Abstract
The state of the brain and body constantly varies on rapid and slow time scales. These variations contribute to the apparent noisiness of sensory responses at both the neural and behavioral level. Recent investigations of rapid state changes in awake, behaving animals have provided insight into the mechanisms by which optimal sensory encoding and behavioral performance are achieved. Fluctuations in state, as indexed by pupillometry, impact both the “signal” (sensory evoked response) and the “noise” (spontaneous activity) of cortical responses. By taking these fluctuations into account, neural response (co-)variability is significantly reduced, revealing the brain to be more reliable and predictable than previously thought.
Introduction
Between the daydreaming of a commuter stuck in traffic and the heightened vigilance of a rock climber executing a difficult move, the brain’s internal dynamics and responsiveness to external stimuli vary widely across different behavioral contexts. Internal brain state can fluctuate even in the absence of overt behavioral changes – most notably in the well-characterized transitions between sleep and waking and within different stages of sleep. Falling asleep, or moving from slow wave sleep into rapid eye movement sleep, results in profound changes in the operation of the brain (Figure 1), including changes in spontaneous activity, the ability to execute motor commands, responses to sensory stimuli and the fidelity of internal representations of the world (Destexhe et al., 1999; Esser et al., 2009; Hennevin et al., 2007; Livingstone and Hubel, 1981; Massimini et al., 2005; Steriade et al., 2001).
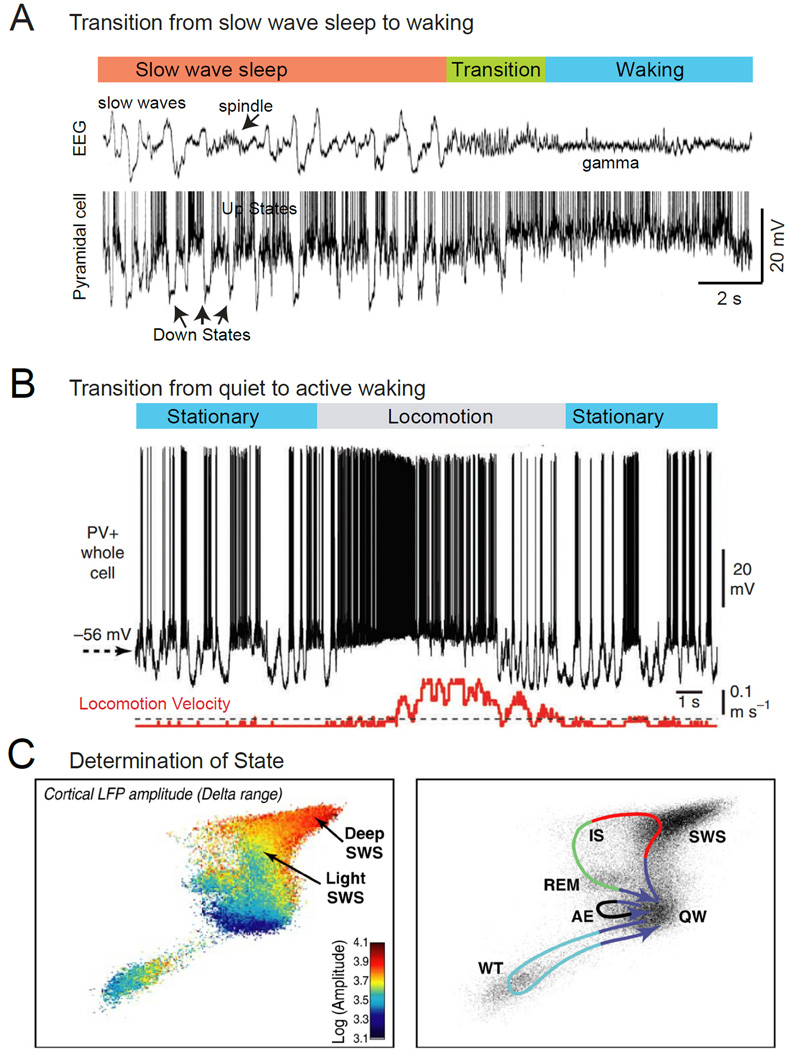
Figure 1.
Characteristics of state change in cortical networks. A. Simultaneous intracellular recording from a cortical pyramidal cell and extracellular cortical field potential in the transition from slow wave sleep to waking. During slow wave sleep, slow waves are prominent in the local field potential and appear as an alternation between depolarized, active, Up states, and hyperpolarized, inactive, Down states. The transition to waking is associated with a suppression of slow rhythms in the local field potential and the loss of Down states, resulting in the persistent depolarization of the pyramidal neuron. B. Whole cell recording from a fast spiking interneuron in the primary visual cortex of an awake mouse reveals that movement (locomotion) is associated with depolarization of the membrane potential and suppression of low frequency fluctuations in synaptic activity. C. Characterization of behavioral state in rodents by principle component analysis of the activity of multiple brain areas reveals the major sleep-waking states seen behaviorally. Note that although the states exist within their own portions of state-space, they are not completely distinct and separate (left). Movement between states follows repeated paths (right). Abbreviations: AE, active exploration; IS, intermediate stage; REM, rapid eye movement sleep; SWS, slow wave sleep; QW, quiet wake; WT, whisker twitching. A from (Steriade et al., 2001); B from (Polack et al., 2013); C from (Gervasoni et al., 2004).
By comparison, the variations in neural activity within the waking state due to changes in factors such as arousal, attention, and awareness are ostensibly less dramatic and therefore the mechanisms of waking state variations have remained more elusive (reviewed by (Harris and Thiele, 2011; Kanwisher, 2001; Lamme, 2003; Maunsell and Treue, 2006; Reynolds and Heeger, 2009; Zagha and McCormick, 2014)). Recently, the marked impact of apparent spontaneous variations in the human waking state on both cortical responses and perceptual abilities has begun to be more widely appreciated (Boly et al., 2007; Fox and Raichle, 2007; He, 2013; Hesselmann et al., 2008a; Hesselmann et al., 2008b; Palva and Palva, 2011). New animal studies reveal that rapid, ongoing, and behaviorally-relevant variations in waking state are not the exception, but rather the rule (Cohen and Maunsell, 2010, 2011; Goris et al., 2014; Hei et al., 2014; I-Chun et al., 2015; McGinley et al., 2015; Niell and Stryker, 2010; Poulet and Petersen, 2008; Reimer et al., 2014; Scholvinck et al., 2015; Vinck et al., 2015; Zhuang et al., 2014). These variations in state exert an effect on sensory evoked neural activity that can be as significant as variations in the sensory stimulus itself (McGinley et al., 2015; Scholvinck et al., 2015).
If we are to understand how information about the world is represented and processed in the brain, variability in neural computations due to ongoing changes in state must be assessed and their mechanisms understood at both the cellular and network levels. Here we review recent work, largely in behaving mice but including relevant primate and human literature, examining the impact and mechanisms of variations in the waking state on cortical function. This work has revealed that spontaneous and sensory-evoked activity varies continuously and rapidly during wakefulness. These changes are sometimes coupled with overt movements such as whisking or locomotion, and can also be predicted with high accuracy by changes in pupil diameter or muscle tone while animals are sitting quietly. All together, these biomarkers for waking states predict changes in the capability of animals to represent and respond to stimuli, and account for a significant fraction of the variability in spontaneous and stimulus-driven activity and behavior.
Defining and Quantifying Sub-states of Wakefulness
The study of sub-states of waking, the topic of this review, is still in its infancy, and widespread consensus is lacking even on basic factors such as the number and defining features of distinct sub-states. For example, the prevailing approach in rodent research of dividing wakefulness into quiet and active awake periods, depending on whether animals are standing still or exhibiting exploratory movements (e.g. whisking or locomotion) almost certainly fails to capture the full granularity of both behavior and neural activity. In an ideal experiment, the state of an animal could be reliably determined by a set of measurable neural and/or behavioral variables, which, when plotted on a multi-dimensional graph (a state space), would yield statistically-separable clusters, extending what is currently done to coarsely differentiate wakeful and sleeping states (Figure 1C) (Gervasoni et al., 2004). Furthermore, some state variables may exert a graded effect on the character of wakefulness, rather than triggering transitions between discrete states (Brody et al., 2003; Ecker et al., 2014). States that recur frequently and that exert a strong influence on neural activity and behavior are clearly the most pressing to investigate. It is beyond the scope of this review (and current knowledge) to account for all, or even many, of the sub-states of waking. Rather, our purpose is to sound a general “call-to-arms” – to raise awareness of the need for better definition and quantification of the diversity of the waking state by illustrating how rapid variations in waking influence the manner in which the brain operates.
State Dependent Brain Activity
Contrasting wakefulness and sleep provides a useful starting point for defining the neural and somatic signatures of cortical states. Classically, slow wave sleep is characterized by a high density of cortical field potential power at 0.5–4 Hz, reduced muscle tone, and rolling eye movements, while waking is associated with a relative suppression of low frequency activity (usually termed “activation” or “desynchronization”), increased muscle tone, and behaviorally relevant eye movements (e.g. fixation, saccades, and smooth pursuit) (Kryger et al., 2010). Intracellular recordings in cortical neurons reveal that the low-frequency components of the field potential during slow-wave sleep correspond to alternating active (Up) and inactive (Down) periods, recurring at 0.2–2 Hz. This slow oscillation is cortically generated but influences activity throughout the brain (Figure 1A) (Ros et al., 2009; Steriade et al., 1993; Steriade et al., 2001). Intracellular recordings in cortical neurons of cats reveal that the waking state involves suppression of the inactive, or Down, periods of cortical activity, resulting in a continuous depolarization of neurons towards firing threshold, even in quietly awake animals (Figure 1A) (Steriade et al., 2001).
This simplistic association of slow oscillations with slow wave sleep and cortical activation with waking has been challenged by intracellular recordings in awake rodents habituated to head fixation. During periods of quiet wakefulness (lacking overt movement), large low-frequency (< 10 Hz) fluctuations in the synaptic activity of somatosensory, visual, and auditory cortical neurons are frequently observed, and these oscillations are strongly suppressed by the initiation of whisking or locomotion (Bennett et al., 2013; Crochet and Petersen, 2006; Gentet et al., 2010; McGinley et al., 2015; Polack et al., 2013; Reimer et al., 2014; Zagha et al., 2013). This discovery led to a refinement in the definition of the waking state into quiet awake (non-movement) periods when low frequencies are prevalent, and active awake (locomotion or other movements) epochs associated with cortical activation (Figure 1B).
In recent years, these two (quiet and active) sub-states of waking have been the subject of intense investigation, and the results of these studies have further refined our taxonomy of states. It has become increasingly clear that the waking state contains continuous transitions between numerous sub-states that are associated with significant changes in sensory-motor processing and behavioral performance (Buzsaki, 2006; Cohen and Maunsell, 2010, 2011; Gervasoni et al., 2004; Goris et al., 2014; I-Chun et al., 2015; McGinley et al., 2015; Niell and Stryker, 2010; Polack et al., 2013; Poulet and Petersen, 2008; Reimer et al., 2014; Vinck et al., 2015; Vyazovskiy et al., 2011). These fluctuations contribute to variability in experimental results between studies, cortical areas, and even different time points within a single recording. The development of useful biomarkers for variations in the waking state, therefore, is of utmost importance for interpretation of experimental results.
Variations in Pupil Diameter Track Changes in Brain State
For more than a century, a defining feature of brain state has been the frequency and spatiotemporal pattern of local field potentials in the neocortex and hippocampus (Buzsaki, 2006). In parallel work, over the last 50 years, waking brain state has been assessed in psychophysical experiments by monitoring the diameter of the pupil (Hess and Polt, 1960, 1964; Kahneman and Beatty, 1966). Human and animal studies have shown that changes in pupil diameter (after controlling for changes associated with luminance and depth accommodation) are correlated with arousal, attention, emotion, cognitive perception, “brain gain”, as well as heart rate and galvanic skin reflex, indicating a tight coupling between the state of the central and peripheral nervous systems (Alnaes et al., 2014; Bradley et al., 2008; de Gee et al., 2014; Einhauser et al., 2010; Einhauser et al., 2008; Eldar et al., 2013; Gilzenrat et al., 2010; Hess and Polt, 1960, 1964; Iriki et al., 1996; Jepma and Nieuwenhuis, 2011; Kahneman and Beatty, 1966; Murphy et al., 2011; Murphy et al., 2014b; Nassar et al., 2012; Nishiyama et al., 2007; Onorati et al., 2013; Preuschoff et al., 2011; Tursky et al., 1969; Wierda et al., 2012; Wilhelm et al., 2001). In human studies, drowsiness during the performance of a routine task, such as simulated driving, can be associated with wide variations in arousal and performance that are reflected by large fluctuations in pupil diameter (Kristjansson et al., 2009; Wilhelm et al., 2001). In addition to arousal, emotions, such as fear, stress, and anxiety, are associated with large pupillary changes (Loewenfeld, 1999). Charles Darwin included pupil diameter as one of many indicators of emotional state in his Expressions of Emotion in Man and Animals. Even in seemingly stable emotional states, pupil diameter can fluctuate with effort on a task (e.g. difficulty of performing a mathematical calculation) (Kahneman and Beatty, 1966), the timing of mental decisions (Einhauser et al., 2010), or changes in perception (e.g. with binocular rivalry) (Einhauser et al., 2008). In one interesting study, different snippets of music were simultaneously delivered to both ears and the subject was required to attend to one or the other musical piece. The pupil diameter changes during listening revealed which snippet of music the person attended (Kang and Wheatley, 2015). Thus, pupil diameter is influenced by a variety of emotional and cognitive factors, including arousal and attention. Remarkably, the relationship between internal brain dynamics and pupil changes that index behavioral state remained largely unknown.
