IgM derived from salivary B cells exhibits distinct repertoire characteristics, and is enriched for specific autoantibodies in a murine model of SS.
Keywords: salivary gland, sialadenitis, B cell repertoire, Id3−/−
Abstract
This study tested the hypothesis that B cells from salivary tissue are distinct in terms of proliferative capacity, immunoglobulin M secretion, repertoire, and autoantibody enrichment in Sjögren’s syndrome. We sorted purified B cells from the spleen, cervical lymph nodes, and submandibular glands of a primary Sjögren’s syndrome mouse model (Id3−/−). Enzyme-linked immunospot and proliferation assays were performed with stimulated B cells. We single-cell sorted B cells from the spleen, cervical lymph nodes, and submandibular gland tissue from Sjögren’s syndrome mice and sequenced immunoglobulin M heavy-chain variable regions. Finally, autoantigen arrays were performed using immunoglobulin M derived from sera, cervical lymph nodes, spleens, and submandibular gland tissue of Id3−/− animals. Results suggest B cells from salivary tissue of Sjögren’s syndrome mice are similar to those from secondary immune sites in terms of proliferative and secretory capacity. However, differences in repertoire usage, heavy chain complementarity-determining region 3 length, mutational frequency, and N region addition were observed among B cells derived from submandibular gland, cervical lymph node, and spleen tissue. Moreover, autoantigen array data show immunoglobulin M from salivary B cells have enriched specificity for Ro (Sjögren’s syndrome A) and La (Sjögren’s syndrome B). All together, these data suggest salivary B cells have unique repertoire characteristics that likely influence autoantigen binding and contribute to Sjögren’s syndrome disease in a tissue-specific manner.
Introduction
SS is an autoimmune disease that primarily affects women. The disease occurs in 2 forms, termed pSS and secondary SS. In pSS, the disease targets exocrine tissue, resulting in debilitating loss of glandular secretions. In addition, many other serious systemic disease manifestations are observed [1]. In secondary SS, another autoimmune connective tissue disease is present as well, most commonly systemic lupus erythematosus or rheumatoid arthritis [2].
One of the hallmarks of SS is inflammation of the salivary tissue, termed focal lymphocytic sialadenitis [3]. The lymphocytic infiltrate in SS comprises both B and T cells, and the percentage of B cells in diseased salivary tissue increases over time [4]. B cell dysfunction is well established in SS, and there are several lines of evidence that implicate B cells in SS development. Notably, numerous autoantibodies are seen in patients with SS [5], and a recent study [6] shows these may be present even before the onset of symptoms. Detection of anti-Ro (anti-Sjögren’s syndrome A) and anti-La (anti-Sjögren’s syndrome B) antibodies aid in diagnosis [7], and antibodies directed against nuclear antigens and RF are included in the SS diagnostic algorithm [8].
For this study, we chose to examine the B cell repertoire in the pSS model Id3−/− [9]. We used Id3−/− animals because they develop SS in the absence of other autoimmune diseases; therefore, they represent a good model to study systemic immune changes resulting from SS specifically. Although the phenotypic properties of B cells from Id3−/− animals have not been studied extensively, work by Pan et al. [10] demonstrates normal percentages of B cells in lymphoid tissues, although a more recent study [11] shows a decrease in the fraction of splenic marginal zone B cells in this model. Importantly, these animals show equivalent levels of serum IgM, IgG2b, IgG3, and IgA when compared with C57BL/6 wild-type controls, and Id3−/− B cells proliferate normally in response to LPS, anti-IgM + IL-4, and CD40 ligand [10]. However, B cells from Id3-deficient animals show reduced serum IgG1 and IgG2a titers, and B cell proliferation is decreased following BCR cross-linking with F(ab′)2 fragments. In addition, Id3−/− B cells demonstrate diminished responsiveness to T dependent and T independent antigens following immunization [10]. Despite these differences, B cells derived from Id3−/− animals facilitate the study of humoral immunity in pSS because they reliably produce anti-Ro and anti-La autoantibodies [9] and have IgM levels within reference range. In addition, salivary gland infiltrates in this model are composed of both B and T cells, allowing for analyses of B cells in salivary tissue in the context of disease [9].
Although many studies show B cells contribute to SS pathology, it is not clear where these autoreactive B cells reside and whether B cells within salivary tissue have distinct functional properties as compared with B cells derived from secondary immune sites. Accordingly, it is not known whether pathogenic B cells enter the salivary tissue in a stochastic manner or whether defects in B cell development or selection give rise to a population of cells with certain repertoire characteristics that target salivary gland tissue preferentially. Because the B cell compartment expressing IgM is clearly dysregulated in human SS [12], we chose to focus on these cells.
Several studies show an important role for IgM+ B cells in SS disease pathogenesis. Specifically, patients with pSS show significantly more mutated CD27+ memory Cμ transcripts as compared with corresponding Cγ or Cα transcripts [13]. In addition, patients with SS have a high risk of developing non-Hodgkin lymphomas [14]. Such malignancies frequently show clonal expansion of B cells expressing IgM [15, 16], and 2 studies of patients with SS and lymphoma demonstrate clonal IgM+ RF B cells [17, 18]. Importantly, results from the Sjogren’s International Collaborative Clinical Alliance study [8] suggest serologic RF positivity should be included as part of the new diagnostic criteria for SS. These findings indicate that development of lymphomas may be driven by chronic autoantigen engagement of IgM-expressing B cells [19]. Therefore, several lines of evidence point to disturbed IgM+ B cells in SS that are associated with autoimmunity and malignancy. Thus, it is important to identify the underlying reasons for IgM abnormalities in patients with SS and also to understand the triggers for autoantibody production in this population to prevent lymphoma development and mitigate B cell–driven pathology in SS.
This study used a pSS murine model to determine whether B cells within salivary tissues have unique characteristics that may be amenable to therapeutic manipulation. We performed extensive analyses on the IgM heavy-chain variable region of sequences from the SMG, cLN, and spleen in a pSS model, and we used an autoantigen array to examine differences in autoantibody binding specificities among IgM derived from sera, SMG, spleen, and cLNs in these animals. Importantly, IgM derived from salivary B cells exhibits unique repertoire features and is enriched for anti-Ro and anti-La reactivity, suggesting distinguishing characteristics inherent in IgM derived from B cells in salivary tissue. Such findings have important implications for understanding SS pathogenesis and will aid in the design of therapeutics that target B cells in SS.
MATERIALS AND METHODS
Mice
B6.129S-Id3tm1Zhu/J (Id3−/−) and C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Id3−/− animals were backcrossed to the C57BL/6 strain for 11 generations (http://jaxmice.jax.org/strain/006301.html). Both male and female C57BL/6 and Id3−/− animals were used because this strain demonstrates SS disease with equal sex predilection. All C57BL/6 and Id3−/− animals were >6 months old at the time of death, consistent with the time of disease development [9]. Animals were killed using CO2. Mice were cared for and handled in accordance with Institutional Animal Care and Use Committee and U.S. National Institutes of Health guidelines.
Cell isolation, flow cytometry, and sorting
B cells were isolated from spleens, cLNs, and SMG tissue of pSS (Id3−/−) mice. Appropriate age- and sex-matched controls were used. Splenocytes and cLN cells were isolated by mechanical disruption. SMG tissue was isolated as previously described [20]. Splenocytes were stained by immunofluorescence for B220 (BD Biosciences, Franklin Lakes, NJ, USA), CD23 (Abcam, Cambridge, MA, USA), CD21/35 (BD Biosciences), and AA4.1 (BioLegend, San Diego, CA, USA) or IgM (BioLegend). Fo B cells (B220+, CD23+, CD21/35int) from splenic tissue were sorted and purified using Influx or FACS Aria instruments (BD Biosciences). cLN B cells (B220+, CD23+, CD21/35int) were sort-purified similarly. B cells from SMG tissue were stained fluorescently with F4/80 (eBioscience, San Diego, CA, USA), B220, and CD4 (BD Biosciences). B cells (B220+, CD4-, F4/80-) from SMG samples were sort-purified as described for spleen and cLN samples. All incubations were performed on ice. Briefly, cells were incubated with CD16/32 (BD Biosciences) in staining buffer (2% FBS in PBS) for 30 min, followed by addition of fluorescently labeled antibodies at a 1:1000 dilution for 30 min. Cells were washed once in staining buffer, and sorted at a concentration of 1 × 107/ml. Gating strategies are shown in Supplemental Fig. 1.
