac events.
Methods
Study Population
We retrospectively identified 226 subjects with biopsy proven extracardiac sarcoidosis referred for CMR as part of their clinical care to evaluate for suspected CS or associated myocardial damage who had LVEF >50% measured by CMR. This study was performed at a tertiary care referral center; as such, many patients included in this cohort have more complex sarcoidosis. A significant portion of the patients underwent the CMR examination as part of widespread screening, despite the absence of symptoms or ECG abnormalities. This study is an extension of our previously published report.10 Individuals with incomplete CMR data sets (n=9) were excluded. Medical and device records were reviewed to determine patient demographics and identify death (any cause), sustained ventricular arrhythmia (ie, lasting ≥30 s or any polymorphic VT), or appropriate ICD shock. Of note, antitachycardia pacing was not considered as a reason for meeting the end point. The Social Security Death Index was used to identify deaths that are not documented in medical records. Attempts were made to contact patients without documented medical record follow-up at least 12 months after CMR. Although the vital status of all patients was confirmed, those with no follow-up via the medical record or direct telephone call were excluded (n=12, all alive), leaving 205 subjects. Institutional Review Board approval was obtained for this study.
CMR Protocol
CMR was performed using a 1.5T scanner (Achieva, Philips Healthcare) using a 5-channel surface coil. Steady-state free-precession cine CMR of the left ventricle in 3 long-axis (2, 3, and 4 chamber views) planes were acquired, along with a stack of short-axis slices spanning the LV base to apex (retrospectively gated, temporal resolution 25–40 ms). LGE images of the same views were obtained 10 minutes after infusion of gadodiamide or gadobenate dimeglumine (0.1–0.2 mmol/kg) using a T1-weighted gradient-echo pulse sequence with a phase-sensitive inversion recovery reconstruction (typical inversion time 200–300 ms, voxel size 2×2×10 mm, sense factor 1–2). Commercially available software was used to quantify CMR volumetric data, such as LV end-diastolic volume index, LV end-systolic volume index, LV mass index (LVMi), LVEF, right ventricular (RV) EDVi, RVESVi, and RVEF. CS or other associated myocardial damage was defined as the presence of any myocardial LGE, that is, if any LV myocardium had a signal intensity >5 SDs above the mean signal intensity of normal remote myocardium, irrespective of LGE pattern or location in the LV. Total amount of LGE was calculated as a percentage of LV mass (%LGE) using Virtue (Diagnosoft, Durham, NC), Figure 1.
Figure 1.
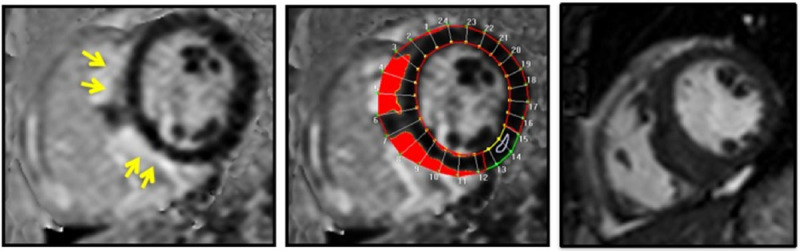
Detection and quantification of late gadolinium enhancement. Images from patient with a history of transient binocular diplopia of unclear pathogenesis presenting with dyspnea on exertion and palpitations. Coronary angiography without obstructive coronary artery disease but with basal inferior wall motion abnormality. Cardiac magnetic resonance reveals mediastinal lymphadenopathy, normal left ventricular ejection fraction (63%), and late gadolinium enhancement as shown above. Lymph node biopsy with non-necrotizing granulomas. Left, A T1-weighted gradient-echo pulse sequence with a phase-sensitive inversion recovery reconstruction showing subepicardial distribution of late gadolinium enhancement in the septum (yellow arrows). Middle, Commercially available software was used to delineate late gadolinium enhancement. Right, Corresponding diastolic frame from steady-state free-precession cine imaging. Red areas denote regions in which the myocardial signal intensity is ≥5 SDs above the region designated as normal (white arrow) by the operator.
Staging and Matching Patients for Pulmonary Sarcoidosis
To exclude the confounding effects of lung disease on patient outcomes, the severity of lung disease was determined by a pulmonologist for the entire cohort using the following parameters: severity of pulmonary sarcoidosis (using Scadding stage14), and pulmonary function test parameters (%predicted total lung capacity, forced expiratory volume 1, forced vital capacity, forced expiratory volume 1/forced vital capacity ratio, and diffusing capacity for carbon monoxide). Subsequently, a subgroup of patients without LGE identified from the entire cohort were matched to the 41 patients with LGE based on age, sex, and the above parameters.
