Abstract
Coronary artery (CA) abnormalities influence exercise capacity (EC) of patients with Kawasaki disease (KD), and Z-score of CA is a well established method for detecting CA aneurysm. We studied the influence of KD on cardiopulmonary function and EC; meanwhile we analyzed echocardiographic findings of KD patients. We also assessed the correlation between CA Z-score and EC of KD patients to see if CA Z-score of KD patients could reflect EC during exercise.
Sixty-three KD patients were recruited as KD group 1 from children (aged 5–18 y) who received transthoracic echocardiographic examinations and symptom-limited treadmill exercise test for regular follow-up of KD from January 2010 to October 2014 in 1 medical center. We then divided KD group 1 into KD group 2 (<5 y, n = 12) and KD group 3 (≥5 y, n = 51) according to time interval between KD onset to when patients received test. Control groups were matched by age, sex, and body mass index. Max-Z of CA was defined as the maximal Z-score of the proximal LCA or RCA by Dalliarre equation or Fuse calculator.
All routine parameters measured during standard exercise test were similar between KD and control groups, except that peak rate pressure products (PRPPs) in KD group 1 to 3 were all lower than corresponding control groups significantly (P = 0.010, 0.020, and 0.049, respectively). PRPPs correlated with Max-Z of CA by both equations modest inversely (by Dallaire, P = 0.017, Spearman rho = −0.301; by Fuse, P = 0.014, Spearman rho = −0.309).
Our study recruited larger number of KD patients and provided a newer data of EC of KD patients. Our finding suggests that after acute stage of KD, patients could maintain normal cardiorespiratory fitness. Therefore, we believe that it is important to promote cardiovascular health to KD patients and KD patients should exercise as normal peers. However, since KD patients might still have compromised coronary perfusion during exercise, it remains crucial to assess and monitor cardiovascular risk of KD patients. Max-Z of CA correlates with PRPP modest inversely and might be used as a follow-up indicator of CA reserve during exercise after acute stage of KD.
INTRODUCTION
Kawasaki disease (KD), first described in Japan in 1967 by Tomisaku Kawasaki,1 is the leading cause of acquired coronary artery (CA) disease in children. CA aneurysms (CAAs) or ectasia develop in 15% to 25%, and 5% of untreated and treated children, respectively.2 The common forms of CAA were in small and medium size, 80% of which might be regression within 5 years after onset of KD. However, 1% of the CAA will eventually lead to the development of thrombosis, ischemic heart disease, myocardial infarction, or even sudden death.3,4 Therefore, routine echocardiographic coronary examination for patients with KD to evaluate the presence of CAA is recommended by the American Heart Association and the Japanese Ministry of Health and welfare.5
Only a few studies evaluated exercise capacity (EC) among KD patients and normal controls. Results of these studies were controversial. Most of them suggested that KD patients have normal cardiorespiratory fitness, whereas patients may have decreased cardiorespiratory fitness with the development of ischemic heart disease.6–8 However, sample sizes of these studies were small and these studies were published more than 5 years ago. Our study included larger number of KD patients who took standard gradual exercise testing in recent 5 years to provide a newer data of the EC, and we also analyzed the correlation between echocardiography findings and EC of KD patients.
The initial recommendations for the definition of CA dilatation were issued by the Japan Ministry of Health. However, their use was limited because of the lack of a correlation with body habitus and the nondifferentiation between the right and the left CAs. As a result, multiple CA Z-score regression equations, derived from a large heterogeneous population of children undergoing echocardiography, both linear9 and exponential10 functions with body surface area, were proposed to provide objective determination of CA size abnormalities. The Z-score describes how many standard deviations above or below a size or age-specific population mean a given measurement lies. Most Z-scores determine the optimal definition of CAA to be small if the Z-score is ≥2.5 to <5.0, large if the Z-score is ≥5.0 to <10.0, and giant if the Z-score is ≥10.0.11 The Fuse Z-scoring calculator is based on measurements from over 4000 normal Japanese children.12 The CA Z-score proposed by Dallaire and Dahdah13 is derived from 1033 healthy children and has been proved that it could estimate CA size accurately. Both Fuse and Dallaire equations could be used in children.14 Therefore, we chose Fuse Z-scoring calculator and Dallaire equation for our analysis.