IgM secretion
IgM secretion was measured by ELISPOT assay, as previously described [21]. B cells were isolated from spleen, cLN, and SMG tissue of SS mice and sort-purified, as described above. Splenic and cLN samples from individual mice were evaluated, whereas SMG samples were pooled because of the limited number of B cells recovered per gland. B cells from spleen and cLNs of age- and sex-matched control mice were also harvested. Cells were cultured in RPMI 1640 medium containing 5% heat-inactivated FBS, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin in duplicate in 96-well U-bottom plates. B cells were stimulated with LPS (25 μg/ml) for 72 h before assay. B cells were counted with an automated cell counter (ADAM, Bulldog Bio, Portsmouth, NH, USA) and were plated onto MultiScreen-IP Plates (Millipore, Billerica, MA, USA) (2000 cells/well). Plates were precoated with goat anti-mouse Ig (H + L) (Southern Biotech, Birmingham, AL, USA) and then incubated for 3 h at 37°C and 5% CO2. Plates were treated with alkaline phosphatase–conjugated goat anti-mouse IgM (Southern Biotech) and developed with 5-bromo-4-chloro-3-indolyl phosphate/p-NBT chloride substrate (KPL, Gaithersburg, MD, USA). Each sample was plated in duplicate. The total number of ELISPOTs for each sample and mean spot-size calculations were carried out using ImmunoSpot (CTL, Shaker Heights, OH, US) or KS ELISPOT software and an Axiocam MRc camera (Zeiss, Pleasanton, CA, USA).
Proliferation
B cells were isolated from spleen, cLN, and pooled SMG tissue of SS and control mice, as described above. Proliferation was measured by thymidine incorporation, as previously described [22]. Cells (2.5 × 104/well) were cultured as described above and stimulated for 72 h with LPS (25 μg/ml). Cultures were pulsed with 0.5 μCi of [3H]thymidine (Amersham, Pittsburgh, PA, USA) or BrdU for 6 h before the end of the 72-h culture. Thymidine incorporation was measured by scintillation counting of β-particle emissions (Beckman Coulter, Brea, CA, USA). BrdU incorporation was measured using a BrdU cell proliferation kit (EMD Millipore, Billerica, MA, USA).
Single cell sorting, PCR, and sequences
Splenic and cLN Fo B cells (B220+, CD23+, CD21/35int, IgM+) and B cells from SMG tissue (B220+, CD4−, F4/80−) were fluorescently stained and were single-cell sorted onto AmpliGrid slides (Beckman Coulter) or 96 well plates (Denville, South Plainfield, NJ, USA) using an Influx sorter or FACSAria, respectively (BD Biosciences). OneStep reverse transcription and PCR (Qiagen, Valencia, CA, USA) were carried out with 2 primers [23], each at 0.6 μM (MsVH E = GGGAATTCGAGGTGCAGCTGCAGGAGTCTGG; MsCμ E = ATGGCCACCGAATTC TTATCAGA), as follows: 49°C for 30 min; 94°C for 15 min; 35 cycles at 93°C for 30 s, 49°C for 30 s, 71°C for 30 s; and then a final extension at 71°C for 10 min (AmpliSpeed PCR cycler, Advalytix or C1000, BioRad, Hercules, CA, USA). Contents of the reaction were then removed from the center of each well and diluted with distilled H2O. A second, seminested amplification was then performed using the same MsVH E primer and an internal, constant-region primer (MsCμ N = TGTAAAACGACGGCCAGTCATTTGGGAAGGACTGA) plus the first-round PCR product. The PCR reaction was as follows: 95°C for 15 min, 40 cycles at 94°C for 30 s, 50°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 10 min. Sequencing was performed by Biotic Solutions (TACGen, San Pablo, CA, USA) or Genewhiz (South Plainfield, NJ, USA).
Repertoire analyses
The International ImMunoGenecTics Information System (IMGT, Montpellier Cedex, France) was used to determine IgM heavy chain (H) usage of variable (V), diversity (D), and joining (J) regions [24, 25]. Analysis of the CDR-H3 sequence, length, mutational frequency, and N region addition was also performed using IMGT. The hydrophobicity and the charge of the CDR-H3 regions were determined using HeliQuest (http://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParamsV2.py). In-frame sequences were used for analysis.
Autoantigen arrays
B cells were sort-purified from spleen, cLN, and SMG tissue of Id3−/− mice as described above. Cells (1 × 105/well) were cultured in 5% RPMI 1640 medium, stimulated with LPS (25 μg/ml) for 6 d, and supernatants were harvested. These undiluted supernatant samples and sera were used for protein array analysis at the Microarray Core Facility of the University of Texas Southwestern Medical Center (UTSW; Dallas, Texas, USA). Autoantigen arrays bearing 90 autoantigens and 8 control proteins (human IgG, human IgM, mouse IgG, mouse IgM, anti-human IgG, anti-human IgM, anti-mouse IgG, and anti-mouse IgM) were printed in duplicate on nitrocellulose film–coated ONCYTE SuperNOVA 16 slides (Grace Bio-Labs, Bend, OR, USA). The samples were incubated with the autoantigen arrays, and autoantibodies were detected with cy5-labeled anti-mouse IgM using a Genepix 4200A scanner (Molecular Devices, Sunnyvale, CA, USA) with laser wavelengths of 532 nm and 635 nm. The resulting images were analyzed using Genepix Pro 6.0 software (Molecular Devices). The median signal intensity for each spot was calculated and subtracted from the local background around the spot, and data obtained from duplicate spots were averaged. Internally spotted IgG and IgM were used as controls for normalization across the arrays. The anti-IgG and anti-IgM antibodies were used for measuring the total IgG and IgM bound to the anti-IgG and anti-IgM antibodies on the arrays. Finally, the net fluorescence intensity for each antigen was calculated by subtracting a PBS control that was included for each experiment as the negative control. The signal-to-noise ratio was used as a quantitative measurement of the ability to resolve the true signal from background noise. Signal-to-noise ratio values ≥3 were considered significantly greater than background and, therefore, were true signals [26, 27]. The autoantigens and controls on the autoantigen array are listed on the UTSW Microarray Core Facility core website: (http://microarray.swmed.edu/products/product/autoantigen-microarray-panel-i/). The purified autoantigens were ordered from different commercial sources, including Sigma Aldrich (St. Louis, MO, USA), Life Technologies (Waltham, MA, USA), BD Biosciences, SurModics (Eden Prairie, MN, USA), Meridian Bioscience (Cincinnati, OH, USA), R&D Systems (Minneapolis, MN, USA), and Abcam. The validation of the autoantigen microarrays is described in several previous publications [26–33]. Individual autoantigen array assay values for IgM are shown in Supplemental Table 1. The background-subtracted, median signal intensity of each antigen was normalized by the Microarray Core Facility using standard practices, (i.e., with the appropriate internal controls for each data set and the average intensity of total mouse IgM). The heat map figures and statistical tests were generated and performed by D.P.G. using R software (https://www.r-project.org/). The autoantigen array accession number is GSE62176. The data can be accessed at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE62176.
Statistical analysis
Mann-Whitney and χ2 tests were done using Prism (GraphPad Software Inc., La Jolla, CA, USA). Analysis of the array data revealed a batch effect (i.e., 2 sets of samples were run more than a year apart on different versions of the array) that could not be easily corrected by a linear or simple nonlinear transformation. Hence, analysis of the array data was conducted stratified according to the 2 “runs.” Expression of Ro-52, Ro-60, and La signals were hypothesized to be greatest in SMG samples. Simple rank-sum tests were performed to test this hypothesis. The ranks (across samples for each run) of each of 3 autoantigens were computed for the SMG sample (for each run). The total rank-sum values were then compared with the rank-sum values from all samples (within run), whereby the samples were permuted with respect to their assay values.
