Abstract
Both obesity and hepatitis B virus (HBV) infection increase the risk of death. We investigate the association between general and central obesity and all-cause mortality among adult Taiwanese HBV versus non-HBV carriers.
A total of 19,850 HBV carriers and non-hepatitis C virus (HCV) carriers, aged 20 years and older at enrollment in 1998 to 1999 in Taiwan, were matched to 79,400 non-HBV and non-HCV carriers (1:4). Cox proportional-hazards models were used to estimate the relative risks for all-cause mortality during a maximum follow-up period of 10 years. Four obesity-related anthropometric indices—body mass index (BMI), waist circumference, waist-to-hip ratio, and waist-to-height ratio—were the main variables of interest.
During the follow-up period, 628 and 2366 participants died among HBV and non-HBV carriers, respectively. Both underweight and general obesity were associated with an increased risk of death. The highest risk of all-cause death in relation to BMI was found in the HBV carriers with underweight (BMI <18.5 kg/m2) and non-HBV carriers with obesity (BMI ≥30 kg/m2). The lowest risks of all-cause death in relation to abdominal adiposity were found at the third quartiles of waist circumference, waist-to-hip ratio, and waist-to-height ratio among HBV carriers, but in the second quartiles among non-HBV carriers. For those with pre-existing liver disease among HBV carriers, patients with underweight have higher risk of death than those with obesity.
Hepatitis B virus carriers with underweight have higher risk of death than non-HBV carriers. HBV carriers with mild abdominal obesity have the lowest risk of death, but not in the non-HBV carriers.
INTRODUCTION
It is well established that chronic hepatitis B virus (HBV) infection increases risk of liver-related morbidity and mortality,1 and has recently been shown to be associated with increased risk of metabolic syndrome (MetS).2,3 It is also recognized that nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of MetS. Taiwan is an area with high prevalence of HBV infection, and obesity and MetS. The national prevalence rates among adult Taiwanese for HBV infection is 15% to 20%.4 According to the survey from Department of Health and Welfare in Taiwan, the prevalence of HBV infection is 15–20%5 which represented about 2.5 to 3 million carriers of HBV in Taiwan. The prevalence of overweight and obesity (defined as body mass index [BMI] ≥24 kg/m2)6 rapidly increased in Taiwanese men and women, from 33.4% and 31.7% to 50.8% and 36.9%, respectively, in the past decade.7 The prevalence of MetS in men and women also increased from 13.6% and 25.5% to 26.4% and 31.5%, respectively.7 Obesity has been recognized as an important and independent risk factor for many of the chronic diseases that are components of the MetS, such as diabetes, hypertension, stroke, cardiovascular disease, and fatty liver disease.8–14 Furthermore, excess body mass increases risk of developing hepatocellular carcinoma (HCC), colon, endometrial, and other cancers.10,15–19 Our study group found that HBV infection is associated with increased risk of MetS.2 Wong et al20 found that MetS increased the risk of liver cirrhosis in chronic hepatitis B. Yu et al18 reported that excess body weight is associated with an increased rate of HCC occurrence and liver-related death among HBV carriers. Finelli et al21 also found that reducing the visceral fat may be a potential treatment strategy to prevent or delay age-related diseases and to increase longevity. Previous studies indicated that the relationship between BMI (a marker of general obesity) and all-cause death among general adults was a U or J-shaped association.9,22–25 Jacobs et al26 also found that waist circumference (WC, a marker of central obesity) was positively associated with all-cause mortality. Both HBV infection and obesity result in increasing risk for all-cause mortality, but there is no study to clarify the association between obesity and all-cause mortality among HBV carriers versus non-HBV carriers. Therefore, we aimed to assess the difference between HBV carriers and non-HBV carriers about the association between general and abdominal obesity and death in Taiwan. A prospective population-based cohort study of HBV carriers matched to non-HBV carriers was conducted to investigate the association between BMI, WC, waist-to-hip ratio (WHipR), and waist-to-height ratio (WHeiR), and all-cause mortality.
METHODS
Study Cohort and Study Population
The data collection and patient recruit were reported in previous studies.25,27–29. In brief, the data were collected in Taiwan from 1998 to 1999. In all, 124,513 patients, aged 20 years and above, were recruited. The population structure in our study was similar to the national data of adults published by the Taiwanese government.30 We excluded 452 participants for whom data on measured height, weight, WC, or hip circumference were missing. Out of the 124,061 adults, 19,934 were identified as HBV surface antigen (HBsAg)-positive and anti-hepatitis C virus (HCV) antibody-negative, and 99,995 patients with non-HBV, non-HCV carriers were included for individual matching process.
