Supplemental Digital Content is available in the text
Abstract
No study has evaluated whether subnormal estimated glomerular filtration rate (eGFR) (between 61 and 90 mL/min) and high normal albumin–creatinine ratio (ACR) (<30 mg/g) are associated with cardiovascular (CV) events and mortality in type 2 diabetic (T2DM) patients with normoalbuminuria.
We observed a longitudinal cohort study of 1291 T2DM patients with normoalbuminuria who were receiving intensified multifactorial treatment from 2004 to 2008. Cox regression models were used to evaluate eGFR and ACR as the risk factors of major CV events (nonfatal myocardial infarction and stroke) and mortality.
During the 4-year period, 56 patients died and 159 patients developed major CV events. We found eGFR, but not ACR, to be associated with major CV events. Compared to those with eGFR higher than 90 mL/min, patients with subnormal eGFR (HR: 3.133, 1.402–7.002, P = 0.005) were at greater risk of incident major CV events. Extremely low eGFR (<30 mL/min) was associated with mortality only in patients under 65 years old.
Subnormal eGFR was a strong predictor of major CV events in diabetic patients with normoalbuminuria. Normoalbuminuric diabetic patients with subnormal eGFR may need intensive CV risk factor intervention to prevent and treat CV events.
INTRODUCTION
Microalbuninuria, a prognostic factor for cardiovascular (CV) morbidity and mortality, is defined as having a urinary albumin–creatinine ratio (ACR) ranging from 30 to 300 mg/g.1–4 American Diabetes Association (ADA) position statement5 announced that microalbuminuria and macroalbuminuria were no longer used but rather referred to as persistent albuminuira at levels 30 to 299 mg/24 hours and levels ≧300 mg/24 hours. The new nomenclature was intended to emphasize the continuous nature of albuminuira as a risk factor. Recent studies have found an association between elevated urinary albumin excretion levels, even within the normal range (ACR < 30 mg/g) (defined as high normal ACR) and greater risk of cardiovascular disease (CVD).1,6,7
Chronic kidney disease (CKD), defined as having an estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1.73 m2, has also been found to be a major risk factor for CV morbidity and mortality.8–10 This increased CKD-CV risk, however, appears to diminish when there is no microalbuminuria or proteinuria.11–13 Studies have found decreased GFR in the absence of increased urine albumin excretion in a substantial percentage of adults with diabetes.14 Ninomiya et al15 have recently reported high albuminuria and low eGFR to be independent risk factors for CV events in patients with type 2 diabetes (T2DM). However, there is no study to evaluate the association between subnormal eGFR (between 61 and 90 mL/min) and CVD and mortality risk particularly in diabetic patients with normoalbuminuria. One of our latest studies16 has reported that diabetic nephropathy can be delayed by helping patients reach many of the treatment goals outlined by ADA at the same time. In this observational study, we investigate the possible association between subnormal eGFR and high normal ACR and the risk of CV events and mortality in patients who have T2DM and normoalbuminuria.
METHODS
Population
This study included all patients with T2DM who visited the diabetic clinic in the Metabolism Division at Kaohsiung Medical University Hospital and Changhua Christian Hospital between January 2004 and April 2005. The follow-up period lasted until December 2008. We included patients1 who had been diagnosed with T2DM with no history of acute myocardial infarction (MI), heart failure, or stroke within 1 year, and had be followed up regularly every 3 to 6 months at the outpatient departments of the 2 hospitals and2 who had normoalbuminuria (defined as having a least 2 times of urinary ACR < 30 mg/g). The protocol for this study was approved by the Human Research Ethics Committees at both hospitals, and informed consent was obtained from each patient.
Baseline Investigation
An interview with all-inclusive appraisal of risk factors, complications, and disease status were accomplished in each patient. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were obtained as the average of 2 mensurations in sitting positions after 10 minutes of relaxation. We estimated body mass index (BMI) by using body weight divided by body height squared (kg/m2) from each patient. We obtained blood samples of each patient at early morning after overnight fast. The plasma glucose, total cholesterol (Chol), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and creatinine levels were interpreted by using a biochemistry automatic analyzer (Bechman-Coulter Inc. Fullerton, CA). We used ion exchange high-performance liquid chromatography (Bio-Rad, Varianttm II Turbo, CA) to figure out HbA1c in whole blood. All patients were followed up regularly and took part in a strict intervention program aimed to meet the treatment goals recommended by ADA: HbA1c < 7%, SBP < 130 mmHg, DBP < 80 mmHg, LDL-C < 100 mg/dL, TG < 150 mg/dL, HDL-C > 40 mg/dL for men, and > 50 mg/dL for women. A diabetes care team consisted of physicians, nurses, and dietitians modified patients’ behavior through counseling and educational programs, and various pharmacologic regimens in order to assist the patient achieve the goals.
Kidney Function Tests
Fasting venous blood and an overnight first-void urine sample were collected to measure creatinine and urine albumin in the clinic. The urinary ACR was calculated from urine albumin and creatinine levels. The definition of normoalbuminuria was as an ACR of <30 mg/g. All cases in this study had normoalbuminuria at baseline and the baseline ACR levels were divided into 4 quartiles. The GFR was estimated by using the equations recommended the National Kidney Foundation in the Modified Diet in Renal Disease (MDRD)17 and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI).18
Outcome Assessment
During the follow-up, we collected information major CV endpoints and all-cause mortality until December 2008. The major CV endpoint was defined as the incidence of nonfatal MI or stroke. Death by any cause was identified from the Data File of Death Causes which published by Department of Health in Taiwan, based on the International Classification of Disease 9th revision.