The product of heart rate (HR) and systolic blood pressure (SBP), termed as rate-pressure product (RPP), is a very reliable indicator of myocardial oxygen demand and is widely used. Peak RPP (PRPP) gives an accurate reflection of the myocardial oxygen demand and myocardial workload during exercise.15 Low value of the PRPP suggests significant compromise of coronary perfusion and decreased left ventricular function.16,17
Previous studies had proved that children with KD had lower myocardial flow reserve and higher total coronary resistance compared with normal controls, even though there was no evidence of CA lesion.18 Therefore, CA diameter of KD patients might influence cardiac blood flow and thus affects the EC. To our best knowledge, there is no study to evaluate the correlation of EC and CA Z-score of KD patients to date. In this study, we evaluated if CA Z-score could be representative of myocardial perfusion of KD patients during exercise.
MATERIALS AND METHODS
Patient Characteristics
We performed a retrospective cohort study at Kaohsiung Veterans General Hospital, Taiwan. All children (aged from 5 to 18 y) referred to the pediatric cardiology outpatient clinic of VGHKS between January 2010 and October 2014 for regular follow-up of KD, who underwent completed transthoracic echocardiographic examinations and symptom-limited treadmill exercise test, were considered for inclusion (KD group 1). Exclusion criteria consisted of the presence of significant structural heart disease; moderate to severe cardiac valvular disease; significant arrhythmia; ventricular hypertrophy; concurrent known pulmonary disease; and lack of record of KD onset date. The basic characteristics including sex, age, body weight, height, date of KD onset, and body fat were recorded. Meanwhile, age, sex, and body mass index (BMI)-matched children referred to the pediatric cardiology outpatient clinic of VGHKS between the same period for chest pain or dyspnea on exertion, who underwent the above 2 examinations without abnormal findings, were recruited as control group 1. KD patients with normal CAs had been found to have less distensible and thicker CA wall and increased CA stiffness.19,20 A recent study even proved that KD patients already had arterial endothelial dysfunction within 5 years after the onset of illness.21 Therefore, we also divided our KD group 1 into 2 subgroups according to the time interval between KD onset to the time the exercise test was performed (KD group 2: the interval was within 5 y, KD group 3: the interval was more than 5 y) and 2 age, sex, and BMI-matched control groups (control group 2 and control group 3) were retrieved from control group 1. All study participants and their parents provided written informed consent. This study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital (number: VGHKS15-CT7-05).
Exercise Test
We used symptom-limited treadmill exercise test to measure the patients’ EC. This testing system was composed by a treadmill, a flow module, a gas analyzer, and an electrocardiographic monitor (Metamax 3B, Cortex Biophysik GmbH Co., Germany). Both groups underwent the exercise test according to the Bruce protocol that is suggested by American College of Sports Medicine. We terminated the test when the children demonstrated subjective unbearable symptoms.22 The oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured by the breath-by-breath method during the testing. In addition, metabolic equivalent (MET), minute ventilation (VE), BP, and HR were measured throughout the exercise test. The anaerobic threshold (AT) was determined by the VE/VO2 and VE/VCO2 methods.23
Pulmonary Function Test
Pulmonary function test was performed by the spirometry at rest in both groups. Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and maximal voluntary ventilation (MVV) were measured.
Echocardiography and Coronary Artery Measurements
All KD patients underwent complete 2-dimensional echocardiographic studies with color flow and spectral Doppler examination. The intraluminal diameters of CA segments were measured from inner edge to inner edge. The right CA (RCA) and left anterior descending CA (LCA) were measured 3 to 5 mm distal to their origins in the parasternal short-axis view.24 Routine examined cardiac structures like valves, left ventricular (LV) diameter, aortic root (AO) diameter, LV diameter at end diastole (LVIDd), and LV diameter at end systole (LVIDs) were also measured according to the guidelines and standards for performance of a pediatric Echocardiogram by American Society of Echocardiography.25
Equation of Coronary Artery Z-score
The body surface area (BSA) was computed by Haycock equations.26 The CA Z-score was computed by Dallaire equation13 and Fuse Z-scoring calculator.12 Max-Z of CA was defined as the maximal Z-score of the proximal LCA or RCA measured by echocardiography.
Statistical Analysis
We used SPSS for Windows version 19.0 (Released 2010; Armonk, NY: IBM Corp) for all analyses. Data were expressed as mean ± standard deviation. Because the variables we analyzed are not all in normal distribution, the Mann–Whitney U test was used to compare the demographic characteristics, EC, pulmonary function, and echocardiographic findings between KD and control groups. Spearman correlation analysis was used to determine the associations between EC and echocardiographic measurable variables (including CA Z-score). A P value ≤0.05 was considered statistically significant.
