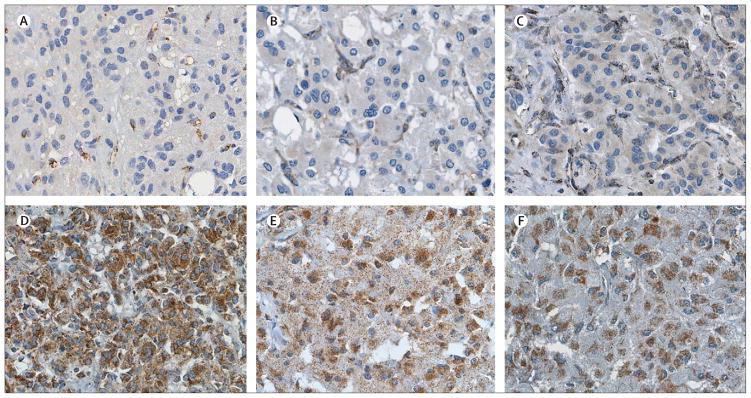
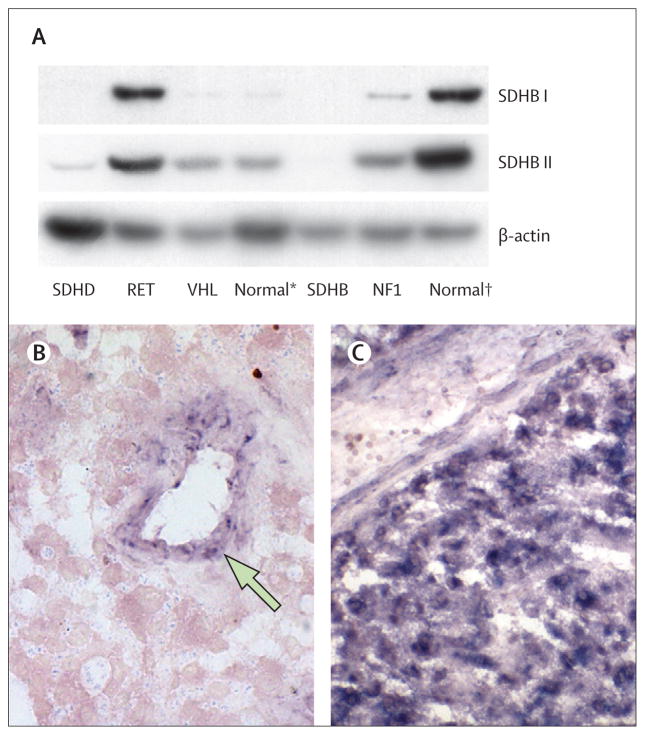
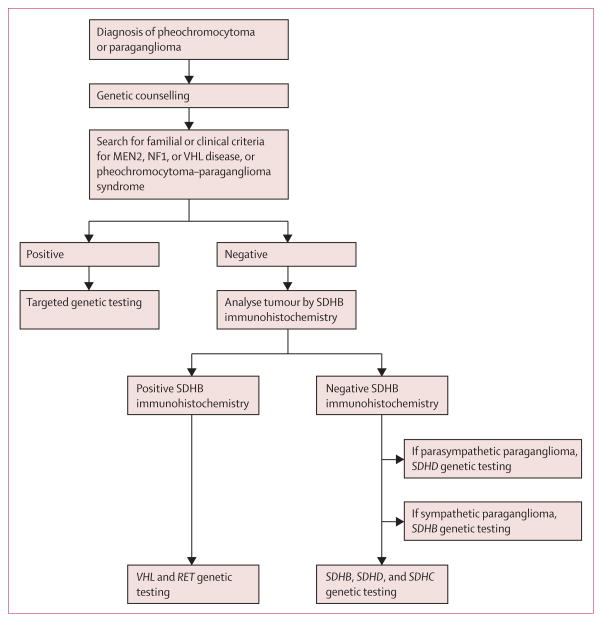
Francien H van Nederveen
Francien H van Nederveen, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,*,
José Gaal
José Gaal, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,*,
Judith Favier
Judith Favier, PhD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,*,
Esther Korpershoek
Esther Korpershoek, BSc
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Rogier A Oldenburg
Rogier A Oldenburg, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Elly M C A de Bruyn
Elly M C A de Bruyn, BSc
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Hein F B M Sleddens
Hein F B M Sleddens, BSc
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Pieter Derkx
Pieter Derkx, BSc
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Julie Rivière
Julie Rivière, BSc
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Hilde Dannenberg
Hilde Dannenberg, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Bart-Jeroen Petri
Bart-Jeroen Petri, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Paul Komminoth
Prof Paul Komminoth, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Karel Pacak
Prof Karel Pacak, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Wim C J Hop
Wim C J Hop, PhD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Patrick J Pollard
Patrick J Pollard, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Massimo Mannelli
Prof Massimo Mannelli, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Jean-Pierre Bayley
Jean-Pierre Bayley, PhD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Aurel Perren
Aurel Perren, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Stephan Niemann
Stephan Niemann, PhD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Albert A Verhofstad
Albert A Verhofstad, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,†,
Adriaan P de Bruïne
Prof Adriaan P de Bruïne, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Eamonn R Maher
Prof Eamonn R Maher, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Frédérique Tissier
Frédérique Tissier, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Tchao Méatchi
Tchao Méatchi, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Cécile Badoual
Cécile Badoual, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Jérôme Bertherat
Prof Jérôme Bertherat, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Laurence Amar
Laurence Amar, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Despoina Alataki
Despoina Alataki, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Eric Van Marck
Prof Eric Van Marck, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Francesco Ferrau
Francesco Ferrau, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Jerney François
Jerney François, BSc
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Wouter W de Herder
Wouter W de Herder, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Mark-Paul F M Vrancken Peeters
Mark-Paul F M Vrancken Peeters, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Anne van Linge
Anne van Linge, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Jacques W M Lenders
