Supplemental Digital Content is available in the text
Abstract
Efavirenz-based antiretroviral therapy (ART) has been associated with dyslipidemia and dysglycemia, risk factors for cardiovascular disease. However, the pathogenesis is not well understood. We characterized relationships between plasma efavirenz concentrations and lipid and glucose concentrations in HIV-infected South Africans.
Participants on efavirenz-based ART were enrolled into a cross-sectional study. The oral glucose tolerance test was performed after an overnight fast, and plasma drawn for mid-dosing interval efavirenz, fasting total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, and triglycerides concentrations.
Among 106 participants (77 women), median age was 38 years, median CD4 + T-cell count was 322 cells/μL, median duration on ART was 18 months, and median (interquartile range) efavirenz concentration was 2.23 (1.66 to 4.10) μg/mL. On multivariable analyses (adjusting for age, sex, body mass index, and ART duration) doubling of efavirenz concentrations resulted in mean changes in mmol/L (95%CI) of: total cholesterol (0.40 [0.22 to 0.59]), LDL cholesterol (0.19 [0.04 to 0.30]), HDL cholesterol (0.14 [0.07 to 0.20]), triglycerides (0.17 [0.03 to 0.33]), fasting glucose (0.18 [0.03 to 0.33]), and 2-h glucose concentrations (0.33 [0.08 to 0.60]). Among 57 participants with CYP2B6 genotype data, associations between slow metabolizer genotypes and metabolic profiles were generally consistent with those for measured efavirenz concentrations.
Higher plasma efavirenz concentrations are associated with higher plasma lipid and glucose concentrations. This may have implications for long-term cardiovascular complications of efavirenz-based ART, particularly among populations with high prevalence of CYP2B6 slow metabolizer genotypes.
INTRODUCTION
The non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz is extensively prescribed and is included in the World Health Organization's preferred first-line ART regimens for HIV-1-infected adults, adolescents, and children at least 3 years of age.1 Efavirenz-based ART has been associated with the development of dysglycemia2 and dyslipidemia,3–5 specifically increases in total cholesterol: HDL cholesterol ratio, LDL cholesterol, and triglycerides.6,7 The pathogenesis of these metabolic effects are unclear, although it has been suggested that efavirenz may contribute to mitochondrial toxicity caused by concomitant thymidine analog nucleoside reverse transcriptase inhibitors (NRTI).8
Data are scant regarding relationships between plasma efavirenz concentrations and plasma glucose or lipid concentrations. There is considerable interindividual variability in plasma efavirenz exposure, which is largely explained by 3 CYP2B6 loss-of-function polymorphisms.9,10 The 2 polymorphisms with the greatest effect, CYP2B6 516G→T and CYP2B6 983T→C, are particularly frequent with African ancestry.11,12 The CYP2B6 516G→T polymorphism is also frequent in Thai and Cambodian populations.13,14
We investigated whether plasma efavirenz concentrations correlated with plasma lipid and/or glucose concentrations in HIV-infected South Africans. We hypothesized that higher plasma efavirenz concentrations would be associated with higher lipid and glucose concentrations.
MATERIALS AND METHODS
Study Design and Participants
We conducted a prospective cross-sectional study of consecutive HIV-infected African adults who presented for routine follow-up visits at 1 community-based (Crossroads) and 1 hospital-based (Groote Schuur) ART clinic in Cape Town, South Africa. Participants were recruited by convenient sampling between February 2007 and September 2008. South African ART guidelines at the time of this study recommended an NNRTI plus 2 NRTIs (stavudine or zidovudine, each with lamivudine) as first-line therapy. Eligible participants were on efavirenz-based ART for at least 6 months. Exclusion criteria included pregnancy, renal or hepatic disease, active opportunistic infections, treatment for diabetes or dyslipidemia, and self-reported non-adherence. The study was conducted in accordance with the Declaration of Helsinki and the South African Good Clinical Practice. The University of Cape Town Research Ethics Committee approved the study (REC REF 128/2007). All participants gave written informed consent.