RESULTS
Seventy patients met the inclusion criteria. Among them, 3 patients did not have medical records about the onset of KD, 2 patients had valvular disease, 1 patient had significant cardiac structural problems, and 1 patient had significant arrhythmia. Therefore, 63 KD patients were retrieved as KD group 1. Sixty-three age, sex, and BMI-matched healthy participants were thus retrieved as control group 1. Twelve KD patients were defined as KD group 2, whereas 51 KD patients were defined as KD group 3, according to the interval from KD onset to the time patients received exercise test. On the basis of age, sex, and BMI of KD group 2 and KD group 3, 12 and 51 healthy participants were retrieved from control group 1 as control group 2 and control group 3, respectively (Figure 1).
FIGURE 1.

Inclusion algorithm. Seventy patients with history of Kawasaki disease met inclusion criteria and 63 patients were recruited after exclusion. KD group 2 (<5 y, n = 12) and KD group 3 (≥5 y, n = 51) were defined according to time interval between disease onset to when patients received test. Control groups (control groups 1, 2, 3) were matched by age, sex, and BMI of the corresponding KD groups (KD groups 1, 2, 3). BMI = body mass index, KD = Kawasaki disease.
Demographic Characteristics
Table 1 demonstrated demographic characteristics of KD groups and control groups in this study. Thirty-eight males and 25 females were included in each group. The mean age of KD patients was 12.27 ± 3.76 years old. There was no significant difference in sex, age, weight, height, BMI, body fat, SBP, diastolic BP (DBP), resting HR, percentage of predicted FVC, percentage of predicted FEV1, and percentage of predicted MVV between 2 groups.
TABLE 1.
Demographic Characteristics of Kawasaki Disease Groups and Control Groups
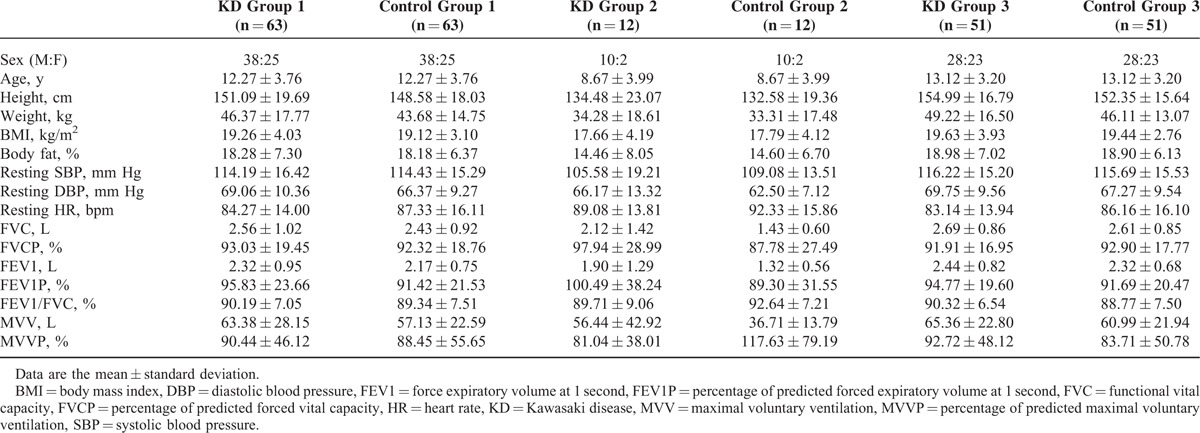
Subgroup analysis demonstrated the similar results. No significant difference was noted among all the basic characteristics presented.
Performance of Exercise Test Between KD and Control Groups
Table 2 showed the performance of exercise test between KD groups and control groups. All the routine parameters we measured during standard exercise test, including MET at the point of AT (AT MET), HR at the point of AT, peak MET, peak VE, peak respiratory exchange ratio, peak SBP/DBP, and HR reserve (HRR) at 1 minute after termination of the test, showed no statistical significance except that PRPP was significantly lower in KD group 1 than in control group 1 (28704.81 ± 5979.16 vs 31031.00 ± 7119.50; P = 0.010).
TABLE 2.