Statistical Methods
Normally distributed continuous variables are presented as mean±SD. Non-normally distributed variables are presented as median (interquartile range). Patients were divided into 2 groups: those with and without LGE on CMR (LGE+ and LGE−, respectively). The LGE+ group was further subdivided into those who did and did not die or have sustained ventricular arrhythmia. Each group was tested for normal distribution using the Kolmogorov–Smirnov test and comparisons between groups were performed using unpaired t-tests. Pearson correlation was used to assess relationships between parameters that predicted adverse events. Composite end point was defined as death or VT. Patients not experiencing the end point were censored at the last known follow-up. Kaplan–Meier method was used to estimate the survival curves in the LGE+ and LGE− groups. In addition, logrank test was used to compare the survival time between the 2 groups. Univariate Cox proportional hazard model was used to assess the relationship between covariates and the survival in the entire study group and in the LGE+ group. Time-dependent receiver–operator characteristics analysis based on the Cox models was performed in the LGE+ group for each of the covariates, and used to evaluate the predictive accuracy for the end point at a mean follow-up duration of 36 months. The optimal cut-off value was identified as the covariate value closest to the point (0.1), representing 100% sensitivity and 100% specificity. Statistical calculations were performed using SPSS (version 22.0, Armonk, NY) and R statistical software (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Population Demographics
This was a middle-aged, predominantly female population (mean age, 56±7 years; 69% female) at moderate cardiovascular risk; 9% had coronary disease, almost half had hypertension, over a fifth had diabetes mellitus and almost a third had dyslipidemia (Table 1). Corticosteroids had been used at some point in almost 60%, whereas just under half were documented as being treated with immunomodulators. Of the 205 study patients, 41 (20%) had evidence of LGE (LGE+ group) and are detailed in Table 2. There was no significant difference in the demographics between those with and without LGE, although there was a trend toward more immunomodulator use in the LGE+ group. There was a significant difference in LVEF, RVEF, and RVESVi, but not in LVMi, LV end-diastolic volume index, LV end-systolic