Abstract
Sitagliptin has been reported to improve lipid profiles, but findings from these studies are conflicting. We conducted this meta-analysis to evaluate the effects of sitagliptin on serum lipids in patients with type 2 diabetes mellitus.
We made a comprehensive literature search in PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure, Wanfang, and VIP database until June 2015. Eligible studies were randomized clinical trials (RCTs) that investigated the effect of sitagliptin on serum triglycerides (TGs), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), or high-density lipoprotein cholesterol (HDL-C).
Eleven RCTs with 2338 patients were identified. Compared with controls, sitagliptin alone or in combination significantly improved serum TG (weighted mean difference [WMD] −0.24 mmol/L; 95% confidence interval [CI] −0.40 to −0.09; P = 0.002) and HDL-C (WMD 0.05 mmol/L; 95% CI 0.02–0.07; P < 0.001).However, no statistical significances were observed in LDL-C (WMD −0.07 mmol/L; 95% CI −0.22 to 0.08; P = 0.337) and TC (WMD −0.14; 95% CI −0.33 to 0.06; P = 0.177). Subgroup analyses revealed that sitagliptin alone achieved greater improvement in serum TG, TC, and HDL-C levels.
These findings suggested that sitagliptin alone or in combination significantly improved serum TG and HDL-C levels in patients with type 2 diabetes mellitus.
INTRODUCTION
Diabetes mellitus is one of the most common chronic diseases worldwide. Diabetes mellitus is a well-known risk factor for cardiovascular disease. Dyslipidemia is an essential determinant of cardiovascular risk in type 2 diabetes.1 Patients with type 2 diabetes are more likely to be dyslipidemic than the general population.2 Lipid abnormalities in patients with type 2 diabetes are characterized as high serum triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), as well as total cholesterol (TC),and reduced serum high-density lipoprotein cholesterol (HDL-C) concentration.3 The coexistence of diabetes and lipid abnormalities can further increase the risk of cardiovascular disease. Antihyperglycemic agents had positive effects on the dyslipidemia and may contribute to reduce the excessive risks of cardiovascular events in a person with diabetes.
Dipeptidyl peptidase-4 (DPP-4) inhibitors are a class of oral antihyperglycemic agents for treatment of type 2 diabetes.4 Treatment with DPP-4 inhibitor alone or in combination with other antihyperglycemic agents can improve the success in achieving and/or maintaining glycemic control in patients with type 2 diabetes. In addition to an improvement in glycemic control, DPP-4 inhibitor appeared to have a beneficial effect on dyslipidemia. On the basis of previous clinical evidence, a well-designed meta-analysis5 confirmed that DPP-4 inhibitors as a class drug had beneficial effects on TC and TG and different DPP-4 inhibitors had differential effects on the lipid profile. Sitagliptin is the first selective DPP-4 inhibitor clinically used in 2006.6 Sitagliptin had pleiotropic effects in the treatment of patients with type 2 diabetes, including improved lipid parameters,7 but findings are inconsistent across studies. Therefore, whether sitagliptin could be effective in improving lipid abnormalities is controversial.
Here, we conducted a meta-analysis of the available randomized controlled trials (RCTs) to determine the positive benefits of sitagliptin on lipid abnormalities in patients with type 2 diabetes.
METHODS
Literature Search
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.8 We searched PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure, Wanfang, and VIP database from inception until July 2015. We used the following search terms with various combinations: sitagliptin [Medical Subject Headings (MeSH)] OR DPP-4 inhibitor [Mesh] OR dipeptidyl peptidase-4[Mesh] AND diabetes mellitus [Mesh] OR type 2 diabetes [Mesh] AND (randomized [Free Item] OR randomized [Free Item] OR randomization [Free Item]. In addition, we also manually searched reference lists from articles to identify additional eligible papers.
Study Selection
Studies were included according to the following criteria: RCTs enrolling patients with 2 diabetes; patients who received sitagliptin alone or in combination with other antihyperglycemic agents; and least reported the changes of serum TG, LDL-C, TC, or HDL-C change as an outcome measure. Studies were excluded if lipid profile indexes were not reported and the study design was review, abstracts, or duplicated publication.