RESULTS
B cells from salivary tissue, spleen, and cLNs secrete similar levels of IgM
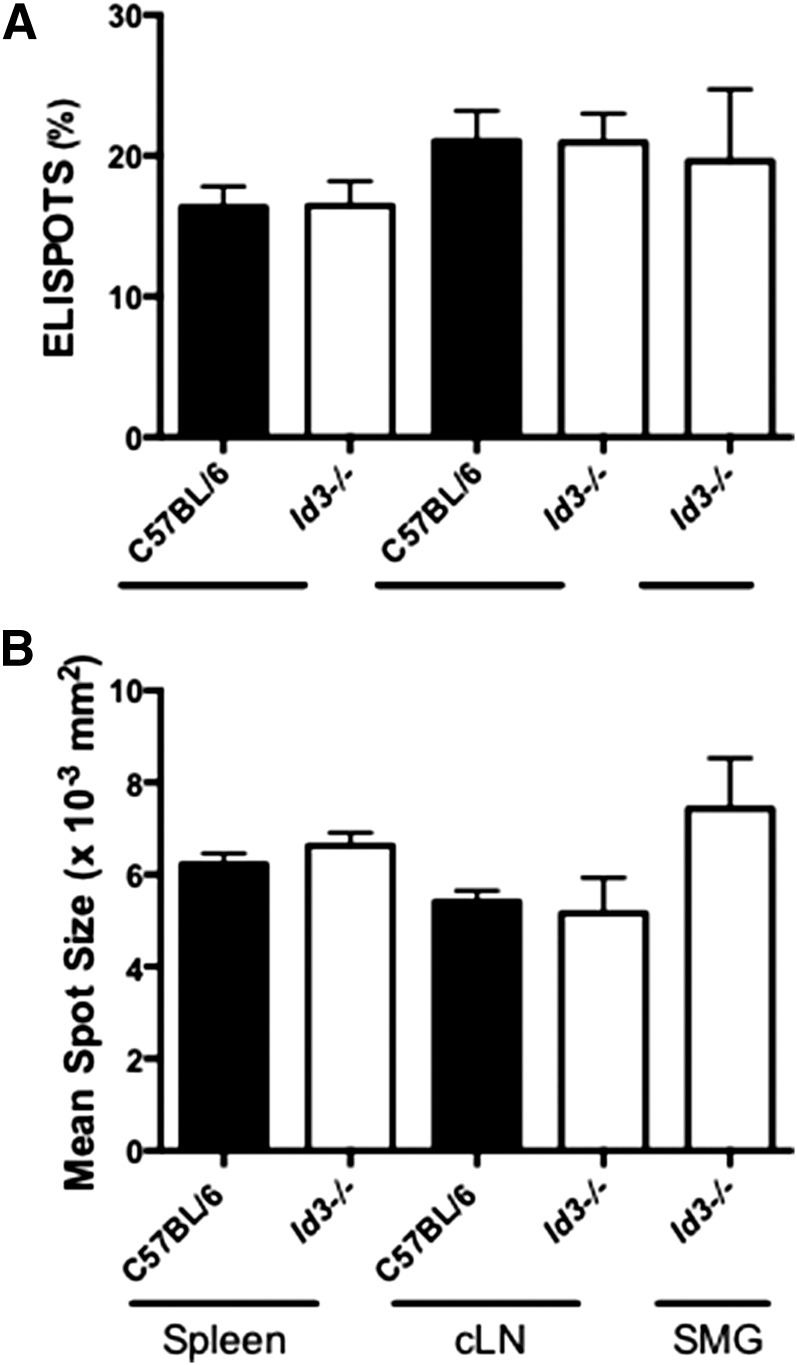
To determine whether B cells from salivary tissue secrete enhanced levels of Ig as compared with B cells isolated from other immune sites, we stimulated sort-purified Id3−/− B cells from cLNs, spleen, and SMG salivary tissue with LPS and performed ELISPOT assays. We found no differences in the percentage of B cells secreting IgM from SMG, spleen, and cLNs in the Id3−/− and control C57BL/6 animals (Fig. 1A). Healthy animals do not have lymphocytes within salivary tissue, so we could not compare SMG lymphocytes from healthy control animals with those with SS. There were no differences when we compared the percentage of IgM secretion-positive salivary B cells with those from cLN and spleen, suggesting that B cells in salivary tissue in SS are similar in the percentage of cells that produce antibodies as compared with those from secondary immune sites.
Figure 1. B cells derived from spleen, cLN, and SMG show similar IgM secretion characteristics.
B cells were sort-purified from the indicated sites and stimulated with LPS (25 μg/ml) for 72 h before the ELISPOT assay (A) Total IgM secretion in Id3−/− (spleen, n = 10; cLN, n = 9; SMG, n = 10) and C57BL/6 B cells (spleen, n = 9; cLN, n = 7). Data are shown as the percentage of total B cells secreting IgM. (B) Mean spot size of Id3−/− and C57BL/6 B cell data in (A). Data from 2 independent experiments are shown.
To determine whether antibody secretion per cell is enhanced in the SMG population, we examined the mean spot size for each sample. Analysis of Id3−/− and C57BL/6 B cells isolated from the spleen, cLN, and SMG revealed no differences in the amount of IgM secreted (Fig. 1B). Together, these findings suggest salivary B cells are similar to those from other immune sites in the percentage of cells that secrete IgM and also in the amount of antibody secreted per B cell in pSS.
B cells from salivary tissue are not as hyperproliferative in response to LPS as compared to those from other immune sites
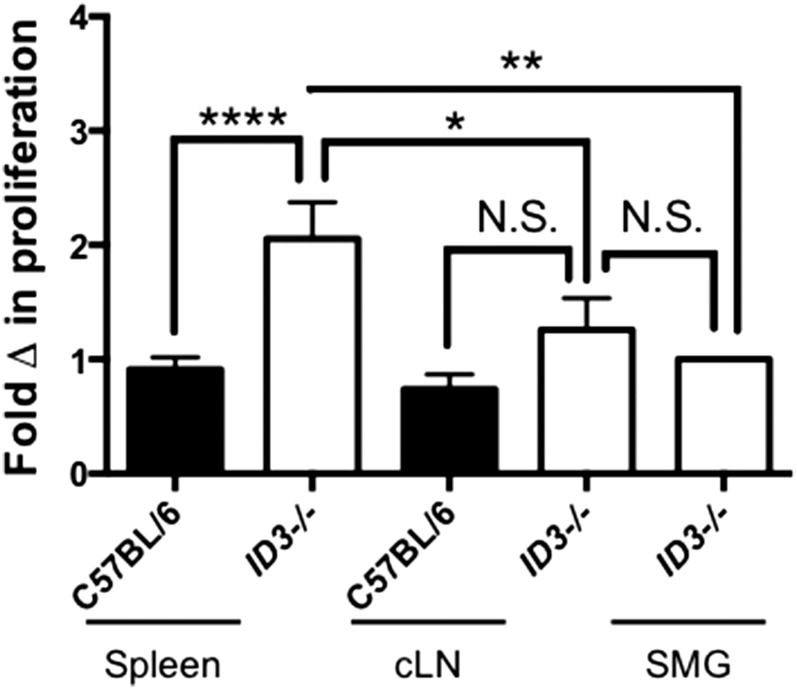
To test whether salivary gland B cells are hyperproliferative, we stimulated B cells from Id3−/− animals and measured proliferation (Fig. 2). B cells derived from splenic tissue showed greater proliferation following LPS stimulation than did splenic B cells from C57BL/6 animals (P < 0.0001) and Id3−/− cLNs (P = 0.01). Interestingly, Id3−/− B cells derived from salivary tissue proliferated similar to B cells derived from cLNs (P = 0.6) but showed reduced proliferation when compared with those from the spleen (P = 0.04). B cell proliferation was similar in cLN B cells derived from Id3−/− and C57BL/6 control animals (P = 0.2). Thus, results from our proliferation studies show salivary gland B cells do not display a hyperproliferative phenotype in response to LPS stimulation and, thus, are not distinguishable from B cells isolated from other sites based on Tlr4-mediated proliferation.
Figure 2. Proliferative capacity of SMG B cells is similar or reduced as compared with B cells isolated from secondary lymphoid organs.
B cells were sort-purified from the indicated site and stimulated with LPS (25 μg/ml) for 72 h before proliferation assay. Cells were incubated with tritium or BrdU for 6 h or before harvesting. Results from 3 experiments are pooled and normalized to SMG data from each experiment; Id3−/− (spleen, n = 13; cLN, n = 13; pooled SMG, n = 3) and C57BL/6 B cells (spleen, n = 11; cLN, n = 12). (N.S., not significant; *P < 0.05, **P < 0.01, and ****P < 0.001).