volume index, or RVEDVi between those who had LGE and those who did not (Table 1). The extent of pulmonary disease in our patient cohort was severe in almost half of the 82 (41 LGE+ and 41 LGE−) subjects matched by age, sex, and lung disease severity; 36 (43.9%) of these patients were assigned Scadding stage IV, 29 (35.4%) stage II or III, and only a fifth (17, 20.7%) stage 0 or I. The percent predicted pulmonary function testing parameters for this subgroup were total lung capacity, 82±19%; forced vital capacity, 74±21%; forced expiratory volume 1, 78±24%; forced expiratory volume 1:forced vital capacity, 77±10%; and diffusing capacity for carbon monoxide, 76±29%).
Table 1.
Patient Demographics and Imaging Variables: Comparison of Patients With and Without LGE
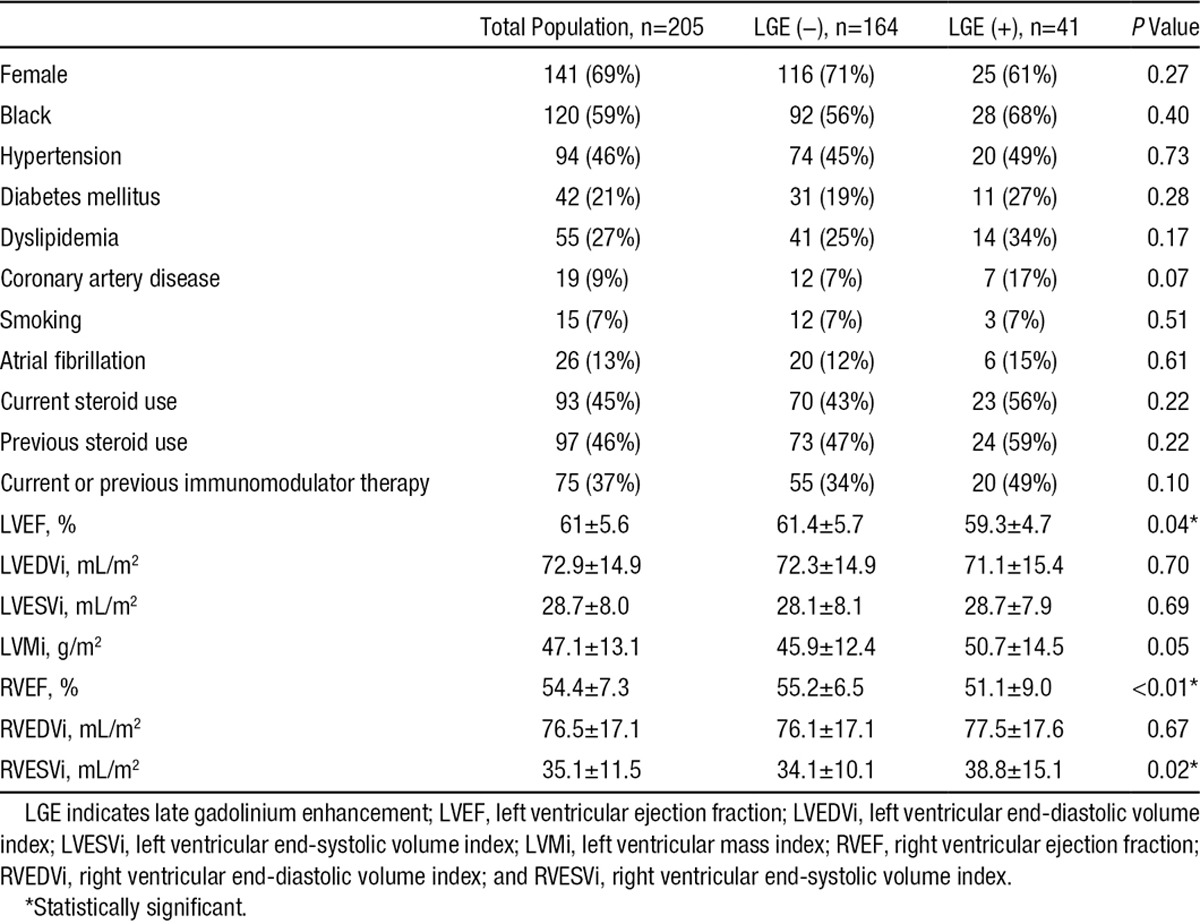
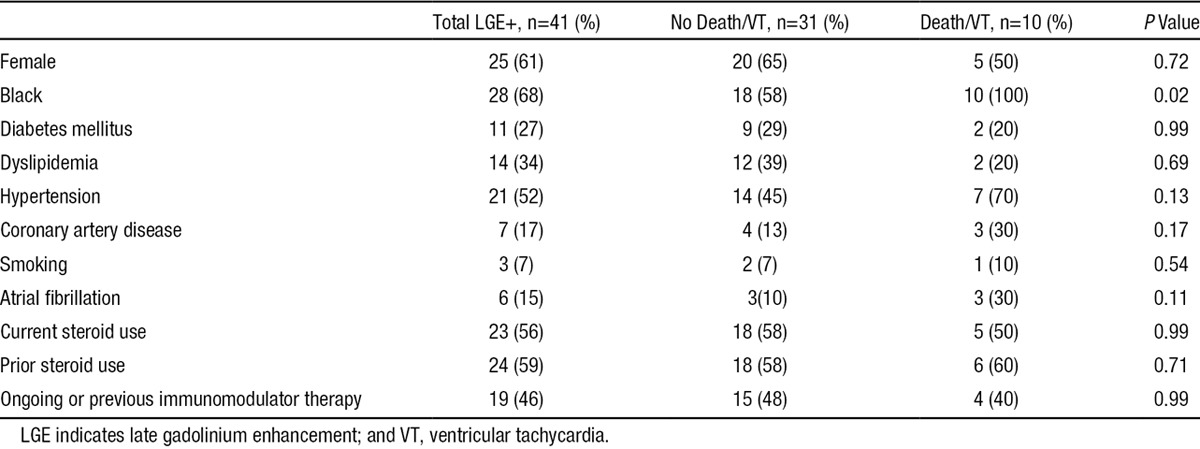
Table 2.
Comparison of Patient Demographics in Those With LGE Who Died or Sustained VT Against Those Who Did Not
Outcomes
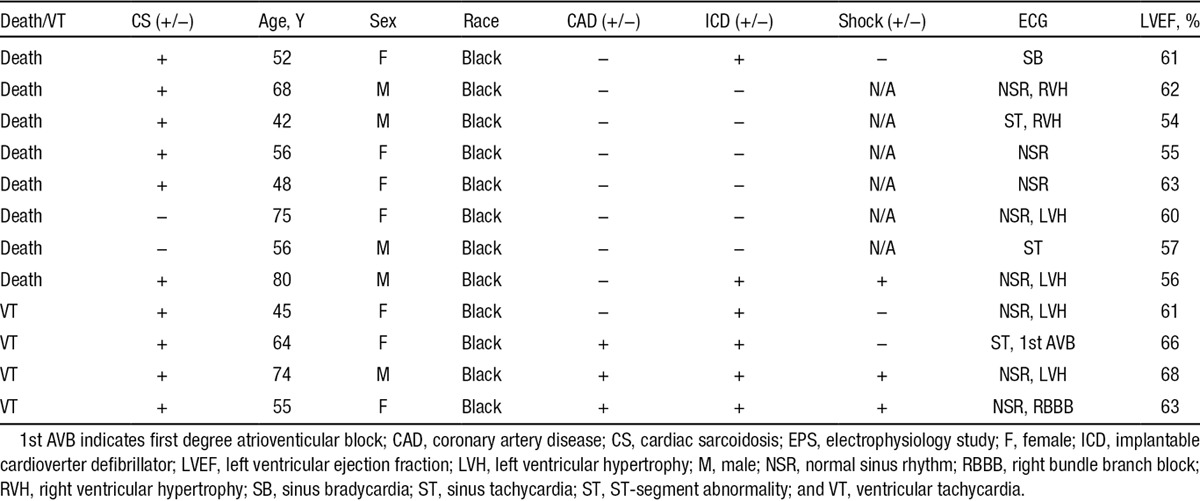
Patients were followed for a mean of 36±18 months. Despite having preserved LVEF (61±5.6%), 12 (5.9%) patients had a cardiac event (8 [3.9%] patients died and 4 [2.0%] had sustained VT) during follow-up. Ten of these 12 (83.3%) patients were in the LGE+ group. Details about the 12 individual who had an event are presented in Table 3. Of the patients with LGE who had events, only a minority of patients had an LGE pattern that might suggest the presence of a confounding disorder, such as pulmonary hypertension or myocardial infarction. Specifically, 7 individuals had a nonmyocardial infarction pattern (suggesting sarcoid infiltration or other some other form of associated myocardial damage), 2 had RV insertion point pattern suggesting pulmonary hypertension, and 1 had a myocardial infarction pattern. Of the 31 patients who had LGE but no event, 26 had a nonmyocardial infarction pattern of LGE, 2 had RV insertion point pattern, and 3 had a myocardial infarction pattern.
Table 3.
Detailed Demographics of Patients Who Died or Sustained VT
The rate of death or VT per year within the LGE+ group was >20× higher than that of the LGE− group (annualized event rate 4.93% versus 0.24%, respectively; P<0.05; Figure 2). Cox proportional hazard models (Figure 3 showed that the presence of LGE (hazard ratio [HR], 24.5), the percent amount of LGE (HR, 1.14), RVESVi (HR, 1.07), and RVEF (HR, 0.88) were significantly associated with the end point.
Figure 2.