Statistical Analysis
The baseline clinical and biochemical features of the population are presented as mean ± SD, or percentages. Cox regression analysis was used to examine the eGFR and ACR that might predict major CV endpoints and all-cause mortality. The eGFR levels were divided into groups >90 mL/min, 61 to 90 mL/min, 46 to 60 mL/min, 30 to 45 mL/min, and <30 mL/min; the ACR levels were divided into 4 quartiles. Variables including sex, age, durations of diabetes, SBP, DBP, BMI, glycosylated hemoglobin (A1C), Chol, TG, LDL-C, and HDL-C at baseline were used for adjustment in Cox regression models by forward selection. A P value of 0.2 or less was used to determine whether a variable would be entered into the analysis, and a P value of 0.05 or less was used to determine whether the variable would remain in the models. Because the distribution of TG was highly right skewed, it was natural log-transformed for analyses. Results are described as hazard ratios (HRs) and their 95% confidence intervals (CIs). All statistical operations were performed using SAS 9.4 (SAS Institute, Cary, NC) except that XLSTAT (Addinsoft, New York, NY) was performed for calculation of power in estimating HRs.
RESULTS
Initially, we enrolled 1303 patients. Twelve patients were lost to follow-up in the initial 6 months and were excluded from our study. After exclusion, we had a total of 1291 diabetic patients with normoalbuminuria (578 men and 713 women). The mean age of the cohort was 59.8 years. Clinical and biochemical characteristics of the patients at baseline are shown in Table 1. Compared to patients who did not receive antihypertensive medication, those who did had a higher mean ACR (11.5 ± 7.6 vs 10.5 ± 7.4, P = 0.013) and a lower mean eGFR (67.1 ± 19.1 vs 76.8 ± 16.8, P < 0.001). During the follow-up period, 56 patients died (4.3%) and 159 patients (12.3%) developed major CV events, including acute MI in 100 patients (7.7%) and stroke in 81 patients (6.3%). By the end of the study period, our patient population had a mean HbA1c of 7.3%, SBP of 129.3 mmHg, DBP of 74.4 mmHg, LDL-C of 98.6 mg/dL, HDL-C of 53.6 mg/dL, and TG of 116.0 mg/dL.
TABLE 1.
Baseline Characteristics of Participants
Major CV Endpoints
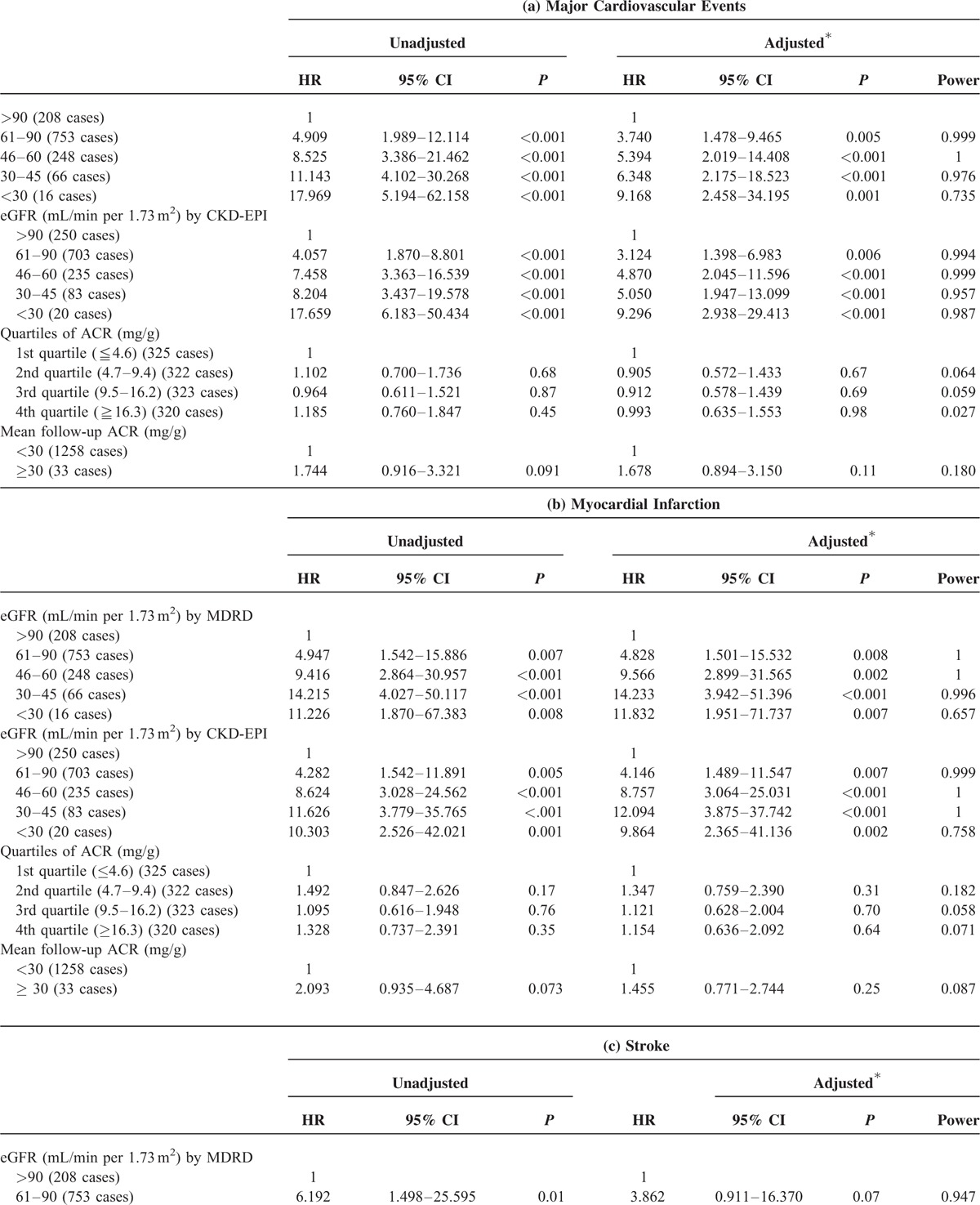