Performance of Exercise Test Between Kawasaki Disease Groups and Control Groups
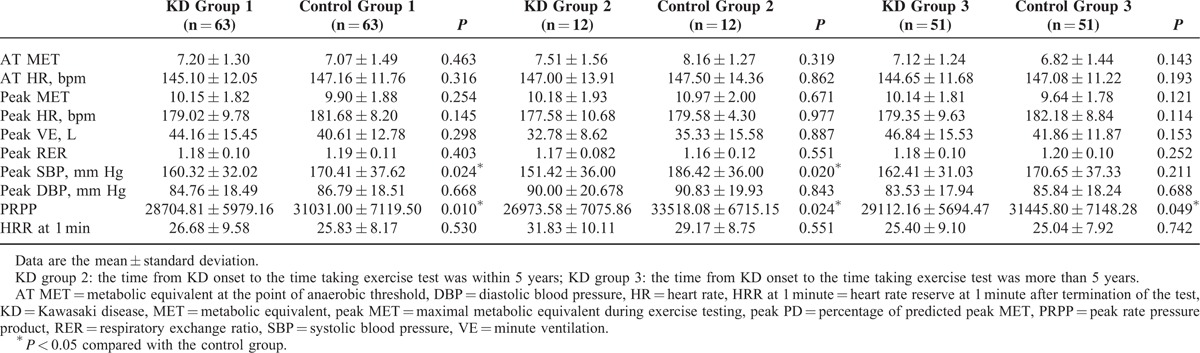
Subgroup analysis showed that peak SBP (151.42 ± 36.00 vs 186.42 ± 36.00; P = 0.020) and PRPP (26973.58 ± 7075.86 vs 33518.08 ± 6715.15; P = 0.024) were significantly lower in KD group 2 as compared with control group 2. PRPP in KD group 3 was also significantly lower than control group 3 (29112.16 ± 5694.47 vs 31445.80 ± 7148.28; P = 0.049). The other parameters we measured routinely during standard exercise test showed no significantly statistical difference.
Echocardiographic Findings
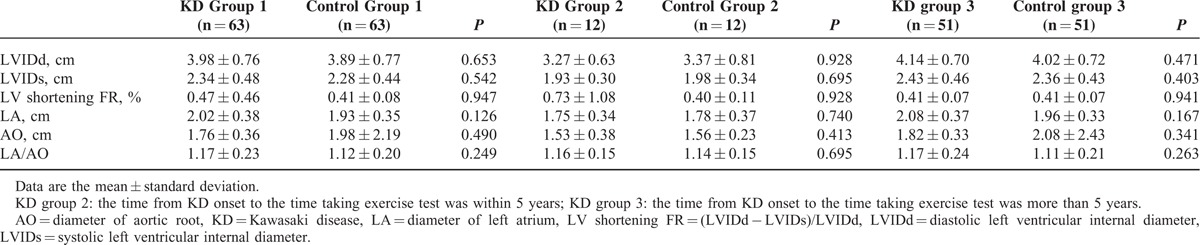
Table 3 demonstrated the echocardiographic findings between KD and control groups. All echocardiographic findings we measured during routine examination, including LVIDd, LVIDs, LV-shortening fraction, diameter of left atrium, and diameter of AO, showed no significant difference.
TABLE 3.
Echocardiographic Findings Between Kawasaki Disease Groups and Control Groups
Among all KD patients of our study, according to the guideline of American Heart Association in 2004,27 only 1 small aneurysm of RCA was noted by echocardiography in KD group 2, whereas 1 giant aneurysm of RCA and 3 small plus 1 giant aneurysms of LCA were noted in KD group 3. As Table 4 shows, the mean diameter of proximal RCA and proximal LCA in KD group 1 were 0.29 ± 0.10 and 0.32 ± 0.11 cm, whereas the Max-Z of CA (by Dallaire equation/by Fuse calculator) was 0.56 ± 1.90/0.99 ± 1.60. In subgroup analysis, the mean diameter of proximal RCA and proximal LCA in KD group 2 were 0.24 ± 0.10 and 0.31 ± 0.08 cm, whereas the Max-Z of CA (by Dallaire equation/by Fuse calculator) was 0.65 ± 1.39/1.06 ± 1.28. The mean diameter of proximal RCA and proximal LCA in KD group 3 were 0.30 ± 0.10 and 0.33 ± 0.12 cm, whereas the Max-Z of CA (by Dallaire equation/by Fuse calculator) of proximal RCA and proximal LCA in KD group 3 was 0.54 ± 2.01/and 0.97 ± 1.68.