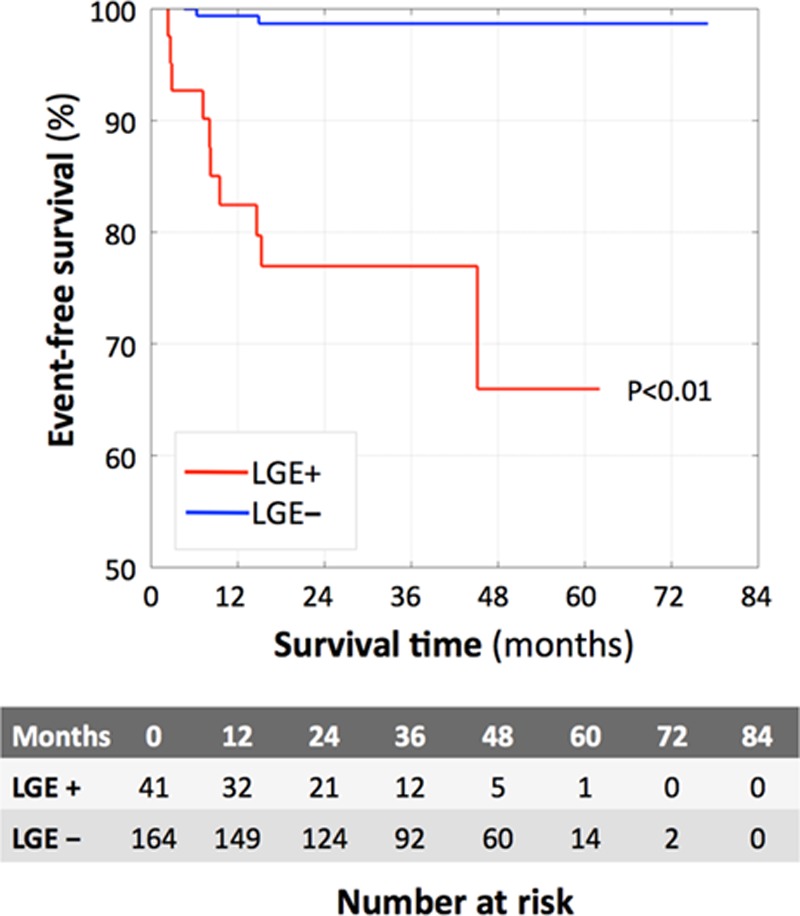
Kaplan–Meier curves demonstrating the impact of cardiac sarcoidosis on survival in the late gadolinium enhancement (LGE)+ (red) and LGE− (blue) groups. P value refers to logrank test LGE+ vs LGE− survival.
Figure 3.
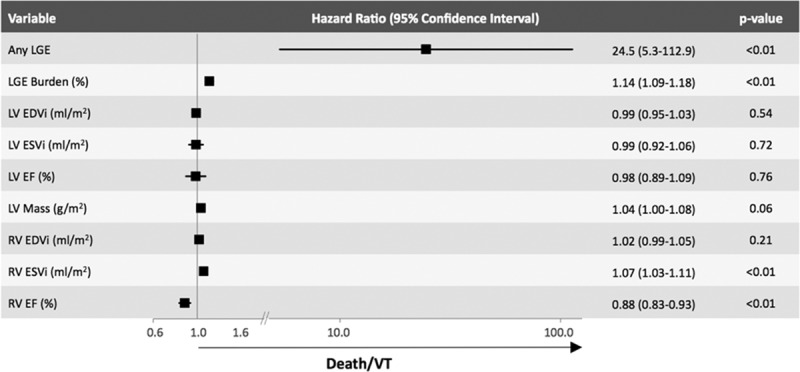
Univariate Cox proportional hazard models for the total population demonstrating that the presence of LGE has a hazard ratio of 24.5 (5.3–112.9; P<0.01) for death or ventricular tachycardia (VT). LVEDVi indicates left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume index; RVEF, right ventricular ejection fraction; RVEDVi, right ventricular end-diastolic volume index; and RVESVi, right ventricular end-systolic volume index.
The specific cause of death could not be determined in the majority of patients who died. Notably, 6 of the 10 patients in the LGE+ group who had a cardiac event had an ICD. Of these, 3 had received appropriate shocks for ventricular arrhythmias, 2 for VT who survived, and 1 for VT/ventricular fibrillation who died without receiving therapy for VT/ventricular fibrillation. Of the 2 patients who died in the LGE− group, one had a pulseless electric activity arrest and the other had an unknown cause of death. Of note, 17 of the 41 (41%) LGE+ patients had a Holter Monitor; whereas only 43 of 164 (26%) LGE− patients did.
When 41 LGE+ patients were blindly matched with 41 LGE− patients by age, sex, and severity of lung disease, no cases of death/VT were found in the LGE− subjects, suggesting that the adverse outcomes in the LGE+ group were not explained by severity of pulmonary disease. Similarly, when we excluded patients with documented coronary artery disease from our cohort, 7 of the 34 (20.6%) LGE+ patients still had death/VT; whereas only 2 of 152 (1.3%) LGE− patients did, P<0.0001, suggesting that the adverse events occur even in the absence of coronary disease. Although 3 of the patients who died had coronary disease, it is difficult to draw any meaningful conclusions from our cohort about the influence of concomitant coronary disease on outcomes in patients with CS given the small number of patients who were known to have both conditions and because a coronary artery disease evaluation was performed at the discretion of the treating physician and not as part of a systematic protocol. Of the 3 patients who had documented coronary artery disease and a cardiac event, their LGE pattern was: 1 subendocardial pattern typical for previous myocardial infarction, 1 focal subendocardial pattern atypical for previous myocardial infarction, and 1 subepicardial pattern atypical for previous myocardial infarction. In the subgroup of patients who had both preserved LVEF and RVEF ≥50% (n=154), 27 (17.5%) had LGE involving the LV and 127 (82.5%) were LGE−, whereas 38% of patients with RVEF <50% had LGE involving the LV. Of the patients with LVEF and RVEF ≥50%, 5 (18.5%) of the LGE+ had death/VT (2=VT and 3=death), whereas only 1 (0.8%) LGE− patient in this cohort died and none had VT (P=0.0006).