Clinical and Laboratory Evaluations
Participants were instructed to fast overnight and to document the time of the evening dose of efavirenz on the day preceding the study visit. On the study day, participants underwent an oral glucose tolerance test (OGTT). Blood was drawn at 0 and 120 min after ingesting 75 g of glucose in 250 mL of water, and kept on ice until centrifuged within 4 h. Plasma for efavirenz quantification was collected into 4 mL lithium heparin tubes, kept on ice until centrifuged within 4 h, and was aliquotted and promptly frozen at −20°C, then stored at −70°C until analysis at the end of recruitment in 2008. Plasma efavirenz, fasting glucose, cholesterol, and triglyceride were quantified using the 0 min OGTT samples.
Efavirenz was quantified by a validated method using liquid chromatography-tandem mass spectrometry (LC-MS/MS) on an Applied Biosystems MDS Sciex API 4000 tandem mass spectrometer at our ISO17025 compliant and accredited analytical laboratory as previously described.15 The assay range of quantification was 0.05 to 20 μg/mL. Accuracy ranged from 94 to 103%. Serum glucose and lipid concentrations were determined by standard methods using the ACE Alera Clinical Chemistry System (Alfa Wassermann Diagnostic Technologies, Woerden, Netherlands). Diabetes, impaired fasting glucose, and impaired glucose tolerance were defined according to American Diabetes Association criteria.16 Hypercholesterolaemia, high LDL cholesterol, low or high HDL, and hypertriglyceridaemia were defined according to the NCEP III criteria.17
Medical records were reviewed to determine duration on ART, plasma HIV-1 RNA concentrations, and CD4 + T-cell counts. Baseline CD4 + T-cell counts were recorded, and current CD4 + T-cell count was defined as the most recent value within 3 months of enrolment. Adherence was assessed using a validated 4-day adherence questionnaire administered by trained staff.18
Pharmacokinetic and Statistical Analysis
Medians (interquartile ranges) and proportions or ratios were used to describe continuous and categorical data, respectively. Scatter plots to visualize relationships between plasma efavirenz concentrations and metabolic parameters were generated using Prism version 6 (GraphPad Software, Inc., La Jolla, CA). Plasma efavirenz concentrations were log10 transformed to approximate normality. Associations between log10 transformed plasma efavirenz concentrations and cholesterol, triglycerides, and glucose were determined using univariate and multivariate linear regression analyses on Stata (version 11, StataCorp, College Station, TX). Univariate linear regression analyses characterized associations between log10 transformed association as an independent variable and several dependent variables: fasting total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, glucose, and 2-h OGTT glucose. Multivariate linear regression analyses adjusted for age, sex, body mass index (BMI), and total duration on ART. These potential confounders were chosen a priori. Sensitivity analyses included adjusting for current stavudine use. Case sensitivity analyses with the participant with the highest plasma concentrations were done. Missing data were not imputed.
In a subset of participants with available CYP2B6 genotype data, we explored relationships between CYP2B6 polymorphisms known to predict increased plasma efavirenz concentrations, CYP2B6 516G>T (rs3745274), 983T>C (rs28399499), and 15582C>T (rs4803419), and metabolic parameters using linear regression in PLINK.19 We did not correct for multiple comparisons. Genotyping was done in the Vanderbilt DNA Resources Core as described elsewhere.20 Composite 516/983 or 15582/516/983 genotypes were as assigned as previously described.20
RESULTS
A total of 107 participants were recruited into the study. One participant with an efavirenz concentration below the limit of assay quantification was excluded from analyses for presumed nonadherence. Characteristics of the 106 evaluable participants are provided in Table 1. All participants were black Africans and most were women, reflecting the patient population at these clinics. More than 90% had been exposed to stavudine. All participants reported 100% adherence. Only 34 participants had plasma HIV-1 RNA data available within the previous 3 months. Metabolic abnormalities, as defined by NCEP III and ADA criteria, were common: hypercholesterolaemia was present in 40%, hypertriglyceridaemia in 26%, low HDL cholesterol in 49%, high LDL cholesterol in 42%, impaired fasting glucose in 24%, impaired glucose tolerance in 10%, and diabetes in 2%.
TABLE 1.