Table 2 summarizes the results of Cox regression analysis for major CV events with baseline eGFR and ACR as risk factors. Diabetic patients with low baseline eGFR were found to be at increased risk of major CV events. After adjusting for sex, age, and baseline data including BMI, A1C, cholesterol, LDL-C, HDL-C, and TG, we found that, compared to those with eGFR calculated by MDRD more than 90 mL/min, patients with subnormal eGFR (between 61 and 90 mL/min) (HR: 3.740, 1.478–9.465, P = 0.005), eGFR between 46 and 60 mL/min (HR: 5.394, 2.019–14.408, P < 0.001), eGFR between 30 and 45 mL/min (HR: 6.348, 2.175–18.523, P < 0.001), and eGFR less than 30 mL/min (HR: 9.168, 2.458–34.195, P = 0.001) were at greater risk of incident major CV event. The subnormal eGFR was significantly associated with elevated risk of incident MI but not stroke after adjusting for sex, age, and baseline data including BMI, A1C, cholesterol, LDL-C, HDL-C, and TG (Table 2 ). Using another often used method of calculating eGFR known as the CKD-EPI produced similar results. Neither baseline ACR nor mean follow-up ACR was associated with major CV events, MI, or stroke.
TABLE 2.
Hazard Ratio of Major Cardiovascular Events, Myocardial Infarction, and Stroke in Patients With Type 2 Diabetes Stratified by eGFR and ACR Categories
All-Cause and CV Mortality
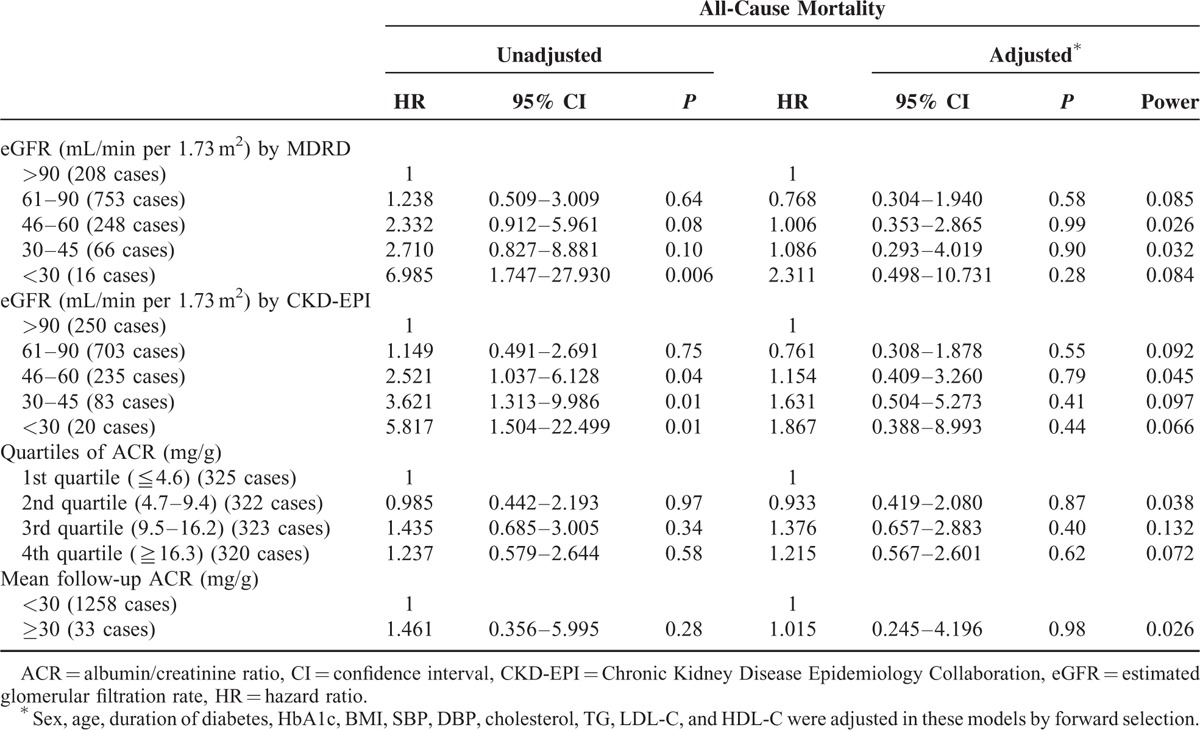
Table 3 showed the results of Cox regression analysis for all-cause mortality with eGFR and ACR as risk factors. Patients with eGFR less than 30 mL/min (HR: 6.985, 1.747–27.930, P = 0.006) were associated with all-cause mortality as compared to those with eGFR more than 90 mL/min calculated by MDRD in simple regression analysis, though the result was not significant in multiple regression after adjusted for other factors. The findings remained similar when calculated eGFR by CKD-EPI. Neither baseline ACR nor mean follow-up ACR was associated with mortality in patients with T2DM. The results of Cox regression analysis for CV mortality with eGFR and ACR as risk factors are shown in Supplementary Table 1. Neither eGFR nor ACR was significantly associated with CV mortality in patients with T2DM. The statistical power for CV mortality, however, was low because the number of CV mortality was small in this cohort, and in some categories of eGFR or quartiles of ACR there was even no CV mortality.