Recently, intracellular membrane potential (Vm) and local field potential (LFP) recordings from cortical neurons collected simultaneously with pupil diameter in head-fixed, spontaneously locomoting or whisking mice revealed a marked relationship between 1) pupil size, 2) low frequency (2–10 Hz) fluctuations in membrane potential/LFP, and 3) exploratory behaviors (whisking and locomotion) (Figure 2) (McGinley et al., 2015; Reimer et al., 2014; Vinck et al., 2015). Previous studies had shown that active behaviors such as locomotion and whisking were associated with a reduction in low-frequency rhythmic activity (Bennett et al., 2013; Crochet and Petersen, 2006; Gentet et al., 2010; Niell and Stryker, 2010; Polack et al., 2013; Zagha et al., 2013). The observation that large pupil dilations occurred around locomotion and whisking prompted interest in the use of the pupil as a relatively independent measure of arousal. Even in the absence of movement, increases in pupil diameter (dilation) were found to be associated with increases in cortical activation and suppression of low frequency rhythms. Likewise, low-frequency cortical activity is enhanced during pupillary constriction, especially below a critical level of pupil diameter. This striking relationship between changes in pupil diameter and cortical network activity, either at the local field potential level (Vinck et al., 2015) or membrane potential level (McGinley et al., 2015; Reimer et al., 2014) has been observed in visual, somatosensory, and auditory cortical areas (Figures 2, 3). The correlation between pupil diameter and the rate of sharp-wave ripples in the hippocampus (Figure 2D), further underscores the generality of this effect.
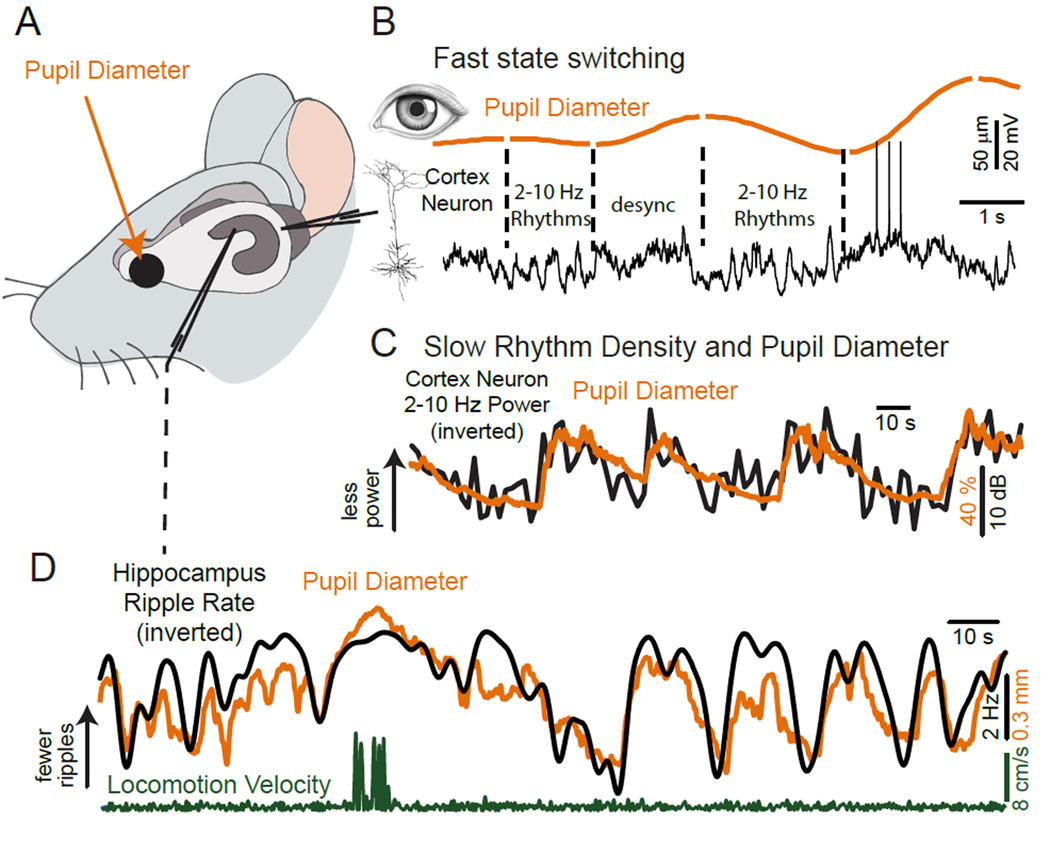
Figure 2.
Pupil diameter is an accurate predictor of variations in multiple parameters related to brain state. A. Schematic diagram of experimental protocol. Whole cell recordings are obtained from a cortical neuron while simultaneously monitoring pupil diameter, locomotion on a cylindrical treadmill, and, in some cases, hippocampal local field potential. B. Simultaneous recording of pupil diameter and cortical neuron membrane potential in layer 2/3 of the mouse primary visual cortex. Pupil diameter exhibits spontaneous variations in size even in the “quiet awake” state and in the absence of overt locomotion. Note the strong relationship between slow (2–10 Hz) rhythmic synaptic activity and constriction and the suppression of this activity with dilation (labeled ‘desync’). C. Comparison of pupil diameter and the density of low frequency (2–10 Hz) rhythmic synaptic activity (up indicates decreased 2–10 Hz power) in an auditory cortical layer 5 pyramidal cell. Note the tight anti-correlation between these two variables, with increases in pupil diameter being associated with prominent suppression of low frequency synaptic activity. D. Comparison of pupil diameter and the rate of occurrence of ripples in the CA1 field of the hippocampus. Note the tight anti-correlation between ripple rate (up indicates decreased ripples) and pupil diameter. Increases in pupil diameter are associated with suppression of ripples in the hippocampus. B from (Reimer et al., 2014); C, D from (McGinley et al., 2015).
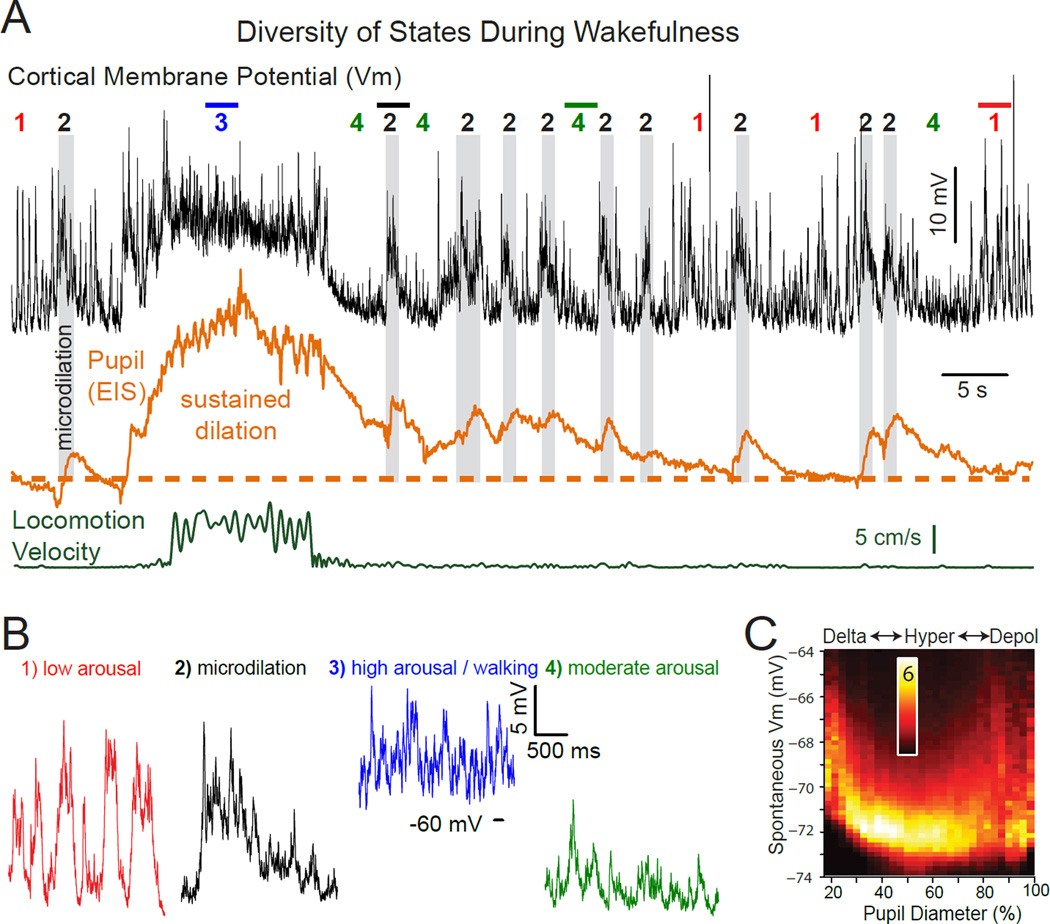
Figure 3.
Waking state rapidly varies between multiple levels of arousal. A. Whole cell recoding from a layer 5 pyramidal neuron in auditory cortex of an awake mouse. No sound was being presented. The second trace is eye-indexed state (EIS; a proxy for pupil diameter determined by measuring reflected infrared light from the exposed surface of the eye) (McGinley et al., 2015). The bottom trace is locomotion velocity on a treadmill. At least 4 waking sub-states can be readily identified: 1) quiescence associated with small pupil diameter and prominent low frequency rhythmic synaptic activity (expanded in B1); 2) brief dilations of the pupil, termed microdilations, are highlighted in gray and are associated with a suppression of the low frequency activity and a depolarization of this neuron; 3) locomotion, associated with a strong suppression of lower frequency synaptic activity, depolarization, pupil dilation, and an increase in higher frequency components of synaptic potential activity; 4) intermediate levels of arousal, as indicated by intermediate pupil diameter, suppression of lower frequency rhythmic activity, a hyperpolarized and relatively quiet membrane potential. Note that the animal spends only seconds within each state and that the level of arousal, as indicated by pupil diameter, rhythmic activity, and membrane potential, is constantly varying even though the animal is awake the entire period. B. Expansion of indicated portions of the trace in A for detail. C. Membrane potential of deep lying pyramidal neurons (n=9) exhibits a U-shaped relationship with pupil diameter. As pupil diameter increases from small (e.g. 20% dilated) to intermediate (e.g. 50–60% dilated), slow fluctuations in synaptic activity (e.g. state 1) are suppressed and therefore the membrane potential is more hyperpolarized and variance is decreased (e.g. state 4 above). Further increases in pupil dilation (e.g. > 60%), such as with locomotion, result in an average depolarization and the increased appearance of barrages of synaptic activity (e.g. states 2 or 3 above). Scale bar is % of time at that membrane potential for that pupil diameter bin. From (McGinley et al., 2015).
In addition to predicting changes in oscillatory network dynamics, changes in pupil diameter are reliably correlated with the average subthreshold membrane potential of at least some subtypes of neurons of the cortex, even in the absence of overt movement (Figure 3). Additionally, the relationship between cortical membrane potential and pupil diameter is influenced by the presence of slow oscillations at low arousal states and strong barrages of synaptic activity and/or tonic depolarization at high arousal states (Figure 3A, B). For example, the presence of these different types of synaptic and membrane potential dynamics at low and high arousal states results in a U-shaped relationship between arousal and average cortical membrane potential in deep layer neurons of the auditory cortex (McGinley et al., 2015). At low levels of arousal, cortical neurons exhibit a high density of large, slow, rhythmic fluctuations in synaptic activity (Figure 2, 3). Increases in arousal from low to intermediate levels result in a suppression of these low (1–10 Hz) frequency activities, a decrease in membrane potential fluctuations, and thus a decrease in spontaneous activity (Figure 2). At these intermediate arousal levels, the average membrane potential of infragranular cortical neurons is at its most hyperpolarized, and least variable, level (Figure 3B, C). Increases from intermediate to high arousal associated with brief (microdilations) or long-lasting increases in pupil diameter (often associated with locomotion), result in a depolarization of the membrane potential and increases in high-frequency membrane potential fluctuations from synaptic barrages (Figure 3). This complex relationship between arousal, network activities, and membrane potential has predictive power to which we will return later (see below).
Pupil diameter has proven to be a remarkably accurate and easily obtained index of a wide variety of continuously and rapidly fluctuating neural variables, including cortical neuronal membrane potential, synaptic and local field potential rhythms (McGinley et al., 2015; Reimer et al., 2014; Vinck et al., 2015) and hippocampal activity (McGinley et al., 2015) (Figure 2). Thus, in addition to overt behavior, the pupil can serve as a highly useful index for more accurately characterizing sub-states of wakefulness.