Covariates That Predicted Death/VT Within the LGE±Group
Within the LGE+ subgroup, all patients in our cohort who experienced death or VT were black; whereas only 58% of patients who did not experience death or VT were black (P=0.02). Demographics and cardiovascular risk factors were otherwise similar. When compared with those without death or VT, individuals who died or had VT demonstrated a higher burden of LGE (14.1±10.7 versus 5.1±4.7%, P=0.004), as well as more RV dysfunction (RVEF, 45.4±12.3 versus 52.7±27.5%; P=0.037) and RV end-systolic dilation (RVESVi, 50.1±17.9 versus 36.2±13.1 mL/m2; P=0.048; Table 4). Covariates associates with the end point in the LGE+ group (Figure 4) were the same as in the entire study group, with a 8% increase in the risk of death or VT for each 1% increase in LGE burden (HR, =1.08 [1.02–1.14]; P=0.01; Figure 4).
Table 4.
Comparison of Imaging Variables in Patients With LGE Who Died or Sustained VT Against Those Who Did Not Die or Have VT
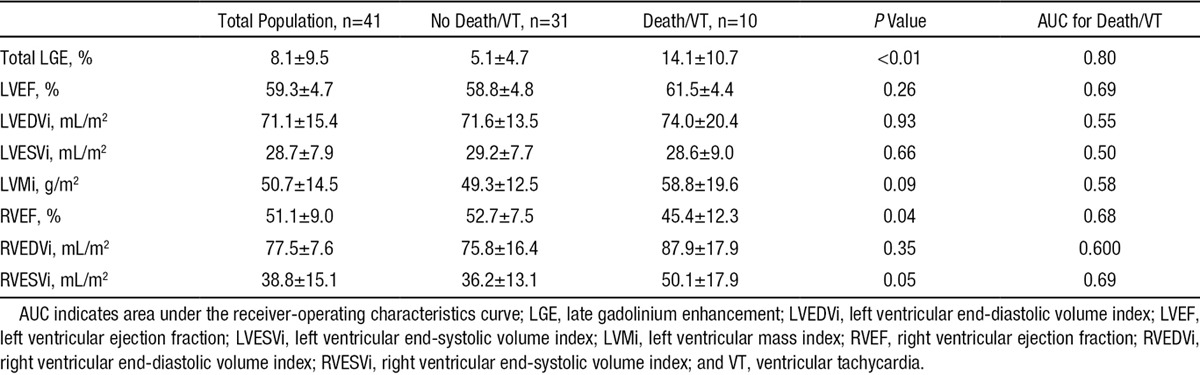
Figure 4.
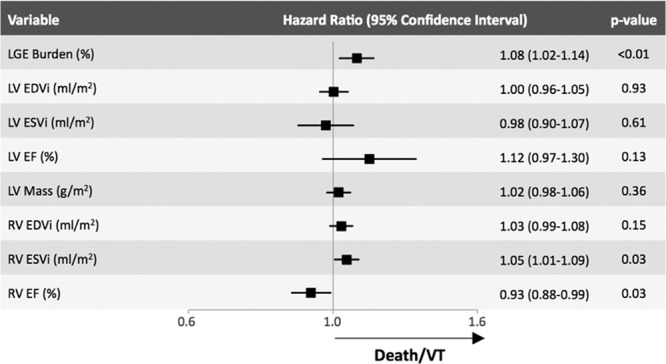
Univariate Cox proportional hazard models for the late gadolinium enhancement (LGE)+ group, demonstrating that hazard ratio of the burden of LGE for predicting death or ventricular tachycardia (VT) was 1.08 (1.02–1.14; P=0.01) equivalent to a 8% increase in the hazard of death or VT for each 1% increase in burden of LGE. LVEDVi indicates left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume index; RVEF, right ventricular ejection fraction; RVEDVi, right ventricular end-diastolic volume index; and RVESVi, right ventricular end-systolic volume index.
In patients with LGE, receiver–operator characteristics analysis indicated that LGE burden was the best predictor of adverse events in the mean follow-up (36 months), with an area under the receiver-operating characteristics curve of 0.79. In this cohort, the optimal level of LGE burden for the detection of such events was 5.7%, which resulted in a sensitivity of 87% and specificity 62% for identifying individuals with CS or other forms of associated myocardial damage who were at highest risk of death or VT, despite preserved LVEF. Similarly, Crawford et al15 found that in patients with CS and an LVEF ≥35%, and LGE burden of >6% was associated with an increased risk of death or VT. However, these cut-off values should be further prospectively validated in other cohorts before being widely used in clinical practice.
In the subgroup of LGE− patients matched to the LGE+ patients for severity of lung disease, the LGE+ group had significantly lower RVEF (50.4±9 versus 54.4±6.7%, P=0.026) and a trend toward higher RVESVi (39.6±14.7 versus 34.3±11.1 mL/m2; P=0.07) suggesting that direct myocardial involvement of the right ventricle may be responsible for added risk of death/VT, rather than RV remodeling related to underlying lung disease.