TABLE 2 (Continued).
Hazard Ratio of Major Cardiovascular Events, Myocardial Infarction, and Stroke in Patients With Type 2 Diabetes Stratified by eGFR and ACR Categories
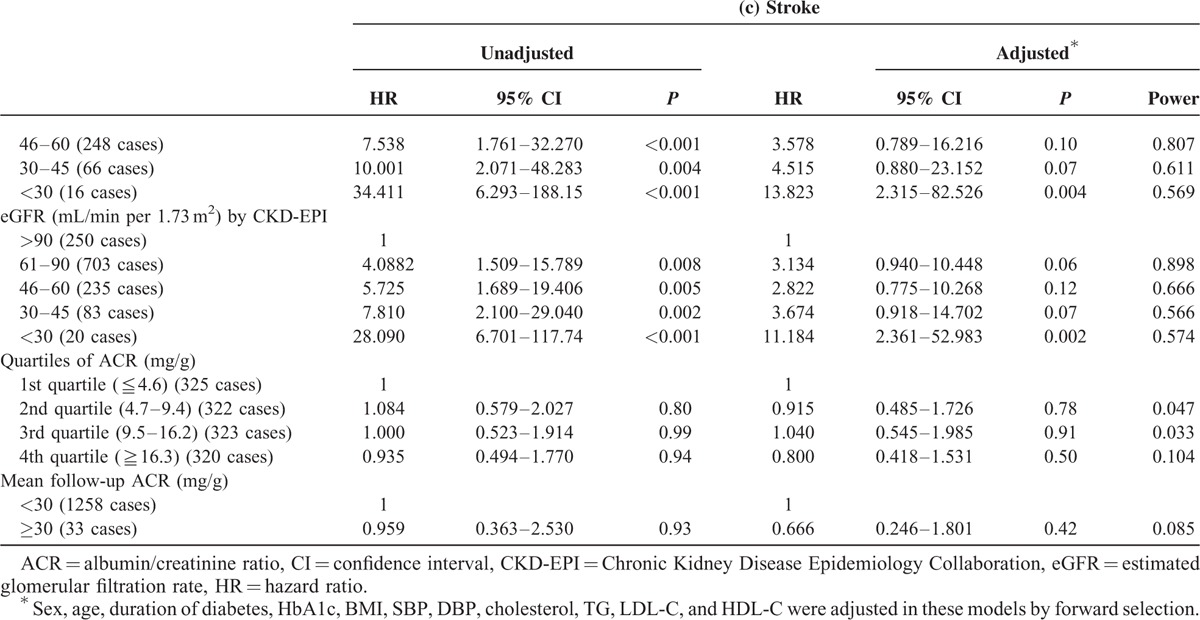
TABLE 3.
Hazard Ratios of All-Cause Mortality in Patients with Type 2 Diabetes Stratified by eGFR and ACR Categories
Older Patients Versus Younger Patients
We analyzed major CV events and mortality by eGFR in older patients (≧65 years old) and younger patients (<65 years old), respectively. Figures 1 and 2 showed the results of Cox regression analysis with adjustment of confounding factors in 2 different age groups. The risk of major CV events increased with decreasing eGFR in both older and younger subjects after adjusted for confounding factors, with even higher magnitude of HR in the younger groups (Figure 1A). Low eGFR was significantly associated with MI in younger patients, while this relationship was not as strong in older ones (Figure 1B). Younger patients with eGFR less than 45 mL/min were at significantly higher risk for stroke (Figure 1C). Younger patients, not older ones, with extremely low eGFRs (<30 mL/min) were found to be at significantly high risk for all-cause mortality (Figure 2). ACR was not related to CV events or all-cause mortality in either the older subjects or the younger ones.
FIGURE 1.