Data Extraction and Quality Assessment
Data for meta-analysis were extracted independently by two authors (M.H.F. and Y.L.L.). The following data were extracted from the included RCTs: first author's surname, country, year of publication, study design, sample size, mean age or age range of participants, regimen of sitagliptin and controls, follow-up period, and outcomes (serum TG, LDL-C, TC, or HDL-C levels). Any disagreement was solved through discussion. The quality of the included trials was assessed using the Cochrane risk bias tools (Revman 5.2; www.cochrane.org/training/cochrane-handbook). The risk of bias tools included selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome data), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other sources of bias.
Data Synthesis and Analysis
Lipid parameters were pooled as the weighted mean difference (WMD) with 95% confidence interval (CI). All the comparisons were made as sitagliptin alone or in combination with other antihyperglycemic agents versus control or other antihyperglycemic agents. Heterogeneity was assessed using the I2 statistics and Cochrane's Q test. On the basis of the Cochrane Handbook for Systematic Review of Interventions, I2 statistics >50% or P < 0.10 in Cochrane's Q test were defined as substantial heterogeneity.9 A random effect model was chosen when substantial heterogeneity was observed. Otherwise, we selected a fixed-effect model. We conducted subgroup analyses on the basis of the type of intervention (sitagliptin alone or in combination with other antihyperglycemic agents) and treatment duration (<18 weeks or ≥18 weeks). To estimate possible publication bias, we conducted both Begg rank correlation test10 and Egger regression test.11 All the analyses were performed using STATA statistical software (version 11.0; StataCorp, College Station, TX). A P value <0.05 was used to indicate statistical significance.
RESULTS
Search Results
The initial search yielded 312 potential citations. Of these, 281 were discarded after reviewing titles and abstracts or removing duplicate publication. After reading the full-text articles, we further excluded 22 papers for various reasons. Finally, a total of 11 RCTs12–22 satisfied the inclusion criteria. A flow chart showing a detailed process of trial selection is provided in Figure 1.
FIGURE 1.
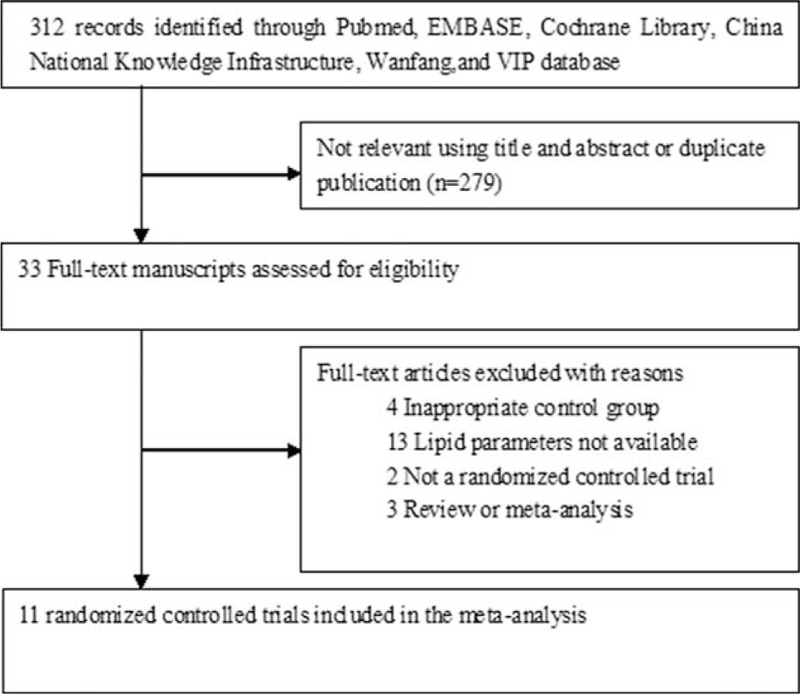
Flow diagram of trials selection process.
Characteristics of Eligible Studies
The characteristics of the included RCTs are presented in Table 1 . A total of 2338 persons with diabetes were included in this meta-analysis. Of these patients, 1283 received sitagliptin alone or in combination with other antihyperglycemic agents, and 1055 assigned to controls. There was no significant difference in baseline serum lipid parameters between the 2 groups. Treatment period varied from 12 to 104 weeks. Overall, the included trials were generally classified as moderate to high quality. However, randomization and sequence concealment was not reported in most of the included studies, and the selection bias could not be excluded. The detailed quality assessment of individual trial is presented in Figure 2.
