ABSTRACT
OBJECTIVE:
A new epidermal harvesting tool (CelluTome; Kinetic Concepts, Inc, San Antonio, Texas) created epidermal micrografts with minimal donor site damage, increased expansion ratios, and did not require the use of an operating room. The tool, which applies both heat and suction concurrently to normal skin, was used to produce epidermal micrografts that were assessed for uniform viability, donor-site healing, and discomfort during and after the epidermal harvesting procedure.
DESIGN:
This study was a prospective, noncomparative institutional review board–approved healthy human study to assess epidermal graft viability, donor-site morbidity, and patient experience.
SETTING:
These studies were conducted at the multispecialty research facility, Clinical Trials of Texas, Inc, San Antonio.
PATIENTS:
The participants were 15 healthy human volunteers.
RESULTS:
The average viability of epidermal micrografts was 99.5%. Skin assessment determined that 76% to 100% of the area of all donor sites was the same in appearance as the surrounding skin within 14 days after epidermal harvest. A mean pain of 1.3 (on a scale of 1 to 5) was reported throughout the harvesting process.
CONCLUSIONS:
Use of this automated, minimally invasive harvesting system provided a simple, low-cost method of producing uniformly viable autologous epidermal micrografts with minimal patient discomfort and superficial donor-site wound healing within 2 weeks.
KEYWORDS: donor site, epidermal graft, split-thickness skin graft, suction blister
INTRODUCTION
For centuries, skin grafts have been successfully used as coverage over acute and postacute wounds. This wide application has made skin the most transplanted organ in the body.1 Split-thickness skin grafts (STSGs) are commonly used to help close burn wounds, melanomas, and chronic wounds, including venous leg ulcers, diabetic foot ulcers, and pressure ulcers; however, STSGs are often associated with donor-site damage and low expansion ratios, and often necessitate the use of an anesthetic and operating room.2,3 Wound healing of an STSG donor site can give rise to complications that cause the patient more detriment than the condition for which the graft was indicated. Problems with the donor site may include excessive pain, pruritus, infection, dyschromia, delayed healing, and hypertrophic scarring, particularly in patients with poor healing properties due to various comorbidities.4,5
Another drawback to traditional skin grafting techniques is the cosmetic outcome, including hypopigmentation, hyperpigmentation, and hypertrophic scarring.6,7 Development and differentiation of the epidermis are regulated by the underlying dermis.8,9–11 Therefore, for techniques such as STSG and pinch grafting, where both the epidermis and dermis are captured, the graft retains some of the characteristics of the donor site; hence, the cosmetic outcome is not always an exact match compared with the surrounding skin.
Epidermal grafting, or using autologous epidermis that has been minced to expand and cover wounds much larger than the donor site, offers an alternative to traditional autografts and uses only a minimal amount of autologous tissue from the donor site.3 Only the epidermal portion of the donor area is grafted, and consequently, the graft acquires the epidermal architecture and characteristics of the recipient site, not the donor site, potentially leading to better color match and cosmetic outcome.11
Epidermal grafting using suction blisters has been previously shown to be an effective treatment alternative for vitiligo and closure of hard-to-heal wounds, including burns and lower-extremity ulcers.12–14 However, use of conventional suction methods that achieve dermoepidermal separation has been limited in clinical practice because of the lack of a reliable and automated method for harvesting patient epidermal skin. Previous harvesting methods have also been described as cumbersome and time consuming.14–18
An automated epidermal harvesting technique is now commercially available and involves a tool that applies both heat and suction concurrently to normal skin to induce epidermal micrograft formation. For this institutional review board (IRB)–approved study, the new automated technique for suction blister epidermal harvesting was used to create micrografts from the desired donor sites of 15 healthy adult volunteers. The purpose of the healthy human study was to determine viability of the epidermal grafts, pain associated with use of the epidermal harvesting tool, and the appearance of donor-site healing postharvest.
MATERIALS AND METHODS
Selection of Healthy Human Study Population and Overall Experimental Design
The healthy human study was prospectively conducted at a single site (Clinical Trials of Texas [CTT], San Antonio) and enrolled 15 healthy human subjects. All procedures were performed under a protocol approved by an IRB (IntegReview Ethical Review Board, Austin, Texas). Informed consent was obtained, and all subjects were assessed for pain and dermal response scores throughout the study as described below. Subjects were compensated directly by CTT and interacted solely with the independent clinical site staff throughout the study. The duration of study participation per subject was as follows: visit 1 screening: 28 days prior to and including day 0; visit 2 epidermal harvesting visit: day 0; visit 3 follow-up: day 7 (± 2) days; visit 4 follow-up: day 14 (± 2) days; and visit 5 follow-up: day 28 (± 2) days. The primary study end point was the viability of harvested epidermal microdome arrays. The secondary end points were pain assessments during and after harvesting (described below) and donor-site appearance after microdome harvesting as assessed by a board-certified clinician at CTT immediately after harvest, as well as at 7, 14, and 28 days. The study was conducted following the International Conference on Harmonisation, Good Clinical Practice guidelines, and Food and Drug Administration regulations and has been audited by an independent clinical and regulatory consulting firm (Health Policy Associates, Inc, Westwood, Massachusetts).