Matching Procedure
Age and sex matched with 1:4 ratios were used between HBV carriers and non-HBV carriers. Finally, 19,850 HBV and non-HCV carriers were matched to 79,400 non-HBV and non-HCV carriers.
Data Collection
The methodology for collecting anthropometric measurements in this population has been described previously.25,31 In brief, trained staff measured height, WC, and hip circumference (measured to the nearest 0.1 cm) and weight (measured to the nearest 0.1 kg) of each participant using an auto-anthropometer (KN-5000A, Nakamura, Tokyo, Japan). Blood was drawn with minimal trauma from an antecubital vein in the morning after a 12-hour overnight fast. Serum HBsAg and anti-HCV antibody were tested by an auto-immunoassay (Abbott Laboratories, North Chicago, IL). Liver function (SGOT (Serum Glutamic-oxaloacetic Transaminase), SGPT (Serum Glutamic-Pyruvic Transaminase), platelet count, albumin) was measured by automated chemistry analyzer (Hitachi Ltd., Tokyo, Japan) and automated hematology analyzer (Abbott Diagnostics, Abbott Park, IL). BMI was calculated as weight divided by height squared. WHipR was calculated as WC divided by hip circumference. WHeiR was calculated as WC divided by height. BMI was divided into 5 levels according to the obesity definition using WHO for Asians criteria—underweight (less than 18.5), normal weight (18.5–22.9), overweight (23.0–24.9), mild obesity (25.0–29.9), and moderate/severe obesity (30.0 or more). WC, WHipR, and WHeiR were divided into quartiles within each sex as follows: for HBV carriers: WC quartiles I to IV were <75.1, 75.1 to 81.0, 81.1 to 87.0, >87.1 cm in men and <66.1, 66.2 to 71.0, 71.1 to 77.0, >77.0 cm in women; WHipR quartiles I to IV were <0.82, 0.82 to 0.86, 0.87 to 0.91, >0.91 in men and <0.73, 0.73 to 0.76, 0.77 to 0.81, >0.81 in women; WHeiR quartiles I to IV were <0.45, 0.45 to 0.48, 0.49 to 0.52, >0.52 in men and <0.42, 0.42 to 0.45, 0.46 to 0.50, >0.50 in women; for non-HBV carriers: WC quartiles I to IV were <75.5, 75.6 to 81.5, 81.6 to 87.5, >87.6 cm in men and <66.1, 66.2 to 71.5, 71.6 to 77.5, >77.6 cm in women; WHipR quartiles I to IV were <0.82, 0.82 to 0.87, 0.88 to 0.91, >0.91 in men and <0.73, 0.73 to 0.77, 0.78 to 0.81, >0.81 in women; WHeiR quartiles I to IV were <0.45, 0.45 to 0.48, 0.49 to 0.52, >0.52 in men and <0.42, 0.42 to 0.45, 0.46 to 0.50, >0.50 in women.
We used the lowest BMI or central obesity-related mortality (especially, all-cause mortality and liver-related mortality) in all subgroups as our reference group. Using this point, the reference groups are overweight group (BMI 23.0–24.9). Since WC has been widely used for the definition of central obesity, we chose WC as our major target reference group. We found that the third quartile of WC has the lowest mortality rate. Therefore, it became our reference group.
Questionnaire
The detailed questionnaire has been reported in previous studies.25,27–29 In brief, past medical histories, socioeconomic status (income and education levels), and lifestyle factors such as smoking, alcohol drinking, and betel nut chewing were recorded. Participants with pre-existing liver diseases were those who reported a previous history of liver cancer, liver cirrhosis, hepatitis, or other liver disease by questionnaire. Others were defined as nonpre-existing liver diseases.
Follow-up
The vital status of cohort members was determined from 1998 to 1999 through December 31, 2008. Deaths were ascertained by annual linkage of the cohort to the national death registry using ID number in Taiwan. Deaths with International Classification of Disease, Ninth Revision (ICD-9) codes 070, 155, and 570 to 573 were classified as liver-related death. All other causes of death were defined as deaths other than liver-related death. Approval for patient recruitment and data analyses was obtained from the MJ Research Foundation Review Committee in Taiwan.