Brain State and Exploratory Behaviors
States defined by pupillometry and locomotion in head-fixed mice
Recent work in head-fixed mice has examined the effects of active and quiet wakefulness on cortical and subcortical activity at synaptic, cellular, and circuit levels (Bennett et al., 2013; Crochet and Petersen, 2006; Ferezou et al., 2007; Gentet et al., 2010; Haider et al., 2013; Lee et al., 2013; McGinley et al., 2015; Nelson et al., 2013; Niell and Stryker, 2010; Polack et al., 2013; Poulet and Petersen, 2008; Reimer et al., 2014; Scholvinck et al., 2015; Vinck et al., 2015; Zagha et al., 2013; Zhou et al., 2014). The transition from sitting quietly to exploratory behaviors (whisking and locomotion) is intertwined with changes in arousal tracked by pupillometry. In short, locomotion and whisking require arousal, but arousal does not necessitate movement (Figures 2–4). In other words, exploratory movement appears to be a sub-state of the general state of heightened arousal. Monitoring treadmill activity, pupil size, and neural activity has made it possible to partially disambiguate the effects of locomotion from the changes in arousal (Figures 2–4).
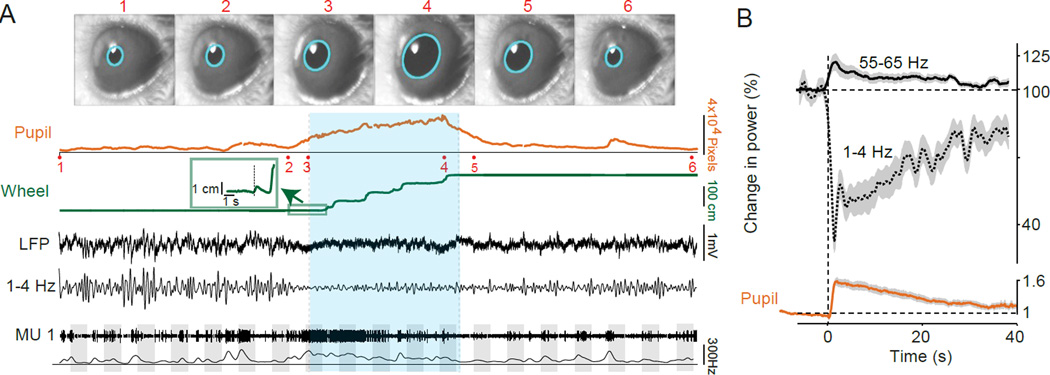
Figure 4.
Locomotion and arousal strongly modulate multiple properties of cortical activity. A. Example of the effects of locomotion on mouse visual cortical activity. Video frame images of the mouse’s eye (1–6) are shown and where acquired at the times indicated in the pupil recording trace. Pupil diameter was recorded on video and extracted post hoc via a fitted ellipse (cyan). The average pupil diameter in pixel units is shown as a function of time. Locomotion is shown as a linearized version of the wheel position. Locomotion onset point is shown in the inset. The locomotion period is indicated by green shading. Local field potential (LFP) recording from layer 2/3 of the primary visual cortex is shown as a raw broadband LFP signal, together with the 1–4 Hz filtered signals. Thresholded multi-unit traces and spike densities (1 s Gaussian smoothing kernel with SD of 0.25 s) are shown for a layer 2/3 multiple unit recording. Grey shadings indicate visual stimuli at 100% contrast and varying orientations. B. Increasing arousal with a puff of air delivered to the back of a quiescent mouse results in suppression of low (1–4 Hz) frequency rhythms and enhancement of higher (55–65 Hz) rhythms in the LFP, together with a significant increase in pupil diameter in the absence of locomotion. Note that the alterations in cortical power track changes in pupil diameter following the arousing stimulus. From (Vinck et al., 2015).
The transition from the quiet awake state to movement (locomotion and/or whisking) in head-fixed mice is always preceded by a strong suppression of cortical slow rhythms at the action potential, membrane potential, and field potential, levels (see Figures 1B, 2D, 3A, 4) (Bennett et al., 2013; Buzsaki, 2006; Crochet and Petersen, 2006; Gentet et al., 2010; McGinley et al., 2015; Niell and Stryker, 2010; Polack et al., 2013; Reimer et al., 2014; Vinck et al., 2015). Locomotion is also associated with a simultaneous increase in 30–80 Hz gamma band activity. These striking effects are consistent with those previously reported to occur in the transition from quiet rest to exploration in freely moving animals (reviewed in (Buzsaki, 2006; Murthy and Fetz, 1996; Vanderwolf et al., 1975)). Interestingly, the initiation of locomotion is preceded by a substantial increase in pupil diameter (Figures 2–4), concurrent with the reduction of low-frequency cortical local field potential synchronization and reduced hippocampal sharp wave and ripple oscillations. These changes in pupil diameter and cortical/hippocampal network activity indicate that an important component of these anticipatory changes may be related to increases in arousal.
At the level of single-cell spiking, an increase in firing rate can be observed in a subset of neurons just prior (~1 s) to the onset of locomotion (Figure 4A). In addition to arousal effects, this increase in activity may also represent pre-movement preparatory signals (Bastos et al., 2012; Bruce and Goldberg, 1985; Carvell et al., 1996; Georgopoulos et al., 1982). During the period of movement (whisking or locomotion), neurons in somatosensory, visual, motor, and auditory cortex maintain an elevated firing rate, the degree of which varies across cell types. For example, a subset of layer 5 pyramidal neurons throughout the cortex is activated by movement (Beloozerova et al., 2003; de Kock and Sakmann, 2009; McGinley et al., 2015). However, the effects of locomotion on the spontaneous discharge rates of presumed pyramidal neurons recorded in other layers is more diverse (Ayaz et al., 2013; Beloozerova et al., 2003; Bennett et al., 2013; Nelson et al., 2013; Saleem et al., 2013; Schneider et al., 2014; Vinck et al., 2015; Zhou et al., 2014). One possible explanation for this heterogeneity is that the change in firing rate depends upon the role of each cell type in unique circuits associated with different behaviors. For example, only a subset of motor cortical neurons that project to auditory cortex modulate their discharge in relation to locomotion under head fixation (Nelson et al., 2013; Schneider et al., 2014) and it is hypothesized that these cells contribute to the locomotion-associated changes of auditory cortical responses.
Following the cessation of locomotion, head-fixed mice typically exhibit a transition from high arousal to moderate or low arousal, as measured by pupil diameter, over a period of seconds or longer. This gradual decrease in pupil diameter is associated with an increase in the prevalence of low-frequency oscillations (Figure 2A, Figure 4A). In some studies, cortical neurons have been observed to transition to slow oscillatory activity quickly following a bout of locomotion or whisking (e.g. (Crochet and Petersen, 2006; Gentet et al., 2010; Polack et al., 2013)) (Figures 1B), even though the pupil usually is still large at the end of running periods (e.g. Figures 2–4) (McGinley et al., 2015; Reimer et al., 2014; Vinck et al., 2015). This suggests that motor processes themselves may also regulate low-frequency synchronization in sensory cortex (Schneider et al., 2014; Zagha et al., 2013) or that states associated with constricting and dilating pupils are distinct, even at the same pupil diameter (Reimer et al., 2014). The finding that motor activity has additional predictive power also applies to synchronization of other cortical patterns. Transitions from locomotion to quiescence, or from externally oriented movements (e.g. exploration) towards internally oriented movements (e.g. grooming, consuming) are associated with a rapid decrease of theta activity in the hippocampus (Buzsaki, 2006; Buzsaki et al., 2003). In rodents, brief (seconds) periods of 6–10 Hz thalamocortical oscillations known as high voltage spindles can appear in the quiet resting state and are enhanced by the cessation of locomotion (Jando et al., 1995). Movement or arousal results in a pronounced suppression of this thalamocortical rhythm (Buzsaki, 2006; Buzsaki et al., 2003).
As described above, even in the quiescent state devoid of overt movements, pupil dilation is associated with suppression of low-frequency, and enhancement of gamma-frequency, cortical network activities that can occur as either a rapid switching (Figure 2A) or as a graded and continuous change (Figure 2B, 3). Consistent with the effects observed during spontaneous fluctuations in pupil size (Figures 1–3), eliciting an increase in arousal through the delivery of an air puff to a quiescent mouse results in a significant increase in pupil diameter and strong suppression of slow rhythmic activity (Figure 4B). However, in contrast with the increase in neuronal activity period prior to movement, air puff-induced arousal elicits an average decrease in the discharge rate of many cortical neurons. Application of the same arousing stimulus during locomotion, when average neuronal activity is elevated, does not result in a suppression of firing, whereas a transition to locomotion that occurs following induced arousal results in an increase in neuronal discharge (Vinck et al., 2015). These results indicate that arousal and locomotion, although intimately interrelated, have some distinct influences on cortical activity.
Mice Exhibit Large, Rapid and Frequent State Changes
Our understanding of the circuit and cellular mechanisms associated with different brain states has been greatly enhanced by the ability to study state transitions in the head-fixed awake mouse, initially by comparing quiet and active awake epochs and, more recently, by monitoring changes in pupil size. In fact, the mouse may be especially well suited for investigations into the full range of states spanning sleep and waking. Although many mammalian species have periods of exploratory inactivity accompanied by drowsiness or naps punctuating their waking periods (Campbell and Tobler, 1984; Rachalski et al., 2014), head-fixed mice may be particularly prone to this phenomenon. In general, free ranging mice exhibit over 100 transitions between sleep and wakefulness per 24 hour period, with the average sleep or waking period lasting <10 minutes (Van Twyver, 1969). The length of sleep or active periods and the rate of transition between these depends on time relative to the light cycle, with the frequency and duration of bouts of exploratory activity being much greater at the beginning, than the ending, of the active period (lights off). Likewise, the intensity of slow waves in the cortical field potential slowly increases over the active period, in direct relationship to more frequent and longer sleep bouts (Curie et al., 2013; Hasan et al., 2012; Vyazovskiy et al., 2011). The variety and speed of transitions between these states and the ability to manipulate the probability of certain states by varying the timing of the experiments relative to the light cycle make mice a useful species for further investigation along these lines.
The highly dynamic state changes in the mouse emphasize the importance of monitoring and controlling for state, even in experiments in awake mice for which state is not of primary interest. In addition to the phenomena described above, the degree of habituation to an experimental situation may also change the prevalence of low frequency rhythms in the cortex (Tang et al., 2005; Vyazovskiy et al., 2011). Thus, experiments involving mice that are habituated to sit quietly, head-fixed, under a microscope (the objective of which can cause significant cooling of the cortex (Kalmbach and Waters, 2012) may be expected to exhibit a high incidence of slow oscillatory network activity associated with drowsiness or inattention (Figures 1–3). On the other hand, head fixation itself may promote a baseline level of arousal that prevents complete transitions to sleep, especially in unhabituated mice. As the use of the mouse as an experimental animal increases, the propensity for rapid and large state transitions in this species must be taken into account.
Brain State Shapes Sensory Responses
In awake, attentive animals, sensory stimuli trigger a neural cascade that ultimately leads to sensory perception or action. In contrast, in states with prominent low frequencies in cortical network activity, sensory stimuli may trigger responses that are either inappropriately weak or strong, and which do not optimally represent the spatiotemporal features of the stimulus (Hasenstaub et al., 2007; Livingstone and Hubel, 1981; Massimini et al., 2005; Petersen et al., 2003; Worgotter et al., 1998; Zagha et al., 2013). Given the large and rapid changes in spontaneous cortical activity during waking that we have outlined above, one would expect to observe similarly dramatic effects on sensory evoked responses. State dependent modulation of sensory evoked responses have been observed to occur along five dimensions: 1) response magnitude; 2) signal-to-noise (S/N) ratio; 3) precise timing; 4) variability; 5) the correlation of this variability across cells (‘noise correlations’).
Effects of State on Response Magnitude
Variations in behavioral state strongly affect the magnitude of evoked cortical activity. Particularly interesting are multiplicative amplitude modulations of the response tuning function since they preserve feature selectivity (Desimone and Duncan, 1995; McAdams and Maunsell, 1999a, b; Reynolds and Heeger, 2009). The degradation of sensory responses by slow wave sleep and anesthesia, in comparison to waking, are well known (e.g. (Livingstone and Hubel, 1981; Marguet and Harris, 2011)). Recent work has also addressed the modulation of sensory responses across multiple wake states. In V1, locomotion causes both multiplicative and additive enhancements of responses to drifting gratings (Bennett et al., 2013; Niell and Stryker, 2010; Polack et al., 2013; Reimer et al., 2014; Vinck et al., 2015) (Figure 5A) and a subset of neurons show a strong increase in evoked response with causally (i.e. airpuff) induced transition from low to high arousal (Vinck et al., 2015). Pupil dilations during quiet wakefulness also correspond to an increase in visual responsiveness along with an enhancement of selectivity (Figure 5B). Changes in arousal result in marked multiplicative gain modulation of auditory cortical responses, with the largest responses being evoked at intermediate levels of arousal (McGinley et al., 2015). In contrast to these enhanced sensory responses with intermediate to high arousal, punctate whisker deflection stimuli can evoke larger responses in the low frequency synchronized than the activated state in somatosensory cortex (Crochet and Petersen, 2006; Fanselow and Nicolelis, 1999; Ferezou et al., 2007; Hentschke et al., 2006; Krupa et al., 2004). However, the ability of somatosensory cortex to accurately represent complex whisker deflections is facilitated by cortical activation (Hasenstaub et al., 2007; Zagha et al., 2013), suggesting that the enhanced responses to punctate stimuli during low frequency oscillatory states may be unique to this particular form of sensory stimulus and pathway (e.g. see (Haider et al., 2007)). Together, these findings across waking states show substantial modulation of sensory response magnitude that can be well predicted by variations in arousal.