CelluTome Epidermal Micrograft Harvesting Procedure
An automated system (CelluTome Epidermal Harvesting Device; Kinetic Concepts, Inc [KCI], San Antonio, Texas) for suction blister epidermal harvesting was used to create micrografts on all inner thigh donor sites. Body hair was removed using water and disposable razor blades, then the inner thigh donor site was cleaned with 70% isopropyl alcohol wipes. The sterile harvester was secured around the subject’s inner thigh, and the vacuum head was snapped onto the harvester. The automated suction blister epidermal harvesting system was initiated, and vacuum (−400 to −500 mm Hg) and heat (37° C to 41° C) were applied to donor sites until epidermal micrografts were formed (30–45 minutes).
At the time of harvest, the vacuum head was detached from the harvester, and immediately, a transparent film dressing (Tegaderm; 3M, St Paul, Minnesota) was placed on top of the micrografts. The micrografts were harvested by activating the harvester’s blue handle, which sets the blade in motion. A 5 × 5-cm array consisting of 128 epidermal micrografts approximately 1.8 mm in diameter and spaced approximately 2 mm apart was then collected onto the adhesive transparent film (Figures 1 and 2).
Figure 1.
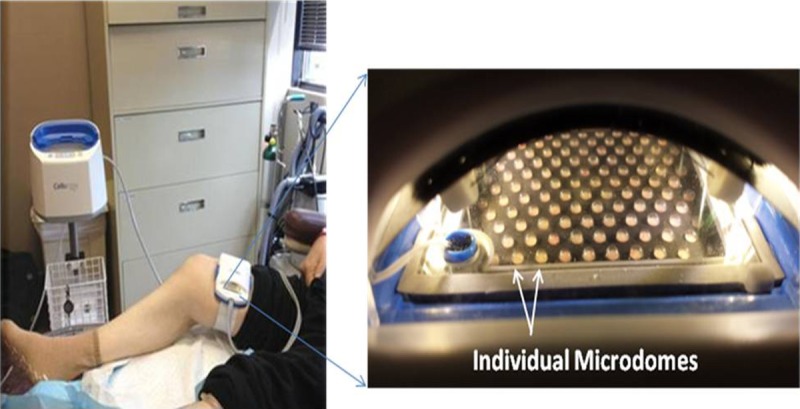
EPIDERMAL HARVESTER
Figure 2.
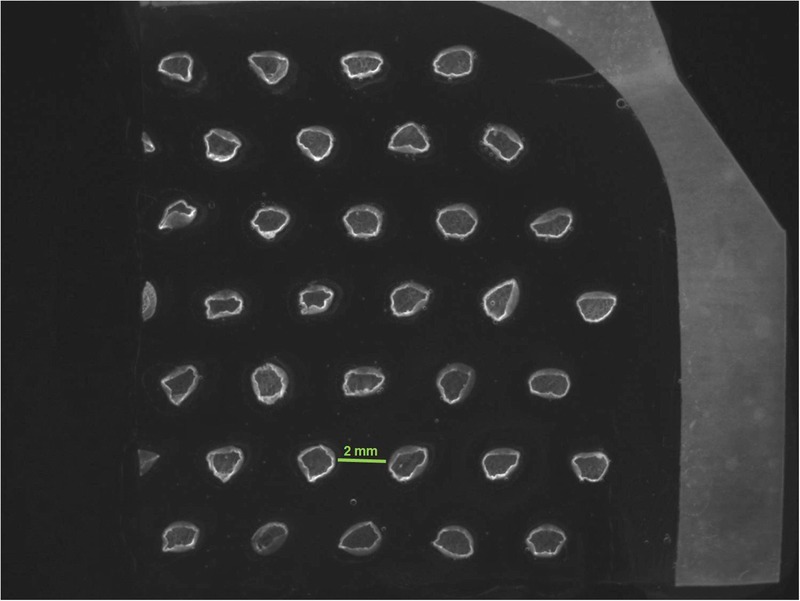
EPIDERMAL MICROGRAFTS TRANSFERRED ONTO TRANSPARENT FILM DRESSING
A stereoscope image of 1 section of original array with 33 intact epidermal micrografts is shown (4.9×). Images and photographs are owned by KCI Licensing Inc.
