Abstract
The role played by vessel disease in stroke-related cognition dysfunction is unclear. We assessed the impact of significant atherosclerotic disease on cognition—even in patients asymptomatic for stroke. We hypothesized that patients would perform poorly relative to controls, but that symptomatic/asymptomatic status (history of stroke/transient ischemic attack) would have no effect. Fifty-two carotid endarterectomy candidates with >60% carotid stenosis and 17 controls underwent a 60-min neuropsychological test protocol. Symptomatic and asymptomatic patients showed deficits in executive function, delayed verbal recall, and general knowledge. Patients symptomatic for stroke also performed worse on tests of language and motor/visuomotor ability. Symptomatic and asymptomatic patients differed in working memory and language task performance. Although all patients showed deficits in executive function and memory, only symptomatic patients showed additional deficits in language and motor function. Cognitive abnormalities in patients viewed as “asymptomatic” for stroke underscore the need for early identification and treatment.
Keywords: Cerebrovascular disease, Accident and stroke
Introduction
Cerebrovascular disease is a major public health problem. The American Heart Association estimates that 800,000 strokes occur per year in the United States, and recent estimates suggest that as many as 11 million “silent strokes” occur per year in the United States (Leary & Saver, 2003). Indeed, for every clinical stroke, some studies report up to five silent strokes evident on neuroimaging (Vermeer et al., 2002). The reasons for the under-recognition of silent strokes and their neurobehavioral consequences are likely multifactorial. Although stroke and cognitive impairment are tightly linked, clinical exams typically focus on classic stroke symptoms including motor paralysis, visual loss, and speech impairment, with considerably less assessment of abnormalities in higher cognitive function including executive function, episodic memory, visuospatial and psychomotor skills, and subtle language impairments. These may not be formally assessed in routine clinical care; furthermore, any detected anomalies may be incorrectly considered to represent normal age-related changes. Large cohort studies have also confirmed a significant association between stroke “risk” and decline in cognitive test performance in samples of both men and women. These findings, even in patients with no overt stroke-like symptoms, support the hypothesis that presence of high-risk factors for cerebrovascular disease can signal subclinical cognitive decline, and sometimes predate the occurrence of more severe cognitive deterioration (Elias et al., 2004; Llewellyn et al., 2008).
Here, we assess the impact of significant atherosclerotic disease on cognitive performance in both patients with a history of stroke/transient ischemic attack (TIA) (“symptomatic”) and those without (“asymptomatic”). In the current study, we included a sample of 52 patients (30 symptomatic and 22 asymptomatic for classic stroke/TIA) with significant carotid stenosis (>60%) who were scheduled to undergo carotid endarterectomy (CEA). Each patient, and 17 control participants, underwent cognitive assessment including tests of executive function/attention, verbal and nonverbal memory, visuospatial construction, and language. We hypothesized that patients would perform poorly on all measures relative to controls. Further, we predicted that symptomatic/asymptomatic status would not have a significant effect on test scores.
Methods
Participants
Clinical research participants consisted of 52 patients undergoing CEA for clinically significant (>60%) carotid stenosis at the University of Wisconsin Hospital and Clinics. Patients were initially screened by the attending neurosurgeon or vascular surgeon, followed by contact with the study coordinator. All patients enrolled in the study met the following inclusion criteria: age 18 years or older, native English speaker, symptomatic carotid stenosis meeting North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria or asymptomatic stenosis meeting Asymptomatic Carotid Atherosclerosis Study (ACAS) criteria, and scheduled to undergo CEA. Patients were excluded if they presented with a previous history of endovascular or open carotid surgery and/or cervical radiation, or were considered unsuitable for CEA or lack informed consent capacity. Participant characteristics are given in Table 1. Of the 52 patients, 30 had a previous stroke or TIA as determined by chart review, clinical imaging results, and patient/family interviews and were therefore considered symptomatic (M age = 69.43; SD = 10.51; range = 43–85) and 22 were identified as asymptomatic (M age = 70.27, SD = 7.61; range = 59–84). Seventeen spouses (inclusion criteria: age 18 years or older; native English speaker) served as control participants in order to match patients on demographic and environmental variables. Control participants were excluded based on previous history of stroke, TIA, or carotid artery surgery. Additionally, one control participant was removed from all analyses after testing revealed severe deficits in memory and IQ, leaving a total of 16 controls (M age = 68.19; SD = 6.55; range = 58–81). Women were overrepresented in the control group relative to both symptomatic (χ2(1, n = 46) = 9.58, p = .002) and asymptomatic (χ2(1, n = 38) = 4.66, p = .030) patients. No differences in gender were found between symptomatic and asymptomatic patient groups, nor were there age differences across all three groups. Control participants had more years of education (t(36) = 2.19, p = .035) and higher estimated full-scale intelligence quotient (t(35) = 2.48, p = .018) than asymptomatic patients, whereas symptomatic patients showed no significant differences from either group on these variables. Finally, patients with left- versus right-sided stenosis were compared; no differences across groups were found for age, years of education, gender distribution, or premorbid IQ. All patients in this sample self-identified as being “White/Caucasian.”