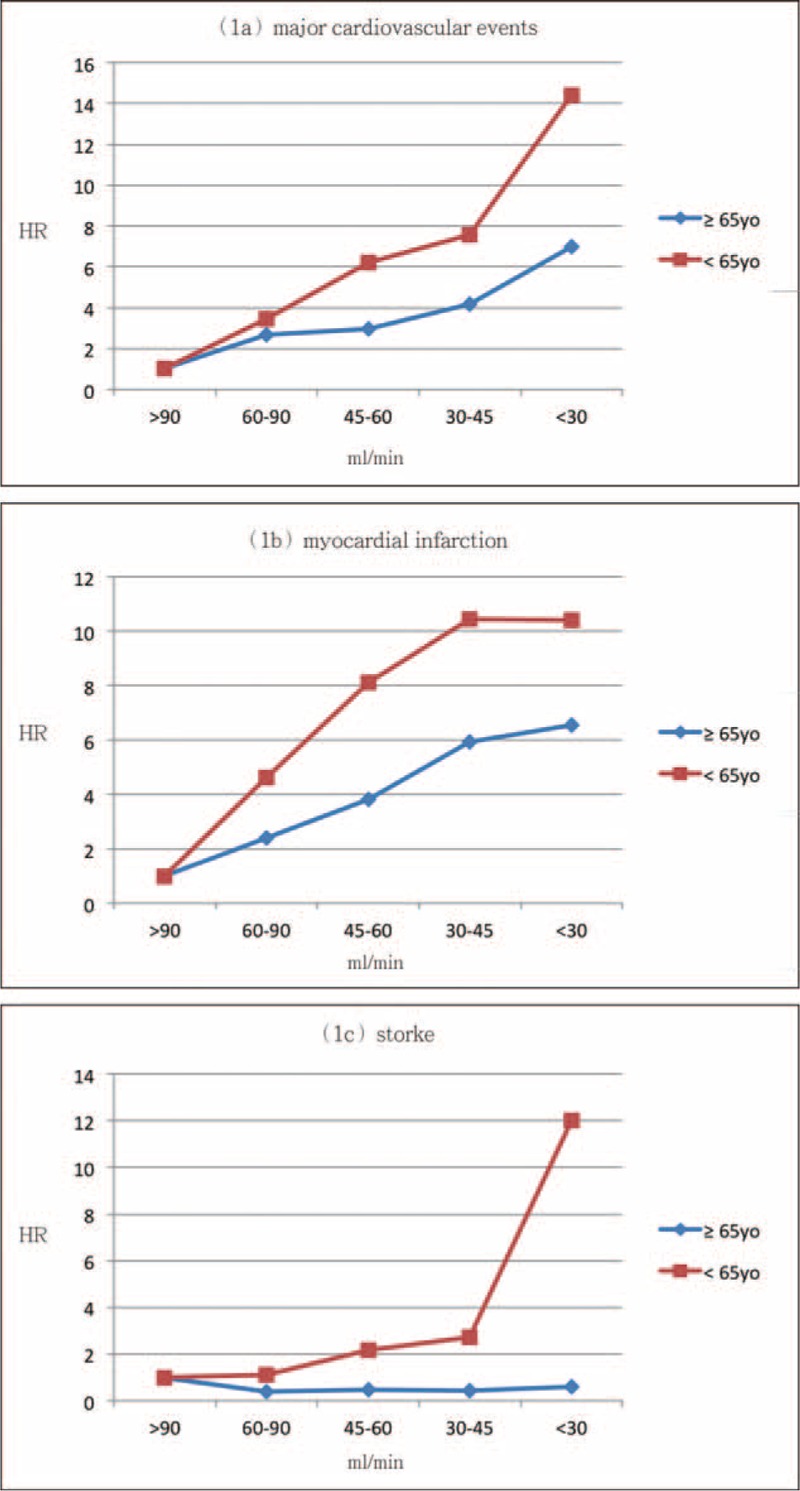
HR for risk of major cardiovascular events (A), myocardial infarction (B), and stroke (C) with eGFR category after adjusting for sex, age, cholesterol, triglyceride, LDL-C, HDL-C, BMI, and ACR in elderly and young patients. Group 1: eGFR > 90 mL/min, Group 2: eGFR 61 to 90 mL/min, Group 3: eGFR 46 to 60 mL/min, Group 4: eGFR 30 to 45 mL/min, and Group 5: eGFR < 30 mL/min. HR = 1 set at reference for Group 1. ACR = albumin-creatinine ratio, BMI = calculated body-mass index, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, HR = hazard ration, LDL-C = low-density lipoprotein cholesterol.
FIGURE 2.
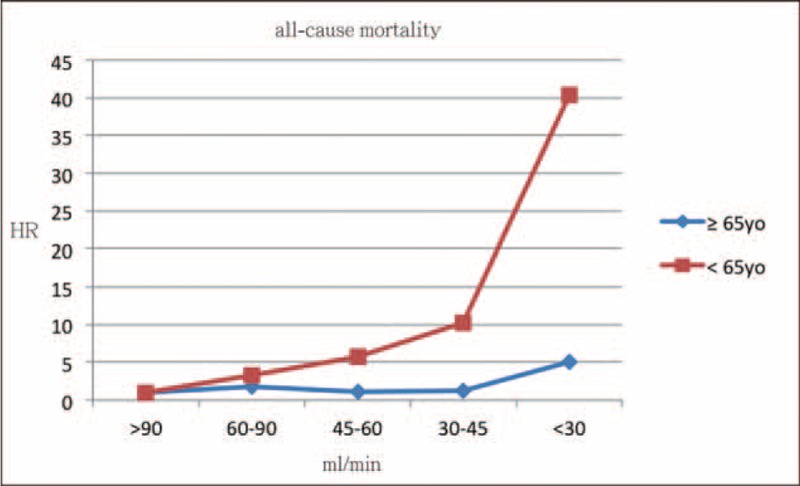
HR for risk of all-cause mortality with eGFR category after adjusting for sex, age, cholesterol, triglyceride, LDL-C, HDL-C, BMI, and ACR in elderly and young patients. Group 1: eGFR > 90 mL/min, Group 2: eGFR 61 to 90 mL/min, Group 3: eGFR 46 to 60 mL/min, Group 4: eGFR 30 to 45 mL/min, and Group 5: eGFR < 30 mL/min. HR = 1 set at reference for Group 1. ACR = albumin-creatinine ratio, BMI = calculated body-mass index, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, HR = hazard ration, LDL-C = low-density lipoprotein cholesterol.
DISCUSSION
Our study found subnormal eGFR but not high normal ACR to be a powerful predictor of major CV events in T2DM patients with normoalbuminuria. Low eGFR was significantly associated with incidental major CV events both in older and younger patients with T2DM, the association being more obvious in younger patients.