Left, Automated system placed on a subject’s inner thigh. Right, Close-up of harvester shows microdomes of epidermal graft formation after 30 minutes. Images and photographs are owned by KCI Licensing Inc, San Antonio, Texas.
Each 5 × 5-cm micrograft array was placed in a sterile 150-cm2 cell culture flask with a recloseable lid (TPP Techno Plastic Products, Trasadingen, Switzerland) with 150 mL of liquid medium (EpiLife Medium; Life Technologies, Carlsbad, California) prepared with 60 μM calcium chloride and immediately transported (for approximately 30 minutes) to a local laboratory for analysis. No local anaesthetics were administered to subjects during or after micrograft formation and harvesting.
Donor-Site Care
While the Tegaderm dressing containing the microdomes was being transferred to the research team, the clinician removed the harvester from the subject’s inner thigh and immediately assessed the donor-site appearance as described below. The clinician also recorded whether any minor superficial bleeding was visible after microdome harvesting. After assessing donor-site appearance, the clinician took a digital photograph of the donor site for the record. After the photograph was taken, the donor site was covered with the Tegaderm dressing. Subjects were instructed to refrain from soaking the donor site in water for prolonged periods and to wear loose-fitting clothing until the follow-up (visit 3) when the transparent film dressing was removed.
Cell Viability Staining
Uniform micrograft viability across the array was assessed using fluorescence-based cell viability assay staining and image analysis. One section of each 5-cm2 micrograft array was labeled with fluorescent probes (Molecular Probes, Eugene, Oregon) from a cell viability/cytotoxicity kit (LIVE/DEAD; Life Technologies) containing calcein AM (green fluorescent marker) and ethidium homodimer 1 (red fluorescent marker) following the manufacturer’s instructions for use and with a nuclear stain (Hoechst 33342, blue fluorescent marker; Molecular Probes). A solution of 2 μM calcein AM and 4 μM ethidium homodimer 1 was prepared in Dulbecco phosphate-buffered saline (Gibco, Carlsbad, California). Separately, a stock solution of Hoechst 33342 was diluted in a liquid medium (EpiLife medium; Life Technologies) prepared with 60 μM calcium chloride to a final concentration of 43 μM.
The epidermal micrograft array was incubated for 30 to 45 minutes in a 37° C humidified chamber with 5% CO2. Following incubation, the arrays were placed in fresh EpiLife medium and were visualized at 4.9× and 82× using a fluorescence stereo microscope (Leica M205 FA; Leica Microsystems, Buffalo Grove, Illinois) with Leica Application Suite 3.5.0 software (Leica Microsystems). Individual micrografts were then assessed using an inverted fluorescence motorized microscope with individual FITC (to assess viable microdomes), TRITC (to assess dead microdomes), and DAPI (to assess cell nuclei) filters (Olympus IX81; Olympus, Center Valley, Pennsylvania); 100× images were collected using Image-Pro Plus 7.0 software (MediaCybernetics, Bethesda, Maryland).
Pain Assessment During Micrograft Formation and After Micrograft Harvesting
The 15 subjects were assessed for pain during micrograft formation and after micrograft harvesting using the Wong-Baker FACES Pain Rating Scale.19 The subjects were asked to select a face on the scale that best represented the amount of pain experienced within 1 minute of device initiation, at 10 ± 1 minutes, 20 ± 1 minutes during micrograft formation, and within 1 minute of micrograft harvesting and at the 7 day follow-up visit. The pain score was recorded as follows: 0 (no hurt), 1 (hurts a little bit), 2 (hurts a little more), 3 (hurts even more), 4 (hurts a whole lot), and 5 (hurts worst).
Donor-Site Healing and Dermal Response Assessment
Donor-site healing immediately after micrograft harvesting was assessed at the clinic using a skin appearance scale and dermal response score. Digital photographs were taken of the subject’s donor site before and after the harvesting procedure and at all follow-up visits. Visits during which digital photographs were captured included visit 1 (before placement of the automated epidermal harvesting system on the inner thigh), visit 2 (immediately after micrograft harvesting, prior to placing transparent film dressing), visit 3 (7 days after harvest and after transparent film dressing removal), visit 4 (14 days after harvesting), and visit 5 (28 days after harvest). The dermal response score assessed by the clinician was based on the following scale: 0 meaning no evidence of irritation; 1 meaning minimal, barely perceptible erythema; 2 meaning definite, readily visible erythema, minimal edema, or minimal papular response; 3 meaning erythema and papules; 4 meaning definite edema; 5 meaning erythema, edema, and papules; 6 meaning vesicular eruption; and 7 meaning strong reaction spreading beyond the donor site. Donor-site skin appearance in comparison with surrounding skin was summarized using frequencies and percentages. Ranges in the skin appearance scale used in this study were based on quadrant measurement ranges provided in the Bates-Jensen Wound Assessment Tool to estimate the percentage of donor-site skin that was the same in appearance as the surrounding tissue.20 At each time point, the estimated percentage of donor site that matched the appearance of the healthy surrounding tissue was noted within the following 4 ranges: less than or equal to 25%, 26% to 50%, 51% to 75%, and 76% to 100%.