TABLE 1.
Characteristics of Clinical Trials Included in Meta-analysis
FIGURE 2.
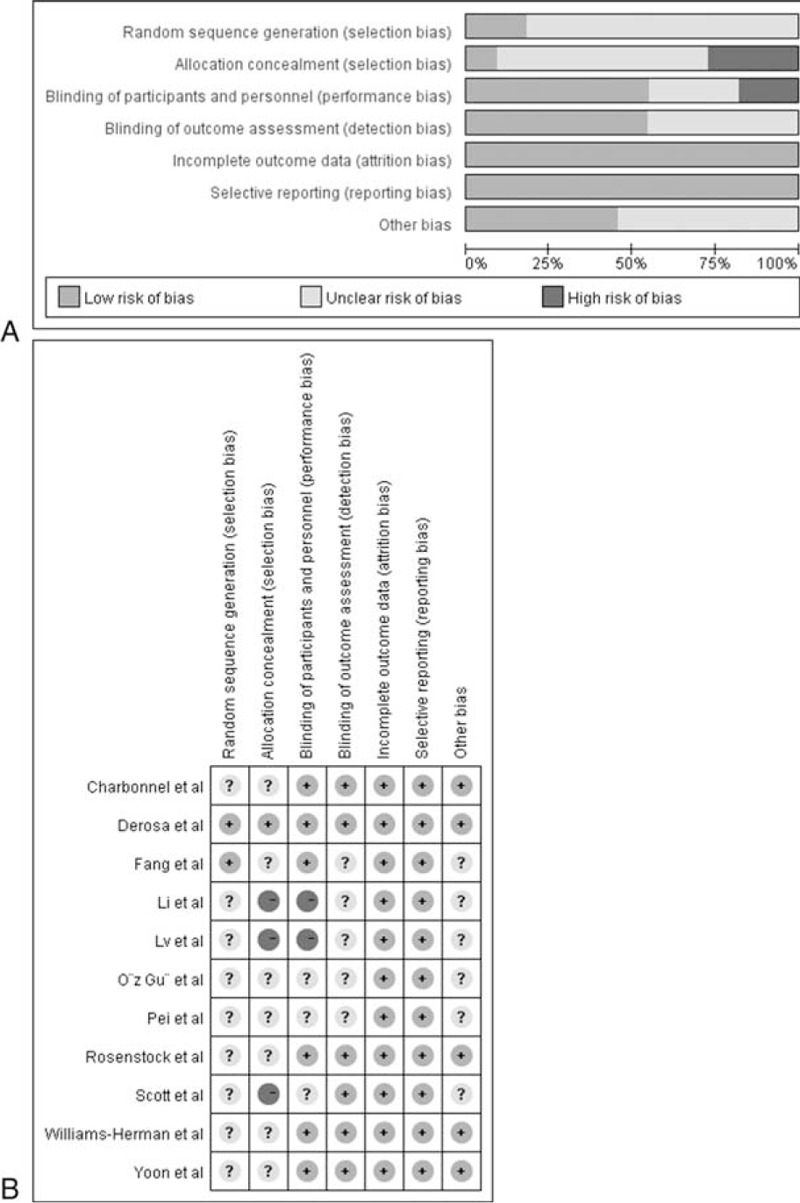
Risk of bias graph (A) and risk of bias summary (B).
Comparison of Serum Lipid Parameters
All the included trials reported the effect of treatment on serum TG levels. As shown in Figure 3, there was substantial heterogeneity among the included trials (I2 = 74.2%, P < 0.001); treatment with sitagliptin was associated with a significant decrease in serum TG levels (WMD −0.24 mmol/L; 95% CI −0.40 to −0.09; P = 0.002) compared with controls in a random effect model. Begg rank correlation test (P = 1.000) and Egger regression test (P = 0.106) did not reveal the evidence of publication bias. Ten trials12–20,22 reported the effect of treatment on serum HDL-C levels. As shown in Figure 4, there was no substantial heterogeneity across the 10 trials (I2 = 40.3%, P = 0.089), so we selected a fixed-effect model. Sitagliptin slightly increased serum HDL levels (WMD 0.05 mmol/L; 95% CI 0.02–0.07; P = 0.002) compared with the controls. Both Begg rank correlation test (P = 1.000) and Egger regression test (P = 0.164) for HDL-C did not suggest significant publication bias. However, there was no statistical significance in the comparison of serum levels of LDL-C (WMD −0.07 mmol/L; 95% CI −0.22 to 0.08; P = 0.337) with substantial heterogeneity (I2 = 75.8%, P < 0.001; Figure 5) and TC (WMD −0.14 mmol/L; 95% CI −0.33 to 0.06; P = 0.177) with obvious heterogeneity (I2 = 76.7%, P < 0.001; Figure 6). Egger tests for LDL-C and TC indicated significant publication bias in the meta-analyses (P = 0.047 and 0.069, respectively) but not in the Begg rank correlation test (P = 0.533 and 1.000, respectively).