Study Participant Characteristics
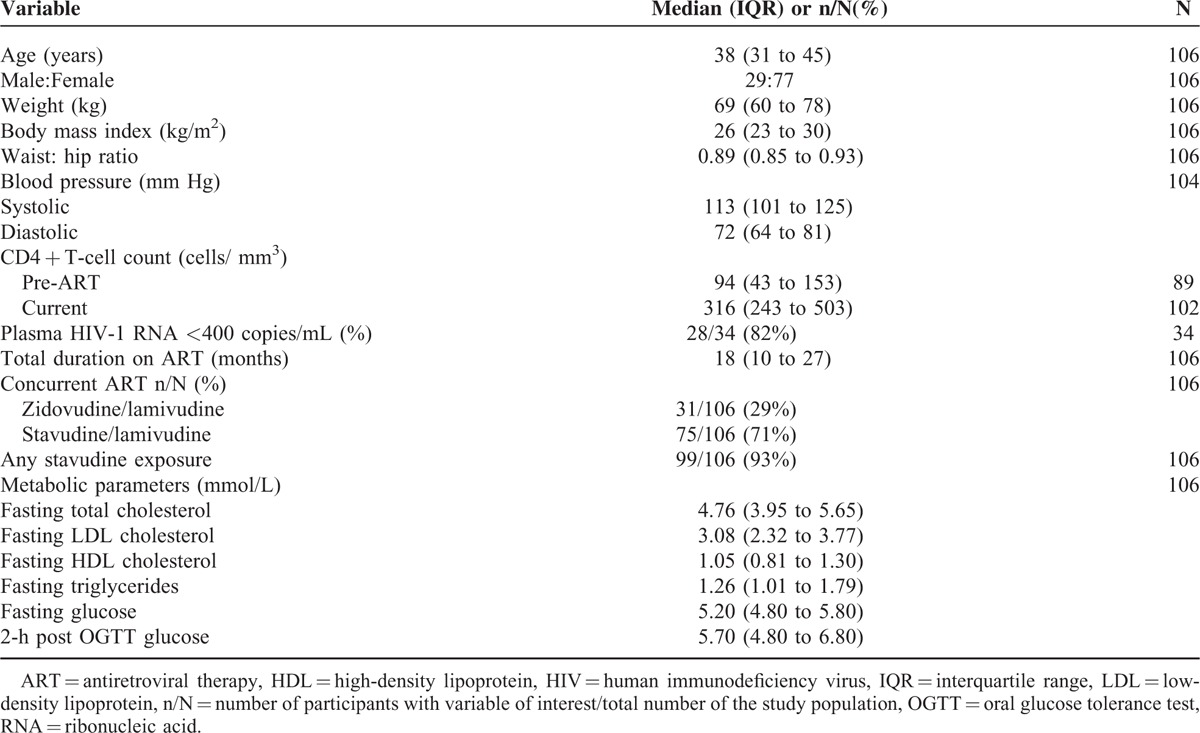
The median efavirenz concentration was 2.23 μg/mL (IQR 1.66 to 4.10 μg/mL). Median time after the last dose was 12.08 h (IQR 11.62 to 12.78 h). Relationships between plasma efavirenz concentrations and metabolic parameters are shown in Figure 1. There was a positive correlation between higher log10 transformed efavirenz concentrations and higher fasting total cholesterol, HDL cholesterol, LDL cholesterol concentrations, but not with triglycerides and 2-h OGTT glucose concentrations. There was a nonsignificant correlation between plasma efavirenz concentrations and fasting glucose concentrations (P = 0.073).
FIGURE 1.