Table 1.
Participant characteristics by group (means and standard deviations)
| Variable | Symptomatic (n = 30) | Asymptomatic (n = 22) | Controls (n = 16) |
|---|---|---|---|
| Age (years) | 69.43 (10.51) | 70.27 (7.61) | 68.19 (6.55) |
| Gender (#/% women)a,b | 12 (40.0%) | 12 (54.5%) | 14 (87.5%) |
| Education (years)a,c | 13.67 (2.80) | 12.55 (2.54) | 14.31 (2.33) |
| Estimated FSIQa,c | 105.68 (8.85) | 102.67 (9.04) | 110.31 (9.65) |
Notes: FSIQ = full-scale intelligence quotient.
aSignificant (p ≤ .05) difference(s) across at least two participant groups.
bControl participants were more likely to be women than either symptomatic or asymptomatic patients.
cControl participants had more years of education and higher estimated FSIQ than did asymptomatic patients.
Neuropsychological Assessment
All patients were assessed 30 days or less before CEA during their hospital visit for mandatory preoperative testing; control participants were tested on the same day as their spouse. The research protocol was performed in accordance with the Health Sciences Institutional Review Boards of the University of Wisconsin–Madison. After giving written consent, each participant was administered the 60-min neuropsychological test protocol recommended by the National Institute of Neurological Disorders (NINDS) and Canadian Stroke Network (CSN). This protocol was selected specifically for stroke patients and assesses several important functional domains with tests of executive function/attention, speeded psychomotor, verbal and nonverbal memory, language, and visuospatial skills (Hachinski et al., 2006). For the current study, widely used verbal and performance IQ measures (Wechsler Adult Intelligence Scale-IV Information, Digit Span, and Block Design; Wechsler, 2008) were added.
Data Analysis and Statistics
All test scores were corrected for age, sex, and level of education using published norms or covariate analysis. First, scores on 4 of 14 measures (semantic and category fluency, Trails A/B) were converted to standardized scores relative to published sex, age, and level of education norms using the Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery (Heaton et al., 2004). Results for 8 of 14 tests were age-normed using materials provided with each test, whereas norms were not available for two tests (figure copy and confrontation naming). In all subsequent analyses, no covariates were used for those tests that were normed for sex, age, and level of education. Those tests that were normed for age only used sex and level of education as covariates (norming for race was impossible due to zero variability in this demographic factor). Finally, tests without norms were examined using sex, age, and level of education as covariates. Analysis of covariance (ANCOVA) was used to first ascertain differences across patient group for each measure. Planned comparisons to the control group for each patient group were then conducted. In the next set of analyses, using the same covariates, the effects of symptomatic status and laterality (side of surgery) on test performance were examined using a series of 2 × 2 ANCOVAs.