Statistical Analysis
Mortality rates are expressed per 1000 person-years, directly standardized within the specified age range of the male or female study population. Cox proportional-hazards regression analyses adjusted for possible confounders were used to estimate the relative risks (RRs) for death. Subgroup analyses were conducted according to strata of age at recruitment and the presence of pre-existing liver diseases. All statistical tests were 2-sided at the 0.05 significance level. These statistical analyses were performed using the PC version of SPSS statistical software (17th version, SPSS Inc., Chicago, IL).
RESULTS
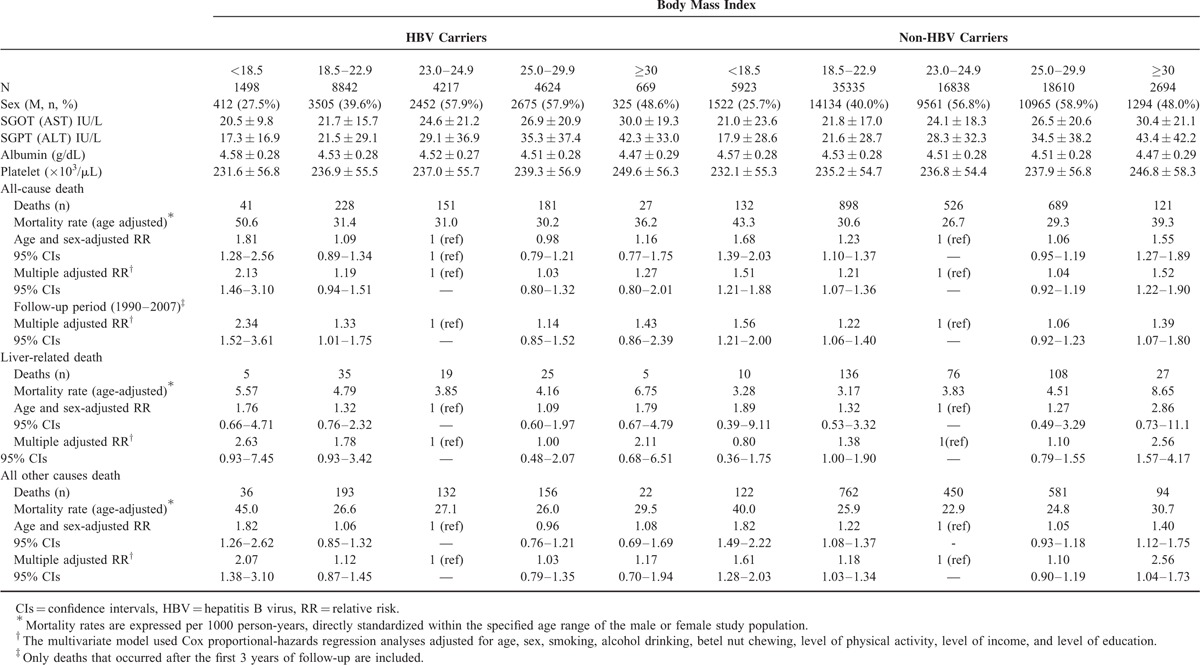
During the follow-up period, 628 and 2366 participants died among HBV carriers and non-HBV carriers, respectively. In Table 1, we show the baseline liver function (SGOT, SGPT, platelet count, albumin) and the RRs for the risk of all-cause death in relation to BMI categories among HBV carriers and non-HBV carriers. There is no statistical significance among liver function between HBV carriers and non-HBV carriers. Both of them pose a nonlinear association between BMI and the risk of all-cause death.
TABLE 1.
Mortality Rates and Hazard Ratios (95% CIs) for Selected Mortality Outcomes in Relation to Body Mass Index in Adult Taiwanese With and Without HBV Carriers
General Obesity and All-cause Mortality
The lowest risk in relation to all-cause death (multiple adjusted RRs) among both HBV carriers and non-HBV carriers were found at a BMI between 23.0 and 24.9 kg/m2. Among HBV carriers, a reverse J-shaped association existed between all-cause death and BMI categories after adjustment for age, sex, smoking status, alcohol intake, betel nut chewing, physical activity, income, and education level (multiple adjusted RR). For non-HBV carriers, however, the association became U-shaped. After excluding study participants who died during the first 3 years of follow-up, the similar findings were also found. The lowest risks in relation to all-cause death (multiple adjusted RRs) were also found at a BMI between 23.0 and 24.9 kg/m2.
General Obesity and Liver-related Mortality
Underweight or obese participants have an increased risk of liver-related death in relation to BMI categories among HBV carriers. However, for non-HBV carriers, only obese participants have an increased risk of liver-related death.