Prof Jacques W M Lenders, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Anne-Paule Gimenez-Roqueplo
Prof Anne-Paule Gimenez-Roqueplo, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,
Ronald R de Krijger
Ronald R de Krijger, MD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,‡,
Winand N M Dinjens
Winand N M Dinjens, PhD
1Department of Pathology, Josephine Nefkens Institute (F H van Nederveen MD, J Gaal MD, E Korpershoek BSc, E M C A de Bruyn BSc, H F B M Sleddens BSc, H Dannenberg MD, B-J Petri MD, P Derkx BSc, F Ferrau MD, J François BSc, R R de Krijger MD, W N M Dinjens PhD), Department of Clinical Genetics (R A Oldenburg MD), Department of Biostatistics (W C J Hop PhD), Department of Internal Medicine, Sector of Endocrinology (W W de Herder MD), Department of Surgery (M-P F M Vrancken Peeters MD), and Department of Otolaryngology (A van Linge MD), Erasmus MC, University Medical Center, Rotterdam, Netherlands; Institut für Pathologie, Stadtspital Triemli, Zürich, Switzerland (Prof P Komminoth MD); INSERM, U970, Paris, France, and Université Paris Descartes, Faculté de Médecine, Paris, France, and Collège de France, Paris, France (J Favier PhD, J Rivière BSc, Prof A-P Gimenez-Roqueplo MD); Reproductive and Adult, Endocrinology Program, National Institutes of Health, Bethesda, MD, USA (Prof K Pacak MD); Henry Wellcome Building for Molecular Physiology, University of Oxford, Oxford, UK (P J Pollard MD); Department of Clinical Physiopathology, University of Florence, Florence, Italy (Prof M Mannelli MD); Department of Human Genetics, Leiden University Medical Center, Leiden, Netherlands (J-P Bayley PhD); Department of Pathology, Klinikum Rechts der Isar, Munich, Germany (A Perren MD); Institut für Humangenetik, Justus-Liebig-Universität, Giessen, Germany (S Niemann PhD); Department of Medicine (Prof J W M Lenders MD), Department of Pathology (A A Verhofstad MD), Radboud University Nijmegen Medical Center, Nijmegen, Netherlands; Department of Pathology, GROW–School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands (Prof A P de Bruïne MD); Department of Medical and Molecular Genetics, Institute of Biomedical Research, University of Birmingham, and West Midlands Region Genetics Service, Birmingham Women’s Hospital, Edgbaston, Birmingham, UK (Prof E R Maher MD); Assistance Publique-Hôpitaux de Paris, Hôpital Cochin, Service d’Anatomie Pathologie (F Tissier MD); Assistance Publique-Hôpitaux de Paris, Hôpital Européen Georges Pompidou, Service d’Anatomo-Pathologie, (T Méatchi MD, C Badoual MD), Service d’Hypertension Artérielle, (L Amar MD), Service de Génétique (Prof A-P Gimenez-Roqueplo), Paris, France; Université Paris Decartes, Faculté de Médecine Paris Descartes, INSERM, U567, Institut Cochin, Paris, France (F Tissier MD); Centre de Reference Maladies Rares de la Surrénale, Hôpital Cochin, Paris, France (Prof J Bertherat MD); Department of Pathology, Hippokration General Hospital of Thessaloniki, Thessaloniki, Greece (D Alataki MD); Laboratory of Pathology, University of Antwerp, Antwerp University Hospital, Edegem, Belgium (Prof E Van Marck MD); and Department of Internal Medicine III, University Hospital Carl Gustav Carus, Dresden, Germany (Prof J W M Lenders MD)
1,‡