FIGURE 3.
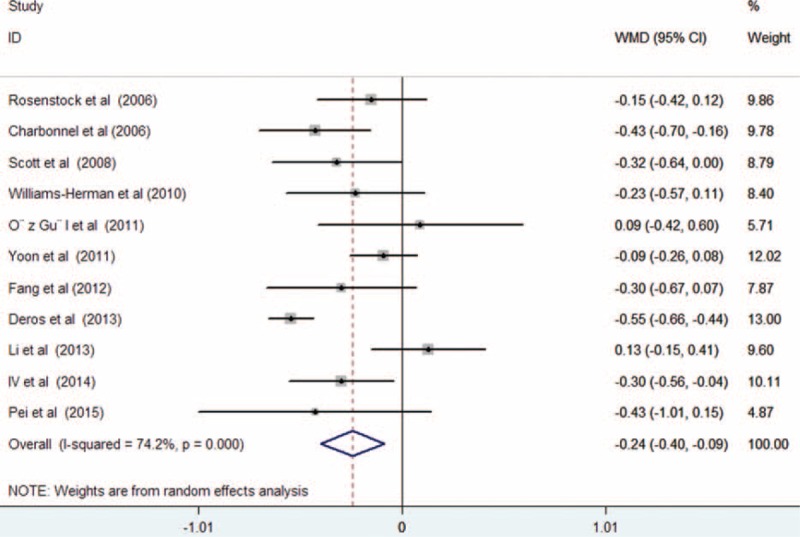
Forest plots showing weighted mean difference and 95% confidence interval for serum triglycerides levels comparing sitagliptin alone or in combination with other antihyperglycemic agents to controls in a random effects model.
FIGURE 4.
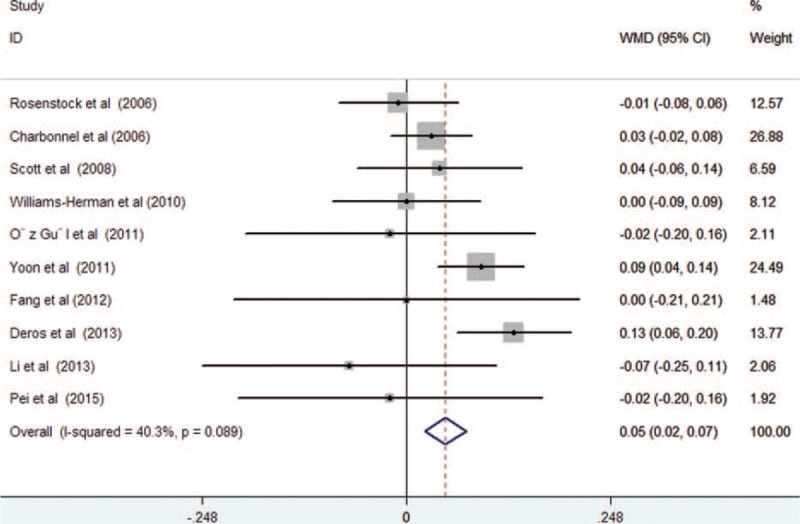
Forest plots showing weighted mean difference and 95% confidence interval for serum high-density lipoprotein cholesterol levels comparing sitagliptin alone or in combination with other antihyperglycemic agents to controls in a random effects model.
FIGURE 5.