Results
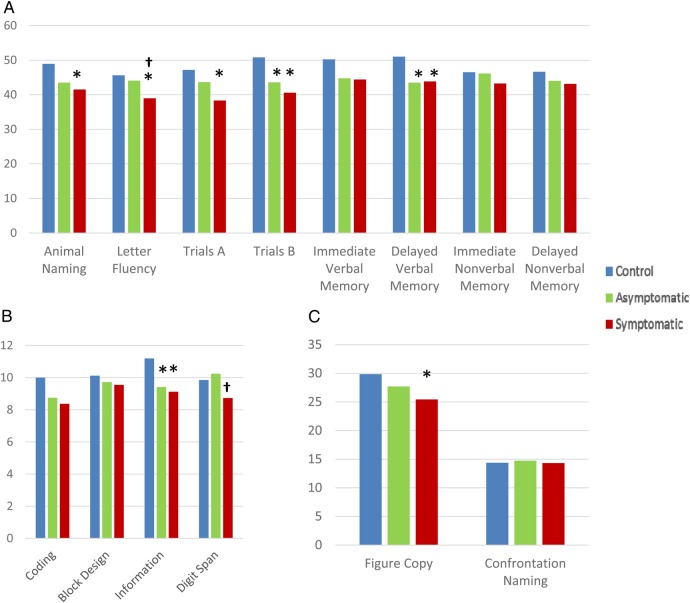
Figure 1 shows scores for patient and control participants across all measures, controlling for sex, age, and level of education. Significant differences were found across groups for five tests: letter (F-A-S: F(2, 64) = 5.12, p = .009, η2 = 0.14) and semantic (Animal Naming: F(2, 65) = 3.76, p = .028, η2 = 0.10) fluency, motor sequencing/executive function (Trails B: F(2, 64) = 4.46, p = .015, η2 = 0.12), general fund of knowledge (WAIS-IV Information: F(2, 62) = 3.81, p = .028, η2 = 0.11), and visuospatial/motor ability (Rey Complex Figure Text [RCFT]—Copy: F(2, 60) = 3.29, p = .044, η2 = 0.10). Fisher's Least Significant Difference tests on the estimated marginal means were used to conduct planned comparisons of the performance of both patient groups relative to controls. Controls (M = 50.81, SE = 2.76) outperformed both symptomatic (M = 40.59, SE = 2.05; p = .004) and asymptomatic (M = 43.59, SE = 2.35; p = .050) patient groups on Trails B. A similar pattern was seen for WAIS-IV Information subtest as controls (M = 11.19, SE = 0.612) outperformed both symptomatic (M = 9.108, SE = 0.44; p = .009) and asymptomatic (M = 9.41, SE = 5.02; p = .031) patient groups. Additionally, despite a nonsignificant analysis of variance (ANOVA) result (F(2, 63) = 2.61, p = .081, η2 = 0.08), both patient groups exhibited deficits in delayed verbal memory (symptomatic: M = 43.86, SE = 1.94, p = .043; asymptomatic: M = 43.50, SE = 2.25, p = .042) relative to controls (M = 51.01, SE = 2.75).
Fig. 1.
Patient and control performance by test. Existing norms have been used when possible to produce T-scores (A) or Scaled Scores (B). (C) Results for non-normed tests. All analyses controlled for age, sex, and level of education. *Differs from controls (p ≤ .05). †Differs from asymptomatic patients (p ≤ .05).
On the test of category fluency, symptomatic (M = 41.54, SE = 1.60) patients performed significantly worse than controls (M = 48.94, SE = 2.19; p = .008), whereas letter (or phonemic) fluency revealed similar performance deficits in symptomatic (M = 38.97, SE = 1.39) patients relative to controls (M = 45.63, SE = 1.87; p = .006). Symptomatic patients (M = 25.44, SE = 1.01) also did significantly worse than controls (M = 29.86, SE = 1.39) on the RCFT Copy task (p = .044). Similar results were obtained for Trails A despite a nonsignificant ANOVA result (F(2, 65) = 3.03, p = .055, η2 = 0.9), with symptomatic patients (M = 38.33, SE = 2.220) performing poorly relative to controls (M = 47.19, SE = 3.03).