TABLE 4.
Coronary Artery Diameter by Echocardiography and Coronary Artery Z-score of Kawasaki Disease Groups

Correlations Between Exercise Capacity and Echocardiographic Measurable Variables
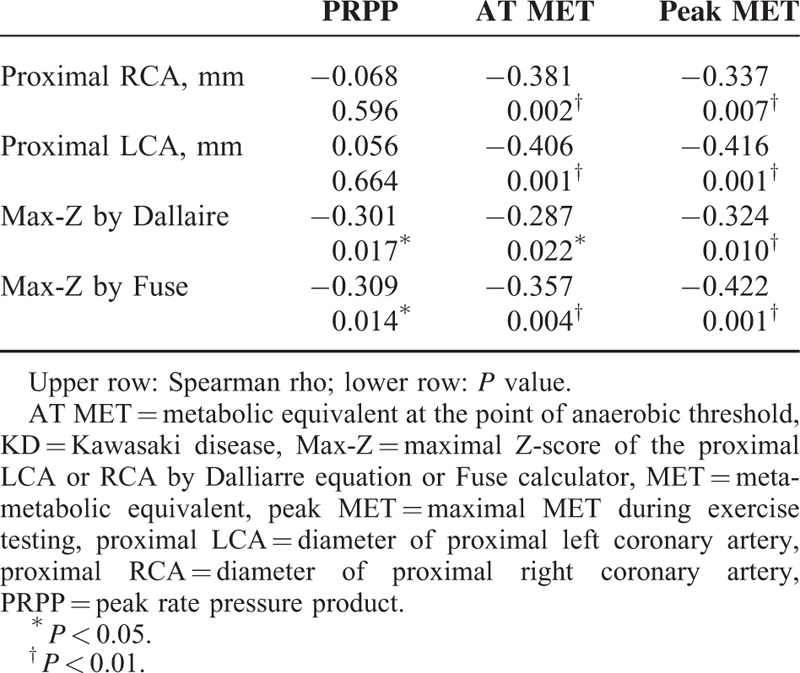
Table 5 demonstrated the correlations between variables of EC (PRPP, AT MET, and peak MET) and echocardiographic measurable variables (diameter of proximal RCA and LCA, Max-Z of CA by Dallaire equation and by Fuse calculator) in KD group 1. PRPP is in inverse association with Max-Z of CA by both equations significantly. The Spearman correlation coefficient was −0.301 (by Dallaire, P = 0.017) and −0.309 (by Fuse, P = 0.014), respectively. Both showed a modest to moderate correlation. AT MET and peak MET versus the other echocardiographic measurable variables is from −0.337 to −0.416, all of which showed a moderate correlation, except that no significant correlation was noted in proximal RCA or proximal LCA with PRPP.
TABLE 5.
Correlation Between Performance of Exercise Test and Echocardiographic Findings in Kawasaki Disease Group 1
DISCUSSION
Exercise Performance of KD Patients
Studies about the exercise performance and aerobic capacity in patients after KD are limited. In 1992, Allen et al6 found that work rate, HRR, and VO2 max of KD patients were within normal range for healthy children. In 1996, Rhodes et al7 showed that KD patients did not differ significantly from the control participants with regard to VO2 max, peak workload, and AT. Wang et al8 in 2008 demonstrated that the VO2 max and the maximal SBP were significantly different between KD children and healthy children, but no significant difference in maximal HR. Sample sizes of these studies were small and no subgroup analyses regarding to the time from the KD onset to the time patients received exercise test were done. Our study showed the similar findings as previous studies, except that PRPP of KD patients was significantly lower than controls. We also did a subgroup analysis and disclosed the same findings as group 1, except that peak SBP were lower in KD group 2 (151.42 ± 36.00 vs 186.42 ± 36.00; P = 0.02) than control group 2.