Statistical Analyses
For the viability of harvested epidermal micrograft arrays, the live rate of micrografts was computed as follows: LIVE rate = [number of live micrografts] / [total number of assessable micrografts]. Summary statistics included n, mean, SD, median, minimum and maximum value, and 95% confidence interval. The frequency and percentage of subjects who achieved uniform viability without clustered unviable grafts were summarized based on the population. The uniform viability rate was calculated as follows: uniform viability rate = [number of subjects whose micrografts achieved uniform viability] / [population]. The population for viability assessment was 12 subjects out of 15, and donor-site samples from 3 subjects out of 15 were allocated for growth factor analyses described in a separate report.21 Viability was defined as the presence of green fluorescence within the micrograft, indicative of active cell metabolism. Uniform viability of the micrograft array was defined as a lack of dead micrograft clusters. A cluster was defined as no more than 2 dead (red) micrografts side-by-side on the array. Summary statistics were provided as follows: n, mean, SD, median and range (minimum, maximum) for treating pain score or skin appearance score as a continuous variable, and frequencies and percentages for treating pain score or skin appearance score as a categorical variable.
RESULTS
Subject Demographics
The epidermal micrograft harvesting technique was performed on 15 healthy human subjects (8 men [53.3%] and 7 women [46.7%]). All subjects were white (6 were Hispanic or Latino), with a mean age of 51.9 years (range, 42–70 years). One subject missed visit 5 (28 days after harvest); otherwise, all visits were attended by all subjects.
Epidermal Micrograft Viability
Donor-site samples from 12 of 15 subjects were allocated for viability, providing 1 donor-site micrograft array with approximately 30 micrografts for each in vitro assessment. Approximately 360 micrografts were assessed for viability from a total of 12 subjects. Of the 12 subjects assessed, 10 subjects demonstrated 100% epidermal micrograft viability. One subject had a total of 38/39 viable micrografts with 1 single nonviable micrograft, resulting in 97.4% viability. A second subject had a total of 29/30 viable micrografts with 1 single nonviable micrograft, resulting in 96.6% viability. Overall for all 12 subjects, there was 99.5% average viability of epidermal micrografts with SD of 1.2%. All 12 subjects demonstrated uniform micrograft viability without evidence of any clusters, defined as greater than or equal to 2 adjacent dead micrografts. Figure 3 (A and B) demonstrates a representative viable micrograft at various magnifications to display the characteristic green network of live basal epidermal cells throughout the micrograft, as well as some red-stained nuclei representing cells from the differentiated, apoptotic granular layer. Blue-stained nuclei were found in all nucleated cells throughout the epidermis. Secreted growth factors were analyzed from 216 micrografts obtained from the remaining 3 subjects in the study and were reported elsewhere.21
Figure 3.
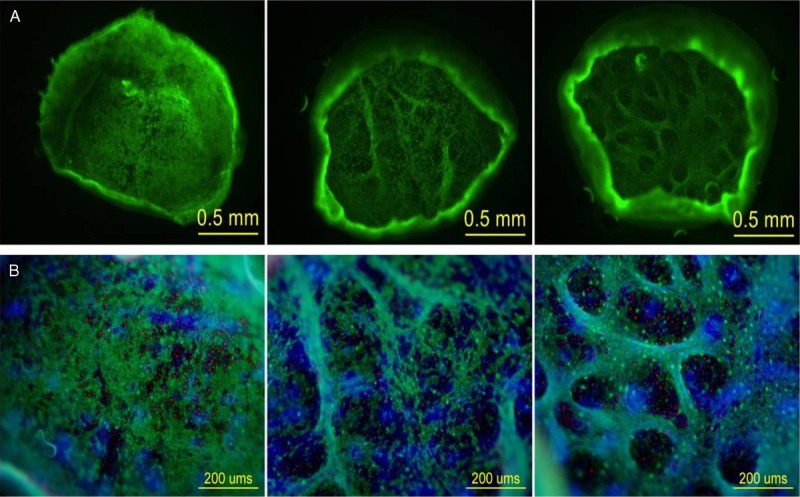
A AND B. MICROGRAFTS AT 82× AND 100× WITH FILTERS
A, A representative viable micrograft from 3 different subjects at 82× to display the characteristic green network of live basal epidermal cells throughout an entire micrograft. B, A magnified (100×) micrograft from 3 different subjects demonstrating different focal planes using an FITC (green), TRITC (red), and DAPI (blue) filter. The green fluorescence displays live basal epidermal cells; the red-stained nuclei represent cells from the differentiated and apoptotic, granular layer; and the blue fluorescence displays cell nuclei. All images and photographs are owned by KCI Licensing Inc.