Central Obesity and Mortality
In Table 2, we show the RRs for the risk of all-cause death in relation to the markers of abdominal adiposity (WC, WhipR, and WheiR quartiles) among HBV carriers and non-HBV carriers. The lowest risks of all-cause death in relation to abdominal adiposity among HBV carriers were found at the third quartile of WC, WHipR, and WHeiR, but they appeared at the second quartiles of WC, WHipR, and WHeiR among non-HBV carriers. The U-shaped relationship between abdominal adiposity (using quartiles of WC, WHipR, and WHeiR) and all-cause death was also found among HBV carriers. However, underweight participants among HBV carriers have highest risk of abdominal adiposity than others. There is no significant association between abdominal adiposity and all-cause death among non-HBV carriers. After excluding study participants who died during the first 3 years of follow-up, the similar U-shaped association between abdominal adiposity and all-cause death was also found. There were significant interactions (P < 0.05) between BMI categories, and pre-existing liver disease status and age group for predicting the risk of all-cause mortality. We, therefore, stratified these groups and presented the results in Figures 1 and 2. Figure 1(A and B) shows the adjusted RRs in relation to all-cause death according to the presence or absence of pre-existing liver diseases. For those participants with pre-existing liver disease, the U-shaped association between all-cause death and BMI categories was found among HBV carriers, but not among non-HBV carriers. Participants aged 20 to 64 years have a similar U-shaped association between all-cause death and BMI categories among both groups (Figure 2A). However, for HBV carriers aged over 65 years, the lowest adjusted RR of all-cause death was found when BMI exceeded 30, demonstrating an “obesity paradox” (Figure 2A).
TABLE 2.
Multivariate Relative Risk of Death Among Participants According to Quartile of Waist Circumference, Waist-to-hip Ratio, Waist-to-height Ratio∗
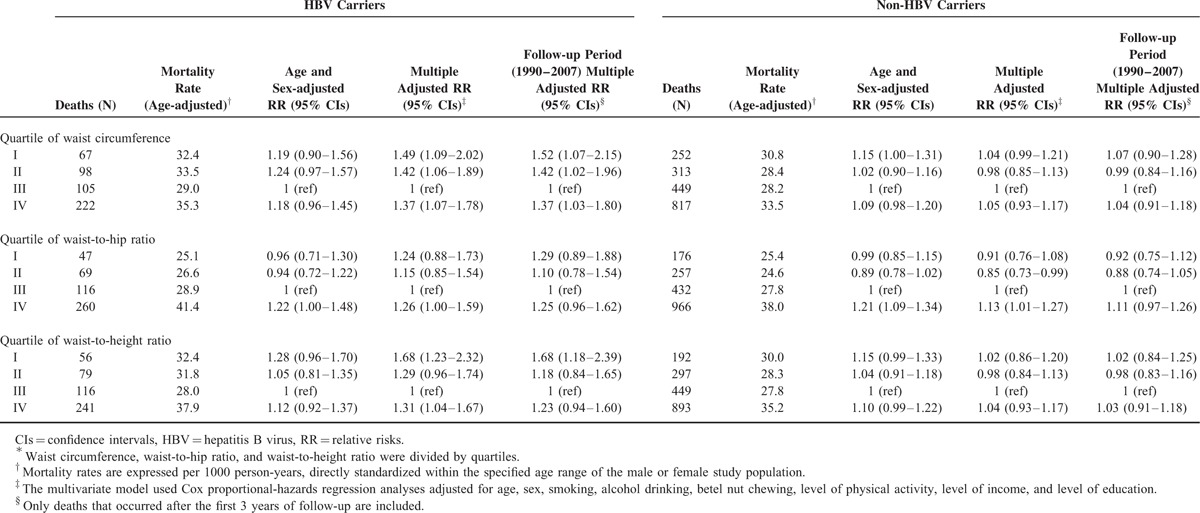
FIGURE 1.