Previous studies using flow-mediated dilatation method to evaluate the endothelial function have shown that systemic endothelial dysfunction can occur late after the onset of KD. Most previous studies assessed endothelial dysfunction in adolescents and adults with KD at 5 years or more after the onset of disease.18–20 To the best of our knowledge, there is only 1 prospective study conducted in 2013 by Ishikawa and Iwashima,21 evaluating endothelial function in children within 5 years after the onset of KD, and it demonstrated that children with KD already had arterial endothelial dysfunction within 5 years after the onset of illness. Our study found that the PRPP of KD group 2 (early KD) and KD group 3 (late KD) were both significantly lower than corresponding control groups. Our study also assessed the exercise performance of early and late KD patients and disclosed that early and late KD patients have similar EC to normal healthy peers. Since PRPP is a good indicator of coronary flow reserve, our finding was compatible with the study by Ishikawa and Iwashima in 2013. Therefore, we could speculate that among KD patients, regardless of the time interval from KD onset, their coronary perfusion during exercise might be compromised, a condition that might be resulted from increased CA resistance and CA dysfunction after KD.18
Echocardiography Characteristics of KD Patients
Our study showed that the diameter of LVIDs, LVIDd, LA, and AO of KD patients were no different from their healthy peers. The LV-shortening fraction, which is an indicator of LV wall motion, of KD patients, was also similar to control group. Previous studies focused on the echocardiography trends of KD patients revealed that LV-shortening fraction was abnormal initially, but became normal by the end of 3 months after KD onset.28,29 The time interval from KD onset to exercise test of KD patients in our KD group 2 was more than 6 months (3.92 ± 1.62 y) and thus the result of our study is in agreement with previous studies.
Kurotobi et al29 in 2002 used 2-dimensional echocardiography to compare the CA diameter of KD children with normal children. They assessed the CA diameter of KD children at different stages from the disease onset (stage 1 and 2 were 43 patients at admission and subsequent 2–3 wks, stage 3 were 62 patients at an average 2.2 y after KD onset). They found that the CA diameters of patient with acute KD (stage 1 and 2) were significantly larger, whereas the CA diameters in the long term after KD group (stage 3) did not show a significant difference compared with normal group.29 Crystal et al28 in 2009 analyzed CA Z-score of 176 KD patients at different time (initial 6–8 wk, and 1 y after) and also found that Z-scores decreased from initial values in a logarithmic manner to a normal range, mostly in the first 2 to 3 months. Our study revealed the same results as these 2 studies. We noticed that Z-score of CA diameter of KD patients after acute stage was within the normal range of healthy people, and the incidence of remaining aneurysm after acute stage was about 3.17% of proximal RCA and 6.35% of proximal LCA. The prevalence of CA aneurysm after KD onset of our study is consistent with previous studies in Taiwan, which showed approximately 5% of patients with KD had coronary complications during the long-term follow-up.30
Correlation Between Performance of Exercise Test and Coronary Artery Z-score
To the best of our knowledge, our study is the first study to evaluate the correlation between CA Z-score and EC. We noticed that Max-Z of CA by both Dallaire equation and Fuse calculator correlates with PRPP inversely and modestly. We also found that Max-Z of CA by both equations correlated with MET at the point of AT and peak MET inversely and modestly. The angiographic picture of CAA is characterized by a striking slow flow, a segmental back flow phenomenon, and a stasis of dye.31 Previous studies also proved that despite the absence of coronary stenosis, myocardial perfusion may be compromised by the blood flow alteration in the CA due to blood stasis and abnormal blood flow pattern in the aneurismal dilatation.18,32 It has also been reported that KD patients may develop a decrease in diastolic function33 or abnormal vascular endothelial function34 after a long period of time, even without evidence of CA dilatation. Therefore, even though the PRPP of the KD patients was significant lower than their health peers, whereas the other variables of EC were similar with control groups, it is reasonable to find that the Max-Z of CA correlates with the PRPP inversely and modestly in the KD patients. The above findings could support our speculation that Max-Z of CA could reflect the myocardial flow reserve, and furthermore the cardiorespiratory fitness during exercise of KD patients to some extent.
Study Limitations
Limitations of this study are as follows. First, no KD patients receive exercise test in the acute stage. Therefore, we could not confirm the normal Max-Z of CA in KD group is due to regression of CA dilatation after acute stage or other reasons such as discrepancies between coronary arterial growth and somatic growth. Second, we did not analyze the exercise performance between KD group 2 and KD group 3 directly since the power after age-matching was poor (only 8 samples in each group after age-matching). We can only conclude that variables of EC except for PRPP of KD patients, regardless of time interval from KD onset, show no significant difference to their normal peers, rather than saying that the EC of KD group 2 is similar with KD group3 directly. Third, there might be some variations when measuring CA diameter even though all the echocardiography examinations were done by well trained cardiologists who followed the current guideline. In addition, at present, we lack a well accepted equation for CA Z-score based on the Taiwanese population. The equations we chose are based on Canadian and Japanese population. The Max-Z by both equations might not reflect the true CA condition of Chinese KD patients fully.