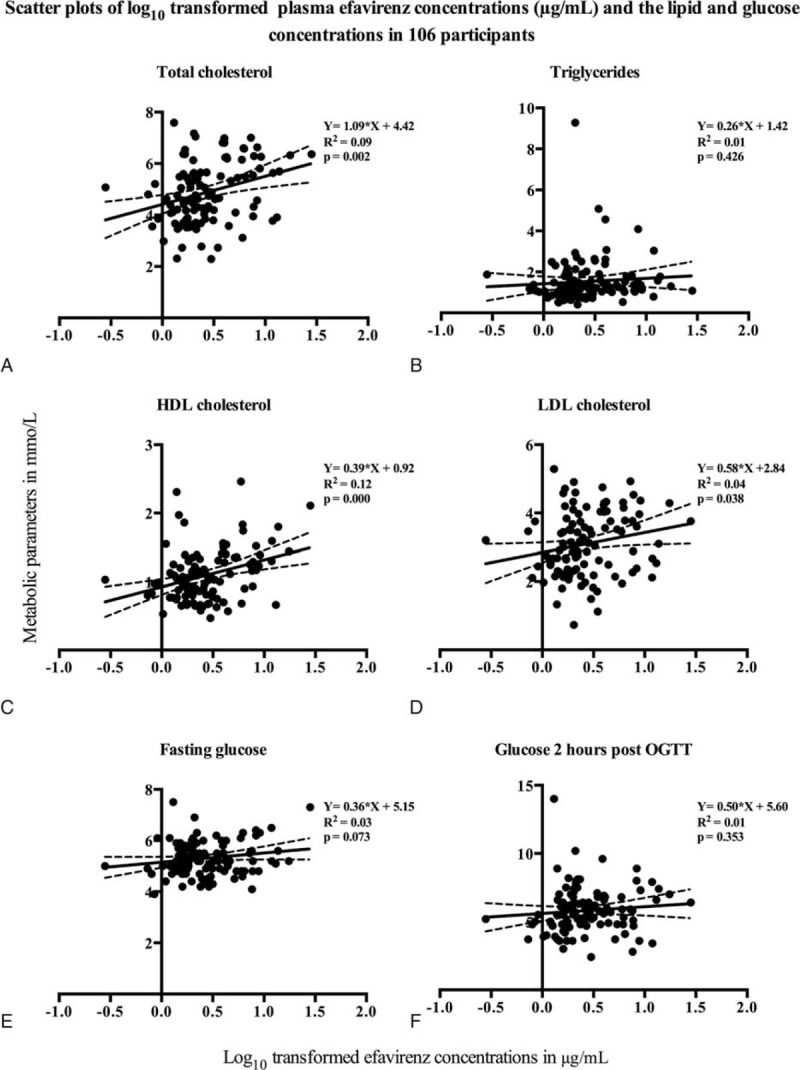
Scatter plots of log10 transformed plasma efavirenz concentrations (μg/mL) and the lipid and glucose concentrations in 106 participants. The x-axes represent log10 transformed plasma efavirenz concentrations. The y-axes represent concentrations of each metabolic parameter—(A) fasting total cholesterol, (B) fasting triglycerides, (C) fasting HDL cholesterol, (D) fasting LDL cholesterol, (E) fasting glucose, and (F) glucose 2 h post oral glucose tolerance test. Each black marker denotes an individual lipid or glucose concentration plotted against the plasma efavirenz concentrations in 106 participants. The solid black line indicates the regression line, with the 95% confidence interval shown in dotted lines. The equations for the regression line (with the slope and y intercept), correlation determinants (R2), and P values are shown on each plot. LDL = low-density lipoprotein, HDL = high-density lipoprotein.
In multivariate analyses that adjusted for age, sex, BMI, and duration on ART, log10 transformed efavirenz concentrations were independently associated with both lipid and glucose concentrations as displayed in Table 2. Table 2 also shows the mean change in each metabolic parameter per doubling of efavirenz concentrations. In these multivariate analyses, advancing age was also independently associated with increasing cholesterol (total cholesterol, HDL and LDL cholesterol) and glucose (fasting and 2 h), but not triglycerides (data not shown). When current stavudine use was included in the multivariate model with age, sex, BMI, and total duration on ART, similar associations between the log10 transformed efavirenz concentrations and both lipid and glucose concentrations were found (see Table, Supplementary Digital Content 1, a table that illustrates the multivariate regression analyses adjusting for age, sex, BMI, total duration on ART, and current stavudine use). Current stavudine use was not included in the final model because it was not a significant variable in all models (data not shown).
TABLE 2.