The IgM heavy-chain repertoire differs in B cells derived from SMG tissue, cLNs, and spleen
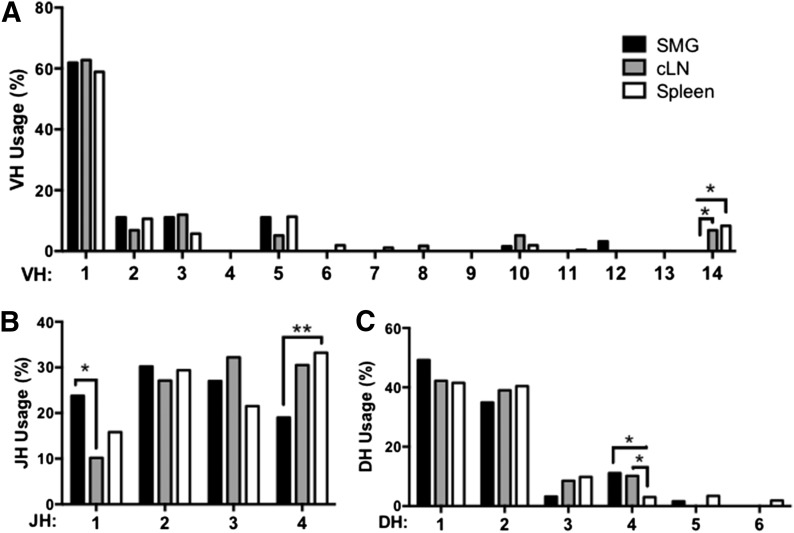
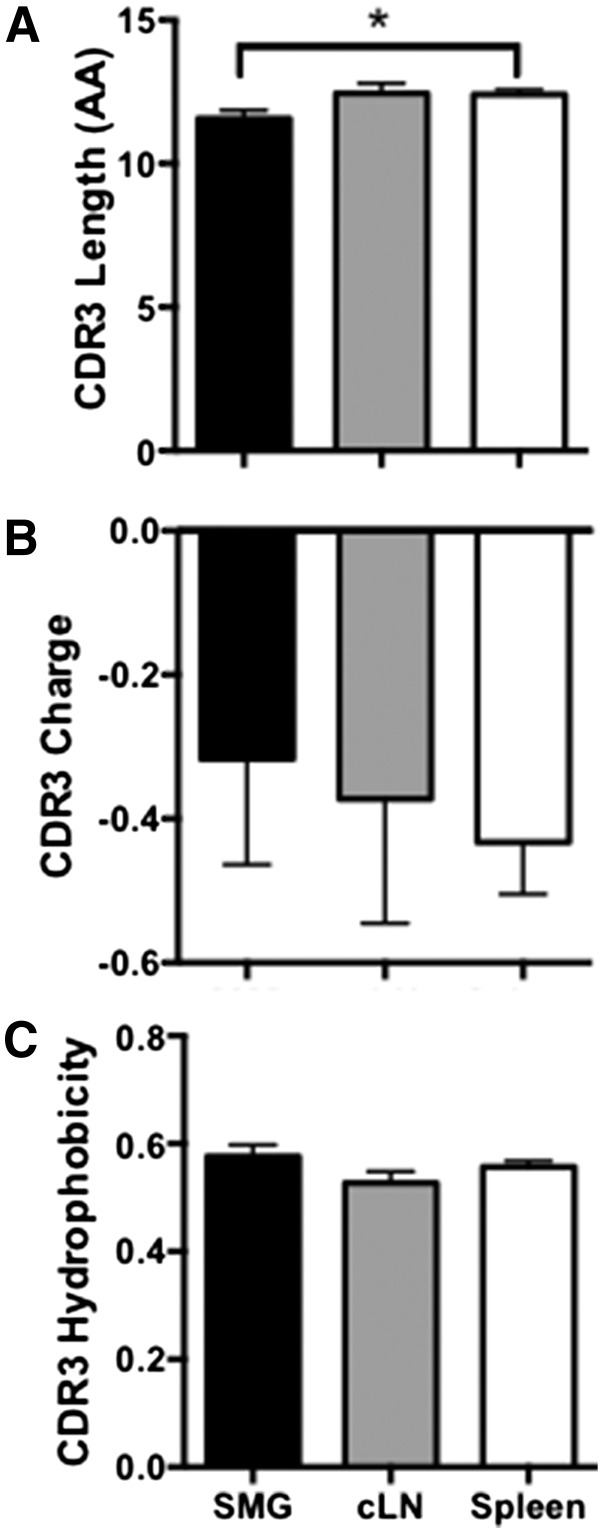
To determine whether B cells in salivary tissue have unique repertoire characteristics, we single-cell sorted B cells from Id3−/− SMG, cLN, and spleen. Splenic and cLN B cell sequences were generated from 4 independent experiments, each with spleens pooled from 4 – 5 animals. SMG B cell sequences were also generated from 4 independent experiments, with SMG tissue from 8–10 animals pooled for each experiment. We performed sequence analysis on IgM from Id3−/− B cells derived from spleen (n = 265), cLN (n = 59), and SMG tissue (n = 63). VH usage was similar among B cells isolated from the 3 sites, with the exception of skewed VH14 usage by Id3−/− splenocytes and cLNs (P = 0.02 and P = 0.01, respectively) (Fig. 3A). Differences were also seen in DH usage. Interestingly, IgM derived from Id3−/− SMG B cells and cLN cells had increased DH4 use as compared with spleen (P = 0.006 and P = 0.01, respectively) (Fig. 3C). JH usage was also skewed because IgM from Id3−/− spleen showed more frequent usage of JH4 than that from salivary tissue (P = 0.03) Moreover, salivary IgM showed increased JH1 usage as compared with cLN (P = 0.05) (Fig. 3B). Of note, the CDR-H3 length of IgM derived from Id3−/− SMG B cells was shorter than that of spleen (average lengths = 11.6 vs. 12.4 amino acids, respectively (P = 0.02) (Fig. 4A). However, CDR-H3 hydrophobicity and charge were similar in each of the sites we examined (Fig. 4B and C, respectively).
Figure 3. Salivary B cells exhibit skewed IgM, VH, DH, and JH usage.
Single-cell PCR was carried out to amplify IgM sequences. (A) VH, (B) JH, and (C) DH usage analysis was performed using IMGT on sequences from Id3−/− SMG (n = 63), cLN (n = 59), and spleen (n = 265). VH, DH, and JH numbers correspond to the gene usage subgroup. Skewing was determined by χ2 test (*P < 0.05, **P < 0.01).
Figure 4. IgM derived from salivary Id3−/− B cells demonstrates short heavy-chain CDR3 lengths but similar charge and hydrophobicity as in cLN and spleen.
Single-cell PCR was performed to amplify and sequence the IgM antibodies. (A) Total CDR-H3 amino acid length, (B) CDR-H3 charge, and (C) CDR-H3 hydrophobicity were analyzed using sequences from Id3−/− SMG (n = 63), cLN (n = 59), and spleen (n = 265). Significance was determined with the Mann-Whitney test (*P < 0.05).
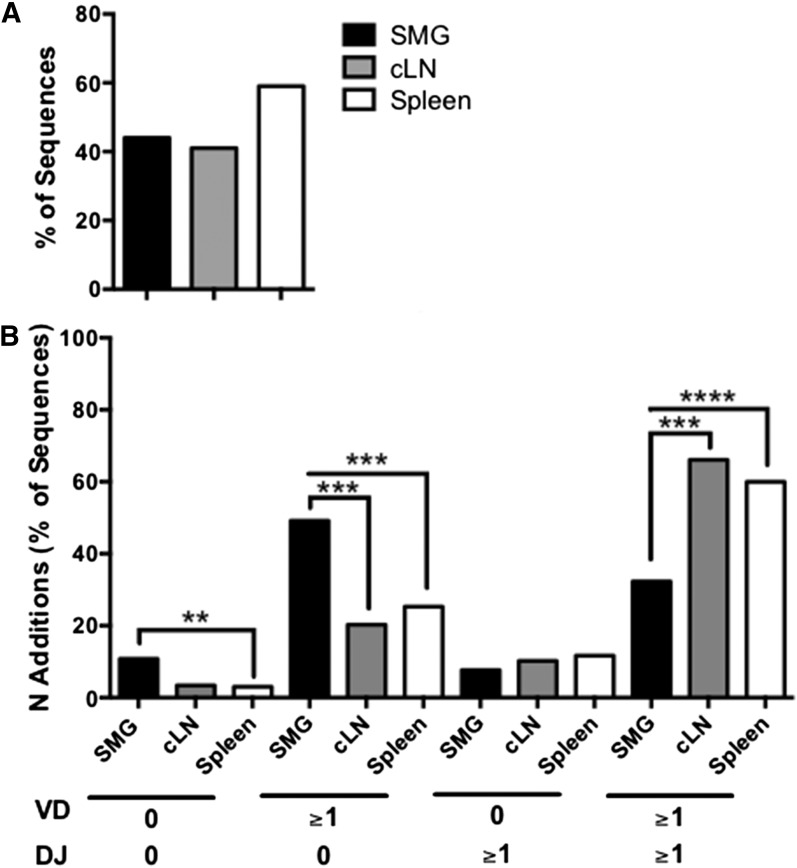
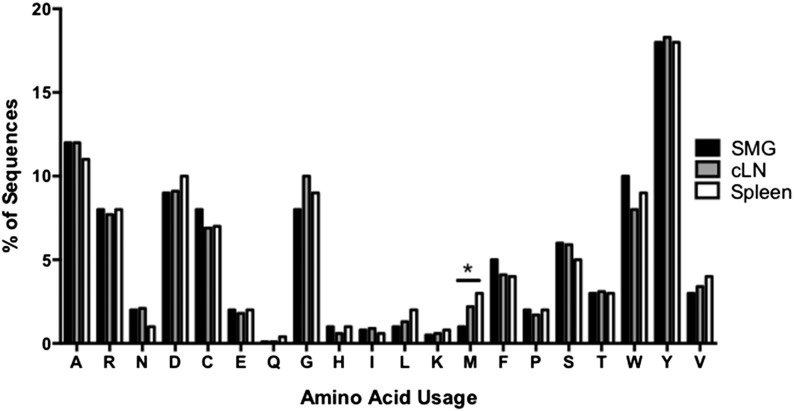
In addition, we evaluated the mutational frequency of the heavy chain variable region from Id3−/− B cells (Fig. 5A). Surprisingly, there were no significant differences seen among salivary, cLN, and splenic IgM. Finally, we examined N region additions. Interestingly, B cells derived from salivary tissue showed significant differences because IgM from SMG B cells was enriched for sequences that lacked N region additions as compared with spleen (P = 0.007). Moreover, salivary B cells had more N region additions at the V–D junction (P = 0.0002 and P = 0.0005) and also fewer sequences that contained N additions at both junctions as compared with spleen and cLN (P < 0.0001 and P = 0.0003, respectively) (Fig. 5B). Finally, we compared CDR-H3 amino acid usage between IgM derived from SMG, cLN, and spleen (Fig. 6). We found that amino acid usage was similar among all populations, although methionine was used more often in the splenic IgM than it was in the salivary IgM (P = 0.01). Taken together, these data suggest that significant differences exist in VH, DH, and JH usage, as well as CDR-H3 length and N region addition of salivary gland–derived IgM that may alter or enhance self-antigen recognition in this population.
Figure 5. IgM derived from salivary B cells has similar mutational frequency but fewer N region additions compared with cLN and spleen.