CONCLUSIONS
Our study revealed that after acute stage of KD, regardless of early or late KD, patients’ EC and Max-Z of their CAs are normal except for PRPP, which is significantly lower than normal peers. This finding suggests that KD patients might have compromised coronary perfusion during exercise while maintaining normal cardiorespiratory fitness and without dilatation of CA. Our study is the first to evaluate the relationship between Max-Z of CA and EC. We showed that Max-Z correlates with EC and the PRPP modest-moderate inversely. Because KD patients could maintain normal exercise fitness after acute stage, we believe that it is important to promote cardiovascular health in KD patients. However, since they might still have compromised coronary perfusion during exercise, it remains crucial to assess and monitor cardiovascular risk of KD patients. Max-Z of CA might reflect the ability of KD patients to achieve coronary perfusion during exercise and could be used as an indicator for long-term follow-up.
Footnotes
Abbreviations: AO = aortic root; AT MET = metabolic equivalent at the point of anaerobic threshold; AT = anaerobic threshold; BMI = body mass index; BSA = body surface area; CA = coronary artery; CAA = coronary artery aneurysm; DBP = diastolic blood pressure; EC = exercise capacity; FEV1 = forced expiratory volume in 1second; FVC = forced vital capacity; HR = heart rate; HRR = heart rate reserve; KD = Kawasaki disease; LCA = left anterior descending coronary artery; LV = left ventricular; LVIDd = left ventricular diameter at end diastole; LVIDs = left ventricular diameter at end systole; Max-Z of coronary artery = maximal Z-score of the proximal LCA or RCA by Dalliarre equation or Fuse calculator; MET = metabolic equivalent; MVV = maximal voluntary ventilation; PRPP = peak rate-pressure product; RCA = right coronary artery; RPP = rate-pressure product; SBP = systolic blood pressure; VCO2 = carbon dioxide production; VE = minute ventilation; VO2 = oxygen consumption.
Disclosure: The results of the present study do not constitute endorsement and this work disclosed no financial support from any foundation. All authors declared that there is no conflict of interest.
REFERENCES
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children [Article in Japanese. Arerugi 1967; 16:178–222. [PubMed] [Google Scholar]
- 2.Burns JC, Glode MP. Kawasaki syndrome. Lancet 2004; 364:533–544. [DOI] [PubMed] [Google Scholar]
- 3.Falcini F. Kawasaki disease. Curr Opin Rheumatol 2006; 18:33–38. [DOI] [PubMed] [Google Scholar]
- 4.Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996; 94:1379–1385. [DOI] [PubMed] [Google Scholar]
- 5.Lin MT, Chang CH, Hsieh WC, et al. Coronary diameters in Taiwanese children younger than 6 years old: Z-score regression equations derived from body surface area. Acta Cardiol Sin 2014; 30:266–273. [PMC free article] [PubMed] [Google Scholar]
- 6.Allen SW, Shaffer EM, Harrigan LA, et al. Maximal voluntary work and cardiorespiratory fitness in patients who have had Kawasaki syndrome. J Pediatr 1992; 121:221–225. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes J, Hijazi ZM, Marx GR, et al. Aerobic exercise function of patients with persistent coronary artery aneurysms secondary to Kawasaki disease. Pediatr Cardiol 1996; 17:226–230. [DOI] [PubMed] [Google Scholar]
- 8.Wang YL, Yang AL, Wang JL, et al. Cardiopulmonary function and exercise capacity in children with Kawasaki disease. Tw J Phys Med Rehabil 2008; 36:209–215. [Google Scholar]
- 9.Tan TH, Wong KY, Cheng TK, et al. Coronary normograms and the coronary-aorta index: objective determinants of coronary artery dilatation. Pediatr Cardiol 2003; 24:328–335. [DOI] [PubMed] [Google Scholar]
- 10.Olivieri L, Arling B, Friberg M, et al. Coronary artery Z score regression equations and calculators derived from a large heterogeneous population of children undergoing echocardiography. J Am Soc Echocardiog 2009; 22:159–164. [DOI] [PubMed] [Google Scholar]
- 11.Manlhiot C, Millar K, Golding F, et al. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardio 2010; 31:242–249. [DOI] [PubMed] [Google Scholar]