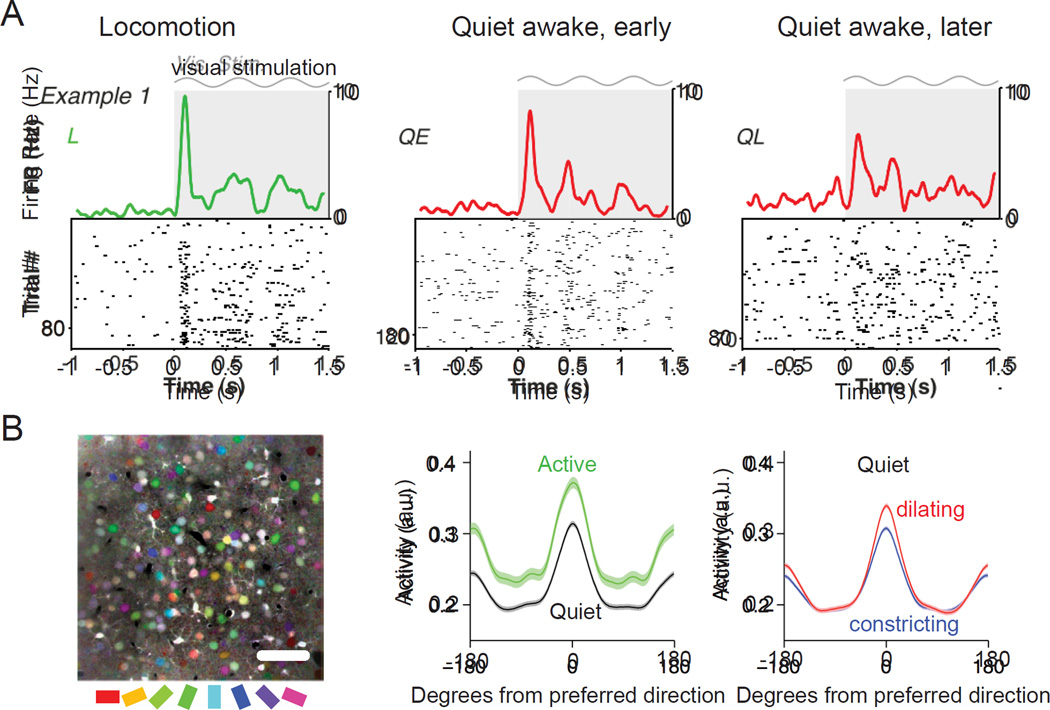
Figure 5.
Alterations in visual responses by state and locomotion. A. Raster plots of the visual responses of an example layer 2/3 pyramidal cell with associated firing rate density (computed using ± 0.025 s Gaussian kernels with SD of 0.0125 s) during locomotion, quiet awake early (3–20 s after locomotion offset) and quiet awake, later (>40 s after locomotion offset). Gray shading and sinusoid indicate visual stimulation. B. Orientation tuning is enhanced during pupil dilation. B, left. Mean fluorescence image colored by orientation preferences of individual pixels; scale bar, 50 µm. B, middle. Average tuning curves aligned to cells’ preferred direction for active (running and/or whisking) periods (green) and quiet (black) periods. Peak responses are increased (20%, p < 10−12) and orientation selectivity is unchanged (7% decrease, p = 0.07). Error bands are SEM computed over cells (n = 516). B, right. Average tuning during pupil dilation (red) and constriction (blue) during quiet periods (excluding running and whisking). In contrast with the effects of locomotion, orientation selectivity is significantly increased during dilation compared to constriction (16% increase in mean OSI, p < 10−6). Cells also respond more reliably during dilation compared to constriction (28% increase in mean binned R2 values of stimulus responses of individual cells, p < 10−15). A from (Vinck et al., 2015); B from (Reimer et al., 2014).
Effect of State on Signal-to-Noise Ratio
The quality and character of sensory responses depends not only on the evoked response, but also on the nature of ongoing (i.e. spontaneous) activity upon which sensory responses occur. In some ways, spontaneous activity may be considered a source of “noise”, which sets the baseline against which sensory signals must be detected. However, spontaneous activity is not random and is determined by the operation of similar or the same neural circuits involved in sensory responses (Arieli et al., 1996; Luczak et al., 2009). Indeed, it has been proposed that spontaneous activity may in fact reflect important internal processes like memory and sensory gating (Hoffman and McNaughton, 2002; Luczak et al., 2009). Even so, it is intuitively appealing to consider the evoked responses that are largest in comparison to ongoing “spontaneous” activity as having particularly high impact on cortical circuits. In cat V1, the signal-to-noise ratio (S/N; evoked/spontaneous activity) increases during waking versus sleeping, due in part to increases in spontaneous activity during sleep (Livingstone and Hubel, 1981). In mouse visual cortex, the S/N is small at low arousal levels, and increases at high arousal levels and during locomotion (Figures 5, 6) (Bennett et al., 2013; Niell and Stryker, 2010; Vinck et al., 2015). In contrast, in auditory cortex, the S/N peaks at intermediate arousal levels and may decrease strongly at high arousal levels and during locomotion (see Figure 7D; (McGinley et al., 2015); but see (Zhou et al., 2014)). Thus, across sensory areas, the highest evoked/spontaneous activity ratio is not necessarily found during the active state, but rather at specific sub-states of waking and determined in part by levels of arousal and attention.
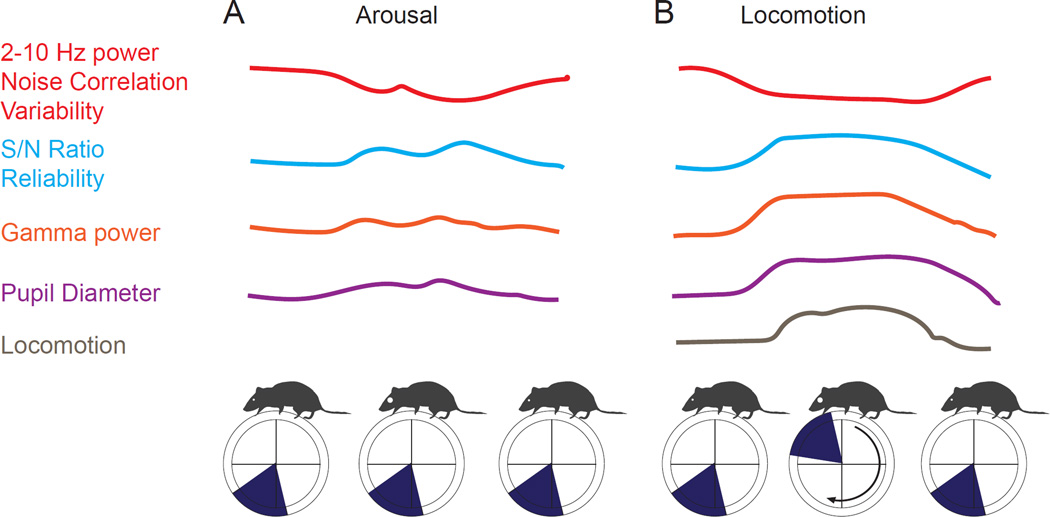
Figure 6.
Schematic illustration of the effects of locomotion and arousal on cortical neuronal activities and responses. A. Increases in pupil dilation in the absence of overt locomotion result in decreased low (2–10 Hz) frequency power, decreased correlations between activity in neighboring neurons, and decreased variability of sensory evoked responses. In addition, pupillary dilation is associated with an increase in signal-to-noise (evoked/spontaneous) ratio, reliability of visually evoked responses and an increase in power in the gamma band. B. Locomotion is also associated with marked decreases in 2–10 Hz power, noise correlations, and variability of visually evoked responses. Locomotion is also associated with an enhancement of S/N ratio and reliability of visually evoked responses, in comparison to the average non-locomotion state, and an increase in power in the gamma frequencies. Locomotion also has effects that differ from arousal without locomotion (see text). (Figure to be redrawn by Neuron Artist.)
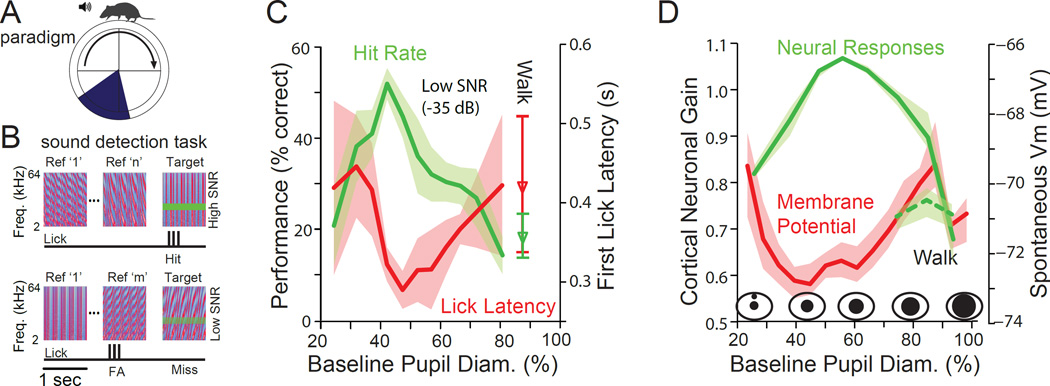
Figure 7.
Optimal performance on an auditory detection task occurs at intermediate levels of arousal. A. Mice spontaneously walked or sat quietly while performing the auditory detection task. Locomotion did not affect reward or trial structure. B. Animals were presented with 1 second periods of complex sounds (temporally orthogonal ripple combinations), in which was occasionally placed a pure tone. If the animal licked during the presentation of the tone, a Hit was recorded and the animal received a liquid reward. If the animal licked when no tone was present, a false alarm (FA) was recorded, while if the animal missed the presence of the tone, a Miss was recorded. C. Performance on the detection task varies with pupil diameter (non-locomotion periods). Hit rate (green) peaked at intermediate pupil diameters, while lick latency (red) was shortest, also at intermediate pupil diameters. Small or large pupil diameters, indicating low or high arousal levels, were associated with non-optimal task performance. Tone level in this example was 35 dB below average sound level of the complex sound, making it a challenging detection task. Responses obtained during walking (locomotion) are illustrated separately from those obtained during stillness. Walking had an effect similar to the high aroused, non-walking state, with a decrease in performance and an increase in lick latency. D. Cortical gain peaks at intermediate levels of arousal (pupil diameter). Walking is associated with a marked decrease in cortical gain in auditory cortex. The optimal state for sensory evoked responses and behavioral performance corresponds to a hyperpolarized average membrane potential in layer 5 auditory cortical neurons. From (McGinley et al., 2015).
Effects of State on Response Timing
Sensory evoked responses may be modulated not only through controlling the amplitude, but also the timing, of neuronal activity. Increases in temporal precision and synchronization, on a millisecond time scale, of interacting networks of neurons can dramatically increase the efficacy of interneuronal communication (reviewed by (Ainsworth et al., 2012; Fries, 2009)). Several theoretical and empirical studies have suggested that one important temporal feature of cortical sensory processing is gamma-band (30–80 Hz) synchronization, which is beneficial for information coding (Vinck et al., 2010; Womelsdorf et al., 2012), inter-areal transmission (Bosman et al., 2012; Womelsdorf et al., 2007) and behavioral performance (Siegle et al., 2014; Womelsdorf et al., 2006). Behavioral state is an important determinant of both the temporal precision of sensory responses and gamma-band synchronization. Spontaneous or causally induced increases in arousal are associated with a monotonic increase in gamma-band synchronization both in terms of local field potential and relative membrane potential power (Lee et al., 2014; Lima et al., 2011; McGinley et al., 2015; Reimer et al., 2014; Vinck et al., 2015), and spike-local field potential phase locking (Munk et al., 1996; Vinck et al., 2015).
Effects of State on Neuronal Variability
Neuronal variability is typically defined as the variability in the neuronal response amplitude and timing across identical stimulus representations. When it is of unknown origin, this variability is typically regarded as ‘noise,’ but may actually reflect internally generated signals (e.g. (Ecker et al., 2014; Ecker et al., 2010; Ecker et al., 2011; Goris et al., 2014; I-Chun et al., 2015; Scholvinck et al., 2015; Shadlen and Newsome, 1998)). Many non-sensory factors affect sensory processing, including behavioral state, motor activity and cross-modal influences (Gur et al., 1997), and their effects may collectively promote response variability (Ecker and Tolias, 2014; Goris et al., 2014; I-Chun et al., 2015). Because firing rates are highly dependent on behavioral state, pooling neuronal responses across states is likely to dramatically increase observed neuronal variability. Neuronal variability in firing rates is particularly high during anesthesia, possibly as a result of enhanced slow fluctuations (Arieli et al., 1996; Ecker and Tolias, 2014; Goard and Dan, 2009; Goris et al., 2014; Hasenstaub et al., 2007; I-Chun et al., 2015; Marguet and Harris, 2011; Scholvinck et al., 2015; Zagha et al., 2013). Locomotion increases response amplitude and decreases response variability in V1 (Figure 5) (Erisken et al., 2014) whereas in L5 of auditory cortex, variability is increased during low and high arousal (either during quiet or active waking), but reduced at intermediate arousal (McGinley et al., 2015). Monitoring of state through pupillometry reveals rapid (seconds) and continuous changes (Figure 2–4), indicating that a significant fraction of trial-to-trial variability in sensory evoked responses may be the result of variations in state. Indeed, compensating for these rapid variations in state can significantly reduce variability in the amplitude and timing of both sensory evoked and behavioral responses (McGinley et al., 2015; Reimer et al., 2014).