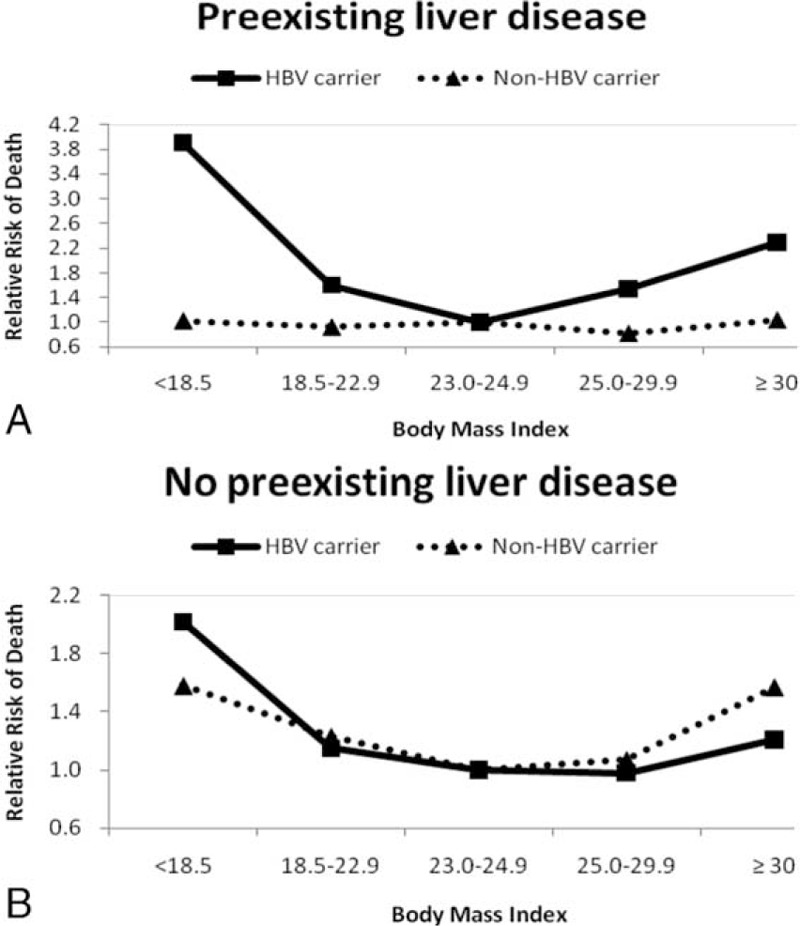
Multivariate relative risks of mortality according to BMI categories among HBV carriers vs. non-HBV carriers. Figure (1A). Participants with preexisting liver diseases (previous history of liver cancer, liver cirrhosis, hepatitis or other liver disease) for all-cause death. Figure (1B). Participants without preexisting liver diseases for all-cause death. HBV carriers and non-HBV carriers were presented with solid line and dashed line. The relative risks were adjusted for age, gender, smoking status, alcohol intake, betel nut chewing, physical activity, income, and education level.
FIGURE 2.
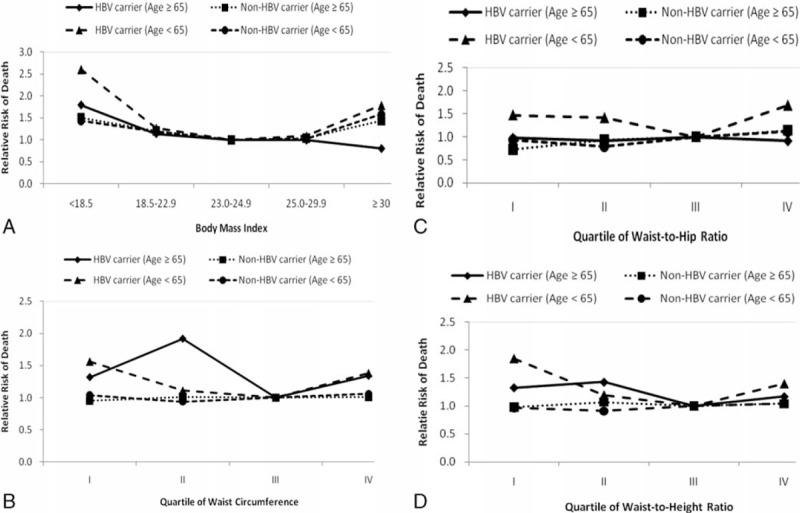
Multivariate relative risks of mortality of HBV carrier vs. non-HBV carriers among different age categories according to BMI category and quartiles of waist circumference, waist-to-hip ratio, and waist-to-height ratio. Age was divided into two categories: <65 years old and ≥ 65 years old. Figure (2A). BMI category. Figure (2B). Quartile of waist circumference. Figure (2C). Quartile of waist-to-hip ratio. Figure (2D). Quartile of waist-to-height ratio. The relative risks were adjusted for age, gender, smoking status, alcohol intake, betel nut chewing, physical activity, income, and education level.