ANCOVAs were next used to test the effects of symptomatic status and laterality (side of surgery) on each cognitive measure. Two tests showed a main effect for symptomatic status: F-A-S (F(1, 47) = 4.88, p = .032, η2 = 0.09) and WAIS-IV Digit Span (F(1, 45) = 4.97, p = .031, η2 = 0.10). In both cases, patients who were symptomatic for stroke (F-A-S: M = 39.33, SE = 1.44; Digit Span: M = 8.48, SE = 0.48) performed poorly relative to their asymptomatic counterparts (F-A-S: M = 44.09, SE = 1.61; Digit Span: M = 10.11, SE = 0.54; see Fig. 1). Neither test showed a main effect for laterality or a significant interaction term. However, two other tests showed a laterality main effect: Rey Complex Figure Task—3′ (F(1, 43) = 4.05, p = .05, η2 = 0.09) and 30′ (F(1, 43) = 7.01, p = .011, η2 = 0.14) recall scores. Patients with right-sided carotid stenosis (3′ recall: M = 41.16, SE = 2.48; 30′ recall: M = 39.14, SE = 2.46) showed nonverbal memory deficits relative to left-sided (3′ recall: M = 48.59, SE = 2.70; 30′ recall: M = 49.09, SE = 2.79) surgery candidates (see Fig. 1). Neither test showed a main effect for symptomatic status or interaction between the two factors.
Discussion
Carotid artery disease has been associated with an increased risk of cognitive deficits associated with bilateral (Buratti et al., 2014; Casas-Hernanz et al., 2012) and left-sided (Johnston et al., 2004) stenosis, although other studies have found no cognitive abnormalities in patients with moderate to severe stenosis (Aleksic et al., 2006). These inconsistent findings may be due at least in part to differences in control groups used (if used at all), discrepancies in quantification of stenosis, underpowered cognitive assessments, and lack of formal statistical tests assessing the role of laterality (side of stenosis) in cognition. In the current study, which expressly took these criticisms into account, both symptomatic and asymptomatic patient performances were significantly worse than those of control participants on tests of executive function (Trails B), delayed verbal memory, and general knowledge fund. However, for four other tests, the results differed as a function of patient group, with symptomatic patients showing deficits compared with controls in motor/visuospatial ability (Figure Copy, Trails A) and language function (letter and category fluency). These results partly supported our hypothesis that both patient groups would show similar deficits relative to controls. We found increased support when directly comparing the two patients groups; significant differences between symptomatic and asymptomatic patients were obtained in only 2 of 14 tests: Digit Span and Animal Naming (category fluency). Put another way, on 12 of 14 tests, patient performance did not differ as a function of symptomatic status.
Compared with controls, the lowest scores across tests of language, working memory, and motor function were associated with a history of stroke/TIA, in addition to the deficits that were associated with carotid stenosis regardless of symptomatic status: executive function, verbal memory, and general knowledge. Although our sample size is modest and caution in interpreting these findings is warranted, these data in part mirror the common functional impairments in speech and movement often seen in stroke patients. Carotid stenosis, however, even in the absence of an acute hypoxic event, was associated with impairments in mental status. Problems in executive function (specifically difficulty shifting set) and verbal memory, which are not assessed as a part of routine clinical care, are perhaps less likely to be noticed by caretakers and family members—particularly in the face of more basic impairments in language and motor function. Similarly, poor performance on the Information subtest may reflect difficulties in memory retrieval. Although this subtest is typically not prone to aging effects, it is nevertheless striking that both patient groups performed poorly relative to controls, with no differences between patient groups. It should be pointed that performance of the controls (M = 11.19) on this test, while slightly higher than expected, was well within the average range for the general population. Regardless, replication of this effect with a larger control sample is necessary.
The finding of significant cognitive decline in asymptomatic patients compared with controls, with few differences when compared with symptomatic patient (only 2 of 14 measures), casts new light on the cognitive status of “asymptomatic” patients. An asymptomatic patient with large plaques without frank neurological deficits may continue to have an ongoing microvascular disease with associated cognitive decline that may be unappreciated in the absence of a major sentinel event, which would bring them to medical attention for a possible intervention. This point is underscored by the varied functional domains in which impairment was found to be associated not with stroke, but with carotid stenosis. The presence of such decline in patients who are asymptomatic suggests that cerebrovascular disorders cause significant cognitive consequences far beyond that of classic symptoms of hemiparesis, visual or speech loss (Droste et al., 1996; Siebler et al., 1996). Indeed, executive functional decline may be a far more important and debilitating consequence of cerebrovascular disease as it robs people of independence and employability, accelerating loss of independence.