Multivariate Linear Regression Analyses (Each Adjusted for Age, Body Mass Index, and Total Duration on ART) Between log10 Transformed Efavirenz Mid-Dosing Interval Concentrations and Each Metabolic Parameter (N = 106)
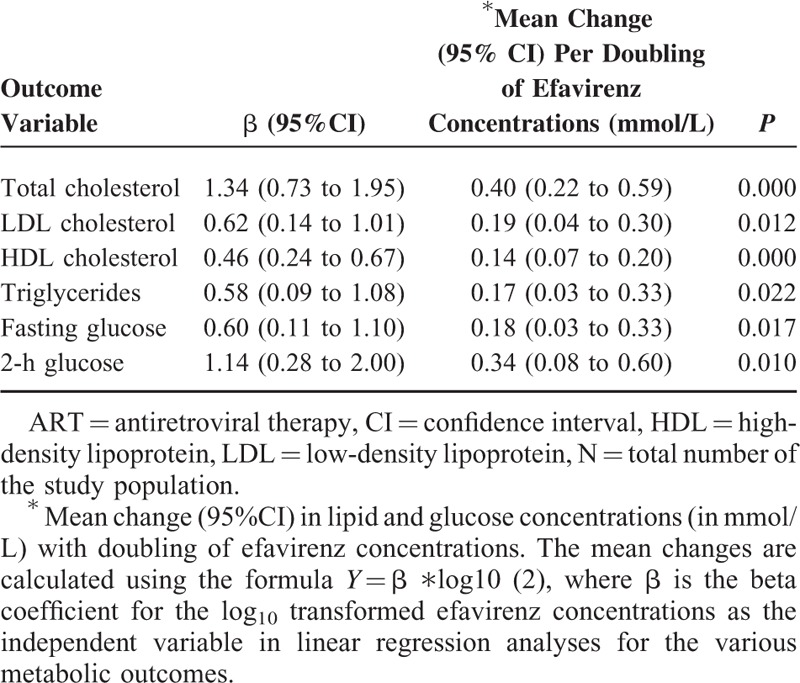
In the final multivariate regression model that included log10 transformed efavirenz concentrations, age, sex, BMI, and total duration on ART, the log10 transformed efavirenz concentrations explained 26% of interindividual variability for total cholesterol, 23% for HDL cholesterol, 20% for triglycerides, 19% for 2-h glucose, 17% for fasting glucose, and 16% for LDL cholesterol.
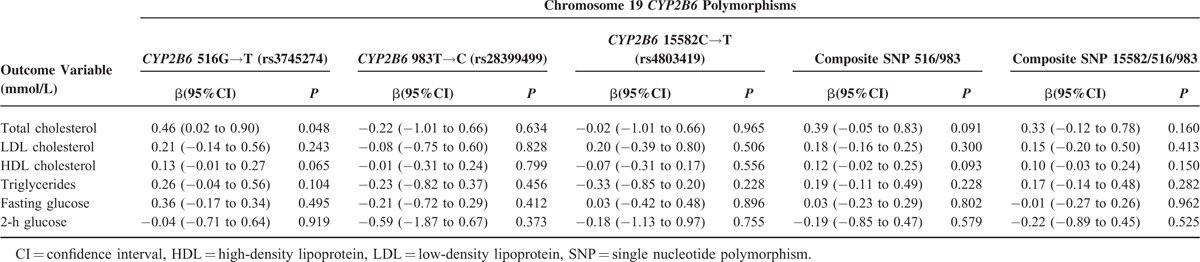
Exploratory analysis examined relationships between CYP2B6 genotypes and metabolic parameters among 57 participants with available genotype data. Allelic frequencies of CYP2B6 516G→T, 983T→C, and 15582C→T were 0.35, 0.08, and 0.08, respectively. Genotype frequencies are displayed in Supplemental Digital Content 2 (see figure, Supplemental Figure 1, that illustrates a bar graph displaying CYP2B6 genotype frequencies in 57 South African adults). There was a significant association between CYP2B6 516G→T and total cholesterol concentrations (P = 0.048) as shown in Table 3. In addition, for CYP2B6 516G→T, beta coefficients for total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were generally consistent and in the same direction as beta coefficients for log10 plasma efavirenz concentrations for these parameters. There were trends toward significant associations between CYP2B6 516G→T and HDL cholesterol concentrations, and between composite 516/983 genotype and total and HDL cholesterol concentrations.
TABLE 3.