Single-cell PCR was performed to amplify and sequence IgM heavy-chain variable regions. (A) The number of sequences with 1 or more mutations was determined for the variable regions. (B) The number of N region additions at the V–D and D–J junction from Id3−/− SMG (n = 63), cLN (n = 59), and spleen (n = 265) was determined. The percentages of the sequences with no N region additions, ≥1 at the V–D junction, ≥1 at the D–J junction, and ≥1 N additions at both junctions are shown. Significance was determined with the χ2 test. (0, no N region additions; ≥1, ≥1 N additions; **P < 0.01, ***P < 0.001, ****P < 0.0001).
Figure 6. CDR-3H amino acid usage is similar in IgM derived from spleen, cLN, and salivary B cells.
Single-cell PCR was performed to amplify and sequence IgM heavy-chain variable regions, and the CDR-H3 amino acid usage from IgM derived from Id3−/− spleen (n = 265), cLN (n = 59), and SMG (n = 63) B cells was determined. Differences in usage were determined with the χ2 test. (*P < 0.05).
SS autoantibodies are enriched in IgM derived from salivary gland B cells in Id3−/− mice
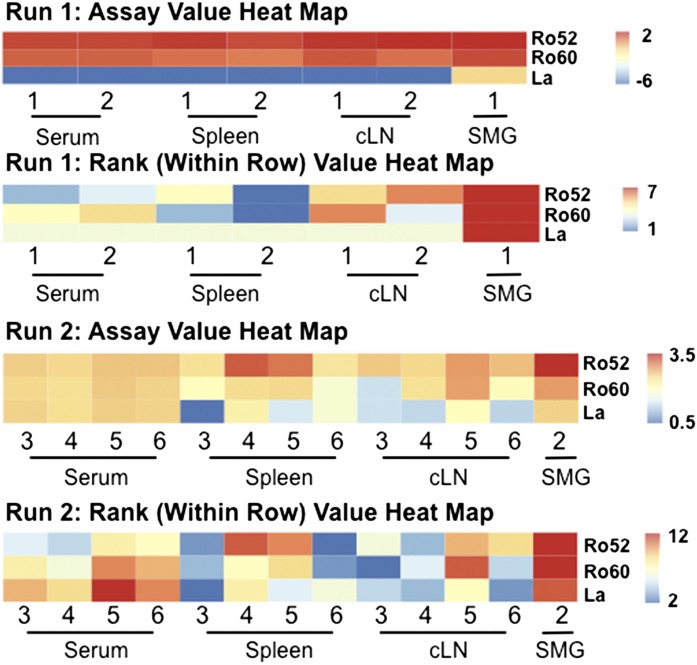
Although Ig sequencing studies reveal fundamental differences in the repertoire, they do not allow for predictions regarding antigen specificity. To investigate cognate antibody interactions, we collected sera and sort-purified B cells from spleen, cLNs, and SMG tissue from Id3−/− animals. We cultured those cells with LPS as described in “Materials and Methods” and collected the supernatants. We then performed autoantigen arrays to detect IgM reactivity to the SS autoantigens Ro and La. Antibodies to La and both Ro isoforms (Ro60 and Ro52) are screened for as part of the diagnostic algorithm for SS [34]. Significantly, IgM derived from salivary B cells showed enriched reactivity for Ro60, Ro52, and La/SSB autoantigens, whereas a diminished signal was present when IgM from other sites and control animals was incubated with these autoantigens (Fig. 7). Taken together, these data demonstrate that reactivity to the SS autoantigens Ro and La lies preferentially in IgM derived from salivary gland B cells.
Figure 7. Salivary IgM from Id3−/− B cells is enriched for Ro and La reactivity.
B cells were sort-purified from Id3−/− spleen (n = 6), cLN (n = 6), and SMG (2 separate pooled samples, n = 9 and n = 10) and stimulated with LPS (25 μg/ml) for 6 d. Supernatant and sera were harvested for autoantigen array. Heat maps of the log10, normalized autoantigen assay values and rank-sum analysis for each run are shown. The observed log10 assay values are color-coded per the legend immediately to the right of the figure. The assay heat maps are color-coded across the entire set of assays depicted for each run, and the rank-sum analysis heat maps are color-coded across each row for each run. Sample type and run are indicated.
DISCUSSION
We used a well-established pSS model (Id3−/−) to explore differences in IgM+ B cell function and repertoire between secondary immune sites and salivary tissue. Importantly, this model has many similarities to human SS because Id3−/− mice exhibit spontaneous disease development, lymphocytic infiltration of salivary tissue, and loss of salivary flow. In addition, Id3−/− mice have autoantibodies to Ro and La [9, 35]. Results from this study show salivary B cells share similar functional characteristics with B cells from other immune sites in pSS. However, we observed skewing of VH, DH, and JH gene usage, shorter CDR-H3 lengths, fewer N region additions in salivary gland B cells, and enriched anti-Ro and anti-La binding specificity in IgM produced by salivary gland B cells. In summary, these data show there are significant differences between IgM antibodies in salivary tissue and those from other immune sites, and this suggests tissue specificity of the immune response that may arise during development or as a result of antigen-driven selection or both. These findings strengthen our current understanding of IgM dysregulation in SS pathogenesis.
B cells are emerging as important therapeutic targets in several types of autoimmunity because they promote disease through interactions with T cells, secrete proinflammatory cytokines and autoantibodies, and migrate to diseased tissue where they may drive or exacerbate inflammation.
B cells are clearly dysregulated in SS disease. Although the presence of antibodies directed against Ro and La are included in the current diagnostic criteria [7], many reports have documented numerous autoantibodies in SS [5]. Although many of the autoantibodies identified in SS are common to other autoimmune connective tissue diseases, such as antinuclear antibodies and RF [36, 37], some are unique and are reported to have functional consequences [38]. For example, anti-M3 muscarinic acetylcholine receptor M3 autoantibodies are identified in patients with SS and these diminish salivary flow in human salivary gland cells [39]. Notably, a complementary study using a mouse model of SS that was deficient in autoantibody production maintained normal salivary flow, suggesting a pathogenic role for B cells in SS-induced xerostomia [40]. Thus, autoantibodies are clearly hallmarks of disease and are likely directly involved in disease progression and symptomology.
Surprisingly, we did not observe a hyperproliferative phenotype in salivary B cells in response to LPS stimulation. However, it is possible that Tlr4 expression differs between salivary B cells and those from secondary immune sites, and further studies are needed to determine whether Id3−/− salivary B cells are hyporesponsive to LPS. Of note, our results differ from a previously published study that examined LPS-induced proliferation of splenic B cells in Id3−/− animals [10]. Variances between the experimental approaches may account for the incongruous results. In the previous study, proliferation assays were performed using mice derived from the 129/SV–C57BL/6 mixed background, whereas our mice were derived from the C57BL/6 strain [10]. It is also important to note differences in B cell isolation. In the previous study, total splenic B cells (including several subpopulations, such as marginal zone, germinal center, and B-1 in addition to Fo) were purified by negative selection, whereas we isolated Fo B cells using positive selection [10]. Thus, differences in genetic background and population heterogeneity could contribute to the disparate results.
Several studies have examined the B cell antibody repertoire in both health and disease. Such studies have focused on the CDR3 region because sequence diversity in this region is particularly important for antigen recognition [41]. In-depth analysis of the CDR-H3 region in murine splenic B cells shows that the average length, charge, and hydrophobicity are tightly controlled during development [42], and there is a loss of charged and hydrophobic CDR-H3s in the mature B cell repertoire in healthy animals [43]. Results from our study show that B cells derived from salivary tissue do not show any differences in CDR-H3 hydrophobicity or charge. However, shorter CDR-H3 lengths were observed in IgM derived from salivary tissue as compared with those from spleen. This is consistent with our N region addition data because IgM from salivary B cells showed fewer N region additions as compared with spleen and cLN, and the number of N region additions is known to influence CDR3 length [23]. We found amino acid usage in the CDR-H3 region was similar among salivary, cLN, and splenic B cells, although methionine was used more commonly by the splenic B cells as compared with those from salivary tissue. Although the significance of this is not known, a study comparing the CDR-H3 regions from Ig derived from bone marrow from TdT-deficient mice to wild type found that the TdT−/− adult bone marrow Ig (which parallels fetal liver in lacking TdT) was virtually devoid of several amino acid residues, including methionine [44]. The paucity of N region addition, increased usage of DH4, and the decreased use of methionine suggest that the IgM repertoire of salivary B cells is reminiscent of early B fetal cells. This finding is surprising because the germline repertoire is generally thought to confer protection from autoimmunity [45]. Thus, it is possible that these “natural” IgM antibodies in salivary tissue are produced as part of a compensatory protective mechanism [46].