Pain Assessment
One minute after device initiation, the mean recorded pain was 0.1 on the Wong-Baker FACES pain rating scale of 0 (no hurt) to 5 (hurts worst). At 10 minutes and during micrograft harvesting procedure, the mean recorded pain was 1.0 (hurts a little bit). At 20 minutes, subjects reported the highest mean pain score of 1.3. Immediately after micrograft harvesting, the mean recorded pain was again 1.0, and at visit 3 (7 days after micrograft harvesting), 100% of all 15 subjects reported pain scores of 0 (no hurt) (Figure 4). Pain scores of 4 (hurts a whole lot) or 5 (hurts worst) were not reported during micrograft formation or after micrograft harvesting.
Figure 4.
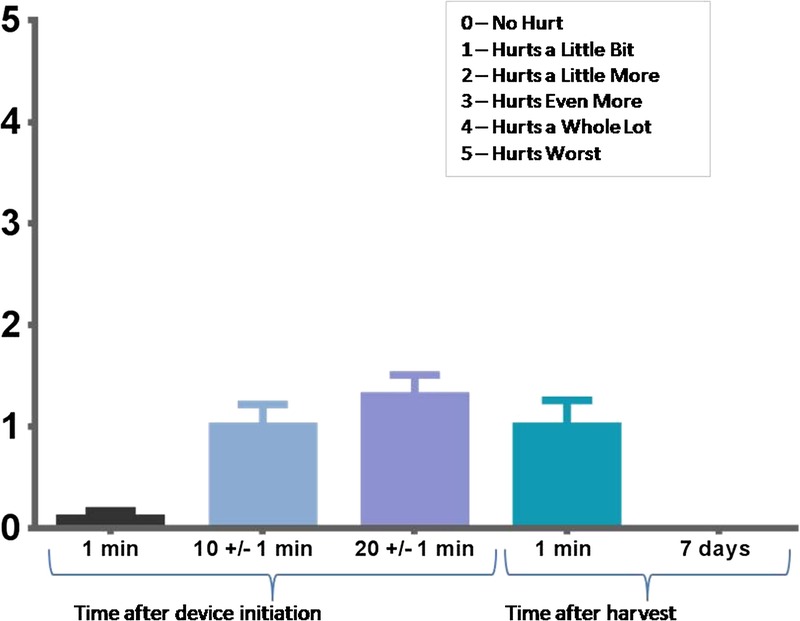
AVERAGE PAIN (WONG-BAKER FACES PAIN RATING SCALE) REPORTED BY SUBJECTS DURING AND AFTER EPIDERMAL MICROGRAFT HARVEST PROCEDURE
Dermal Response Scores
A mean dermal response score of 1.7, corresponding to minimal erythema (barely perceptible) and definite erythema (readily visible), was observed immediately after micrograft harvesting from all 15 subjects. At visit 3 (7 days after micrograft harvesting), the mean dermal score was 0.8, between minimal erythema (barely perceptible) and no evidence of irritation. At visit 4 (14 days after micrograft harvesting), the mean dermal response score was 0.5. At visit 5 (28 days after micrograft harvesting), the mean dermal response score was 0.1 (Figure 5). A score of 1 (minimal erythema, barely perceptible) was reported for 1 subject, and a score of 0 (no evidence of irritation) was reported for all other subjects. Immediately after micrograft harvesting, approximate percentage of donor site that was same in appearance as surrounding skin was 26 to 50 in 10 subjects (66.7%) and 51 to 75 in 5 subjects (33.3%). At visit 3 (7 days after micrograft harvesting), 4 subjects (26.7%) had a skin appearance of 0 to 25, 1 subject (6.7%) had a skin appearance of 26 to 50, 1 subject (6.7%) had a skin appearance of 51 to 75, and the remaining 9 subjects (60.0%) had a skin appearance of 76 to 100. At visit 4 (14 days after micrograft harvesting), 100% of all 15 subjects had a skin appearance of 76 to 100, demonstrating that 76% to 100% of the area of each donor site was the same in appearance as the surrounding skin for all 15 subjects (Figure 6). The donor-site healing trajectory of 1 representative subject immediately following harvest as well as after 7, 14, and 26 days is shown in Figure 7.
Figure 5.