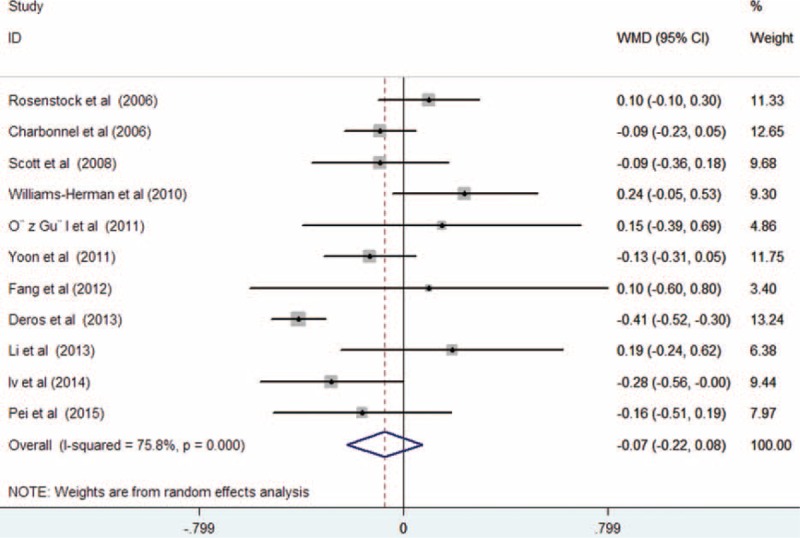
Forest plots showing weighted mean difference and 95% confidence interval for serum low-density lipoprotein cholesterol levels comparing sitagliptin alone or in combination with other antihyperglycemic agents to controls in a fixed-effect model.
FIGURE 6.
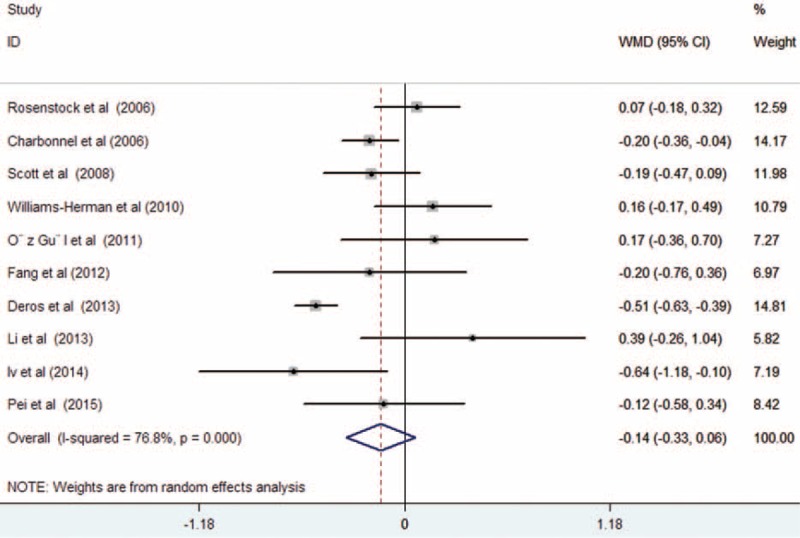
Forest plots showing weighted mean difference and 95% confidence interval for serum total cholesterol levels comparing sitagliptin alone or in combination with other antihyperglycemic agents to controls in a random effects model.