Natural antibodies are produced primarily by marginal zone and B-1 cells [46–48]. Although several studies have examined B cell subsets in SS [13, 49, 50], it remains unclear as to which subset or subsets provide the most significant source of autoantibodies in this disease. Although we focused on follicular B cells in this report, it is also possible that marginal zone or B-1 cells contribute to IgM autoantibody production in SS and may be present within salivary tissue. Studies using BAFF transgenic animals show evidence of a lupus-like disease and SS. These animals have an expanded marginal-zone compartments, and marginal zone and B-1 cells are identified in salivary tissue, suggesting these subsets may have a role in SS [51]. However, Id3−/− animals have reduced marginal-zone B cells and also develop disease, so the role of the marginal-zone subset in SS is unclear at present [11]. In addition, B-1 cells are implicated in autoimmune disease in mice and humans [52–54], although evidence for this subset in autoimmunity is limited. Thus, further studies are needed to determine whether the autoreactive IgM detected in salivary tissue is derived, at least in part, from B-1 or marginal-zone B cells in SS disease.
Although the significance of the shorter CDR-H3s in salivary tissue is not clear, alterations in this region are likely to modify antibody specificity in a pathogenic manner [41]. Accordingly, short IgM CDR-H3 regions are seen in both murine and human lupus [55, 56], and antibodies isolated from parotid tissue of a patient with SS showed shorter CDR-H3 regions as compared with peripheral blood [57]. Although studies suggest short CDR-H3 regions may be indicative of autoreactivity, there is evidence that longer CDR3 regions may also correlate with polyreactivity or autoreactivity [58–60]. Because CDR3 length is tightly controlled during development [42, 43], any deviation from this constraint may be indicative of pathology. Importantly, there are no CDR-H3 sequence characteristics that are known to confer reactivity for Ro or La, so it is not clear whether these differences influence binding of these autoantigens specifically.
The B cell repertoire in SS models and patients has not been studied extensively, likely because of the inherent difficulty in isolating B cells from salivary gland tissue in sufficient amounts for these types of analyses. Moreover, it is difficult to acquire tissue from secondary immune sites of patients with SS. To our knowledge, no studies to date have compared repertoire specificity of IgM derived from SMG to secondary immune sites, although, one recent study [61] used a pSS model to show greater IgG1 isotype specificity against salivary gland lysates from antibody derived from salivary tissue as compared with that from cLNs. Limited studies in patients with SS have compared the salivary gland B cell heavy-chain repertoire to other sites. Results of these studies demonstrated most Ig isolated from lymph nodes and salivary tissue of 2 patients with pSS is polyclonal in nature [62]. Importantly, significant differences were seen in the proportion of cells expressing mutated VH rearrangements, and those in salivary tissue were increased as compared with those in peripheral blood [57, 62].
Although the numbers of patients analyzed in these aforementioned studies is small, together these data suggest that patients with SS have a polyclonal B cell repertoire, and repertoire differences may exist between salivary gland and peripheral B cells in humans [63]. Results from our study herein are consistent with the limited human repertoire analyses available to date. Although we did not see differences in somatic hypermutation between IgM derived from the spleen and salivary tissue, we found the vast number of sequences from our pSS model were polyclonal (data not shown), and our results show IgM derived from salivary B cells has relatively short CDR-H3 regions. At present, it is not clear why the mutational frequency we observed in salivary tissue in mice differs from that reported in humans. This may be due to differences between murine and human SS. Alternatively, our repertoire analyses focused solely on IgM, whereas human studies examined all Ig isotypes in the tissue. Thus, it is possible that the difference in our results may reflect the variance in mutational frequencies between IgG and IgM classes in SS.
The significance of the repertoire differences observed between IgM derived from salivary and splenic B cells is poorly understood at present. However, there are discrete changes that affect small numbers of B cells that may, in fact, be the pathogenic B cells in our model. It is clear that even slight repertoire skewing can have a dramatic effect on the biologic function of a particular B cell subset. For example, the B-1a cell repertoire is skewed toward VH11 and VH12 [64–66]. Although only 5–10% of the population exhibits this VH usage, this results in preferential recognition of phosphatidylcholine by B-1a cells [67]. Studies show that this phosphatidylcholine specificity has an important protective role in a model of sepsis [68]. Thus, B-1a cells provide a notable example of the importance of repertoire skewing in innate defense, and this has biologic significance, even though the actual percentage of VH11/12-expressing cells in the population is low. Therefore, it may well be that the skewed repertoire observed in salivary-derived IgM confers self-reactivity and is significant in SS disease pathogenesis, and further studies are needed to determine how such molecular differences may be directly related to the increased reactivity to autoantigens Ro and La observed in the salivary-derived IgM.
Strikingly, we show for the first time that the salivary IgM repertoire is distinct in Id3−/− mice and IgM reactivity to Ro52, Ro60, and La specifically is concentrated in the B cells from salivary tissue in these animals. Although the reasons for this observation are unclear at present, IgM+ B cells may bind autoantigens in the periphery where they become activated. They then may migrate to salivary tissue in a stochastic manner, where they are retained in the tissue. Alternatively, IgM+ B cells with specificity for SS autoantigens may be preferentially recruited to glandular tissue, where they then become activated. Thus, although it is clear that IgM+ B cells with reactivity to SS autoantigens preferentially populate salivary tissue in our disease model, it remains to be determined whether B cells with antinuclear antigen specificity undergo directional migration to salivary tissue or enter stochastically and are then retained at that site. Studies in humans provide limited data to support both possibilities. One study demonstrated clonal expansion of B cells derived from salivary tissue and lymph nodes of a patient with SS, although most of the Ig examined was polyclonal in nature [57]. Additional work shows salivary gland accumulation of CXCR4+, CXCR5+, CD27+ memory B cells in patients with SS [69], suggesting B cells expressing high levels of these receptors are preferentially recruited to salivary tissue by CXCL12 and CXCL13. However, whether these B cells have specificity for Ro and La is unknown at present.
The importance of ID3 in human SS is unclear, as a study using human minor salivary gland tissue derived from patients with SS did not reveal any significant differences in ID3 expression or any genetic abnormalities in ID3 itself [70]. Nonetheless, it is intriguing to speculate that TCR-mediated T cell selection during T cell development may alter B cell characteristics and drive SS pathogenesis. Importantly, mice that are genetically deficient in Id3 show defects in both positive and negative T cell selection [71]. Elegant experiments by Li et al. [9] suggest that defects in thymic T cell selection are responsible for the generation and accumulation of autoreactive T cells in Id3−/− mice because animals that receive thymectomies at 3 d of age are protected from SS development. Many studies show autoreactive T cells can promote loss of tolerance in B cells [72, 73], although the way in which T cells influence B cells in the Id3−/− model is poorly understood. Of note, autoantibodies are observed only in aged Id3−/− animals, and they correlate with exocrine histopathology [9]. These findings suggest pathogenic T cells induce B cell autoreactivity, which is crucial for disease progression.
In summary, B cells derived from salivary tissue in SS mice share similar functional characteristics with those derived from secondary immune sites. However, differences are seen in the repertoire of salivary B cells, suggesting a role for salivary tissue in the progression of the B cell response in SS. Although further studies in humans are needed to fully elucidate the role of the salivary tissue to shaping of the adaptive immune response in disease, the current study suggests that salivary tissue contributes to the development and selection of the B cell repertoire in SS.
AUTHORSHIP
J.M.K., N.E.H., T.C.V., and T.L.R. conceived of, designed, and performed the experiments related to cell sorting, single-cell PCR, repertoire analysis, secretion, and proliferation. I.R., M.Y., Q.Z.L, and D.P.G. conceived of, designed, and performed the experiments related to the autoantigen array.
ACKNOWLEDGMENTS
This work was supported by a Mentored Clinical Scientist Research Career Development Award (K08 DE020882) from the U.S. National Institutes of Health (NIH) National Institute of Dental and Craniofacial Research (NIDCR) awarded to J.M.K. and Grant R01 AI29690 from the NIH National Institute of Allergy and Infectious Diseases (NIAID) awarded to T.L.R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCR, NIAID, or the NIH. The authors thank Raymond J. Kelleher, PhD, at the University at Buffalo, State University of New York, and Jeremy Kiripolsky, MSc, currently at Roswell Park Cancer Institute in Buffalo, New York, USA, for technical assistance.
Glossary
- CDR-H3
heavy chain complementarity-determining region 3
- cLN
cervical lymph node
- Fo
follicular
- IMGT
ImMunoGenecTics Information System
- pSS
primary Sjögren’s syndrome
- RF
rheumatoid factor
- SMG
submandibular gland
- SS
Sjögren’s syndrome
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interests.