Effects of State on Noise Correlations
Noise correlations in sensory responses are defined as correlations in the response variability of pairs of neurons across repeated presentations of identical sensory input. The level of noise correlations measured experimentally varies widely (e.g. (Ecker et al., 2010; Zohary et al., 1994)) and their impact on the encoding accuracy of a neuronal population is an active field of theoretical research (e.g. (Abbott and Dayan, 1999; Ecker et al., 2011; Moreno-Bote et al., 2014; Sompolinsky et al., 2001)). In contrast to many previous studies (e.g. (Kohn and Smith, 2005; Smith and Kohn, 2008)), Ecker et al. (Ecker et al., 2010) found only very weak pairwise noise correlations between well-isolated single units in V1 in the awake monkey, stimulating lively debate in the field (see (Cohen and Kohn, 2011)). Central to this debate is the question of to what extent this covariability can be attributed to noise in the sensory processing stream or alternatively to meaningful internally generated processes to which the experimenter is ignorant. Recent proposals suggest that elevated interneuronal correlations likely reflect the action of signals internal to the brain (e.g. (Ecker et al., 2014; Ecker et al., 2010; Goris et al., 2014)). For example, noise correlations are generally high during anesthesia or synchronized network states (Ecker et al., 2014; Renart et al., 2010) and low during desynchronized states of wakefulness (Ecker et al., 2010; Ecker and Tolias, 2014; Renart et al., 2010). Further, changes in arousal, attentional state and neuronal excitability modulate the level of correlated variability in sensory cortex (Cohen and Maunsell, 2009; Ecker et al., 2010; Ecker and Tolias, 2014; Goris et al., 2014; I-Chun et al., 2015; Mitchell et al., 2009).
Recent work in the mouse suggests that the overall level of noise correlation varies across different wakeful brain states (e.g. (Gentet et al., 2010; Poulet and Petersen, 2008; Reimer et al., 2014; Vinck et al., 2015). Moreover, pooling different behavioral states together in the analysis of noise correlations introduces shared variability in neuronal responses, over-estimating their magnitude (Vinck et al., 2015). Within wakefulness, noise correlations are lower during locomotion than quiescence (Figure 6B) (Erisken et al., 2014; Vinck et al., 2015). Recent work also shows that noise correlations within wakefulness are, to a significant degree, explained by fluctuations in arousal as indexed by pupil diameter (Reimer et al., 2014; Vinck et al., 2015). Thus, a precise analysis of behavioral state seems to explain considerable variability in noise correlations, and poor control or knowledge of behavioral state can lead to artificial inflation.
Attentional States are Sub-states of Waking
Arousal and selective attention appear to exert overlapping effects on cortical activity and sensory encoding (Harris and Thiele, 2011). Selective attention is conceptually distinguished from arousal in being directed (space, features, modalities, internal/external) and in resolving competition for resources (Desimone and Duncan, 1995). In contrast, the term “arousal” is generally used to signify a change in global brain state, although the possibility of fine-grained arousal in cortical networks has been proposed (Steriade and McCarley, 2005; Vyazovskiy et al., 2011; Zaborszky et al., 2015). In addition, selective attention is largely considered a cognitive function, whereas arousal incorporates emotional and stress responses in addition to cognitive components.
Two distinct lines of investigation have explored the role of attention in regulating cortical computation. One focuses on the information transfer between downstream target and distractor populations (e.g. area V1), who compete for the resources of upstream populations responding to both target and distractor stimuli (e.g. area V4; (Desimone and Duncan, 1995)). Selective inter-areal gamma-band coherence may be a mechanism to resolve this competition (Bosman et al., 2012; Gregoriou et al., 2009), but it remains unknown whether global arousal plays a similar role in regulating inter-areal coherence patterns. The other line of investigation focuses on the effect of selective attention on separate neuronal populations representing either a target or a distractor stimulus. Selective attention reduces neuronal variability (Mitchell et al., 2007), decreases noise correlations (Cohen and Maunsell, 2009; Mitchell et al., 2009), increases the S/N and both spontaneous and evoked firing rates (Luck et al., 1997; McAdams and Maunsell, 1999b; Reynolds et al., 1999; Reynolds and Desimone, 1999), and enhances gamma-synchronization (Fries et al., 2001; Gregoriou et al., 2009). These findings reveal a strong convergence between the effects of attention and global arousal and suggest that the underlying cellular/network mechanisms may be related.
Neural Correlates of State-Dependent Optimal Performance
Given that the waking state constantly fluctuates, even on a second by second basis, it is imperative to examine the relationship between the state of the brain/animal and its performance on behavioral tasks. More than a century ago, Robert Yerkes and John Dodson (Yerkes and Dodson, 1908) proposed that increases in arousal/stress from low to moderate levels can facilitate performance on a difficult behavioral task, whereas further increases from moderate to severe can impair performance. This relationship between arousal or stress and task performance on difficult or complex tasks has been characterized as an inverted U, because performance (percent correct, latency, discriminability) peaks at some intermediate level of arousal or stress and has been the subject of intense investigation (reviewed in (Diamond et al., 2007)). The precise shape of the inverted U-relationship depends upon multiple factors, including the type and difficulty of the task, baseline state of the test subject, expectancy, context, experience, and stress-response interactions. Indeed, in the case of simple tasks (e.g. those that do not require high level cognition), increases in performance may be observed with nearly all increases in arousal or stress, thereby resulting in a more linear relationship between arousal and performance (Diamond et al., 2007; Yerkes and Dodson, 1908). It is likely that the relationship between arousal and performance can take multiple forms, anywhere from curvilinear (e.g. U-shaped) to linear.
Arousal, stress, and their effects on behavior are interrelated but separable. In tasks that require prefrontal cortical (PFC) function, there is an inverted U relationship between stress and performance. Pharmacological investigations indicate that this relationship depends upon the level of release of neuromodulators, such as dopamine and norepinephrine (Arnsten, 2009; Arnsten et al., 2012). Activation of receptors for these neuromodulators at intermediate levels may enhance performance on PFC dependent tasks, whereas further increases may result in a decrement in behavioral performance and reduced neural representation of that performance in the PFC (reviewed in (Arnsten, 2009; Arnsten et al., 2012)).
The relationship between norepinephrine release by the locus coeruleus (LC) and forebrain function and behavior has likewise been interpreted as an inverted-U with an optimal state at intermediate levels. Moderate increases in LC activity enhance the cortical S/N ratio and set the conditions for optimal neural and behavioral performance, but further increasing LC discharge rates to high levels can have detrimental effects (Aston-Jones and Cohen, 2005).
Extracellular and intracellular recording investigations are beginning to reveal the cortical mechanisms of neural/behavioral optimization. For example, in rodents the density and prevalence of low frequency rhythms (Figure 1B) increase over the course of the 12 hour active period since lights off. Is prevalence of these lower frequency events associated with degradation of behavioral task performance? In one recent study, the ability of animals to perform a difficult pellet retrieval task was significantly impaired during the appearance of Off periods in cortical networks, supporting the hypothesis that these sudden, but brief, cessations of cortical activity are detrimental to behaviorally relevant cortical operation (Vyazovskiy et al., 2011) (however, in another recent study the presence of slow oscillatory events in the somatosensory cortex was not detrimental to the performance of a touch detection task, although the strength of stimulus used was supramaximal at all arousal levels) (Sachidhanandam et al., 2013). At the high end of the arousal-performance relationship, increases in arousal (as measured by pupil diameter) increased the ability of a distractor (e.g. a flashed picture of a monkey face on the screen during the delay period) to disrupt performance on a Go/No-Go saccade task in primates (Ebitz et al., 2014). Similarly, large pupil diameter, indicating high arousal, is also associated with increased variability (e.g. degraded accuracy) in performance on a motion detection task (Murphy et al., 2014b). Together, these studies suggest that for at least some studies, optimal performance may occur at intermediate levels of arousal (see Figure 2A in (Ebitz et al., 2014)).
In a visual response task, mice trained to discriminate the orientation of moving bars exhibited improvement in performance when the animals were locomoting in comparison to when they were sitting quietly (Bennett et al., 2013). Locomotion, as we have mentioned, is associated with a suppression of slow rhythmic activity in cortical networks, a depolarization of cortical neurons closer to action potential threshold, and enhanced visually evoked responses (Bennett et al., 2013; Polack et al., 2013; Reimer et al., 2014). Because sub-states of wakefulness during stillness were not parsed in this study, it remains to be determined if the optimal state for visual responses is indeed the locomotive state, or an undetermined sub-state of stillness or locomotion.
To examine the relationship between alterations in the waking state, task performance, and cortical activity, McGinley et al. recorded auditory cortical and medial geniculate responses during variations in state and compared this to the ability of animals to perform a demanding auditory signal detection task (McGinley et al., 2015). Mice were trained on a Go/No-Go task in which they were given a liquid reward upon licking as a response to detection of the presence of a pure tone embedded in a complex noise stimulus (Figure 7A,B) (Atiani et al., 2009). The sound level of the tone varied on a trial-by-trial basis, with stimulus intensities ranging from threshold for behavioral detection up to just suprathreshold for maximal performance. Importantly, the performance of mice on this auditory detection task was highly state dependent, with maximal hit rate, shortest lick latency, maximal discrimination, and least behavioral bias all occurring at intermediate pupil diameters (Figure 7C). Increases in arousal from low to intermediate, as indicated by increases in pupil diameter from small to medium, were associated with a large increase in task performance, while further increases in pupil diameter from intermediate to large were associated with a strong decrement in performance, whether or not the animal was walking at these large pupil diameters (Figure 7C). Interestingly, locomotion, as a sub-state of hyper-arousal, was associated with a strong decrease in behavioral performance, in comparison to intermediate arousal levels, which always occurred during stillness.
These results indicate that arousal level robustly influences behavioral performance, and this influence can take the form of an inverted U in this task, as predicted by the curvilinear component of the Yerkes-Dodson relationship (Figure 7C). Interestingly, sound evoked responses in the auditory cortex also exhibited a strong relationship between arousal (pupil diameter) and the gain, amplitude, and reliability of these responses (Figure 7D). All three measures exhibited an inverted U relationship with arousal, with the largest, most reliable, and highest S/N ratio responses occurring at the same intermediate pupil diameters that revealed optimal performance on the auditory tone-in-complex sound detection task (Figure 7). The influence of sub-states during quiet waking and locomotion on behavioral performance or cortical responses has not yet been examined in visual or somatosensory tasks in mice, making it difficult to compare between sensory systems. However, given the prominence of the influence of fluctuations in the waking state (e.g. attention) on visually guided performance (Maunsell and Treue, 2006; Reynolds and Heeger, 2009), it is expected that further research will reveal strong influences of arousal and attention on the ability of mice to perform visually and/or somatosensory guided tasks.
Are these findings relevant to similar tasks in human and monkeys? In an auditory odd-ball task, response latency upon detection of the correct stimulus was shortest at mid-pupil diameters in human subjects and the P3 evoked potential was also maximal at this level of arousal, resulting in U (latency) and inverted-U (evoked cortical field potential P3 amplitude) relationships with pupil-indexed arousal (Murphy et al., 2011). Variations in spontaneous activity, as inferred from functional magnetic resonance imaging (fMRI) and/or electrocorticogram (ECoG) recordings, in awake humans has strong influences on task performance (Boly et al., 2007; Hesselmann et al., 2008a). In a visual detection task, the reaction time of human subjects exhibited an inverted-U relationship with the degree to which ECoG activity prior to cue onset was similar to the average ECoG level. The more similar the pattern of ECoG activity to the average of all trials, the better the performance on the visual detection task, with significant decrements in performance when the ECoG activity deviated in either direction from average (He and Zempel, 2013). Finally, the ability of primates to perform a delayed saccade to target task was optimal at intermediate pupil diameters, with degradation in performance at either low or high levels of arousal (Ebitz et al., 2014). These results indicate that variations in human/primate arousal and task engagement during the waking state can result in an optimal zone for neural responses on particular types of tasks.
Cellular and Network Mechanisms of State Control
Intracellular Mechanisms of Variations in the Waking State
Variations in the waking state strongly modulate the patterns of spontaneous cortical network activity (e.g. synchronized, desynchronized, slow rhythms) and the amplitude, timing, and variability of sensory evoked responses (see above). Here we examine the synaptic, cellular, and network mechanisms that may underlie these effects.