The relationship between cognitive decline and vascular disease is clear, both in terms of macro- and microvascular disorders. Postmortem studies suggest that small vessel disease can heavily influence the perforating vessels of the middle cerebral and basilar arteries, especially prevalent issues in patients with complications such as chronic hypertension causing stereotypic loss of function related to duration of exposure to these risk factors (Droste et al., 2003; Ergul et al., 2012). Our earlier pathologic studies have suggested that the carotid artery wall, specifically its media, may be an important nexus of the disease (Shi et al., 2008; Türeyen et al., 2006). The process in the carotid artery may also be a window to microvascular disorders, which may be present in brain and other organs systemically. We and others have previously shown the relationship of angiogenesis and fragile new vessel formation within the carotid artery media to be a marker for symptomatic cerebrovascular disease above and beyond the mere presence of plaque (Chowdhury et al., 2010; Türeyen et al., 2006). Indeed it is a potential destabilizer of the plaque within the carotid wall (Moller et al., 2012). The same microvascular changes seen in the carotid wall mirrored those seen in the diseased brain. For this reason, advanced atherosclerotic disease at the carotid bifurcation may well be a marker not only for systemic disease but also for cerebral dysfunction. The pathophysiology of this could be multifactorial: cerebral small vessel disease, carotid to cerebral vessel thrombo-microembolism from unstable carotid plaques, and diminished cerebral blood flow, all of which are testable and may be affected by treatments aimed at the detection or elimination of the carotid disease. Finally, changes in arterial stiffness over time are associated with increased carotid pressure and flow pulsatility and have been related to changes in brain structure/function as well as problems in cognition (Mitchell et al., 2011).
The relationship of cerebrovascular disease and cognitive decline is extraordinarily important. A careful review of Alzheimer's disease and cerebrovascular disease has shown a key relationship between the two in common risk factors (Purandare et al., 2006). The presentation, however, suggests that cerebrovascular disease and its cognitive decline may precede the memory loss and potential dementia in the same patients. Other key studies such as the Nun Study have shown that cerebrovascular disease may be an important trigger to cause expression of the clinical phenotype in a patient population with pathologic changes—that is, the percentage of Nuns who had pathologic changes of Alzheimer's disease was higher than those that had expressed Alzheimer's clinical dementia. The relationship became direct in those Nuns who had suffered clinical strokes suggesting a causative or triggering relationship of cerebrovascular disease for these declines (Snowdon et al., 1997).
Limitations and Future Directions
There are several limitations to the current study. First, our control group was quite small and was unbalanced with regard to sex of participants, with a much higher percentage of women than either patient group. We note that our controls performed very much as expected in terms of the distribution and mean of test scores (i.e., T-scores of 50 ± 10, scaled scores of 10 ± 1). We also controlled for sex in all analyses. Still, a larger sample is needed to ensure reliability and external validity. For future analyses, we are intensifying our recruiting procedures for controls, particularly for men. Second, we lack data on vascular risk factors and other comorbidities such as smoking history, diabetes, hypertension, and hyperlipidemia. We are currently expanding our research protocol to allow us to interrogate these data, in the hopes that they will help us to characterize phenotypes of CEA candidates. This information could ultimately help us to predict who will respond well to surgery. Third, our sample of surgical candidates is racially and ethnically homogenous, with all patients self-identifying as “White/Caucasian.” Increasing patient diversity is another important goal of our intensified recruiting efforts. Finally, it is difficult to interpret our findings of poor nonverbal memory in patients with right-sided stenosis, given that we have no information on stenosis of the contralateral carotid. This information will be necessary if future studies are to refine our knowledge of the effects of carotid stenosis on cognition.