REFERENCES
- 1.Malladi A. S., Sack K. E., Shiboski S. C., Shiboski C. H., Baer A. N., Banushree R., Dong Y., Helin P., Kirkham B. W., Li M., Sugai S., Umehara H., Vivino F. B., Vollenweider C. F., Zhang W., Zhao Y., Greenspan J. S., Daniels T. E., Criswell L. A. (2012) Primary Sjögren’s syndrome as a systemic disease: a study of participants enrolled in an international Sjögren’s syndrome registry. Arthritis Care Res. (Hoboken) 64, 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox R. I. (2005) Sjögren’s syndrome. Lancet 366, 321–331. [DOI] [PubMed] [Google Scholar]
- 3.Daniels T. E., Cox D., Shiboski C. H., Schiødt M., Wu A., Lanfranchi H., Umehara H., Zhao Y., Challacombe S., Lam M. Y., De Souza Y., Schiødt J., Holm H., Bisio P. A., Gandolfo M. S., Sawaki T., Li M., Zhang W., Varghese-Jacob B., Ibsen P., Keszler A., Kurose N., Nojima T., Odell E., Criswell L. A., Jordan R., Greenspan J. S.; Sjögren’s International Collaborative Clinical Alliance Research Groups (2011) Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome among 1,726 registry participants. Arthritis Rheum. 63, 2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christodoulou M. I., Kapsogeorgou E. K., Moutsopoulos H. M. (2010) Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J. Autoimmun. 34, 400–407. [DOI] [PubMed] [Google Scholar]
- 5.Tzioufas A. G., Tatouli I. P., Moutsopoulos H. M. (2012) Autoantibodies in Sjögren’s syndrome: clinical presentation and regulatory mechanisms. Presse Med. 41, e451–e460. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson R., Theander E., Sjöström B., Brokstad K., Henriksson G. (2013) Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA 310, 1854–1855. [DOI] [PubMed] [Google Scholar]
- 7.Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H. M., Alexander E. L., Carsons S. E., Daniels T. E., Fox P. C., Fox R. I., Kassan S. S., Pillemer S. R., Talal N., Weisman M. H.; European Study Group on Classification Criteria for Sjögren’s Syndrome (2002) Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 61, 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiboski S. C., Shiboski C. H., Criswell L., Baer A., Challacombe S., Lanfranchi H., Schiødt M., Umehara H., Vivino F., Zhao Y., Dong Y., Greenspan D., Heidenreich A. M., Helin P., Kirkham B., Kitagawa K., Larkin G., Li M., Lietman T., Lindegaard J., McNamara N., Sack K., Shirlaw P., Sugai S., Vollenweider C., Whitcher J., Wu A., Zhang S., Zhang W., Greenspan J., Daniels T.; Sjögren’s International Collaborative Clinical Alliance (SICCA) Research Groups (2012) American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res. (Hoboken) 64, 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H., Dai M., Zhuang Y. (2004) A T cell intrinsic role of Id3 in a mouse model for primary Sjogren’s syndrome. Immunity 21, 551–560. [DOI] [PubMed] [Google Scholar]
- 10.Pan L., Sato S., Frederick J. P., Sun X. H., Zhuang Y. (1999) Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol. Cell. Biol. 19, 5969–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quong M. W., Martensson A., Langerak A. W., Rivera R. R., Nemazee D., Murre C. (2004) Receptor editing and marginal zone B cell development are regulated by the helix-loop-helix protein, E2A. J. Exp. Med. 199, 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen A., Lipsky P. E., Dörner T. (2007) B cells in Sjögren’s syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res. Ther. 9, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen A., Gosemann M., Pruss A., Reiter K., Ruzickova S., Lipsky P. E., Dörner T. (2004) Abnormalities in peripheral B cell memory of patients with primary Sjögren’s syndrome. Arthritis Rheum. 50, 1897–1908. [DOI] [PubMed] [Google Scholar]
- 14.Ekström Smedby K., Vajdic C. M., Falster M., Engels E. A., Martínez-Maza O., Turner J., Hjalgrim H., Vineis P., Seniori Costantini A., Bracci P. M., Holly E. A., Willett E., Spinelli J. J., La Vecchia C., Zheng T., Becker N., De Sanjosé S., Chiu B. C., Dal Maso L., Cocco P., Maynadié M., Foretova L., Staines A., Brennan P., Davis S., Severson R., Cerhan J. R., Breen E. C., Birmann B., Grulich A. E., Cozen W. (2008) Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 111, 4029–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papiris S. A., Kalomenidis I., Malagari K., Kapotsis G. E., Harhalakis N., Manali E. D., Rontogianni D., Roussos C., Moutsopoulos H. M. (2007) Extranodal marginal zone B-cell lymphoma of the lung in Sjögren’s syndrome patients: reappraisal of clinical, radiological, and pathology findings. Respir. Med. 101, 84–92. [DOI] [PubMed] [Google Scholar]
- 16.Risselada A. P., Kruize A. A., Bijlsma J. W. (2013) Clinical features distinguishing lymphoma development in primary Sjögren’s Syndrome—a retrospective cohort study. Semin. Arthritis Rheum. 43, 171–177. [DOI] [PubMed] [Google Scholar]
- 17.Martin T., Weber J. C., Levallois H., Labouret N., Soley A., Koenig S., Korganow A. S., Pasquali J. L. (2000) Salivary gland lymphomas in patients with Sjögren’s syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum. 43, 908–916. [DOI] [PubMed] [Google Scholar]
- 18.Hansen A., Reiter K., Pruss A., Loddenkemper C., Kaufmann O., Jacobi A. M., Scholze J., Lipsky P. E., Dörner T. (2006) Dissemination of a Sjögren’s syndrome-associated extranodal marginal-zone B cell lymphoma: circulating lymphoma cells and invariant mutation pattern of nodal Ig heavy- and light-chain variable-region gene rearrangements. Arthritis Rheum. 54, 127–137. [DOI] [PubMed] [Google Scholar]
- 19.Du M., Diss T. C., Xu C., Peng H., Isaacson P. G., Pan L. (1996) Ongoing mutation in MALT lymphoma immunoglobulin gene suggests that antigen stimulation plays a role in the clonal expansion. Leukemia 10, 1190–1197. [PubMed] [Google Scholar]
- 20.Nauntofte B., Dissing S. (1987) Stimulation-induced changes in cytosolic calcium in rat parotid acini. Am. J. Physiol. 253, G290–G297. [DOI] [PubMed] [Google Scholar]
- 21.Tumang J. R., Francés R., Yeo S. G., Rothstein T. L. (2005) Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J. Immunol. 174, 3173–3177. [DOI] [PubMed] [Google Scholar]
- 22.Tumang J. R., Hastings W. D., Bai C., Rothstein T. L. (2004) Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur. J. Immunol. 34, 2158–2167. [DOI] [PubMed] [Google Scholar]
- 23.Kantor A. B., Merrill C. E., Herzenberg L. A., Hillson J. L. (1997) An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J. Immunol. 158, 1175–1186. [PubMed] [Google Scholar]
- 24.Lefranc M. P. (2011) IMGT, the International ImMunoGeneTics Information System. Cold Spring Harb. Protoc. 2011, 595–603. [DOI] [PubMed] [Google Scholar]
- 25.Giudicelli V., Brochet X., Lefranc M. P. (2011) IMGT/V-QUEST: IMGT standardized analysis of the immunoglobulin (IG) and T cell receptor (TR) nucleotide sequences. Cold Spring Harb. Protoc. 2011, 695–715. [DOI] [PubMed] [Google Scholar]
- 26.Li Q. Z., Xie C., Wu T., Mackay M., Aranow C., Putterman C., Mohan C. (2005) Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J. Clin. Invest. 115, 3428–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q. Z., Zhou J., Wandstrat A. E., Carr-Johnson F., Branch V., Karp D. R., Mohan C., Wakeland E. K., Olsen N. J. (2007) Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin. Exp. Immunol. 147, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong B. F., Tseng L. C., Lee T., Vasquez R., Li Q. Z., Zhang S., Karp D. R., Olsen N. J., Mohan C. (2012) IgG and IgM autoantibody differences in discoid and systemic lupus patients. J. Invest. Dermatol. 132, 2770–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q. Z., Zhou J., Lian Y., Zhang B., Branch V. K., Carr-Johnson F., Karp D. R., Mohan C., Wakeland E. K., Olsen N. J. (2010) Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin. Exp. Immunol. 159, 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q. Z., Karp D. R., Quan J., Branch V. K., Zhou J., Lian Y., Chong B. F., Wakeland E. K., Olsen N. J. (2011) Risk factors for ANA positivity in healthy persons. Arthritis Res. Ther. 13, R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson S. W., Scharping N. E., Kolhatkar N. S., Khim S., Schwartz M. A., Li Q. Z., Hudkins K. L., Alpers C. E., Liggitt D., Rawlings D. J. (2014) Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 192, 4525–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayeux J., Skaug B., Luo W., Russell L. M., John S., Saelee P., Abbasi H., Li Q. Z., Garrett-Sinha L. A., Satterthwaite A. B. (2015) Genetic Interaction between Lyn, Ets1, and Btk in the control of antibody levels. J. Immunol. 195, 1955–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baum R., Sharma S., Carpenter S., Li Q. Z., Busto P., Fitzgerald K. A., Marshak-Rothstein A., Gravallese E. M. (2015) Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice. J. Immunol. 194, 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulte-Pelkum J., Fritzler M., Mahler M. (2009) Latest update on the Ro/SS-A autoantibody system. Autoimmun. Rev. 8, 632–637. [DOI] [PubMed] [Google Scholar]