Figure 2(B–D) illustrates the relationships between abdominal adiposity and all-cause death among different age groups. Participants aged 20 to 64 years with HBV carriers had the similar U-shaped association between all-cause death, and WC and WheiR quartiles. However, for participants aged 65 years and above, the lowest adjusted RR of all-cause death was also found at the third quartile of WC and WheiR, but the highest adjusted RR appeared at the second quartile of WC and WheiR (Figure 2B and D). For non-HBV carriers aged 65 years and older, there are no significant associations between all-cause mortality and abdominal adiposity (Figure 2B–D).
DISCUSSION
In this study, we demonstrated a reverse J-shaped association between BMI and all-cause death among HBV carriers and a U-shaped association among non-HBV carriers. The U or reverse J-shaped association between abdominal adiposity and all-cause death among HBV carriers was also found. Underweight participants with HBV carriers have highest RR of all-cause death for BMI than others. Underweight and obese HBV carriers with pre-existing liver diseases have higher RR of all-cause death for BMI than normal-weight participants. The lowest RRs of all-cause death in relation to abdominal adiposity were found at the third quartiles of WC, WHipR, and WHeiR among HBV carriers and at the second quartiles of WC, WHipR, and WHeiR among non-HBV carriers.
To our knowledge, this is the first study to compare the associations between general and abdominal adiposity, and all-cause death among HBV carriers versus non-HBV carriers. Both HBV infection and excess body fat have been recognized as an inflammatory status and pose an increased risk of death.13,32–34 The association between excess or less body fat and mortality among HBV carriers was different from the non-HBV carriers. Previous studies among the general population that examined the relationship between BMI and death found a J or U-shaped association, which is similar to our non-HBV carriers.22,23 However, for HBV carriers in our study, we found a reverse J-shaped association with the highest risk of death being observed in the underweight group. On the contrary, whether moderate elevation of BMI (overweight) increases the risk of death is controversial. Years ago, Gu et al22 reported that overweight was not associated with an increased risk of death in China. The authors reported that persons with a BMI between 24.0 and 26.9 have the lowest all-cause death rate. Flegal et al23 also found that overweight was not associated with excess mortality in the nationally representative samples of US adults drawn from the National Health and Nutrition Examination Survey. Our previous study also found that the lowest risk for all-cause death among adults was observed to relate to a BMI between 24.0 and 25.9.25 Our present findings (normal weight was the lowest RRs among both HBV carriers and non-HBV carriers) were different to these large nationally representative cohort studies. Although a study done by Yu et al18 claimed that BMI quartiles were positively associated with the increase of liver-related mortality and HCC occurrence among healthy HBV carriers, they observed an increased rate of all other causes of death and HCC occurrence in those patients with a BMI <18.5 as compared with those with a BMI of 18.5 to 24.9. In our study, we found a reverse J-shaped association between liver-related death and BMI categories among HBV carriers and a J-shaped association among non-HBV carriers. In other words, underweight HBV carriers and obese non-HBV carriers have higher liver-related death for BMI than normal-weight participants. Bigaard et al35 and Heitmann et al36 found that excess body fat and low lean body mass increased all-cause mortality. It is believed that WC, WHipR, and WHeiR reflect abdominal fat and visceral fat, whereas BMI reflects lean body mass. Therefore, underweight patients may have a lower lean body mass than those with normal weight, which is related to the increased risk of mortality. Although obese patients may have a higher lean body mass than those with normal weight, they also have higher abdominal fat, which increases the risk of mortality.18 Previous studies have mentioned that obese patients have higher prevalence of NAFLD. NAFLD has been recognized as liver component of MetS.14,21 In the current study, we found that patients with moderate to severe obesity have higher mortality rate among both groups. This may emphasize that the presence of NAFLD may be one of the future cause of mortality. Patients with chronic HBV infection are under a chronic inflammatory state which may synergistically increase the risks of malnutrition-related liver injury and all-cause mortality.37,38 This synergistic compounding of inflammatory risk is clinically important and merits further study.
Pre-existing liver diseases are related to increased all-cause mortality and decreased body weight. The effect of decreased body weight on all-cause mortality observed in the present study could be mediated through pre-existing liver disease-related weight loss. Two methods including restricting the analysis to nonpre-existing liver diseases participants only and excluding those who died during the first 3 years of follow-up (when deaths may be due to pre-existing diseases) were used to minimize the bias incurred by pre-existing liver diseases. Despite these adjustments, for those participants with or without pre-existing liver diseases and for those who died after the first 3 years of follow-up, we found the similar reverse J-shaped relation between BMI categories and all-cause mortality among HBV carriers. Another interesting finding is that, for those non-HBV carriers with pre-existing liver disease, there was no significant association between all-cause death and BMI categories. This also enhances the possibility that HBV carriers with pre-existing liver diseases may have a synergistically increased risk of all-cause death.