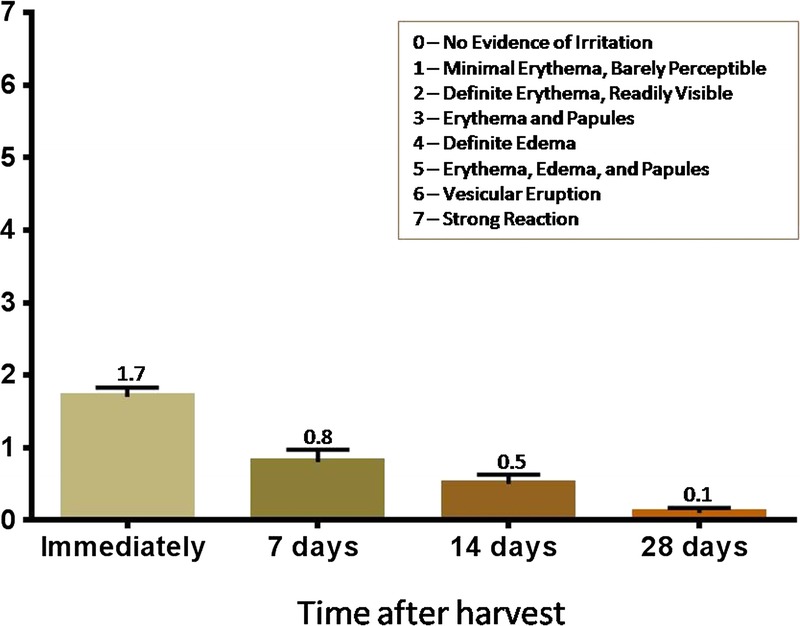
DERMAL RESPONSE SCORES POSTHARVEST AND FOLLOW-UP VISITS (N = 15; MEAN ± SEM)
Figure 6.
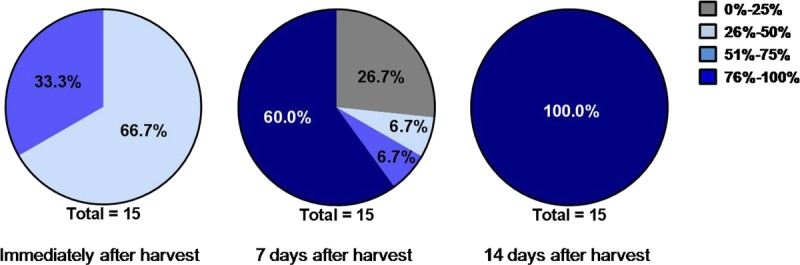
SKIN APPEARANCE RESULTS
Figure 7.
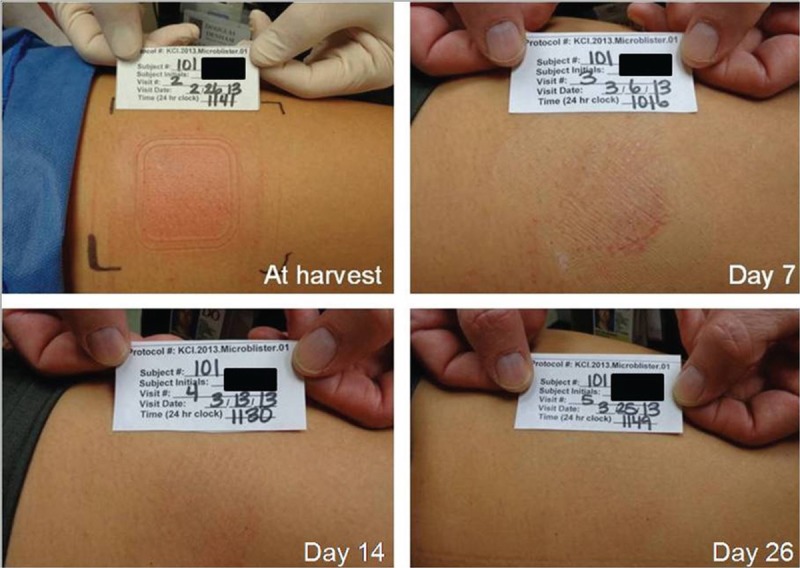
REPRESENTATIVE SUBJECT’S DONOR SITE
The site is shown immediately within minutes of harvest and after 7, 14, and 26 days. Complete healing was achieved within 2 to 4 weeks. All images and photographs are owned by KCI Licensing Inc.
Estimated percentage of donor site was the same in appearance as surrounding skin.
DISCUSSION
Although the majority of literature regarding epidermal grafting has focused on dermatological conditions, successful outcomes in pigmentation disorders have prompted use of epidermal grafts in wound care as well.14,22 To date, the most commonly used method of epidermal harvesting consisted of using syringes to raise epidermal suction blisters23,25; however, this procedure has been described as time consuming and painful when using larger syringes or when the floor of the blisters is touched during manual harvest.26 The syringe method also requires multiple sittings, and inadequate handling of the graft can lead to tearing and improper orientation, causing failure of graft take.