Subgroup Analysis and Sensitivity Analyses
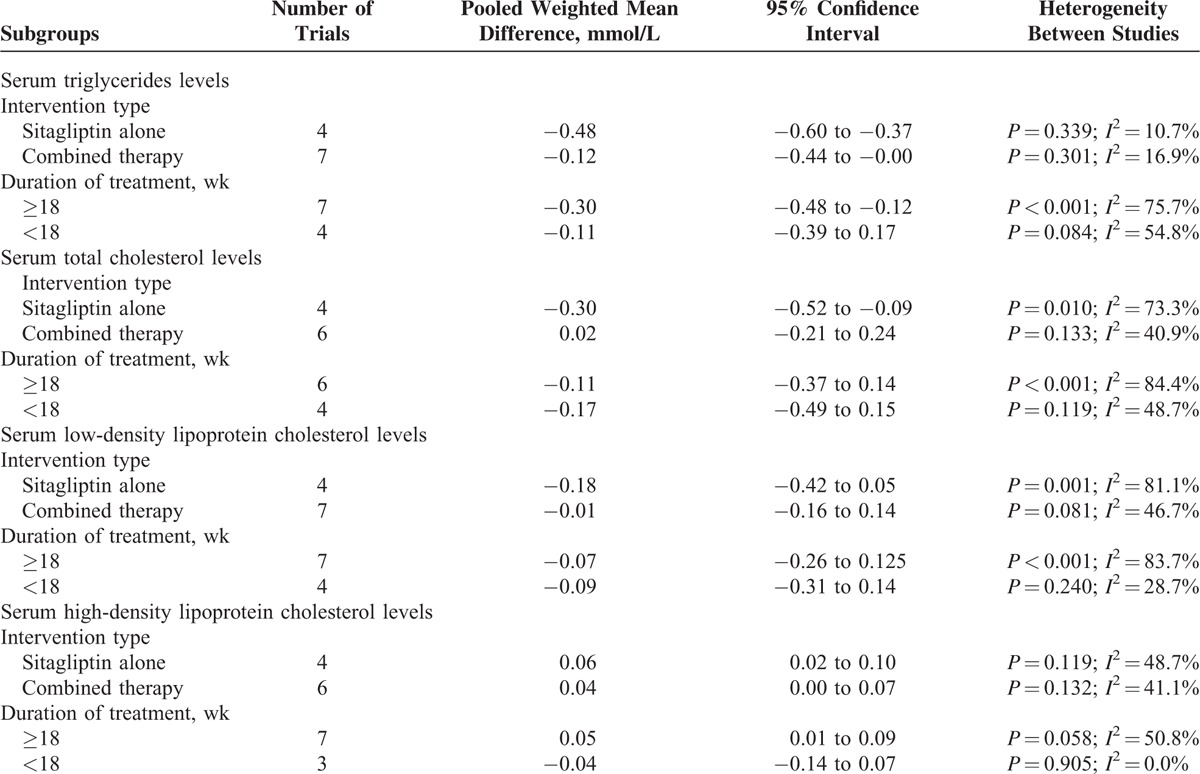
Subgroup analysis (Table 2) was conducted by the intervention type (sitagliptin alone or sitagliptin in combination with other antihyperglycemic agents) and treatment duration (<18 or ≥18 weeks). The results indicated that sitagliptin alone achieved greater improvement in serum TG, TC, and HDL-C levels. Long-term treatment with the sitagliptin appeared to be associated with greater reduction in serum TG and HDL levels. Sensitivity analyses revealed that there was no change in the direction of the pooled WMD of lipid parameters when any one study was excluded (data not shown).
TABLE 1 (Continued).
Characteristics of Clinical Trials Included in Meta-analysis

TABLE 2.
Subgroup Analyses of Serum Lipid Levels
DISCUSSION
This is the first meta-analysis designed specially to assess the effects of sitagliptin on lipid profiles in patients with type 2 diabetes mellitus. The findings of this study suggest that sitagliptin alone or in combination significantly improved TG and HDL-C in patients with type 2 diabetes. Long-term treatment appeared to achieve better effects on serum TG and HDL-C levels. Sitagliptin alone achieves greater improvement in serum levels of TG, TC, and HDL-C than those in combination with other antihyperglycemic agents, which makes it a favorable therapeutic strategy for a person with diabetes along with dyslipidemia. However, the reductions in these variables are relatively small; whether the reduction is clinically relevant needs further investigation.
Low HDL-C level was the most frequent type of lipid abnormality in patients with type 2 diabetes.23 Current guideline on the treatment of blood cholesterol recommended the correction of low HDL-C and high TG levels among a person with type 2 diabetes.24,25 Single antihyperglycemic agent or in combination use could achieve target glycemic control in type 2 diabetic individuals. However, most of antihyperglycemic agents had a little impact on blood glucose levels. Therefore, diabetic patients with dyslipidemia represented a population with an excessive risk of cardiovascular disease.