Discussion
CMR has the ability to identify individuals with CS or other forms of associated myocardial damage, even when it is otherwise not clinically evident. Although it is recognized that patients with CS who have reduced LVEF are at increased risk for heart failure and sudden cardiac death, it is unknown whether those with preserved LVEF also have a poor prognosis. In this study, we found (like others9,10,16,17) that 1 in 5 individuals with sarcoidosis have cardiac involvement based on the presence of LGE, despite having a preserved LVEF. We also found that cardiac involvement in this cohort was associated with a ≈5% annual risk of death or sustained VT. In fact, almost a quarter of LGE+ patients with a preserved LVEF had a significant event during the average 41 months of follow-up, whereas <2% of patients without LGE had an event during the same time period. This suggests that LGE–CMR is a valuable tool for the cardiac risk stratification of patients with extracardiac sarcoidosis. In patients with LGE, death/VT were significantly more frequent among those with a higher burden of LGE or RV dysfunction, even after attempting to eliminate the confounding effects of lung disease.
With increasing use of CMR, it is apparent that cardiac infiltration and its sequela in sarcoidosis affects more patients than was previously recognized. This is important because progressive heart failure and sudden cardiac death are well known manifestations of CS, even in the absence of significant echocardiographic abnormalities.9,17–19 Within our population, all-cause mortality in the LGE+ cohort was 15% (6/41). This is comparable with 2-year all-cause mortality in major trials of ICD placement for primary prevention, such as the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II20 (21%) and Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE21; 11%). The overall event rate for death/VT in our LGE+ population was 24% (10/41). Again this is similar to ICD trials that report the number of appropriate ICD therapies, which varied between 18% in the DEFINITE trial21 and 21% in Sudden Cardiac Death in Heart Failure Trial (ScD-HEFT).22
LGE–CMR is one of the most promising modalities in this regard, allowing for high-resolution tissue characterization, so that small areas of myocardial fibrosis or infiltration that occur before abnormalities of regional or global LV function can be identified.23,24 Previous studies, which included patients with both normal and abnormal LVEF, have shown that LGE–CMR is more sensitive than ECG, echocardiography, or the Japanese Ministry of Health and Welfare criteria in predicting death and adverse events in CS9,25,26 and thus may provide the means by which those who seem low risk by ECG and echocardiography but remain vulnerable to ventricular arrhythmias can be identified. Interestingly, none of the 12 patients who had death or sustained VT within our cohort met the JMHW criteria for the diagnosis of CS. This suggests that the application of the JMHW criteria may not be well-suited for use in those whose LVEF is >50%, and that the other diagnostic tools such as LGE–CMR may be helpful. In fact, based on the most recent expert consensus document published by the Heart Rhythm Society,13 CMR would not have been recommended for the majority of our population given the absence of a concerning abnormality on the ECG, echocardiogram, or obvious cardiac symptoms (of note, we were not able to determine the presence or the absence of symptoms in every single patient because of the retrospective nature of the study). It must be noted that, unlike what we observed in our cohort, 1 recent study of patients with preserved LV function and no clinical evidence of CS did not find an association between LGE and increased rates of adverse events.27 However, the patients included in that study seemed to have milder disease not requiring immunomodulator therapy and the amount of LGE was not quantified. Thus, it is possible that their cohort was less vulnerable to adverse events than the sarcoidosis populations described by us and other groups.
Previous studies have shown that the presence or the absence of LGE is a strong prognostic marker in patients with suspected CS; however, the relationship between the amount of LGE present and the risk of adverse outcomes was not reported. LGE estimation has often been performed in a qualitative rather than quantitative fashion, relying on the presence or the absence of LGE in each ventricular segment as determined visually. In addition, programmed electric stimulation and possible ICD implantation has been advocated for those whose LVEF is >35% and also have a large burden of LGE.13 However, it has not been defined what constitutes a large burden of LGE. In our study, we quantified LGE burden by delineating areas with a signal intensity ≥5 SDs brighter than user-defined normal remote myocardium. We were thus able to refine risk prediction by calculating the increased hazard of death or sustained VT conferred by each additional 1% increase of LGE burden. Not only did an increasing LGE burden predict adverse cardiac events in our population, but conversely the absence of LGE on CMR provided reassurance that the risk of death/VT is extremely low. On the basis of the Kaplan–Meier curves, the warranty period for a negative CMR study in patients with extracardiac sarcoidosis seems to be at least 4 years. The falloff in the LGE− group on the Kaplan–Meier curve may be confounded by the limited follow-up data available after 4 years.
Although LGE is clearly an important prognostic marker, it is also important to recognize that some patients with LGE will not have adverse events. We examined the role of other imaging-based covariates to further refine the risk of death/VT, and found that RV dysfunction may also play an important role. An association between LV LGE and reduced RV systolic function has been noted in previous studies.10,15 Like Crawford et al,15 our results also indicated that these factors independently predicted adverse outcomes; however, our study expands their findings by only including patients with LVEF >50%. Because pulmonary involvement in sarcoidosis is common, it is logical that this could have either a primary (granulomatous infiltration of the RV muscle) or secondary (pulmonary hypertension) effect on the RV. Unfortunately, as a consequence of the limited spatial resolution of the LGE pulse sequence used in our clinical CMR protocol, our image quality is not sufficient enough to reliably determine how often the RV had evidence of LGE. Similarly, a limitation of some previous studies was that the extent of subjects’ pulmonary disease was unaccounted for, and adverse outcomes may have occurred because of more severe lung disease, rather than as an independent effect of CS or other forms of associated myocardial damage. The role played by RV dysfunction in influencing outcomes seems to be independent of lung disease because in the subgroup of LGE− patients matched to the LGE+ patients for severity of lung disease, the LGE+ group had significantly lower RVEF and a trend toward higher RVESVi, suggesting that direct myocardial involvement of the RV may be responsible for added risk of death/VT, rather than adverse RV remodeling related to underlying lung disease. To the best of our knowledge, this is the first study to match patients by severity of lung disease and thus eliminate this confounding factor, demonstrating that the ability of both LGE burden and RV dysfunction to predict death/VT is independent of the severity of pulmonary disease.