Ishani et al19 reported the association of eGFR with risk of CVD to be limited in the absence of microalbuminuria. The Prevention of Renal and Vascular End-stage Disease study13 also found subjects with stage 3 CKD who had a urinary albumin excretion rates <30 mg/24 hours to have a CV risk comparable to those with stage 1 and 2 CKD. The second Nord-Trondelag Health Study11 found no significant trend for increased CV risk in patients with decreased eGFR and lower ACR levels. However, So et al20 found that T2DM Chinese patients with no albuminuria but eGFRs < 60 mL/min to be at significant CV risk. Drury et al21 reported that diabetic patients with an eGFR less than 60 mL/min and normoalbuminuria had higher risk of CV events in a low-risk population mainly of European ancestry. Knobler et al22 also reported an eGFR less than 60 mL/min to predict increased risk for cardiac events independent of albuminuria. However, there has been no study to evaluate the association between subnormal eGFR and major CV events in the absence of microalbuminuria. We studied this association and we found a significant association between eGFR and major CV events in diabetic patients with normoalbuminuria. Patients with eGFRs between 61 and 90 mL/min were at significantly higher risk of incidental major CV events (HR: 2.617, 1.246–5.496, P = .01) than those with normal renal function after adjustment for age and multiple known risk factors. Besides, we also found that there was a strong progressive relationship between lower eGFR and major CV events. Our results suggested eGFR to be a strong predictor of major CV events in normoalbuminuria diabetic patients receiving treatment for multiple factors.
van der Velde et al23 recently reported that most GFR estimates and age together could significantly predict CV outcomes, and eGFR measurement was associated with the risk of CV events in younger subjects but not in the elderly. Two previous studies24,25 reported that as people age, the less influence decreasing eGFR has on the risk of mortality. O’Hare et al26 also found a lower association between eGFR and risk for mortality in older population. Our study revealed that the risk of major CV events increased with decreasing eGFR both in older and younger patients, while the correlation was stronger in younger group. As to the association between eGFR and all-cause mortality, it was found only in younger patients but not in older patients. The possible reason for this decreased risk in the older group is that traditional risk factors have reduced predictive power for CV events and mortality in the elderly.27–29 Danziger et al30 reported that the age-associated decline in GFR is not modulated by changes in blood pressure or CV performance. Of course, the elderly might have many comorbid disorders which can have an influence on the decline rate of GFR over the age-associated decline. However, it may be difficult to sort out the effects of aging per se and the effect of comorbid conditions commonly accompanying the aging process.31 Age itself is not a direct cause of CV events but rather reflects the progression of atherosclerosis. Recently, Hallan et al11 reported that reduced renal function and albuminuria were both risk factors for CV death particularly in the elderly persons.
In a pooled analysis of 4 studies (the Atherosclerosis Risk in Communities, Cardiovascular Health, Framingham Heart, and Framingham Offspring studies),32 it was found that CKD was a risk factor for all-cause mortality in the general population. Another meta-analysis study33 found that diabetic patients with low eGFR (<60 mL/min) and normoalbuminuria had significantly higher all-cause mortality but not CV mortality than those with normal eGFR (≥60 mL/min) and normoalbuminuria. One large study34 of 1,102,581 individuals (75% Asians, 21% Whites, and 4% Blacks) found that low eGFR was independently associated with an increased risk of all-cause mortality and CV mortality in the three major races. However, the National Health and Nutrition Examination Survey (NHANES) I study35 reported that moderate renal insufficiency was not associated with all-cause mortality and CV mortality after adjustment for traditional CV risk factors. The Framingham Heart Study36 did not find baseline kidney disease to be associated with CV mortality in multivariable analyses. Our study also did not find there to be an association between low eGFR and all-cause mortality or CV mortality, although there was a trend toward increased all-cause mortality as the eGFR decreased. Although the first three studies 32–34 were very large and had sufficient statistical power to investigate the relationship between CKD and CV and all-cause mortality, they each had their own definition of normal eGFR as the reference category in the meta-analysis studies. These differences in the definition may have influenced their results. One of them33 evaluated the association between low eGFR and mortality in patients with diabetes, and this meta-analysis concluded that the patient numbers investigating the association between low eGFR and mortality were small. Our study found that eGFR was significantly associated with major CV events but not with CV mortality and all-cause mortality. Therefore, eGFR may be a specific predictor of major CV events but not an unspecific marker of health status.
The traditional definition of microalbuminuria, based on the level of urinary albumin excretion in patients with diabetes, predicts the development of clinical diabetic nephropathy. Although microalbuninuria has been known to be a predictor of CV morbidities and mortality,1–4 it was not until it was discovered that there was an association between low-grade albuminuria and CV events that much attention was paid to it. Gerstein et al1 found any degree of albuminuria, starting well below the microalbuminuria cutoff, to be a risk factor for CV events in individuals with or without diabetes. Klausen et al6,37 reported urinary albumin excretion >4.8 μg/min to be a strong and independent determinant of coronary artery disease and death in the general population and hypertensive subjects. Recently, Huang et al38 found an association between low grade albuminuria, which is below the current cutoff point of microalbuminuria, and carotid intima-media thickness (IMT) in Chinese T2DM. Our current study, however, did not find an association between high normal ACR and incidental major CV events or death in Chinese with normoalbuminuria. The difference between 2 of the previous studies and our current one might be explained by the fact that the subjects of previous studies6,37 came from the general population or were hypertensive subjects with a low prevalence of diabetes. The other study38 focused on the association between albuminuria and carotid IMT, but was not designed to follow progression. Our study evaluated the effect of multiple factorial interventions in a 5-year cohort of normoalbuminuric T2DM patients. Therefore, the difference in our results and those of others may be related in differences in study populations and design. We did not find an association between high normal ACR and risk for major CV events or mortality in normoalbuminuric T2DM Chinese.