While commonly used approaches divide the waking state into binary groups (e.g. quiet vs. active; attentive vs. inattentive), careful examination of synaptic activity and network dynamics reveal a richer, more complex picture (Figures 1–3, 8). Here we propose two distinct models of arousal-dependent control of cortical activities that extend beyond simple dichotomies or linear changes along 1 or 2 axes (Figure 8A).
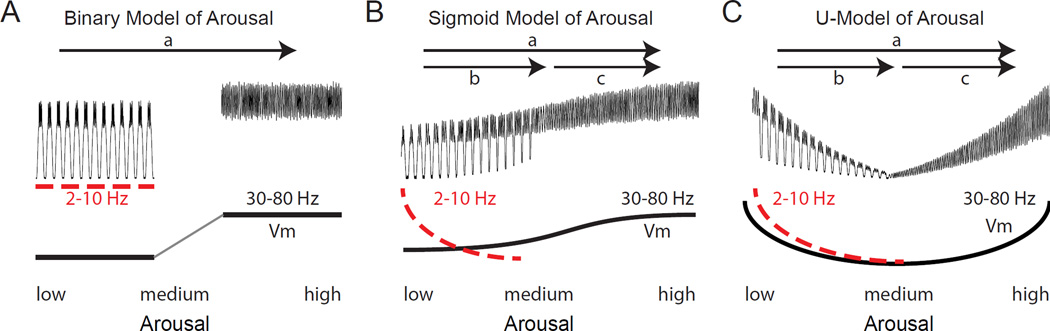
Figure 8.
Alternative idealized relationships between arousal and cortical membrane potential and oscillations. A. Binary model of arousal in which waking is divided into an “inactivated” state with prominent low frequency oscillations and an “activated” state with enhanced higher frequency activities. B. Sigmoidal model of arousal based upon intracellular recordings in the transition from slow wave sleep to waking (Figure 1A) or from quiet waking to movement in somatosensory and visual cortex (Figure 1B). The transition from low to medium arousal (b) is associated with a suppression of low (2–10 Hz) frequency rhythms and a depolarization of some, but not all, cortical neurons. The transition to high arousal (c), particularly with movement, results in a strong enhancement of activity in the gamma frequency band and further depolarization of some neurons. C. The U-shaped model of arousal contrasts with the sigmoid model in that the membrane potential of depolarization-activated cortical neurons exhibits a low point in between low and high arousal. Gamma is drawn as increasing from intermediate to low arousal owing to the increased activation of cortical networks by slow oscillations. These idealized models are only for comparison and it is expected that real cortical networks will operate in a regime that may mix different features of all three models.
Intracellular recordings in cat cortical neurons in the transition from slow wave sleep to waking or rapid eye movement (REM) sleep, or in rodents in the transition from stillness to whisking or locomotion, reveal a flattened sigmoidal relation between average membrane potential and cortical activation (Figures 1, 8B) (Figure 10 in (Steriade et al., 2001)). This sigmoidal relation results in large part from a suppression of slow rhythmic activity and a promotion of higher frequency synaptic barrages (including gamma synchronization) with cortical activation (Figure 8B), although additional direct effects of neuromodulators on neuronal membrane potential (e.g. depolarization) likely contribute (reviewed in (McCormick, 1992; Zagha and McCormick, 2014)).
In contrast to the sigmoidal model of activation, intracellular recordings in awake behaving rodents can reveal a pattern that is more consistent with a U-shaped dependence of average membrane potential and cortical activation on arousal (McGinley et al., 2015) (Figures 3C, 8C). In this model, the left side of the relationship is dominated by a suppression of slow oscillatory activity with arousal, which results in an average hyperpolarization of neuronal membrane potential. Since each activated period (e.g. Up state) of slow oscillatory activity is also associated with higher frequency synaptic barrages, the suppression of lower frequency rhythms can also reduce power in the gamma frequencies (Figure 8C). On the right side of the U-relationship, increases in arousal or locomotion result in an average depolarization of the membrane potential owing either to: 1) increased barrages of synaptic potentials which contain both synchronous (e.g. 30–80 Hz) and asynchronous components (Figure 8C), or 2) from the depolarizing effects of modulatory neurotransmitters such as acetylcholine or norepinephrine(McCormick, 1992).
Both the sigmoidal and U model predict decreases in lower frequency activities and enhancement of higher frequency activities with arousal. However, the U model also predicts that at intermediate levels of arousal, the membrane potential may be hyperpolarized and exhibit reduced variability (Figure 8C). Another prediction of both models is that increases in arousal or locomotion can be associated with a variety of changes in firing rate, even in the same neuron. In a low state of arousal, neurons may exhibit an elevated firing rate owing to the presence of lower frequency rhythmic activities. Activation of the cortex to a more moderate level of arousal may result in either a moderate increase in action potential activity (sigmoid model) or a significant decrease in action potential discharge (U model) (Figure 8Bb, Cb). Further increases in activation may result in an increase in spontaneous discharge, at either asynchronous or gamma-frequency rates, in both models (Figure 8Bc, Cc). Thus, arousal or locomotion could have diverse effects on spontaneous discharge rates, even along the same axis of arousal or activation. This is especially evident when considering that the sigmoidal and U-shaped models are simplified versions of the complex changes that occur with arousal in cortical neurons (McGinley et al., 2015; Steriade et al., 2001). Not tracking the beginning and ending points of changes in arousal may contribute significantly to the wide variations observed between studies in the effects of arousal on spontaneous cortical discharge rates.
At present, intracellular recordings from cortical neurons support both the sigmoidal and U model of arousal (e.g. Figure 1–3). The reasons for this marked variation between recorded cells in their response to arousal or locomotion are not yet known. It is likely that cell type, laminar location, behavioral context, and perhaps region of cortex are important variables (Beloozerova et al., 2003; Gentet et al., 2010; Reimer et al., 2014). A differentiating feature between these two models is in the promotion of prolonged depolarization during intermediate arousal in the sigmoidal model and hyperpolarization in the U model. One possibility is that these two extremes represent differential promotion of depolarization or activation by the release of neuromodulators that underlie arousal. For example, neurons that are strongly depolarized, or neural circuits that are well activated, by acetylcholine during intermediate arousal may be expected to exhibit a depolarized membrane potential at this state, as predicted by the sigmoidal model (e.g. Figures 1A, 8B), in contrast to the U-shaped model (e.g. Figures 3C, 8C). This possibility remains to be explored.
Neural Control of Waking State – Neuromodulatory Influences
Although the precise mechanisms underlying the neural control of state are not fully known, a general picture has emerged, and the development of new tools promises to reveal specific circuit level components. A wide variety of neuromodulatory pathways have been implicated in the neural control of cortical responsiveness in a state dependent manner (reviewed in (Saper et al., 2010; Steriade and McCarley, 2005)). Two of these are the central noradrenergic and cholinergic pathways (perhaps not coincidentally the same neurotransmitters used by the sympathetic and parasympathetic pathways to control pupil diameter) (Figure 9). The locus coeruleus provides the source of noradrenergic, and the basal forebrain the source of cholinergic, innervation of the cerebral cortex. The release of either of these neurotransmitters can potentially modulate the state of cortical activity through cell type, and even subcellular, specific effects. Effects that are consistent with a role in arousal of pyramidal cell networks include depolarization of cortical neurons through the reduction of a variety of membrane K+ currents (McCormick, 1992), modulation of the h-current (Wang et al., 2007), which controls neuronal and dendritic excitability (Magee, 2000), and control of synaptic transmission, through the modulation of presynaptic terminals (Gil et al., 1997). Through the modulation of ionic currents, the release of norephinephrine or acetylcholine can not only control the membrane potential and excitability of cortical neurons, but also strongly suppress the generation of slow oscillations. Layer 5 pyramidal cells, in particular, respond to the activation of α1 adrenergic and muscarinic cholinergic receptors with depolarization towards firing threshold, and an increase in excitability through the reduction of K+ currents that function to dampen the generation of repetitive trains of action potentials (McCormick, 1992). Since the slow rhythmic activities appear to arise within cortical layer 5 (Sanchez-Vives and McCormick, 2000), these actions may be responsible in part for the suppression of lower frequency cortical rhythms with arousal or locomotion.
Figure 9.
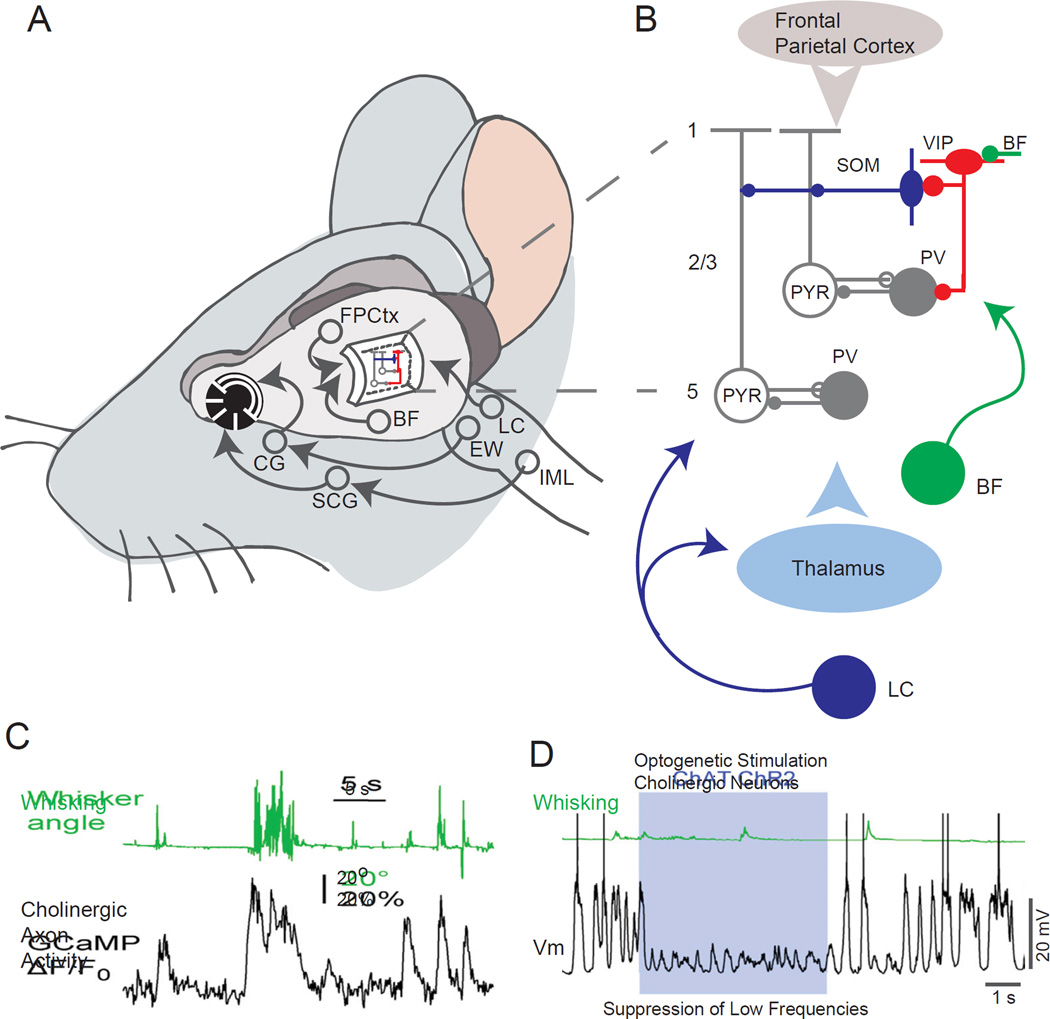
Schematic of proposed mechanisms for state dependent modulation of cortical activity and responsiveness. A. Schematic diagram illustrating the major neural circuits involved in control of brain state and pupil diameter. B. Schematic circuit components illustrating the potentially important roles for disinhibition and modulation in the control of neuronal responses. The release of ACh by basal forebrain (BF) neurons may activate layer 2/3 VIP interneurons through nicotinic receptors. Activation of these cells may decrease the excitability of dendritic targeting interneurons (e.g. somatostatin – SOM – interneurons) or the activity of other types of interneurons (e.g. PV expressing interneurons), resulting in an increase in excitability of pyramidal cells. Alternatively, activity in multiple pathways, including neuromodulators from the locus coeruleus and basal forebrain, corticocortical connections from the frontoparietal (e.g. motor) cortex, or excitatory inputs from the thalamus may modulate multiple components of the cortical circuit. Through as of yet unknown mechanisms, the state of the cortex is coupled together with the state of the peripheral nervous system, resulting in a high correlation between cortical state and pupil diameter. One possibility is that the activity of the locus coeruleus is intimately involved in both. C. Time-course of cholinergic fiber activity observed with 2-photon monitoring of GCamp6 labeled cholinergic fibers in the superficial layers of the somatosensory cortex. Note that whisker movement is associated with large changes in cholinergic axonal activity. D. Vm (black) and quantified whisker movement (green) during control period and during blue light illumination to stimulate basal forebrain cholinergic neurons expressing ChR2 (ChAT ChR2) in a thalamus-inactivated mouse. Abbreviations: BF – Basal Forebrain; CG – Ciliary Ganglion; EW - Edinger-Westphal nucleus; FPCtx – Frontal-Parietal Cortex; LC - Locus Coeruleus; IML – Intralaminar neurons of the spinal cord; PV – Parvalbumin containing interneurons; SCG – Superior Cervical Ganglion; SOM – somatostatin containing interneurons; VIP – vasoactive intestinal peptide containing interneurons; C and D from (Eggermann et al., 2014).