Our findings suggest that in sarcoidosis patients with LGE and preserved LVEF, the presence of RV dysfunction or a higher LGE burden involving the myocardium may identify patients who are at highest risk of death/VT. This could have significant clinical implications because recent recommendations13 acknowledge that invasive testing such as programmed electric stimulation may be required in patients with preserved LVEF needing further risk stratification. Although previous studies involving programmed electric stimulation in patients with CS have suggested that the greatest yield in terms of a positive test was in those with reduced LVEF,28 preserved LV systolic function did not rule out the possibility of significant inducible arrhythmia. Our results demonstrate that the presence of LGE is an important risk factor for death/VT and that the burden of LGE is an important modulator of risk. Further studies are needed to better define the interplay between CMR findings and programmed electric stimulation for identifying which patients are most likely to benefit from ICD implantation.
Although LGE–CMR seems to be a promising technique for the evaluation of patients with suspected CS, it is limited in its ability to differentiate CS in the inflammatory stage versus those in the fibrotic stage. As such, there is an increasing interest in the evaluation T2-mapping techniques, which are thought to be more sensitive for detecting inflammation.29 A comprehensive technique that includes both LGE imaging and T2 mapping may prove to be useful for not only identifying individuals at increased risk for ventricular arrhythmia but also those individuals with CS in the inflammatory stage, which might benefit from treatment with immunomodulator therapy. In addition to CMR, cardiac F(18)-fluorodeoxyglucose positron emission tomography also clearly has role to not only detect the presence of CS but also to potentially guide titration of immunosuppressive therapy.30 It has recently also been shown to be an important tool to risk stratify these patients.31 The relationship between positron emission tomography and CMR for the evaluation of CS has yet to be fully elucidated but the 2 modalities are likely complementary with each providing unique information to be used in the care of these patients.
Although not a focus of this article, another interesting finding that cannot be ignored is that within the LGE+ subgroup, all patients in our cohort who experienced death or VT were black; whereas only 58% of patients who did not experience death or VT were black (P=0.02). Although we have no doubt that whites with CS can also die or have VT, our cohort provides a strong signal that blacks may be at particularly increased risk. Unfortunately, most other studies related to the use of CMR in patients with sarcoidosis have not reported patient race, so we do not know how this finding compares to other cohorts. However, an outcomes study similar to this but using positron emission tomography also reported a higher risk of major cardiovascular events in blacks.31 Interestingly, data from The Black Women’s Health Study have suggested that women with sarcoidosis were nearly twice as likely to die prematurely as women without the disease.32 Further work is needed to better understand the mechanism underlying this racial difference.
Limitations of this study include the fact that it was retrospective, and it was performed in a single tertiary center, thus referral bias is likely present. Our findings need to be validated prospectively in another cohort before being adopted widely in clinical care. Another limitation of our study is that Holter Monitoring was not performed consistently in all patients and some patients with VT could have been missed. In addition, the cause of death is not always known in our cohort. Because of the slice thickness, we used on our LGE pulse sequence, we were also not able to reliably assess for RV LGE. In addition, although we tried to account for the confounding effects of pulmonary hypertension and coronary artery disease as best as possible, right heart catheterization and coronary angiography were not routinely performed in this cohort, which limits our understanding of these potential confounders. Finally, because of the low event rate and the retrospective nature of our study, we are unable to reliably determine if results from the CMR examination were used to modify immunomodulator therapy and whether such changes might have influenced patient outcomes.
Conclusions
CS is associated with an increased rate of death/VT even in patients with preserved LV function. The burden of LGE and the severity of RV dysfunction further refine the risk of death/VT in patients with CS.
Disclosures
Dr Murtagh is currently an employee of Abbott Diagnostics (since 7/6/15); however, she was used by the University of Chicago at the time of the study. Abbott did not provide any funding and had no role in study design, research, analysis, writing, or approving of the publication.
Supplementary Material
CLINICAL PERSPECTIVE
Cardiac sarcoidosis is associated with an increased risk of heart failure and sudden death, especially when associated with reduced left ventricular ejection fraction. In this study, we show that myocardial damage, as detected by cardiovascular magnetic resonance late gadolinium enhancement imaging, is present in a fifth of individuals with known extracardiac sarcoidosis, despite having a preserved left ventricular ejection fraction. Furthermore, the presence of myocardial damage in these patients is associated with a significantly increased risk of death and sustained ventricular tachycardia and that the risk is further modulated by the burden of late gadolinium enhancement and the presence of concomitant right ventricular dysfunction. Our data also show that the absence of late gadolinium enhancement in these patients is associated with a low risk of death or sustained ventricular tachycardia. It is well known that cardiac sarcoidosis is difficult to diagnose and not readily detected by electrocardiography, echocardiography, or even endomyocardial biopsy. Our findings continue to build on the growing body of data establishing the important role of cardiovascular magnetic resonance in the assessment and risk stratification of patients with suspected cardiac sarcoidosis, even those who have a preserved left ventricular ejection fraction. Sarcoidosis patients who have evidence of a significant amount of myocardial damage and associated right ventricular abnormalities should be evaluated for possible implantable cardioverter defibrillator insertion; however, the role of cardiovascular magnetic resonance to guide immunosuppressive therapy in these patients is yet to be determined.