The automated, minimally invasive epidermal harvesting tool used in this study consisted of a control unit, vacuum head, and harvester. The system was simple to operate and created viable epidermal micrografts in less than 40 minutes. Unlike other epidermal harvesting methods, the tool is designed to achieve a 6× expansion ratio by maintaining micrograft spacing orientation on the transparent film used to transfer the microdomes spaced approximately 2 mm apart onto the recipient site. The 99.5% average viability of epidermal micrografts achieved in the study showed reproducibility in generating uniform viable micrografts with this system (Figure 3, A and B).
For this study, viability was defined as the presence of green fluorescence within the micrograft, indicative of active cell metabolism. The 2-color, fluorescence-based assay used in this study targets 2 of the recognized parameters of cell viability: intracellular esterase activity and plasma membrane integrity.27 Using this method, live cells have been shown to be represented as a bright green network, and dead cells have been shown to exhibit a bright red fluorescence.28 These fluorescent probes have previously been used as viability markers with epithelial sheets harvested from suction blisters, which is consistent with the findings of this study.29
Pain during conventional skin grafting is largely caused by severing the sensory nerve endings in the dermis. During epidermal harvesting, only superficial epidermis is removed from the donor site; thus, sensory organs as well as hair follicles that exist in the dermis remain undisturbed.30 Lack of contact with the dermal layer during this procedure resulted in minimal to no bleeding (data not shown), no scarring (Figures 6 and 7), and minimal donor-site pain (Figure 7). The mean pain score did not exceed 1.3 for any of the measured time points during or after the procedure, and there was no need for anesthetics.
Within 14 days (visit 4) of micrograft harvesting, at least 76% of the area of all donor sites was estimated to be the same in appearance as the surrounding skin. Day 28 data points of 1 subject were not recorded because of subject no-show; otherwise, 14 subject donor sites were found to be improved in appearance (Figure 7) compared with day 14 when the donor site was already 76% to 100% similar in appearance to the surrounding skin. These results differ from other reports of STSG donor-site morbidity, which is often associated with prolonged healing times, fluid loss, pain, and undesirable cosmesis.4,5 Outcomes of this study suggest that the epidermal micrograft system may improve patient experience as compared with traditional STSG and pinch grafting procedures that harvest both epidermis and dermis.
CONCLUSIONS
The automated and minimally invasive epidermal harvesting system used in this IRB-approved study yielded uniformly viable grafts. This method of epidermal harvesting produced immediate and ready-for-use autologous micrografts while causing minimal discomfort to the patient during harvesting and allowing for rapid healing of the superficial donor-site wounds without scarring. Future, well-designed clinical trials that include representative patients of all demographics will need to be performed to confirm the effectiveness of epidermal grafting.
REFERENCES
- 1. Dasgupta S, Sanyal S, Gupta P, et al. A modification of split-skin graft. Burns 1997; 23: 509- 11. [DOI] [PubMed] [Google Scholar]
- 2. Leung JJ, Fish J. Skin grafts. Univ Toronto Med J 2009; 86: 61- 4. [Google Scholar]
- 3. Biswas A, Bharara M, Hurst C, et al. The micrograft concept for wound healing: strategies and applications. J Diabetes Sci Technol 2010; 4: 808- 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chuenkongkaew T. Modification of split-thickness skin graft: cosmetic donor site and better recipient site. Ann Plast Surg 2003; 50: 212- 4. [DOI] [PubMed] [Google Scholar]
- 5. Edwards J. Management of skin grafts and donor sites. Nurs Times 2007; 103: 52- 3. [PubMed] [Google Scholar]
- 6. Simizu R, Kishi K, Okabe K, et al. Recruited minced skin grafting for improving the skin appearance of the donor site of a split-thickness skin graft. Dermatol Surg 2012; 38: 654- 60. [DOI] [PubMed] [Google Scholar]
- 7. Demirtas Y, Yagmur C, Soylemez F, et al. Management of split-thickness skin graft donor site: a prospective clinical trial for comparison of five different dressing materials. Burns 2010; 36: 999- 1005. [DOI] [PubMed] [Google Scholar]