- 12.Ogata S, Tremoulet AH, Sato Y, et al. Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int J Cardiol 2013; 168:3825–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr 2011; 24:60–74. [DOI] [PubMed] [Google Scholar]
- 14.Chubb H, Simpson JM. The use of Z-scores in paediatric cardiology. Ann Pediatr Cardiol 2012; 5:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gobel FL, Norstrom LA, Nelson RR, et al. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 1978; 57:549–556. [DOI] [PubMed] [Google Scholar]
- 16.Ansari M, Javadi H, Pourbehi M, et al. The association of rate pressure product (RPP) and myocardial perfusion imaging (MPI) findings: a preliminary study. Perfusion 2012; 27:207–213. [DOI] [PubMed] [Google Scholar]
- 17.Nagpal S, Walia L, Lata H, et al. Effect of exercise on rate pressure product in premenopausal and postmenopausal women with coronary artery disease. Indian J Physiol Pharmacol 2007; 51:279–283. [PubMed] [Google Scholar]
- 18.Crystal MA, Syan SK, Yeung RS, et al. Echocardiographic and electrocardiographic trends in children with acute Kawasaki disease. Can J Cardiol 2008; 24:776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung YF, Yung TC, Tam SC, et al. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: implications for premature atherosclerosis. J Am Coll Cardiol 2004; 43:120–124. [DOI] [PubMed] [Google Scholar]
- 20.Noto N, Okada T, Yamasuge M, et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics 2001; 107:1095–1099. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa T, Iwashima S. Endothelial dysfunction in children within 5 years after onset of Kawasaki disease. J Pediatr 2013; 163:1117–1121. [DOI] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th ed.2013; Philadelphia: Lippincott Williams & Wilkins, 114–131. [DOI] [PubMed] [Google Scholar]
- 23.Washington RL. Cardiorespiratory testing: anaerobic threshold/respiratory threshold. Pediatr Cardiol 1999; 20:12–15.discussion 16. [DOI] [PubMed] [Google Scholar]
- 24.Weng KP, Hsieh KS, Huang SH, et al. Clinical relevance of the risk factors for coronary artery lesions in Kawasaki disease. Kaohsiung J Med Sci 2012; 28:23–29. [DOI] [PubMed] [Google Scholar]
- 25.Lai WW, Geva T, Shirali GS, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 2006; 19:1413–1430. [DOI] [PubMed] [Google Scholar]
- 26.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 1978; 93:62–66. [DOI] [PubMed] [Google Scholar]
- 27.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004; 114:1708–1733. [DOI] [PubMed] [Google Scholar]
- 28.Crystal MA, Manlhiot C, Yeung RS, et al. Coronary artery dilation after Kawasaki disease for children within the normal range. Int J Cardiol 2009; 136:27–32. [DOI] [PubMed] [Google Scholar]
- 29.Kurotobi S, Nagai T, Kawakami N, et al. Coronary diameter in normal infants, children and patients with Kawasaki disease. Pediatr Int 2002; 44:1–4. [DOI] [PubMed] [Google Scholar]
- 30.Wu MH, Chen HC, Yeh SJ, et al. Prevalence and the long-term coronary risks of patients with Kawasaki disease in a general population <40 years: a national database study. Circ Cardiovasc Qual Outcomes 2012; 5:566–570. [DOI] [PubMed] [Google Scholar]
- 31.Krüger D, Stierle U, Herrmann G, et al. Exercise-induced myocardial ischemia in isolated coronary artery ectasias and aneurysms (‘dilated coronaropathy’). J Am Coll Cardiol 1999; 34:1461–1470. [DOI] [PubMed] [Google Scholar]
- 32.Krüger D, Mokhtari NE, Wieckhorst A, et al. Intravascular ultrasound study and evidence of pathological coronary flow reserve in patients with isolated coronary artery aneurysms. Clin Res Cardiol 2010; 99:157–164. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki A, Yamagishi M, Kimura K, et al. Functional behavior and morphology of the coronary artery wall in patients with Kawasaki disease assessed by intravascular ultrasound. J Am Coll Cardiol 1996; 27:291–296. [DOI] [PubMed] [Google Scholar]
- 34.Yamakawa R, Ishii M, Sugimura T, et al. Coronary endothelial dysfunction after Kawasaki disease: Evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol 1998; 31:1074–1080. [DOI] [PubMed] [Google Scholar]