We are currently testing both the patient and control samples 1 year after CEA, in order to examine the effects of surgery on cognition. Following the controls will allow us examine possible practice effects on the tests used here, and to control for the effects of aging on test performance. Furthermore, analysis of these longitudinal data will allow us to examine factors that contribute to positive cognitive prognosis (or a reduction in decline) following CEA, including demographic and clinical predictors. These will include both behavioral and medical risk factors and should help to us understand the way multiple vulnerabilities to carotid stenosis (and stroke/TIA) affect recovery of function (or cessation of decline) following surgery. Finally, we also plan to continue to examine the relations between vessel strain and plaque, while also examining the stability of plaque tissue itself to identify potential biomarkers for cognitive risk/resilience. We have previously reported an ultrasound-related methodology that assesses the instability of large carotid plaques based on their handling of strain distributed through the vessel with pulsation. By applying this parameter to patients with atherosclerotic disease symptomatic or asymptomatic for stroke/TIA, we have been able to show a direct relationship not only to classic atherosclerotic large emboli but also to cognitive deficits (Wang et al., 2014).
The data from the plaque instability studies have suggested the possible role of microemboli in this cognitive decline. A long-term follow-up to see whether rate of cognitive decline can be stopped or reversed by interventions will do much to determine pathophysiology and suggest treatment options. Furthermore, measurement of changes in stenosis of the contralateral carotid artery should lead to stronger interpretation of any lateralized findings in the neuropsychological data.
Conclusion
Both symptomatic and asymptomatic patients with carotid plaques exhibited cognitive defects relative to age-matched controls, suggesting that the term “asymptomatic” in regard to patients with significant carotid plaques without clear vascular events such as stroke or TIA may be a misnomer. Classic symptoms of motor, speech, or visual deficits do not encompass the full spectrum of neurocognitive outcomes, which can be devastating to this population. Indeed, the presence of significant cognitive deficits in patients deemed to be asymptomatic for stroke or TIA may suggest that the presence of a symptom in a major motor, visual, or speech area such as an emboli would bring the patient to attention for care sooner—strongly underscoring the need to identify and treat these patients in a timely manner.
Funding
This work was supported by National Institutes of Health (grant number RO1 NS064034).
Conflict of Interest
C.C. Mitchell may receive future royalties from Davies Publishing and Elsevier for chapter/book authorship. The other authors report no disclosures.
Acknowledgment
D.C.J. would like to thank C.A. Burghy, H.J. Havens, and G.W.B. Jackson for their timely assistance.
References
- Aleksic M., Huff W., Hoppmann B., Heckenkamp J., Pukrop R., Brunkwall J. (2006). Cognitive function remains unchanged after endarterectomy of unilateral internal carotid artery stenosis under local anaesthesia. Cerebrovascular Diseases (Basel, Switzerland), 22, 276–281. [DOI] [PubMed] [Google Scholar]
- Buratti L., Balucani C., Viticchi G., Falsetti L., Altamura C., Avitabile E. et al. (2014). Cognitive deterioration in bilateral asymptomatic severe carotid stenosis. Stroke, 45, 2072–2077. [DOI] [PubMed] [Google Scholar]
- Casas-Hernanz L., Garolera M., Badenes-Guia D., Cejudo-Bolivar J. C., Royo J., Aguilar M. (2012). The effect of carotid occlusion in cognition before endarectomy. Archives of Clinical Neuropsychology, 27, 879–890. [DOI] [PubMed] [Google Scholar]
- Chowdhury M., Ghosh J., Slevin M., Smyth J. V., Alexander M. Y., Serracino-Inglott F. (2010). A comparative study of carotid atherosclerotic plaque microvessel density and angiogenic growth factor expression in symptomatic versus asymptomatic patients. European Journal of Vascular and Endovascular Surgery, 39, 388–395. [DOI] [PubMed] [Google Scholar]