References
- 1.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 2.Banba K, Kusano KF, Nakamura K, Morita H, Ogawa A, Ohtsuka F, Ogo KO, Nishii N, Watanabe A, Nagase S, Sakuragi S, Ohe T. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm. 2007;4:1292–1299. doi: 10.1016/j.hrthm.2007.06.006. doi: 10.1016/j.hrthm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Viles-Gonzalez JF, Pastori L, Fischer A, Wisnivesky JP, Goldman MG, Mehta D. Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest. 2013;143:1085–1090. doi: 10.1378/chest.11-3214. [DOI] [PubMed] [Google Scholar]
- 4.Cain MA, Metzl MD, Patel AR, Addetia K, Spencer KT, Sweiss NJ, Beshai JF. Cardiac sarcoidosis detected by late gadolinium enhancement and prevalence of atrial arrhythmias. Am J Cardiol. 2014;113:1556–1560. doi: 10.1016/j.amjcard.2014.01.434. doi: 10.1016/j.amjcard.2014.01.434. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Schuller JL, Zipse M, Crawford T, Bogun F, Beshai J, Patel AR, Sweiss NJ, Nguyen DT, Aleong RG, Varosy PD, Weinberger HD, Sauer WH. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2012;23:925–929. doi: 10.1111/j.1540-8167.2012.02350.x. doi: 10.1111/j.1540-8167.2012.02350.x. [DOI] [PubMed] [Google Scholar]
- 7.Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D, Greenspon AJ, Ortman M, Delurgio DB, Valadri R, Narasimhan C, Swapna N, Singh JP, Danik S, Markowitz SM, Almquist AK, Krahn AD, Wolfe LG, Feinstein S, Ellenbogen KA. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15:347–354. doi: 10.1093/europace/eus316. doi: 10.1093/europace/eus316. [DOI] [PubMed] [Google Scholar]
- 8.Freeman AM, Curran-Everett D, Weinberger HD, Fenster BE, Buckner JK, Gottschall EB, Sauer WH, Maier LA, Hamzeh NY. Predictors of cardiac sarcoidosis using commonly available cardiac studies. Am J Cardiol. 2013;112:280–285. doi: 10.1016/j.amjcard.2013.03.027. doi: 10.1016/j.amjcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AR, Klein MR, Chandra S, Spencer KT, Decara JM, Lang RM, Burke MC, Garrity ER, Hogarth DK, Archer SL, Sweiss NJ, Beshai JF. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail. 2011;13:1231–1237. doi: 10.1093/eurjhf/hfr099. doi: 10.1093/eurjhf/hfr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M Central Japan Heart Study Group. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 12.Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. doi: 10.1016/j.amjcard.2004.08.083. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 13.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 14.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br Med J. 1961;2:1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, Schuller J, Kron J, Nour KA, Cheng A, Ji SY, Feinstein S, Gupta S, Ilg K, Sinno M, Abu-Hashish S, Al-Mallah M, Sauer WH, Ellenbogen K, Morady F, Bogun F. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:1109–1115. doi: 10.1161/CIRCEP.113.000156. doi: 10.1161/CIRCEP.113.000156. [DOI] [PubMed] [Google Scholar]
- 16.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–1690. doi: 10.1016/j.jacc.2005.01.047. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 17.Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S, Ong P, Wagner A, Schneider S, Nassenstein K, Gawaz M, Sechtem U, Bruder O, Mahrholdt H. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–511. doi: 10.1016/j.jcmg.2012.10.021. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Roberts WC, McAllister HA, Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977;63:86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 19.Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119:167–172. [PubMed] [Google Scholar]
- 20.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 21.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, Sanders WE, Schaechter A, Levine JH Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 22.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 23.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 24.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 25.Tadamura E, Yamamuro M, Kubo S, Kanao S, Saga T, Harada M, Ohba M, Hosokawa R, Kimura T, Kita T, Togashi K. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol. 2005;185:110–115. doi: 10.2214/ajr.185.1.01850110. doi: 10.2214/ajr.185.1.01850110. [DOI] [PubMed] [Google Scholar]
- 26.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Cheriex EC, Gorgels AP, Crijns HJ. The additional value of gadolinium-enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis. Chest. 2005;128:1629–1637. doi: 10.1378/chest.128.3.1629. doi: 10.1378/chest.128.3.1629. [DOI] [PubMed] [Google Scholar]
- 27.Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest. 2014;146:1064–1072. doi: 10.1378/chest.14-0139. doi: 10.1378/chest.14-0139. [DOI] [PubMed] [Google Scholar]
- 28.Mehta D, Mori N, Goldbarg SH, Lubitz S, Wisnivesky JP, Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2011;4:43–48. doi: 10.1161/CIRCEP.110.958322. doi: 10.1161/CIRCEP.110.958322. [DOI] [PubMed] [Google Scholar]
- 29.Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014;189:109–112. doi: 10.1164/rccm.201309-1668LE. doi: 10.1164/rccm.201309-1668LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, Hainer J, Murthy VL, Skali H, Dorbala S, Di Carli MF, Blankstein R. Reduction in 18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. doi: 10.1007/s12350-013-9828-6. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 31.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tukey MH, Berman JS, Boggs DA, White LF, Rosenberg L, Cozier YC. Mortality among African American women with sarcoidosis: Data from the Black Women’s Health Study. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:128–133. [PMC free article] [PubMed] [Google Scholar]