Age is another major factor that affects the association between body weight and all-cause death. Our study found that general obesity (represented by BMI) among the older HBV carriers had a protective effect against all-cause death, but abdominal obesity (presented with WC, WHipR, or WHeiR) was harmful. Since BMI may reflect lean mass in the older patients, whereas abdominal adiposity (measured by WC, WHipR, and WHeiR) better reflects total or abdominal fat,21,39,40 abdominal visceral fat may be an important risk factor of all-cause death and higher lean mass may be a protective factor against all-cause death among older HBV carriers. Finelli et al21 also reported that reduced visceral fat leads to increased longevity. This notion also has clinical implications and merits further study.
A major strength of our study is the large prospective cohort that is particularly valuable to determine a more precise dose-response gradient for the association between body weight and the risk of death. The cohort is large enough to allow us to adjust for potential confounders such as lifestyle factors and social economic status. Second, this is the first study to assess the relation between general and abdominal adiposity, and the risk of death among HBV carriers versus non-HBV carriers. On the basis of the present study, clinicians can tailor target weight recommendations for HBV carriers. However, some limitations of this study should be considered. First, although we adjusted for several confounding factors to minimize the bias, we could not rule out all the unknown or unmeasured factors which may account for the association between body weight and all-cause death. Second, the cohort was selected from a general population who attended a health examination rather than from a nationally representative sample. Although the population structure in the present study was similar to the national population structure in Taiwan, external validation is necessary to extrapolate this observation to HBV carriers or non-HBV carriers in general. In this regard, we found that only 14.2% of total deaths among HBV carriers in the study were attributed to liver-related causes, whereas 42.2% of total deaths were liver-related in a study of 2903 male Taiwanese government employees with a mean follow-up period of 14.7 years.13 Conceivably, age, sex, and viral factors might account for the difference in the observed liver-related mortalities, but further studies are warranted to investigate these discrepancies.
In summary, the results of the present study demonstrate a reverse J-shaped or U-shaped association between general and abdominal obesity and all-cause death among HBV carriers. For non-HBV carriers, however, there was a U-shaped association between BMI and all-cause death, and no significant association between abdominal obesity and all-cause death. Mild abdominal obesity seems to be protective against all-cause death among Taiwanese HBV carriers regardless of whether they suffered from pre-existing chronic diseases. Regular measurement of parameters using BMI, WC, WHipR, and WHeiR should be performed routinely in clinical assessment of HBV carriers.
Acknowledgments
We thank all participants in this study.
Footnotes
Abbreviations: BMI = body mass index; HBV = hepatitis B virus; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; MetS = metabolic syndrome; NAFLD = nonalcoholic fatty liver disease; RRs = relative risks; WC = waist circumference; WHeiR = waist-to-height ratio; WHipR = waist-to-hip ratio.
Funding: This study was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002) and China Medical University Hospital (DMR-103-073).
Disclosure: The authors declared no conflict of interest.
REFERENCES
- 1.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008; 48:335–352. [DOI] [PubMed] [Google Scholar]
- 2.Yen SL, Chiu TY, Lin YC, et al. Obesity and hepatitis B infection are associated with increased risk of metabolic syndrome in university freshmen. Int J Obes (Lond) 2008; 32:474–480. [DOI] [PubMed] [Google Scholar]
- 3.Wang CC, Tseng TC, Kao JH. Hepatitis B virus infection and metabolic syndrome: fact or fiction? J Gastroenterol Hepatol 2015; 30:14–20. [DOI] [PubMed] [Google Scholar]
- 4.Sung JL. Prevention of hepatitis B and C virus infection for prevention of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol 1997; 12:S370–S376. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Welfare, Executive Yuan, Taiwan, ROC. Public health report.; 2008. Available at: http://www.mohw.gov.tw/MOHW_Upload/doc/Taiwan_Public_Health_Report2008_0029947001.pdf. [Google Scholar]
- 6.Chu NF. Prevalence of obesity in Taiwan. Obes Rev. 2005;6:271–4 [DOI] [PubMed] [Google Scholar]