DPP-4 inhibitors are a class of oral antihyperglycemic agents for treatment of type 2 diabetes.4 The effects of DPP-4 on lipid profiles in patients with type 2 diabetes have been widely investigated. Sitagliptin is a potent and well tolerable DPP-4 inhibitor. Our meta-analysis indicated that the overall effect of sitagliptin alone or in combination therapy on serum lipid improvement was approximately −0.24 mmol/L for TG and 0.14 mmol/L for HDL-C, respectively. Subgroup analyses showed that treatment with sitagliptin alone achieved greater improvement in serum TG, TC, and HDL-C levels than those in combination with other antihyperglycemic agents. Long-term treatment (≥18 weeks) with the sitagliptin appeared to achieve greater lipid-lowing effects than those with treatment less than 18 weeks. In addition, treatment with sitagliptin was associated with a significant decline in carotid intima-media thickness.26,27 Carotid intima-media thickness is a marker of subclinical atherosclerosis. Together, these findings revealed that sitagliptin had additional specific benefits on lowering TG, TC, and HDL-C levels, and provided potential for cardiovascular prevention.
Several studies not satisfying our inclusion criteria also investigated the effects of sitagliptin and incretin-based therapies on serum lipids. Shigematsu et al28 reported that sitagliptin significantly reduced serum TC, LDL-C, and non-HDL-C, particularly in patients with high baseline TG concentrations after 12 weeks’ treatment. Serum levels of TC and TG decreased after 3 months of treatment with sitagliptin in a prospective, open-label, multicenter trial.29 A retrospective study30 indicated that administration of sitagliptin significantly improved serum levels of TG, TC, and LDL-C except for serum HDL-C levels in patients with type 2 diabetes during 90 to 365 days follow-up period. Collectively, sitagliptin alone or in combination resulted in a more favorable lipid profile. However, the underlying mechanisms of sitagliptin in improving lipid are not fully elucidated. The beneficial effect of sitagliptin on serum lipid parameters could be partly explained by an improvement in glycemic control and insulin resistance, weight loss, or delayed gastric emptying. Incretin-based therapies also exhibited beneficial effects on lipoprotein metabolism.31,32 Apart from the effects on lipid parameters, sitagliptin and incretin-based therapies could improve oxidative stress and heme oxygenase-1, reduce markers of systemic inflammation, and improve endothelial dysfunction.33,34
Several limitations of this study should be considered. First, the primary outcome measures in most trials were glycemic control, and lipid determination may have been not very well-standardized. Second, substantial heterogeneities (I2 from 40.3% to 76.7%) existed in this meta-analysis, which may partly be explained by the patients’ characters, intervention type, and treatment duration. Third, potential publication bias cannot be excluded mainly due to the fact that lipid parameters were not the primary endpoint or not reported in most of the related studies. Moreover, evidence of publication bias was observed in Egger tests for pooled LDL-C and TC (P = 0.047 and 0.069, respectively). Finally, whether administration of sitagliptin achieved a greater reduction in cardiovascular events needs further investigation.
CONCLUSIONS
This meta-analysis reveals that sitagliptin alone or added to other antihyperglycemic agents significantly improved serum TG and HDL-C levels in patients with type 2 diabetes mellitus. Sitagliptin alone achieves greater improvement in serum levels of TG, TC, and HDL-C. However, whether the use of sitagliptin can decrease cardiovascular events remains unclear.
Footnotes
Abbreviations: CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; RCTs = randomized clinical trials; TC = total cholesterol; TG = triglyceride; WMD = weighted mean difference.