Exploratory Analyses Between Known CYP2B6 Polymorphisms and Metabolic Parameters in 57 Participants
DISCUSSION
We investigated whether plasma efavirenz concentrations correlated with plasma lipid and/or glucose concentrations in HIV-infected South Africans. Higher plasma efavirenz concentrations were associated with significantly higher plasma fasting lipid concentrations and higher glucose concentrations in 106 HIV-infected South African adults receiving ART in multivariate analyses. In a subset of 57 participants with available CYP2B6 genotype data, associations between slow metabolizer genotypes and metabolic profiles were generally consistent with associations based on measured efavirenz concentrations.
To our knowledge, this is the first report of positive associations between plasma efavirenz concentrations and LDL cholesterol, or glucose concentrations. Our findings are consistent with a previous study that showed an association between plasma efavirenz concentrations and fasting HDL cholesterol in 34 participants,21 but disagree with a retrospective study that found no associations between plasma efavirenz concentrations and either fasting HDL cholesterol or triglycerides in 59 participants.22
The advent of ART has greatly reduced morbidity and mortality among HIV-infected patients.23 However, the D:A:D (Data Collection on Adverse Events of Anti-HIV Drugs) study showed that patients treated with protease inhibitors or NNRTIs, alone or in combination, have elevated total cholesterol, after controlling for known risk factors such as age, BMI, and sex.24 Some of these abnormalities, notably LDL cholesterol and glucose, are associated with an increased risk of vascular disease in the general population.25,26
The prevalence of metabolic complications in our cohort was high (dysglycemia 37% and dyslipidemia 47%). The commonest dyslipidemia we found was low HDL cholesterol in 49%, which is <71% we found in ART-naive patients drawn from the same clinics as the participants in our study (JA Dave, MBChB PhD, unpublished data, November 2015). However, high LDL cholesterol and high triglycerides, which were common in our study population, were rare in ART-naive patients. High prevalence of both dyslipidemia and dysglycemia in patients on ART has also been reported from other African countries.27–29
There is considerable interindividual variability in plasma efavirenz exposure, ∼34% of which is explained by 3 CYP2B6 loss-of-function polymorphisms, CYP2B6 516G→T, 983T→C, and 15582C→T.10,20 Among South Africans, minor allele frequencies of CYP2B6 516G→T, 983T→C, and 15582C→T have been reported to be 0.36, 0.07, and 0.09, respectively.20 Both 516G→T and 983T→C are more frequent with African than with European ancestry. The CYP2B6 983T→C allele is found almost exclusively with African ancestry,11 and has a somewhat greater effect on efavirenz concentrations per allele than does 516G→T, whereas 15582C→T has a much lesser effect than 516G→T. The lower prevalence of CYP2B6 slow metabolizer genotypes with European ancestry may explain, in part, why some studies conducted largely in Europeans have not shown associations between efavirenz and cardiovascular risk.30,31 In our study, the smaller number of participants with genotype data may have limited our ability to identify statistically significant genetic associations.
Our results have potential public health implications. Efavirenz is the preferred third drug, in combination with NRTIs, as first-line therapy in resource-limited settings where the HIV-1 burden is greatest.1 Efavirenz may be more likely to result in an increased risk of cardiovascular events among populations in whom CYP2B6 slow metabolizer genotypes are prevalent. However, higher efavirenz concentrations were also associated with higher HDL cholesterol in our study, which has been associated with decreased risk of cardiovascular events. Newer agents such as rilpivirine and dolutegravir have little or no effect on lipids,32,33 these drugs are currently not available in most low-middle income countries.
Our study has several limitations. Because it is a cross-sectional study, we cannot compare changes from baseline in lipid or glucose concentrations in patients starting ART. The viral load data was determined by reviewing medical records and only 34 participants had viral load data recorded. Therefore, viral load was not included in our multivariate analyses. Our sample size is relatively small. We investigated associations with mid-dose interval efavirenz concentrations and metabolic parameters, but other pharmacokinetic parameters (eg area under the concentration-time curve) might better describe the relationships with metabolic profiles. Although participants recorded the time of last dose on the day before pharmacokinetic sampling, we cannot exclude incomplete adherence.