- 7.Yeh CJ, Chang HY, Pan WH. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1996 to NAHSIT. Asia Pac J Clin Nutr [Research Support Non-U S Gov’t] 2011; 20:292–300. [PubMed] [Google Scholar]
- 8.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444:881–887. [DOI] [PubMed] [Google Scholar]
- 9.Calle EE, Teras LR, Thun MJ. Obesity and mortality. N Engl J Med 2005; 353:2197–2199. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 11.Lin WY, Yang WS, Lee LT, et al. Insulin resistance, obesity, and metabolic syndrome among non-diabetic pre- and post-menopausal women in North Taiwan. Int J Obes (Lond) 2006; 30:912–917. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 25: 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 13.Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014; 384:1953–1997. [DOI] [PubMed] [Google Scholar]
- 14.Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol 2013; 19:3375–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006; 17:901–909. [DOI] [PubMed] [Google Scholar]
- 16.Calle EE. Obesity and cancer. BMJ 2007; 335:1107–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adami HO, Trichopoulos D. Obesity and mortality from cancer. N Engl J Med 2003; 348:1623–1624. [DOI] [PubMed] [Google Scholar]
- 18.Yu MW, Shih WL, Lin CL, et al. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol 2008; 26:5576–5582. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev 2015; 95:727–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong GL, Wong VW, Choi PC, et al. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut 2009; 58:111–117. [DOI] [PubMed] [Google Scholar]
- 21.Finelli C, Sommella L, Gioia S, et al. Should visceral fat be reduced to increase longevity? Ageing Res Rev 2013; 12:996–1004. [DOI] [PubMed] [Google Scholar]
- 22.Gu D, He J, Duan X, et al. Body weight and mortality among men and women in China. JAMA 2006; 295:776–783. [DOI] [PubMed] [Google Scholar]
- 23.Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005; 293:1861–1867. [DOI] [PubMed] [Google Scholar]
- 24.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999; 341:1097–1105. [DOI] [PubMed] [Google Scholar]
- 25.Lin WY, Tsai SL, Albu JB, et al. Body mass index and all-cause mortality in a large Chinese cohort. Can Med Assoc J[Research Support, N I H, Extramural Research Support, Non-U S Gov’t] 2011; 183:E329–E336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med 2010; 170:1293–1301. [DOI] [PubMed] [Google Scholar]
- 27.Tseng FY, Lin WY, Lin CC, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol 2012; 60:730–737. [DOI] [PubMed] [Google Scholar]
- 28.Tseng FY, Lin WY, Li CI, et al. Subclinical hypothyroidism is associated with increased risk for cancer mortality in adult Taiwanese: a 10 years population-based cohort. PLoS One 2015; 10:e0122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin WY, Chiu TY, Lee LT, et al. Betel nut chewing is associated with increased risk of cardiovascular disease and all-cause mortality in Taiwanese men. Am J Clin Nutr 2008; 87:1204–1211. [DOI] [PubMed] [Google Scholar]
- 30.Department of Health, Executive Yuan, Taiwan. Taiwan Public Health Report 1998-2000. DOH: Taipei. 2001. Available at: http://www.doh.gov.tw. [Google Scholar]
- 31.Lin WY, Lee LT, Chen CY, et al. Optimal cut-off values for obesity: using simple anthropometric indices to predict cardiovascular risk factors in Taiwan. Int J Obes Relat Metab Disord 2002; 26:1232–1238. [DOI] [PubMed] [Google Scholar]
- 32.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 2005; 69:29–35. [DOI] [PubMed] [Google Scholar]
- 33.Haslam DW, James WP. Obesity. Lancet 2005; 366:1197–1209. [DOI] [PubMed] [Google Scholar]
- 34.Fattovich G, Olivari N, Pasino M, et al. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut 2008; 57:84–90. [DOI] [PubMed] [Google Scholar]
- 35.Bigaard J, Frederiksen K, Tjonneland A, et al. Body fat and fat-free mass and all-cause mortality. Obes Res 2004; 12:1042–1049. [DOI] [PubMed] [Google Scholar]
- 36.Heitmann BL, Erikson H, Ellsinger BM, et al. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old swedish men-a 22-year follow-up The study of men born in. Int J Obes Relat Metab Disord 2000; 24:33–37. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien A, Williams R. Nutrition in end-stage liver disease: principles and practice. Gastroenterology [Review] 2008; 134:1729–1740. [DOI] [PubMed] [Google Scholar]
- 38.Alberino F, Gatta A, Amodio P, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition [Research Support, Non-U S Gov’t] 2001; 17:445–450. [DOI] [PubMed] [Google Scholar]
- 39.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc 2005; 53:2112–2118. [DOI] [PubMed] [Google Scholar]
- 40.Bigaard J, Tjonneland A, Thomsen BL, et al. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res 2003; 11:895–903. [DOI] [PubMed] [Google Scholar]