- Droste D. W., Decker W., Siemens H. J., Kaps M., Shulte-Altedorneburg G. (1996). Variability in occurrence of embolic signals in long term transcranial Doppler recordings. Neurological Research, 18, 25–30. [DOI] [PubMed] [Google Scholar]
- Droste D. W., Ritter M. A., Dittrick R., Heidenreich S., Wichter T., Freund M. et al. (2003). Arterial hypertension and ischemic stroke. Acta Neurological Scandinavic, 107, 241–251. [DOI] [PubMed] [Google Scholar]
- Elias M. F., Sullivan L. M., D'Agostino R. B., Elias P. K., Beiser A., Au R. et al. (2004). Framingham stroke risk profile and lowered cognitive performance. Stroke, 35, 404–409. [DOI] [PubMed] [Google Scholar]
- Ergul A., Kelly-Cobbs A., Abdalla M., Fagan S. C. (2012). Cerebrovascular complications of diabetes: Focus on stroke. Endocrine, Metabolic & Immune Disorders-Drug Targets, 12, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski V., Iadecola C., Petersen R. C., Breteler M. M., Nyenhuis D. L., Black S. E. et al. (2006). National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards. Stroke, 37, 2220–2241. [DOI] [PubMed] [Google Scholar]
- Heaton R. K., Miller S. W., Taylor M. J., Grant I. (2004). Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, Florida: Psychological Assessment Resources, 638 pp. [Google Scholar]
- Johnston S. C., O'Meara E. S., Manolio T. A., Lefkowitz D., O'Leary D. H., Goldstein S. et al. (2004). Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Annals of Internal Medicine, 140, 237–247. [DOI] [PubMed] [Google Scholar]
- Leary M. C., Saver J. L. (2003). Annual incidence of first silent stroke in the United States: A preliminary estimate. Cerebrovascular Diseases (Basel, Switzerland), 16, 280–285. [DOI] [PubMed] [Google Scholar]
- Llewellyn D. J., Lang I. A., Xie J., Huppert F. A., Meltzer D., Langa K. M. (2008). Framingham Stroke Risk Profile and poor cognitive function: A population-based study. BMC Neurology, 22, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., van Buchem M. A., Sigurdsson S., Gotal J. D., Jonsdottir M. K., Kjartansson O. et al. (2011). Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility—Reykjavik Study. Brain, 134, 3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller M. J., Qin Z., Toursarkissian B. (2012). Tissue markers in human atherosclerotic carotid artery plaque. Annals of Vascular Surgery, 26, 1160–1165. [DOI] [PubMed] [Google Scholar]
- Purandare N., Burns A., Daly K. J., Hardicre J., Morris J., Macfarlane G. et al. (2006). Cerebral emboli as a potential cause of Alzheimer's disease and vascular dementia: Case-control study. BMJ, 332, 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Varghese T., Dempsey R. J., Salamat M. S., Zagzebski J. A. (2008). Relationship between ultrasonic attenuation, size and axial strain parameters for ex vivo atherosclerotic carotid plaque. Ultrasound in Medicine & Biology, 34, 1666–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebler M., Sitzer M., Rose G., Steinmetz H. (1996). Microembolism in carotid artery disease. Journal of Neuroimaging, 13, 529–536. [DOI] [PubMed] [Google Scholar]
- Snowdon D. A., Greiner L. H., Mortimer J. A., Riley K. P., Greiner P. A., Markesbery W. R. (1997). Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA, 277, 813–817. [PubMed] [Google Scholar]
- Türeyen K., Vemuganti R., Salamat M. S., Dempsey R. J. (2006). Increased angiogenesis and angiogenic gene expression in carotid artery plaques from symptomatic stroke patients. Neurosurgery, 58, 971–977. [DOI] [PubMed] [Google Scholar]
- Vermeer S. E., Koudstaal P. J., Oudkerk M., Hofman A., Breteler M. M. (2002). Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke, 33, 21–25. [DOI] [PubMed] [Google Scholar]
- Wang X., Jackson D. C., Varghese T., Mitchell C. C., Hermann B. P., Kliewer M. A. et al. (2014). Correlation of cognitive function with ultrasound strain indices in carotid plaque. Ultrasound in Medicine & Biology, 40, 78–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (2008). Wechsler Adult Intelligence Scale (4th ed.). San Antonio, TX: Pearson. [Google Scholar]