CONCLUSIONS
In conclusion, higher plasma efavirenz concentrations were associated with higher plasma lipid and glucose concentrations. However, larger cohort studies are needed to replicate these associations. Well-powered studies in Africa and other regions where efavirenz slow metabolizer genotypes are prevalent are needed to assess whether long-term efavirenz use is associated with increased risk of cardiovascular events.
Supplementary Material
Acknowledgments
The authors are grateful to the patients who volunteered for the study. They acknowledge contributions of the study coordinator, Carmen Delport, and her field team in the recruitment process and data collection. They are also grateful to the pharmacology laboratory team for handling and processing pharmacokinetic samples.
Footnotes
Abbreviations: 95%CI = 95% confidence interval; ADA = American Diabetes Association; ART = antiretroviral therapy; BMI = body mass index; CYP2B6 = Cytochrome P450 isoenzyme 2B6; D:A:D = Data Collection on Adverse Events of Anti-HIV Drugs; HDL = high-density lipoprotein; HIV = human immunodeficiency virus; IQR = interquartile range; LDL = low-density lipoprotein; NCEP III = National Cholesterol Education Program III; NNRTI = non-nucleoside reverse transcriptase inhibitor; NRTI = nucleoside reverse transcriptase inhibitor; OGTT = oral glucose tolerance test; RECREF = Research Ethics Committee Reference number; RNA = ribonucleic acid.
Part of this data was presented at the 17th World Congress of Basic and Clinical Pharmacology (WCP2014) held in Cape Town, South Africa from 13 to 18 July 2014.
Author contributions: PZS participated in the study design, acquisition of data, data analysis, and interpretation and drafted the manuscript. HMM participated in the study design, data interpretation, and critically revised the manuscript. PJS performed the analysis of the pharmacokinetic samples and helped to draft the manuscript. JAD participated in the study design, acquisition of data, and critically revised the manuscript. NSL participated in study design and critically revised the manuscript. GM participated in study design, data interpretation, and critically revised the manuscript. DWH participated in the data analysis and interpretation and drafted the manuscript. All authors read and approved the final manuscript.
Funding: this work was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers UM1 AI068634 [HMM], UM1 AI068636 [HMM], UM1 AI106701 [HMM], and RO1 AI077505 [DWH], UL1 TR00045 [DWH], P30 AI110527 [DWH]); the National Research Foundation of South Africa (grant Number 90729[HMM]); World Diabetes Foundation; and the South African Department of Health. PZS received scholarships from Discovery Foundation, Welcome Trust, SA Medical Research Council and the National Health Scholar Program. She attended a manuscript-writing workshop supported by the South African Tuberculosis and AIDS Training (SATBAT) program (National Institute of Health/Fogarty International Center 1U2RTW007370/05). The funding bodies had no role in study design; in collection, analysis, and interpretation of the data; in writing of the manuscript; and in the decision to submit the manuscript for publication.
DWH has been a consultant to Merck. The rest of the authors declare no support from any organization for the submitted work, and there are no other relationships or activities that could appear to have influenced the submitted work.