Both basal forebrain cholinergic and locus coeruleus noradrenergic neurons increase their discharge in relation to increased attention to external stimuli, arousal, and locomotion, in both a graded and transient manner (Figure 9) (Aston-Jones and Cohen, 2005; Eggermann et al., 2014; Steriade and McCarley, 2005). Stimulation of the basal forebrain can have many of the same effects as those associated with arousal and locomotion, including increased amplitude and precision of sensory evoked responses (Goard and Dan, 2009; Pinto et al., 2013). Stimulation of the locus coeruleus can markedly enhance auditory cortical responses and increase learning induced plasticity (Martins and Froemke, 2015). While the pathways that link changes in pupil diameter with arousal are currently unknown, locus coeruleus neurons have been reported to discharge in close relation to pupil diameter on a time scale of tens of seconds (Aston-Jones and Cohen, 2005; Murphy et al., 2014a) and electrical stimulation in the region of the locus coeruleus results in pupil dilation (Liu, Li, Wang, Society for Neuroscience Abstract, 2014). The locus coeruleus receives synaptic inputs from multiple brain regions, such as the frontal cortex, that may be involved in arousal responses to complex stimuli, including those requiring high level cognition(Aston-Jones and Cohen, 2005). The high correlation between movement and basal forebrain cholinergic activity (Eggermann et al., 2014), and neurons in the region of the brainstem cholinergic nuclei (Lee et al., 2014) suggests that there may also be a high correlation between pupil diameter and the activity of these neurons. Indeed, recent studies monitoring the activity of cortical noradrenergic and cholinergic axons and cortical NE/ACh release reveal high correlations between the activity of both of these neuromodulatory systems with both transient and sustained changes in arousal and locomotion (Reimer, McGinley, McCormick, Tolias, unpublished observations).
Pharmacological manipulations of cholinergic and noradrenergic receptors can dramatically alter cortical responsiveness and behavioral performance in a manner consistent with cortical activation by these two neuromodulators (Aston-Jones and Cohen, 2005). Depolarization of layer 2/3 visual cortical neurons with locomotion (e.g. Figure 1B) is suppressed by block of noradrenergic receptors (Polack et al., 2013). Pharmacological block of both cholinergic and noradrenergic receptors locally in the cortex is associated with a strong reduction of the ability of locomotion to modulate cortical state (Eggermann et al., 2014; Polack et al., 2013), suggesting that movement may reduce low frequency, and promote higher frequency, rhythms largely through its association with arousal. These diverse findings strongly support a prominent role for both the central cholinergic and noradrenergic pathways in the rapid modulation of cortical state. However, it is highly likely that other systems are also involved (reviewed in (Lee et al., 2013; Lee and Dan, 2012; Zagha and McCormick, 2014)).
In addition to the modulation of cortical neuronal excitability by ascending noradrenergic and cholinergic systems, recent studies have identified thalamocortical, corticocortical and corticothalamic pathways that can control cortical state and sensory responsiveness with high temporal and spatial specificity, owing to their activation of ionotropic and metabotropic glutamate receptors (Nelson et al., 2013; Schneider et al., 2014; Zagha et al., 2013). Such fast actions may be particularly beneficial where rapid modulations in local or long range network processing are required, such as alterations in attention or in motor planning/execution. Glutamatergic pathways, however, are not the only ones that can act rapidly. Both cholinergic and serotoninergic systems can activate kinetically fast excitatory postsynaptic potentials in postsynaptic targets through nicotinic and 5HT3A receptors, respectively, and the discharges of both cholinergic (e.g. Figure 9C) and serotonergic neurons are strongly modulated by movement (Eggermann et al., 2014; Jacobs and Fornal, 1993) (Reimer, McGinley, McCormick, Tolias, unpublished observations). In cortical networks, nicotinic and 5HT3A receptors are often (but not exclusively) located on particular subpopulations of GABAergic interneurons (Fu et al., 2014; Griguoli and Cherubini, 2012; Jakab and Goldman-Rakic, 2000) and may contribute to modulation of waking state activities through disinhibition.
Neural Control of Waking State – Local Circuit Mechanisms
Several recent studies, in somatosensory, auditory, and visual cortical areas have revealed a disinhibitory pathway, the activation of which can increase the level of excitability of cortical pyramidal cells to sensory stimuli (Fu et al., 2014; Lee et al., 2013; Pi et al., 2013). One disinhibitory circuit centers around layer 2/3 VIP-containing inhibitory interneurons which synapse onto somatostatin (apical dendrite-targeting) and parvalbumin (soma-targeting) interneurons (Figure 9A,B). VIP interneurons become highly active in relation to locomotion, whisking, or reinforcement signals (Fu et al., 2014; Lee et al., 2013; Pi et al., 2013). VIP-containing cortical inhibitory interneurons project to both somatostatin (e.g. Martinotti) and parvalbumin (e.g. basket cells) containing interneurons and have an inhibitory action on these cell types (David et al., 2007; Jiang et al., 2013; Lee et al., 2013; Pi et al., 2013). Somatostatin-containing interneurons decrease their activity during locomotion (Fu et al., 2014; Gentet et al., 2012; Reimer et al., 2014). Parvalbumin-containing interneurons exhibit a more diverse response to locomotion, with some increasing (e.g. Figure 1B) and others decreasing their average firing rate (Fu et al., 2014; Gentet et al., 2010; Polack et al., 2013). As a result, the postsynaptic targets of SOM and PV containing interneurons, namely the apical dendrites and somata of pyramidal neurons, are believed to be disinhibited by movement/reward activation of VIP interneurons (Fu et al., 2014; Lee et al., 2013; Pfeffer et al., 2013; Pi et al., 2013). This circuit likely contributes to state-dependent gain effects related to movement, since lesion of VIP-containing interneurons is reported to decrease movement-related modulation of visual cortical responses (Fu et al., 2014). Interestingly, VIP-containing neurons show rapid depolarization to serotoninergic and cholinergic inputs from subcortical pathways and glutamatergic inputs from higher cortical areas, positioning these interneurons as possible effectors of multiple modulatory inputs. Since serotoninergic, cholinergic, and corticocortical pathways are all modulated by movement and arousal, this disinhibitory pathway is uniquely positioned to contribute to arousal and locomotion based modulation of cortical responsiveness.
Conclusions
The Greek philosopher Heraclitus famously said “No man ever steps in the same river twice, for it’s not the same river and he’s not the same man.” Likewise, neuroscientists are faced with ever-changing patterns of activity in the awake brain, many of which take the form of state changes. Luckily, detailed observation of these rapid state fluctuations can significantly account for variability, and allow for a more accurate exploration of the neural mechanisms of behavior at all levels, from sensory coding, decision, to motor response. As neuroscience moves more and more into experiments in awake, behaving animals, a holistic and integrative approach becomes imperative, requiring careful accounting of uncontrolled variables. We encourage the inclusion of state as a central feature of experimental design.
Acknowledgements
This work was supported by grants NIH 5R01N2026143 and the Kavli Institute for Neuroscience at Yale (D.A.M.), F32 DC012449 (M.J.M.), a NARSAD Young Investigator award, an Alfred P. Sloan Fellowship award, a Whitehall grant, a Klingenstein fellowship award, a McKnight Scholar award, NIH/NEI grants R00 EY018407 and R01 EY022951 (J.A.C.), a Rubicon Grant (Netherlands Organization for Science; to M.V.), a Jane Coffin Childs Fund fellowship award (R.B.B.), DP1EY023176, DP1OD008301, P30EY002520, T32EY07001, Beckman Young Investigator Award and McKnight Scholar Award (A.S.T.). C.R.C was supported by grants F30MH095440, T32GM007330 and T32EB006350.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andreas S. Tolias, Email: atolias@cns.bcm.edu.
Jessica A. Cardin, Email: jess.cardin@yale.edu.
David A. McCormick, Email: david.mccormick@yale.edu.
References
- Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural computation. 1999;11:91–101. doi: 10.1162/089976699300016827. [DOI] [PubMed] [Google Scholar]
- Ainsworth M, Lee S, Cunningham MO, Traub RD, Kopell NJ, Whittington MA. Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron. 2012;75:572–583. doi: 10.1016/j.neuron.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Alnaes D, Sneve MH, Espeseth T, Endestad T, van de Pavert SH, Laeng B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J Vis. 2014:14. doi: 10.1167/14.4.1. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature reviews Neuroscience. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual review of neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz A, Saleem AB, Scholvinck ML, Carandini M. Locomotion controls spatial integration in mouse visual cortex. Curr Biol. 2013;23:890–894. doi: 10.1016/j.cub.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ. Canonical microcircuits for predictive coding. Neuron. 2012;76:695–711. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA. Activity of different classes of neurons of the motor cortex during locomotion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:1087–1097. doi: 10.1523/JNEUROSCI.23-03-01087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody CD, Romo R, Kepecs A. Basic mechanisms for graded persistent activity: discrete attractors, continuous attractors, and dynamic representations. Current opinion in neurobiology. 2003;13:204–211. doi: 10.1016/s0959-4388(03)00050-3. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. Journal of neurophysiology. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- Buzsaki G, Buhl DL, Harris KD, Csicsvari J, Czeh B, Morozov A. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116:201–211. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neuroscience and biobehavioral reviews. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Miller SA, Simons DJ. The relationship of vibrissal motor cortex unit activity to whisking in the awake rat. Somatosens Mot Res. 1996;13:115–127. doi: 10.3109/08990229609051399. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nature neuroscience. 2011;14:811–819. doi: 10.1038/nn.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nature neuroscience. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. A neuronal population measure of attention predicts behavioral performance on individual trials. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15241–15253. doi: 10.1523/JNEUROSCI.2171-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. When attention wanders: how uncontrolled fluctuations in attention affect performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15802–15806. doi: 10.1523/JNEUROSCI.3063-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nature neuroscience. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- Curie T, Mongrain V, Dorsaz S, Mang GM, Emmenegger Y, Franken P. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;36:311–323. doi: 10.5665/sleep.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C, Schleicher A, Zuschratter W, Staiger JF. The innervation of parvalbumin-containing interneurons by VIP-immunopositive interneurons in the primary somatosensory cortex of the adult rat. The European journal of neuroscience. 2007;25:2329–2340. doi: 10.1111/j.1460-9568.2007.05496.x. [DOI] [PubMed] [Google Scholar]
- de Gee JW, Knapen T, Donner TH. Decision-related pupil dilation reflects upcoming choice and individual bias. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E618–E625. doi: 10.1073/pnas.1317557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CP, Sakmann B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16446–16450. doi: 10.1073/pnas.0904143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual review of neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural plasticity. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Pearson JM, Platt ML. Pupil size and social vigilance in rhesus macaques. Frontiers in neuroscience. 2014;8:100. doi: 10.3389/fnins.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Cotton RJ, Subramaniyan M, Denfield GH, Cadwell CR, Smirnakis SM, Bethge M, Tolias AS. State dependence of noise correlations in macaque primary visual cortex. Neuron. 2014;82:235–248. doi: 10.1016/j.neuron.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Keliris GA, Bethge M, Logothetis NK, Tolias AS. Decorrelated neuronal firing in cortical microcircuits. Science. 2010;327:584–587. doi: 10.1126/science.1179867. [DOI] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Tolias AS, Bethge M. The effect of noise correlations in populations of diversely tuned neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14272–14283. doi: 10.1523/JNEUROSCI.2539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker AS, Tolias AS. Is there signal in the noise? Nature neuroscience. 2014;17:750–751. doi: 10.1038/nn.3722. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Kremer Y, Crochet S, Petersen CC. Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep. 2014;9:1654–1660. doi: 10.1016/j.celrep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Einhauser W, Koch C, Carter OL. Pupil dilation betrays the timing of decisions. Front Hum Neurosci. 2010;4:18. doi: 10.3389/fnhum.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhauser W, Stout J, Koch C, Carter O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1704–1709. doi: 10.1073/pnas.0707727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nature neuroscience. 2013;16:1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisken S, Vaiceliunaite A, Jurjut O, Fiorini M, Katzner S, Busse L. Effects of Locomotion Extend throughout the Mouse Early Visual System. Curr Biol. 2014;24:2899–2907. doi: 10.1016/j.cub.2014.10.045. [DOI] [PubMed] [Google Scholar]
- Esser SK, Hill S, Tononi G. Breakdown of effective connectivity during slow wave sleep: investigating the mechanism underlying a cortical gate using large-scale modeling. Journal of neurophysiology. 2009;102:2096–2111. doi: 10.1152/jn.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow EE, Nicolelis MA. Behavioral modulation of tactile responses in the rat somatosensory system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual review of neuroscience. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nature neuroscience. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. Global forebrain dynamics predict rat behavioral states and their transitions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:11137–11147. doi: 10.1523/JNEUROSCI.3524-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn Affect Behav Neurosci. 2010;10:252–269. doi: 10.3758/CABN.10.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nature neuroscience. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris RL, Movshon JA, Simoncelli EP. Partitioning neuronal variability. Nature neuroscience. 2014 doi: 10.1038/nn.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griguoli M, Cherubini E. Regulation of hippocampal inhibitory circuits by nicotinic acetylcholine receptors. The Journal of physiology. 2012;590:655–666. doi: 10.1113/jphysiol.2011.220095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur M, Beylin A, Snodderly DM. Response variability of neurons in primary visual cortex (V1) of alert monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:2914–2920. doi: 10.1523/JNEUROSCI.17-08-02914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, Yu Y, McCormick DA. Enhancement of visual responsiveness by spontaneous local network activity in vivo. Journal of neurophysiology. 2007;97:4186–4202. doi: 10.1152/jn.01114.2006. [DOI] [PubMed] [Google Scholar]