A reduced eGFR may be associated with retention of urotoxins and an increased occurrence of nontraditional risk factors of CV events, including chronic inflammation, hyperhomocysteinemia, and increased oxidative stress, all of which could lead to atherosclerosis.39,40 Albuminuria can indicate damage to glomerular basement membranes and may reflect endothelial dysfunction.41 Recently, Drury et al21 reported reduced eGFR and albuminuria to be independent risk factors for CV events and mortality in patients with T2DM. One collaborative meta-analysis42 has also concluded that low eGFR and high ACR were independent predictors of mortality in the general population. Our prospective cohort has clearly demonstrated an inverse relationship between eGFR and major CV events in diabetic patients with normoalbuminuria. This association was obvious even in subjects with eGFR between 61 and 90 mL/min. On the other hand, we found no association between high normal ACR and major CV events. It is likely that reduced eGFR and albuminuria are markers of different pathologic processes. Our findings are in agreement with the ADA recommendation5 that albuminuria and renal function, as estimated by eGFR, be used to assess CV risk in diabetic patients.
This study has several limitations. First, we have not examined the contribution of nontraditional risk factors, which are complex and not routinely measured in daily clinical practice. Nontraditional risk factors such as oxidant stress, elevated inflammatory markers, and homocystine are associated with atherosclerosis and may be additional contributors to high risk of CV events in CKD.43,44 Second, we did not find an association between ACR and adverse outcomes. This could be due to several possible reasons. One possibility is that there is a threshold effect at or near the ACR decision point of 30 mg/g. Another is that the correlations between ACR and adverse outcomes are small and continuous. The inability to show that small changes in ACR within the reference interval are predictive of an adverse outcome could be due to the small size of our sample and the short follow-up time. A third limitation is that we did not demonstrate a relationship between eGFR and CV mortality or all-cause mortality, possibly also due to the small number of CV mortalities and all-cause mortalities. A large cohort study with a longer follow-up time may be necessary to clarify the association between eGFR and mortality. The strengths of the present study should be acknowledged as well. First, this is the first study to evaluate whether subnormal eGFR and high normal ACR are independently associated with CV events and mortality in T2DM with normoalbuminuria under multifactorial intervention. Second, eGFR was calculated by both MDRD and CKD-EPI equations in this study. Third, our study clarifies the important role of renal function, as estimated by eGFR, showing it can be used in the assessment of CV risk in diabetic patients with normoalbuminuria. Although the ADA recommends that albuminuria and renal function, as estimated by eGFR, to be used to assess CV risk in diabetic patients,5 the awareness of CKD by general practitioners is low in diabetic population.45 One study also reported the low rates of CKD detection at a large hospital.46 Furthermore, some studies reported that eGFR was not a strong predictor of CV events in the absence of microalbuminuria. It is reasonable to hypothesize that unawareness of CKD is common especially in diabetic patients with normoalbuminuria in the real world practice.
In conclusion, this study found subnormal eGFR to be a strong predictor of major CV events in diabetic patients with normoalbuminuria. Renal function, as estimated by eGFR, can be used to assess CV risk in diabetic patients with normoalbuminuria. Normoalbuminuric diabetic patients with subnormal eGFR are at higher risk of CV events and may need intensive CV risk factor intervention to prevent and treat CV events.
Supplementary Material
Footnotes
Abbreviations: A1C = glycosylated hemoglobin; ACR = albumin–creatinine ratio; ADA = American Diabetes Association; BMI = body mass index; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; CV = cardiovascular; CVD = cardiovascular disease; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HDL-Chigh-density = lipoprotein cholesterol; HR = hazard ratio; LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; SBP = systolic blood pressure; T2DM = type 2 diabetes; TG = triglyceride.
Y-TH had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design: Y-TH, S-LS, M-CH; Acquisition of data: Y-TH, M-CH; Analysis and interpretation of data: Y-TH, J-FK, S-LS, J-FC, H-CC, M-CH; Manuscript preparation: Y-TH, J-FK, M-CH; and Statistical analysis: Y-TH, M-CH.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426. [DOI] [PubMed] [Google Scholar]
- 2.De Leeuw PW, Thijs L, Birkenhäger WH, et al. Prognostic significance of renal function in elderly patients with isolated systolic hypertension: results from the Syst-Eur trial. J Am Soc Nephrol 2002; 13:2213–2222. [DOI] [PubMed] [Google Scholar]
- 3.Stehouwer CD, Gall MA, Twisk JW, et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 2002; 51:1157–1165. [DOI] [PubMed] [Google Scholar]
- 4.Valmadrid CT, Klein R, Moss SE, et al. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med 2000; 160:1093–1100. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care in diabetes – 2014. Diabetes Care 2014; 37 Suppl 1:S14–S80. [DOI] [PubMed] [Google Scholar]
- 6.Klausen KP, Scharling H, Jensen G, et al. New definition of microalbuminuria in hypertensive subjects: association with incident coronary heart disease and death. Hypertension 2005; 46:33–37. [DOI] [PubMed] [Google Scholar]
- 7.Forman JP, Fisher ND, Schopick EL, et al. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol 2008; 19:1983–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 9.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 10.Henry RM, Kostense PJ, Bos G, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int 2002; 62:1402–2147. [DOI] [PubMed] [Google Scholar]