REFERENCES
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and prenting HIV infection. 2013; http://www.who.int/hiv/pub/guidelines/arv2013/download/en/. [PubMed] [Google Scholar]
- 2.Dave JA, Lambert EV, Badri M, et al. Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV-infected patients. J Acquir Immune Defic Syndr 2011; 57:284–289. [DOI] [PubMed] [Google Scholar]
- 3.Fontas E, van Leth F, Sabin CA, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis 2004; 189:1056–1074. [DOI] [PubMed] [Google Scholar]
- 4.Tashima KT, Bausserman L, Alt EN, et al. Lipid changes in patients initiating efavirenz- and indinavir-based antiretroviral regimens. HIV Clin Trials 2003; 4:29–36. [DOI] [PubMed] [Google Scholar]
- 5.Williams P, Wu J, Cohn S, et al. Improvement in lipid profiles over 6 years of follow-up in adults with AIDS and immune reconstitution. HIV Med 2009; 10:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Leth F, Phanuphak P, Stroes E, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med 2004; 1:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhoads MP, Lanigan J, Smith CJ, et al. Effect of specific ART drugs on lipid changes and the need for lipid management in children with HIV. J Acquir Immune Defic Syndr 2011; 57:404–412. [DOI] [PubMed] [Google Scholar]
- 8.Carr A, Samaras K, Thorisdottir A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 1999; 353:2093–2099. [DOI] [PubMed] [Google Scholar]
- 9.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004; 18:2391–2400. [PubMed] [Google Scholar]
- 10.Holzinger ER, Grady B, Ritchie MD, et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics 2012; 22:858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.dbSNP. Short Genetic Variations. http://www.ncbi.nlm.nih.gov/projects/SNP/. [Google Scholar]
- 12.Wang J, Sonnerborg A, Rane A, et al. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet Genomics 2006; 16:191–198. [DOI] [PubMed] [Google Scholar]
- 13.Chou M, Bertrand J, Segeral O, et al. Population pharmacokinetic-pharmacogenetic study of nevirapine in HIV-infected Cambodian patients. Antimicrob Agents Chemother 2010; 54:4432–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukasem C, Cressey TR, Prapaithong P, et al. Pharmacogenetic markers of CYP2B6 associated with efavirenz plasma concentrations in HIV-1 infected Thai adults. Br J Clin Pharmacol 2012; 74:1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren Y, Nuttall JJ, Eley BS, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr 2009; 50:439–443. [DOI] [PubMed] [Google Scholar]
- 16.Association AD. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36 Suppl 1:S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cholesterol Education Program (NCEP) Expert Panel on Detection, E., Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 18.AIDS Clinical Trials Group. ACTG Adherence follow-up questionnaire. 2006; http://caps.ucsf.edu/resources/survey-instruments-8. [Google Scholar]
- 19.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinxadi PZ, Leger PD, McIlleron HM, et al. Pharmacogenetics of plasma efavirenz exposure in HIV-infected adults and children in South Africa. Br J Clin Pharmacol 2015; 80:146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira SA, Branco T, Corte-Real RM, et al. Long-term and concentration-dependent beneficial effect of efavirenz on HDL-cholesterol in HIV-infected patients. Br J Clin Pharmacol 2006; 61:601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Autar RS, Boyd MA, Wit FW, et al. Relationships between drug exposure, changes in metabolic parameters and body fat in HIV-infected patients switched to a nucleoside sparing regimen. Antivir Ther 2007; 12:1265–1271. [PubMed] [Google Scholar]
- 23.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–860. [DOI] [PubMed] [Google Scholar]
- 24.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS 2003; 17:1179–1193. [DOI] [PubMed] [Google Scholar]
- 25.Friis-Moller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil 2010; 17:491–501. [DOI] [PubMed] [Google Scholar]
- 26.Anand SS, Islam S, Rosengren A, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 2008; 29:932–940. [DOI] [PubMed] [Google Scholar]
- 27.Zannou DM, Denoeud L, Lacombe K, et al. Incidence of lipodystrophy and metabolic disorders in patients starting non-nucleoside reverse transcriptase inhibitors in Benin. Antivir Ther 2009; 14:371–380. [DOI] [PubMed] [Google Scholar]
- 28.Mutimura E, Stewart A, Rheeder P, et al. Metabolic function and the prevalence of lipodystrophy in a population of HIV-infected African subjects receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2007; 46:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omech B, Sempa J, Castelnuovo B, et al. Prevalence of HIV-associated metabolic abnormalities among patients taking first-line antiretroviral therapy in Uganda. ISRN AIDS 2012; 2012:960178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samaras K, Wand H, Law M, et al. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care 2007; 30:113–119. [DOI] [PubMed] [Google Scholar]
- 31.Lang S, Mary-Krause M, Cotte L, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med 2010; 170:1228–1238. [DOI] [PubMed] [Google Scholar]
- 32.Tebas P, Sension M, Arribas J, et al. Lipid levels and changes in body fat distribution in treatment-naive, HIV-1-Infected adults treated with rilpivirine or efavirenz for 96 weeks in the ECHO and THRIVE trials. Clin Infect Dis 2014; 59:425–434. [DOI] [PubMed] [Google Scholar]
- 33.Quercia R, Roberts J, Martin-Carpenter L, et al. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs. efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig 2015; 35:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