- 8. Haake A, Scott GA, Holbrook KA. Structure and function of the skin: overview of the epidermis and dermis. In: Freinkel RK, Woodley DT, eds. The Biology of the Skin. New York, NY: The Parthenon Publishing Group; 2001; 19- 46. [Google Scholar]
- 9. Rinn JL, Wang JK, Allen N, et al. A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev 2008; 22: 303- 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamaguchi Y, Kubo T, Tarutani M, et al. Epithelial-mesenchymal interactions in wounds: treatment of palmoplantar wounds by nonpalmoplantar pure epidermal sheet grafts. Arch Dermatol 2001; 137: 621- 8. [PubMed] [Google Scholar]
- 11. Yamaguchi Y, Itami S, Tarutani M, et al. Regulation of keratin 9 in nonpalmoplantar keratinocytes by palmoplantar fibroblasts through epithelial-mesenchymal interactions. J Invest Dermatol 1999; 112: 483- 8. [DOI] [PubMed] [Google Scholar]
- 12. Tang WY, Chan LY, Lo KK. Treatment of vitiligo with autologous epidermal transplantation using the roofs of suction blisters. Hong Kong Med J 1998; 4: 219- 24. [PubMed] [Google Scholar]
- 13. Budania A, Parsad D, Kanwar AJ, et al. Comparison between autologous noncultured epidermal cell suspension and suction blister epidermal grafting in stable vitiligo: a randomized study. Br J Dermatol 2012; 167: 1295- 301. [DOI] [PubMed] [Google Scholar]
- 14. Njoo MD, Westerhof W, Bos JD. A systematic review of autologous transplantation methods in vitiligo. Arch Dermatol 1998; 134: 1543- 9. [DOI] [PubMed] [Google Scholar]
- 15. Ichiki Y, Kitajima Y. Successful treatment of scleroderma-related cutaneous ulcer with suction blister grafting. Rheumatol Int 2008; 28: 299- 301. [DOI] [PubMed] [Google Scholar]
- 16. Hanafusa T, Yamaguchi Y, Katayama I. Intractable wounds caused by arteriosclerosis obliterans with end-stage renal disease treated by aggressive debridement and epidermal grafting. J Am Acad Dermatol 2007; 57: 322- 6. [DOI] [PubMed] [Google Scholar]
- 17. Burm JS, Rhee SC, Kim YW. Superficial dermabrasion and suction blister epidermal grafting for postburn dyspigmentation in Asian skin. Dermatol Surg 2007; 33: 326- 32. [DOI] [PubMed] [Google Scholar]
- 18. Costanzo U, Streit M, Braathen LR. Autologous suction blister grafting for chronic leg ulcers. J Eur Acad Dermatol Venereol 2008; 22: 7- 10. [DOI] [PubMed] [Google Scholar]
- 19. Hockenberry MJ, Wilson D. Wong’s Essentials of Pediatric Nursing. 8th ed St Louis, MO: Mosby Elsevier, 2009. [Google Scholar]
- 20. Harris C, Bates-Jensen B, Parslow N, et al. Development of a pictorial guide for training nurses. Wound Care Canada 2009; 7: 33- 4, 36, 38. [Google Scholar]
- 21. Osborne SN, Schmidt MA, Derrick K, Harper JR. Epidermal micrografts produced via an automated and minimally invasive tool form at the dermal/epidermal junction and contain proliferative cells that secrete wound healing growth factors. Adv Skin Wound Care 2015; 28: 397- 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramos JV, Schmidt M, Wu SC. Epidermal skin grafts for the treatment of chronic lower extremity ulcers. Podiatry Management 2013; 95- 6, 98-100, 102, 104. [Google Scholar]
- 23. Gupta S, Jain VK, Saraswat PK, et al. Suction blister epidermal grafting versus punch skin grafting in recalcitrant and stable vitiligo. Dermatol Surg 1999; 25: 955- 8. [DOI] [PubMed] [Google Scholar]
- 24. Burm JS. Simple suction device for autologous epidermal grafting. Plast Reconstr Surg 2000; 106: 1225- 6. [DOI] [PubMed] [Google Scholar]
- 25. Alexis AF, Wilson DC, Todhunter JA, et al. Reassessment of the suction blister model of wound healing: introduction of a new higher pressure device. Int J Dermatol 1999; 38: 613- 7. [PubMed] [Google Scholar]
- 26. Khunger N, Kathuria SD, Ramesh V. Tissue grafts in vitiligo surgery—past, present, and future. Indian J Dermatol 2009; 54: 150- 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Papadopoulos NG, Dedoussis GV, Spanakos G, et al. An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods 1994; 177: 101- 11. [DOI] [PubMed] [Google Scholar]
- 28. Poole CA, Brookes NH, Clover GM. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci 1993; 106: 685- 91. [DOI] [PubMed] [Google Scholar]
- 29. Hladik F, Sakchalathom P, Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 2007; 26: 257- 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Metze D, Luger T. Nervous system in the skin. In: Freinkel RK, Woodley DT, eds. The Biology of the Skin. New York, NY: The Parthenon Publishing Group; 2001; 153- 76. [Google Scholar]