- 11.Hallan S, Astor B, Romundstad S, et al. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 2007; 167:2490–2496. [DOI] [PubMed] [Google Scholar]
- 12.Tonelli M, Jose P, Curhan G, et al. Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. BMJ 2006; 332:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brantsma AH, Bakker SJ, Hillege HL, et al. Cardiovascular and renal outcome in subjects with K/DOQI stage 1-3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant 2008; 23:3851–3858. [DOI] [PubMed] [Google Scholar]
- 14.Kramer HJ, Nguyen QD, Curhan G, et al. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003; 289:3273–3277. [DOI] [PubMed] [Google Scholar]
- 15.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009; 20:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu ST, Chang SJ, Chen JF, et al. Prevention of diabetic nephropathy by tight target control in an asian population with type 2 diabetes mellitus: a 4-year prospective analysis. Arch Intern Med 2010; 170:155–161. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139:137–147. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol 2006; 17:1444–1452. [DOI] [PubMed] [Google Scholar]
- 20.So WY, Kong AP, Ma RC, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care 2006; 29:2046–2052. [DOI] [PubMed] [Google Scholar]
- 21.Drury PL, Ting R, Zannino D, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2011; 54:32–43. [DOI] [PubMed] [Google Scholar]
- 22.Knobler H, Zornitzki T, Vered S, et al. Reduced glomerular filtration rate in asymptomatic diabetic patients: predictor of increased risk for cardiac events independent of albuminuria. J Am Coll Cardiol 2004; 44:2142–2148. [DOI] [PubMed] [Google Scholar]
- 23.van der Velde M, Bakker SJ, de Jong PE, et al. Influence of age and measure of eGFR on the association between renal function and cardiovascular events. Clin J Am Soc Nephrol 2010; 5:2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 2007; 18:2758–2765. [DOI] [PubMed] [Google Scholar]
- 25.Raymond NT, Zehnder D, Smith SC, et al. Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial Transplant 2007; 22:3214–3220. [DOI] [PubMed] [Google Scholar]
- 26.O’Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol 2006; 17:846–853. [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM, Bazzarre T, Cleeman J, et al. Prevention Conference V: Beyond secondary prevention: identifying the high-risk patient for primary prevention: medical office assessment: Writing Group I. Circulation 2002; 101:E3–E11. [DOI] [PubMed] [Google Scholar]
- 28.Abbott RD, Curb JD, Rodriguez BL, et al. Age-related changes in risk factor effects on the incidence of coronary heart disease. Ann Epidemiol 2002; 12:173–181. [DOI] [PubMed] [Google Scholar]
- 29.Störk S, Feelders RA, van den Beld AW, et al. Prediction of mortality risk in the elderly. Am J Med 2006; 119:519–525. [DOI] [PubMed] [Google Scholar]
- 30.Danziger RS, Tobin JD, Becker LC, et al. The age-associated decline in glomerular filtration in healthy normotensive volunteers. Lack of relationship to cardiovascular performance. J Am Geriatr Soc 1990; 38:1127–1132. [DOI] [PubMed] [Google Scholar]
- 31.Zhou XJ, Rakheja D, Yu X, et al. The aging kidney. Kidney Int 2008; 74:710–720. [DOI] [PubMed] [Google Scholar]
- 32.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315. [DOI] [PubMed] [Google Scholar]
- 33.Toyama T, Furuichi K, Ninomiya T, et al. The impacts of albuminuria and low eGFR on the risk of cardiovascular death, all-cause mortality, and renal events in diabetic patients: meta-analysis. PLoS One 2013; 8:e71810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen CP, Matsushita K, Coresh J, et al. Relative risks of chronic kidney disease for mortality and end-stage renal disease across races are similar. Kidney Int 2014; 86:819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garg AX, Clark WF, Haynes RB, et al. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int 2002; 61:1486–1494. [DOI] [PubMed] [Google Scholar]
- 36.Culleton BF, Larson MG, Wilson PW, et al. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int 1999; 56:2214–2219. [DOI] [PubMed] [Google Scholar]
- 37.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004; 110:32–35. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Chen Y, Xu M, et al. Low-grade albuminuria is associated with carotid intima-media thickness in Chinese type 2 diabetic patients. J Clin Endocrinol Metab 2010; 95:5122–5128. [DOI] [PubMed] [Google Scholar]
- 39.Kalantar-Zadeh K, Stenvinkel P, Bross R, et al. Kidney insufficiency and nutrient-based modulation of inflammation. Curr Opin Clin Nutr Metab Care 2005; 8:388–396. [DOI] [PubMed] [Google Scholar]
- 40.Tonelli M, Sacks F, Pfeffer M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 2005; 68:237–245. [DOI] [PubMed] [Google Scholar]
- 41.Hermans MM, Henry RM, Dekker JM, et al. Albuminuria, but not estimated glomerular filtration rate, is associated with maladaptive arterial remodeling: the Hoorn Study. J Hypertens 2008; 26:791–797. [DOI] [PubMed] [Google Scholar]
- 42.Matsushita K, van der Velde M, et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int 2001; 59:407–414. [DOI] [PubMed] [Google Scholar]
- 44.Himmelfarb J, Stenvinkel P, Ikizler TA, et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 2002; 62:1524–1538. [DOI] [PubMed] [Google Scholar]
- 45.Ravera M, Noberasco G, Re M, et al. Chronic kidney disease and cardiovascular risk in hypertensive type 2 diabetics: a primary care perspective. Nephrol Dial Transplant 2009; 24:1528–1533. [DOI] [PubMed] [Google Scholar]
- 46.Gentile G, Postorino M, Mooring RD, et al. Estimated GFR reporting is not sufficient to allow detection of chronic kidney disease in an Italian regional hospital. BMC Nephrol 2009; 10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