The design of this study was a meta-analysis.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591. [DOI] [PubMed] [Google Scholar]
- 2.Tan CE, Chew LS, Chio LF, et al. Cardiovascular risk factors and LDL subfraction profile in Type 2 diabetes mellitus subjects with good glycaemic control. Diabetes Res Clin Pract 2001; 51:107–114. [DOI] [PubMed] [Google Scholar]
- 3.Chehade JM, Gladysz M, Mooradian AD. Dyslipidemia in type 2 diabetes: prevalence, pathophysiology, and management. Drugs 2013; 73:327–339. [DOI] [PubMed] [Google Scholar]
- 4.Deacon CF, Holst JJ. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin Pharmacother 2013; 14:2047–2058. [DOI] [PubMed] [Google Scholar]
- 5.Monami M, Lamanna C, Desideri CM, et al. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther 2012; 29:14–25. [DOI] [PubMed] [Google Scholar]
- 6.Gallwitz B. Sitagliptin: profile of a novel DPP-4 inhibitor for the treatment of type 2 diabetes. Drugs Today (Barc) 2007; 43:13–25. [DOI] [PubMed] [Google Scholar]
- 7.Kubota A, Maeda H, Kanamori A, et al. Pleiotropic effects of sitagliptin in the treatment of type 2 diabetes mellitus patients. J Clin Med Res 2012; 4:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269.W64. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org Accessed July 06, 2015. [Google Scholar]
- 10.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568. [DOI] [PubMed] [Google Scholar]
- 13.Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643. [DOI] [PubMed] [Google Scholar]
- 14.Scott R, Loeys T, Davies MJ, et al. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969. [DOI] [PubMed] [Google Scholar]
- 15.Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab 2010; 12:442–451. [DOI] [PubMed] [Google Scholar]
- 16.Yoon KH, Shockey GR, Teng R, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and pioglitazone on glycemic control and measures of beta-cell function in patients with type 2 diabetes. Int J Clin Pract 2011; 65:154–164. [DOI] [PubMed] [Google Scholar]
- 17.Oz O, Kiyici S, Ersoy C, et al. Effect of sitagliptin monotherapy on serum total ghrelin levels in people with type 2 diabetes. Diabetes Res Clin Pract 2011; 94:212–216. [DOI] [PubMed] [Google Scholar]
- 18.Fang WJ, Jian WX, Hu JF, et al. Effect of sitagliptin combined insulin on 46 patients with patients with type 2 diabetes mellitus. Chinese J New Drugs Clin Remedies 2012; 31:557–560. [Google Scholar]
- 19.Li L, Han P. Hypoglycemic effect of sitagliptin on type 2 diabetes and its protection of islet function. Pract Pharm Clin Remedies 2013; 16:696–698. [Google Scholar]
- 20.Derosa G, Ragonesi PD, Fogari E, et al. Sitagliptin added to previously taken antidiabetic agents on insulin resistance and lipid profile: a 2-year study evaluation. Fundam Clin Pharmacol 2014; 28:221–229. [DOI] [PubMed] [Google Scholar]
- 21.Lv SS, Pan TR, Zhong X, et al. Effect of sitagliptin combined with metformin on insulin resistance, blood lipids and blood pressure in patients with type 2 diabetes mellitus. Acta Univ Med Anhui 2014; 49:103–109. [Google Scholar]
- 22.Pei LL, Sun ZH, Li Z, et al. Effects of sitagliptin combined with high-dose insulin on type 2 diabetes mellitus. J Shandong Univ 2015; 53:39–42. [Google Scholar]
- 23.Karim MN, Ahmed KR, Bukht MS, et al. Pattern and predictors of dyslipidemia in patients with type 2 diabetes mellitus. Diabetes Metab Syndr 2013; 7:95–100. [DOI] [PubMed] [Google Scholar]
- 24.Genest J, Frohlich J, Fodor G, et al. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: summary of the 2003 update. CMAJ 2003; 169:921–924. [PMC free article] [PubMed] [Google Scholar]
- 25.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 26.Barbieri M, Rizzo MR, Marfella R, et al. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis 2013; 227:349–354. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa S, Shimano M, Watarai M, et al. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild diabetes mellitus. Am J Cardiol 2014; 114:384–388. [DOI] [PubMed] [Google Scholar]
- 28.Shigematsu E, Yamakawa T, Kadonosono K, et al. Effect of sitagliptin on lipid profile in patients with type 2 diabetes mellitus. J Clin Med Res 2014; 6:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto Y, Oyama J, Ikeda H, et al. Effects of sitagliptin beyond glycemic control: focus on quality of life. Cardiovasc Diabetol 2013; 12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton ES, Silberman C, Davis KL, et al. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care 2010; 33:1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiquette E, Toth PP, Ramirez G, et al. Treatment with exenatide once weekly or twice daily for 30 weeks is associated with changes in several cardiovascular risk markers. Vasc Health Risk Manag 2012; 8:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matikainen N, Taskinen MR. The effect of vildagliptin therapy on atherogenic postprandial remnant particles and LDL particle size in subjects with type 2 diabetes. Diabet Med 2013; 30:756–757. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo M, Nikolic D, Banach M, et al. Incretin-based therapies, glucometabolic health and endovascular inflammation. Curr Pharm Des 2014; 20:4953–4960. [DOI] [PubMed] [Google Scholar]
- 34.Rizzo M, Abate N, Chandalia M, et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: a prospective pilot study. J Clin Endocrinol Metab 2015; 100:603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]