- 35.Cha S., Peck A. B., Humphreys-Beher M. G. (2002) Progress in understanding autoimmune exocrinopathy using the non-obese diabetic mouse: an update. Crit. Rev. Oral Biol. Med. 13, 5–16. [DOI] [PubMed] [Google Scholar]
- 36.Evans J. (1998) Antinuclear antibody testing in systemic autoimmune disease. Clin. Chest Med. 19, 613–625, vii. [DOI] [PubMed] [Google Scholar]
- 37.Steiner G. (2007) Auto-antibodies and autoreactive T-cells in rheumatoid arthritis: pathogenetic players and diagnostic tools. Clin. Rev. Allergy Immunol. 32, 23–35. [DOI] [PubMed] [Google Scholar]
- 38.Routsias J. G., Tzioufas A. G. (2007) Sjögren’s syndrome—study of autoantigens and autoantibodies. Clin. Rev. Allergy Immunol. 32, 238–251. [DOI] [PubMed] [Google Scholar]
- 39.Lee B. H., Gauna A. E., Perez G., Park Y. J., Pauley K. M., Kawai T., Cha S. (2013) Autoantibodies against muscarinic type 3 receptor in Sjögren’s syndrome inhibit aquaporin 5 trafficking. PLoS One 8, e53113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson C. P., Brayer J., Yamachika S., Esch T. R., Peck A. B., Stewart C. A., Peen E., Jonsson R., Humphreys-Beher M. G. (1998) Transfer of human serum IgG to nonobese diabetic Igμnull mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjögren’s syndrome. Proc. Natl. Acad. Sci. USA 95, 7538–7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J. L., Davis M. M. (2000) Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 13, 37–45. [DOI] [PubMed] [Google Scholar]
- 42.Schelonka R. L., Tanner J., Zhuang Y., Gartland G. L., Zemlin M., Schroeder H. W. Jr (2007) Categorical selection of the antibody repertoire in splenic B cells. Eur. J. Immunol. 37, 1010–1021. [DOI] [PubMed] [Google Scholar]
- 43.Ivanov I. I., Schelonka R. L., Zhuang Y., Gartland G. L., Zemlin M., Schroeder H. W. Jr (2005) Development of the expressed Ig CDR-H3 repertoire is marked by focusing of constraints in length, amino acid use, and charge that are first established in early B cell progenitors. J. Immunol. 174, 7773–7780. [DOI] [PubMed] [Google Scholar]
- 44.Schelonka R. L., Ivanov I. I., Vale A. M., Szymanska E., Zemlin M., Gartland G. L., Schroeder H. W. Jr (2010) The CDR-H3 repertoire from TdT-deficient adult bone marrow is a close, but not exact, homologue of the CDR-H3 repertoire from perinatal liver. J. Immunol. 185, 6075–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robey I. F., Peterson M., Horwitz M. S., Kono D. H., Stratmann T., Theofilopoulos A. N., Sarvetnick N., Teyton L., Feeney A. J. (2004) Terminal deoxynucleotidyltransferase deficiency decreases autoimmune disease in diabetes-prone nonobese diabetic mice and lupus-prone MRL-Fas(lpr) mice. J. Immunol. 172, 4624–4629. [DOI] [PubMed] [Google Scholar]
- 46.Rothstein T. L., Griffin D. O., Holodick N. E., Quach T. D., Kaku H. (2013) Human B-1 cells take the stage. Ann. N. Y. Acad. Sci. 1285, 97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baumgarth N. (2011) The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11, 34–46. [DOI] [PubMed] [Google Scholar]
- 48.Cerutti A., Cols M., Puga I. (2013) Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 13, 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bohnhorst J. Ø., Bjørgan M. B., Thoen J. E., Natvig J. B., Thompson K. M. (2001) Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjögren’s syndrome. J. Immunol. 167, 3610–3618. [DOI] [PubMed] [Google Scholar]
- 50.Szyszko E. A., Brun J. G., Skarstein K., Peck A. B., Jonsson R., Brokstad K. A. (2011) Phenotypic diversity of peripheral blood plasma cells in primary Sjögren’s syndrome. Scand. J. Immunol. 73, 18–28. [DOI] [PubMed] [Google Scholar]
- 51.Groom J., Kalled S. L., Cutler A. H., Olson C., Woodcock S. A., Schneider P., Tschopp J., Cachero T. G., Batten M., Wheway J., Mauri D., Cavill D., Gordon T. P., Mackay C. R., Mackay F. (2002) Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J. Clin. Invest. 109, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T., Ishikawa S., Akadegawa K., Ito T., Yurino H., Kitabatake M., Yoneyama H., Matsushima K. (2004) Aberrant B1 cell migration into the thymus results in activation of CD4 T cells through its potent antigen-presenting activity in the development of murine lupus. Eur. J. Immunol. 34, 3346–3358. [DOI] [PubMed] [Google Scholar]
- 53.Griffin D. O., Rothstein T. L. (2011) A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med. 208, 2591–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Potula H. H., Xu Z., Zeumer L., Sang A., Croker B. P., Morel L. (2012) Cyclin-dependent kinase inhibitor Cdkn2c deficiency promotes B1a cell expansion and autoimmunity in a mouse model of lupus. J. Immunol. 189, 2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipsanen V., Walter B., Emara M., Siminovitch K., Lam J., Kaushik A. (1997) Restricted CDR3 length of the heavy chain is characteristic of six randomly isolated disease-associated VH J558+ IgM autoantibodies in lupus prone motheaten mice. Int. Immunol. 9, 655–664. [DOI] [PubMed] [Google Scholar]
- 56.Yurasov S., Wardemann H., Hammersen J., Tsuiji M., Meffre E., Pascual V., Nussenzweig M. C. (2005) Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen A., Jacobi A., Pruss A., Kaufmann O., Scholze J., Lipsky P. E., Dörner T. (2003) Comparison of immunoglobulin heavy chain rearrangements between peripheral and glandular B cells in a patient with primary Sjögren’s syndrome. Scand. J. Immunol. 57, 470–479. [DOI] [PubMed] [Google Scholar]
- 58.Samuels J., Ng Y. S., Coupillaud C., Paget D., Meffre E. (2005) Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 201, 1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meffre E., Milili M., Blanco-Betancourt C., Antunes H., Nussenzweig M. C., Schiff C. (2001) Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J. Clin. Invest. 108, 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haynes B. F., Verkoczy L. (2014) AIDS/HIV. Host controls of HIV neutralizing antibodies. Science 344, 588–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen C. Q., Ogunniyi A. O., Karabiyik A., Love J. C. (2013) Single-cell analysis reveals isotype-specific autoreactive B cell repertoires in Sjögren’s syndrome. PLoS One 8, e58127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gellrich S., Rutz S., Borkowski A., Golembowski S., Gromnica-Ihle E., Sterry W., Jahn S. (1999) Analysis of V(H)-D-J(H) gene transcripts in B cells infiltrating the salivary glands and lymph node tissues of patients with Sjögren’s syndrome. Arthritis Rheum. 42, 240–247. [DOI] [PubMed] [Google Scholar]
- 63.Dörner T., Hansen A., Jacobi A., Lipsky P. E. (2002) Immunglobulin repertoire analysis provides new insights into the immunopathogenesis of Sjögren’s syndrome. Autoimmun. Rev. 1, 119–124. [DOI] [PubMed] [Google Scholar]
- 64.Mercolino T. J., Arnold L. W., Hawkins L. A., Haughton G. (1988) Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline: relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J. Exp. Med. 168, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang H., Clarke S. H. (2004) Positive selection focuses the VH12 B-cell repertoire towards a single B1 specificity with survival function. Immunol. Rev. 197, 51–59. [DOI] [PubMed] [Google Scholar]
- 66.Carmack C. E., Shinton S. A., Hayakawa K., Hardy R. R. (1990) Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J. Exp. Med. 172, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hardy R. R., Wei C. J., Hayakawa K. (2004) Selection during development of VH11+ B cells: a model for natural autoantibody-producing CD5+ B cells. Immunol. Rev. 197, 60–74. [DOI] [PubMed] [Google Scholar]
- 68.Boes M., Prodeus A. P., Schmidt T., Carroll M. C., Chen J. (1998) A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188, 2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen A., Reiter K., Ziprian T., Jacobi A., Hoffmann A., Gosemann M., Scholze J., Lipsky P. E., Dörner T. (2005) Dysregulation of chemokine receptor expression and function by B cells of patients with primary Sjögren’s syndrome. Arthritis Rheum. 52, 2109–2119. [DOI] [PubMed] [Google Scholar]
- 70.Sellam J., Miceli-Richard C., Gottenberg J. E., Proust A., Ittah M., Lavie F., Loiseau P., Mariette X. (2008) Is inhibitor of differentiation 3 involved in human primary Sjögren’s syndrome? Rheumatology (Oxford) 47, 437–441. [DOI] [PubMed] [Google Scholar]
- 71.Bain G., Cravatt C. B., Loomans C., Alberola-Ila J., Hedrick S. M., Murre C. (2001) Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat. Immunol. 2, 165–171. [DOI] [PubMed] [Google Scholar]
- 72.Shlomchik M. J. (2009) Activating systemic autoimmunity: B’s, T’s, and tolls. Curr. Opin. Immunol. 21, 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pagán A. J., Ramón H. E., Hondowicz B. D., Erikson J. (2006) T cell-mediated activation and regulation of anti-chromatin B cells. Autoimmun. Rev. 5, 373–376. [DOI] [PubMed] [Google Scholar]