- Haider B, Hausser M, Carandini M. Inhibition dominates sensory responses in the awake cortex. Nature. 2013;493:97–100. doi: 10.1038/nature11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nature reviews Neuroscience. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S, Dauvilliers Y, Mongrain V, Franken P, Tafti M. Age-related changes in sleep in inbred mice are genotype dependent. Neurobiol Aging. 2012;33:195 e113–195 e126. doi: 10.1016/j.neurobiolaging.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ. Spontaneous and task-evoked brain activity negatively interact. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4672–4682. doi: 10.1523/JNEUROSCI.2922-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Zempel JM. Average is optimal: an inverted-U relationship between trial-to-trial brain activity and behavioral performance. PLoS Comput Biol. 2013;9:e1003348. doi: 10.1371/journal.pcbi.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei X, Stoelzel CR, Zhuang J, Bereshpolova Y, Huff JM, Alonso JM, Swadlow HA. Directional selective neurons in the awake LGN: response properties and modulation by brain state. Journal of neurophysiology. 2014;112:362–373. doi: 10.1152/jn.00121.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennevin E, Huetz C, Edeline JM. Neural representations during sleep: from sensory processing to memory traces. Neurobiol Learn Mem. 2007;87:416–440. doi: 10.1016/j.nlm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Haiss F, Schwarz C. Central signals rapidly switch tactile processing in rat barrel cortex during whisker movements. Cerebral cortex. 2006;16:1142–1156. doi: 10.1093/cercor/bhj056. [DOI] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil Size in Relation to Mental Activity during Simple Problem-Solving. Science. 1964;143:1190–1192. doi: 10.1126/science.143.3611.1190. [DOI] [PubMed] [Google Scholar]
- Hesselmann G, Kell CA, Eger E, Kleinschmidt A. Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proceedings of the National Academy of Sciences of the United States of America. 2008a;105:10984–10989. doi: 10.1073/pnas.0712043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G, Kell CA, Kleinschmidt A. Ongoing activity fluctuations in hMT+ bias the perception of coherent visual motion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008b;28:14481–14485. doi: 10.1523/JNEUROSCI.4398-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Sleep on it: cortical reorganization after-the-fact. Trends in neurosciences. 2002;25:1–2. doi: 10.1016/s0166-2236(00)02005-1. [DOI] [PubMed] [Google Scholar]
- I-Chun L, Okun M, Carandini M, Harris KD. The nature of shared cortical variability. Neuron. 2015;87:1–13. doi: 10.1016/j.neuron.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Attention-induced neuronal activity in the monkey somatosensory cortex revealed by pupillometrics. Neurosci Res. 1996;25:173–181. doi: 10.1016/0168-0102(96)01043-7. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends in neurosciences. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. The Journal of comparative neurology. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jando G, Carpi D, Kandel A, Urioste R, Horvath Z, Pierre E, Vadi D, Vadasz C, Buzsaki G. Spike-and-wave epilepsy in rats: sex differences and inheritance of physiological traits. Neuroscience. 1995;64:301–317. doi: 10.1016/0306-4522(94)00329-4. [DOI] [PubMed] [Google Scholar]
- Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. J Cogn Neurosci. 2011;23:1587–1596. doi: 10.1162/jocn.2010.21548. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nature neuroscience. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154:1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Kalmbach AS, Waters J. Brain surface temperature under a craniotomy. Journal of neurophysiology. 2012;108:3138–3146. doi: 10.1152/jn.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang O, Wheatley T. Pupil dilation patterns reflect the contents of consciousness. Conscious Cogn. 2015;35:128–135. doi: 10.1016/j.concog.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Neural events and perceptual awareness. Cognition. 2001;79:89–113. doi: 10.1016/s0010-0277(00)00125-6. [DOI] [PubMed] [Google Scholar]
- Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3661–3673. doi: 10.1523/JNEUROSCI.5106-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson SD, Stern JA, Brown TB, Rohrbaugh JW. Detecting phasic lapses in alertness using pupillometric measures. Appl Ergon. 2009;40:978–986. doi: 10.1016/j.apergo.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Wiest MC, Shuler MG, Laubach M, Nicolelis MA. Layer-specific somatosensory cortical activation during active tactile discrimination. Science. 2004;304:1989–1992. doi: 10.1126/science.1093318. [DOI] [PubMed] [Google Scholar]
- Kryger MH, Roth T, Demet WC. Principles and Practice of Sleep Medicine (Saunders) 2010 [Google Scholar]
- Lamme VA. Why visual attention and awareness are different. Trends in cognitive sciences. 2003;7:12–18. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron. 2014;83:455–466. doi: 10.1016/j.neuron.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nature neuroscience. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Dan Y. Neuromodulation of brain states. Neuron. 2012;76:209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B, Singer W, Neuenschwander S. Gamma responses correlate with temporal expectation in monkey primary visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15919–15931. doi: 10.1523/JNEUROSCI.0957-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. Anatomy, Physiology, and Clinical Applications. Vol. 1. Boston: Butterworth Heinemann; 1999. The Pupil. [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. Journal of neurophysiology. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Harris KD. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron. 2009;62:413–425. doi: 10.1016/j.neuron.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nature reviews Neuroscience. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Marguet SL, Harris KD. State-dependent representation of amplitude-modulated noise stimuli in rat auditory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6414–6420. doi: 10.1523/JNEUROSCI.5773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AR, Froemke RC. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nature neuroscience. 2015 doi: 10.1038/nn.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends in neurosciences. 2006;29:317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999a;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron. 1999b;23:765–773. doi: 10.1016/s0896-6273(01)80034-9. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, David SV, McCormick DA. Cortical Membrane Potential Signature of Optimal States for Sensory Signal Detection. Neuron. 2015;87:179–192. doi: 10.1016/j.neuron.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Bote R, Beck J, Kanitscheider I, Pitkow X, Latham P, Pouget A. Information-limiting correlations. Nature neuroscience. 2014;17:1410–1417. doi: 10.1038/nn.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- Murphy PR, O'Connell RG, O'Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum Brain Mapp. 2014a;35:4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, Robertson IH, Balsters JH, O'Connell RG. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology. 2011;48:1532–1543. doi: 10.1111/j.1469-8986.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- Murphy PR, Vandekerckhove J, Nieuwenhuis S. Pupil-linked arousal determines variability in perceptual decision making. PLoS Comput Biol. 2014b;10:e1003854. doi: 10.1371/journal.pcbi.1003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: Synchronization of local field potentials and relation to behavior. Journal of neurophysiology. 1996;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Nassar MR, Rumsey KM, Wilson RC, Parikh K, Heasly B, Gold JI. Rational regulation of learning dynamics by pupil-linked arousal systems. Nature neuroscience. 2012;15:1040–1046. doi: 10.1038/nn.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A, Schneider DM, Takatoh J, Sakurai K, Wang F, Mooney R. A circuit for motor cortical modulation of auditory cortical activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:14342–14353. doi: 10.1523/JNEUROSCI.2275-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama J, Tanida K, Kusumi M, Hirata Y. The pupil as a possible premonitor of drowsiness. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1586–1589. doi: 10.1109/IEMBS.2007.4352608. [DOI] [PubMed] [Google Scholar]
- Onorati F, Barbieri R, Mauri M, Russo V, Mainardi L. Characterization of affective states by pupillary dynamics and autonomic correlates. Front Neuroeng. 2013;6:9. doi: 10.3389/fneng.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva JM, Palva S. Roles of multiscale brain activity fluctuations in shaping the variability and dynamics of psychophysical performance. Prog Brain Res. 2011;193:335–350. doi: 10.1016/B978-0-444-53839-0.00022-3. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nature neuroscience. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature neuroscience. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nature neuroscience. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, t Hart BM, Einhauser W. Pupil Dilation Signals Surprise: Evidence for Noradrenaline's Role in Decision Making. Frontiers in neuroscience. 2011;5:115. doi: 10.3389/fnins.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachalski A, Authier S, Bassett L, Pouliot M, Tremblay G, Mongrain V. Sleep electroencephalographic characteristics of the Cynomolgus monkey measured by telemetry. J Sleep Res. 2014;23:619–627. doi: 10.1111/jsr.12189. [DOI] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron. 2014;84:355–362. doi: 10.1016/j.neuron.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD. The asynchronous state in cortical circuits. Science. 2010;327:587–590. doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. The role of neural mechanisms of attention in solving the binding problem. Neuron. 1999;24:19–29. 111–125. doi: 10.1016/s0896-6273(00)80819-3. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros H, Sachdev RN, Yu Y, Sestan N, McCormick DA. Neocortical networks entrain neuronal circuits in cerebellar cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:10309–10320. doi: 10.1523/JNEUROSCI.2327-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidhanandam S, Sreenivasan V, Kyriakatos A, Kremer Y, Petersen CC. Membrane potential correlates of sensory perception in mouse barrel cortex. Nature neuroscience. 2013;16:1671–1677. doi: 10.1038/nn.3532. [DOI] [PubMed] [Google Scholar]
- Saleem AB, Ayaz A, Jeffery KJ, Harris KD, Carandini M. Integration of visual motion and locomotion in mouse visual cortex. Nature neuroscience. 2013;16:1864–1869. doi: 10.1038/nn.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature neuroscience. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Nelson A, Mooney R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature. 2014;513:189–194. doi: 10.1038/nature13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Saleem AB, Benucci A, Harris KD, Carandini M. Cortical state determines global variability and correlations in visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:170–178. doi: 10.1523/JNEUROSCI.4994-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JH, Pritchett DL, Moore CI. Gamma-range synchronization of fast-spiking interneurons can enhance detection of tactile stimuli. Nature neuroscience. 2014;17:1371–1379. doi: 10.1038/nn.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:12591–12603. doi: 10.1523/JNEUROSCI.2929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky H, Yoon H, Kang K, Shamir M. Population coding in neuronal systems with correlated noise. Physical review E, Statistical, nonlinear, and soft matter physics. 2001;64:051904. doi: 10.1103/PhysRevE.64.051904. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. New York: Springer; 2005. [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Tang X, Xiao J, Parris BS, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/6J mice. Physiology & behavior. 2005;85:419–429. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Tursky B, Shapiro D, Crider A, Kahneman D. Pupillary, heart rate, and skin resistance changes during a mental task. Journal of experimental psychology. 1969;79:164–167. doi: 10.1037/h0026952. [DOI] [PubMed] [Google Scholar]
- Van Twyver H. Sleep patterns of five rodent species. Physiology and Behavior. 1969;4:901–905. [Google Scholar]
- Vanderwolf CH, Kramis R, Gillespie LA, Bland BH. Hippocampal rhythmic slow activity and neocortical low-voltage fast activity: relations to behavior. In: Isaacson RL, Pribram KH, editors. In The Hippocampus Neurophysiology and Behavior. New York: Spinger; 1975. pp. 101–128. [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron. 2015;86:1–15. doi: 10.1016/j.neuron.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Lima B, Womelsdorf T, Oostenveld R, Singer W, Neuenschwander S, Fries P. Gamma-phase shifting in awake monkey visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:1250–1257. doi: 10.1523/JNEUROSCI.1623-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wierda SM, van Rijn H, Taatgen NA, Martens S. Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8456–8460. doi: 10.1073/pnas.1201858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm B, Giedke H, Ludtke H, Bittner E, Hofmann A, Wilhelm H. Daytime variations in central nervous system activation measured by a pupillographic sleepiness test. J Sleep Res. 2001;10:1–7. doi: 10.1046/j.1365-2869.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Lima B, Vinck M, Oostenveld R, Singer W, Neuenschwander S, Fries P. Orientation selectivity and noise correlation in awake monkey area V1 are modulated by the gamma cycle. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4302–4307. doi: 10.1073/pnas.1114223109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Worgotter F, Suder K, Zhao Y, Kerscher N, Eysel UT, Funke K. State-dependent receptive-field restructuring in the visual cortex. Nature. 1998;396:165–168. doi: 10.1038/24157. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimlus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cerebral cortex. 2015;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–578. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, McCormick DA. Neural control of brain state. Current opinion in neurobiology. 2014;29C:178–186. doi: 10.1016/j.conb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Liang F, Xiong XR, Li L, Li H, Xiao Z, Tao HW, Zhang LI. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nature neuroscience. 2014 doi: 10.1038/nn.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Bereshpolova Y, Stoelzel CR, Huff JM, Hei X, Alonso JM, Swadlow HA. Brain state effects on layer 4 of the awake visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:3888–3900. doi: 10.1523/JNEUROSCI.4969-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]