Abstract
Clinicopathologic features and clinical outcomes of gastrointestinal stromal tumors (GISTs) in esophagus are limited, because of the relatively rare incidence of esophageal GISTs. Therefore, the aim of the current study was to investigate the clinicopathologic features and clinical outcomes of esophageal GISTs, and to investigate the potential factors that may predict prognosis.
Esophageal GIST cases were obtained from our center and from case reports and clinical studies extracted from MEDLINE. Clinicopathologic features and survivals were analyzed and compared with gastric GISTs from our center.
The most common location was lower esophagus (86.84%), followed by middle and upper esophagus (11.40% and 1.76%). The majority of esophageal GISTs were classified as high-risk category (70.83%). Mitotic index was correlated with histologic type, mutational status, and tumor size. The 5-year disease-free survival and disease-specific survival were 65.1% and 65.9%, respectively. Tumor size, mitotic index, and National Institutes of Health risk classification were associated with prognosis of esophageal GISTs. Only tumor size, however, was the independent risk factor for the prognosis of esophageal GISTs. In comparison to gastric GISTs, the distribution of tumor size, histologic type, and National Institutes of Health risk classification were significantly different between esophageal GISTs and gastric GISTs. The disease-free survival and disease-specific survival of esophageal GISTs were significantly lower than that of gastric GISTs.
The most common location for esophageal GISTs was lower esophagus, and most of the esophageal GISTs are high-risk category. Tumor size was the independent risk factor for the prognosis of esophageal GISTs. Esophageal GISTs differ significantly from gastric GISTs in respect to clinicopathologic features. The prognosis of esophageal GISTs was worse than that of gastric GISTs.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the alimentary tract. It represents approximately 1% to 2% of all the alimentary malignancies.1 Based on their phenotypic similarities, GISTs are considered to arise from muscularis propria of gastrointestinal tract, and derived from the interstitial cells of Cajal (ICC).2 Histologically, the majority of GISTs display spindle cell morphology (70%), followed by epithelioid morphology (20%), and mixed morphology (10%).3 Most of the GISTs were positive for CD117 and CD34.4 In 1998, gain-of-function mutations in the c-kit proto-oncogene protein (KIT) protooncogene in GISTs were demonstrated by Hirota et al.5
Gastrointestinal stromal tumors can occur anywhere throughout the gastrointestinal tract and are seen most commonly in the stomach (40%–70%), small intestine (20%–40%), and colon and rectum (5%–15%).6 Esophageal GISTs are extremely uncommon, accounting for 0.7% of all GISTs.7 The reporting of esophageal GISTs has been limited to individual case reports and case series of small numbers. Studies involving large numbers of esophageal GISTs are lacking, many questions remain unanswered regarding the clinicopathologic profiles and clinical outcomes. Therefore, the aim of the current study was to explore the clinicopathologic characteristics and clinical outcome of esophageal GISTs, and to investigate the potential factors that may predict postoperative outcomes.
PATIENTS AND METHODS
Gastrointestinal stromal tumor cases of the esophagus were from our center and in addition from the literature. From May 2010 to March 2015, 7 patients of esophageal GISTs were diagnosed and received treatment in our center. Literature search of MEDLINE was performed for all articles in English published from 2000 through 2015. MEDLINE search resulted in 46 case reports,8–53 including 52 patients and 8 case series,54–61 including 76 cases. To this end, a total of 135 esophageal GISTs patients were identified (Figure 1). In addition, the clinicopathologic characteristics and prognosis of 297 patients of gastric GISTs were analyzed and compared with esophageal GISTs. This study was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from the seven patients in our center.
FIGURE 1.
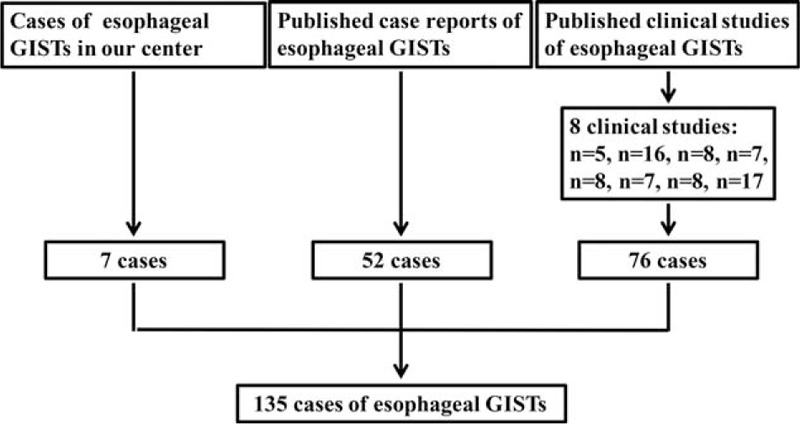
Schematic diagram regarding selection of esophageal gastrointestinal stromal tumors.
Clinicopathologic data, including age, sex, accompanied tumor, symptoms, location, tumor size, surgical intervention, histologic type, lymph node metastasis, mitotic index, immunohistochemical features, mutational status, National Institutes of Health (NIH) risk classification, adjuvant imatinib therapy, tumor recurrence or metastasis, and survival data were recorded from hospital medical records in our center or extracted from published reports and studies. The tumors were categorized into very low, low, intermediate, and high-risk groups according to the modified NIH risk classification criteria reported by Joensuu et al.62 For survival analysis, the exclusion criteria were listed as follows: accompanied with other malignant tumors, accompanied with GISTs in other locations, accompanied with distant metastasis, with neoadjuvant imatinib therapy, not receive R0 resection, with tumor rupture during operation, without follow-up data. Owing to data acquisition, completeness of data is limited.
The clinicophathologic characteristics, including age, sex, tumor size, histologic type, mitotic index, and NIH risk classification were compared with gastric GISTs in our center. For survival analysis between the 2 groups, patients with gastric GISTs in our center were matched with esophageal GISTs based on the following parameters: tumor size: ≤2.0, 2.1 to 5.0, 5.1 to 10.0, or >10.0 cm; mitotic index: 5 or less, or more than 5/50 high power fields (HPFs); and adjuvant imatinib therapy: yes or no.
Data were processed using SPSS 16.0 for Windows (SPSS Inc, Chicago, IL). Discrete variables were analyzed using the χ2 test or Fisher exact test. Numerical variables were expressed as the mean ± SD unless otherwise stated. Significant predictors for survival identified by univariate analysis were further assessed by multivariate analysis using the logistic regression analysis. Evaluation for disease-free survival (DFS) and disease-specific survival (DSS) were obtained by the Kaplan–Meier method and differences between curves were compared using log-rank test. Non-GIST-related deaths were censored for analysis of DSS. The P values were considered to be statistically significant at the 5% level.
RESULTS
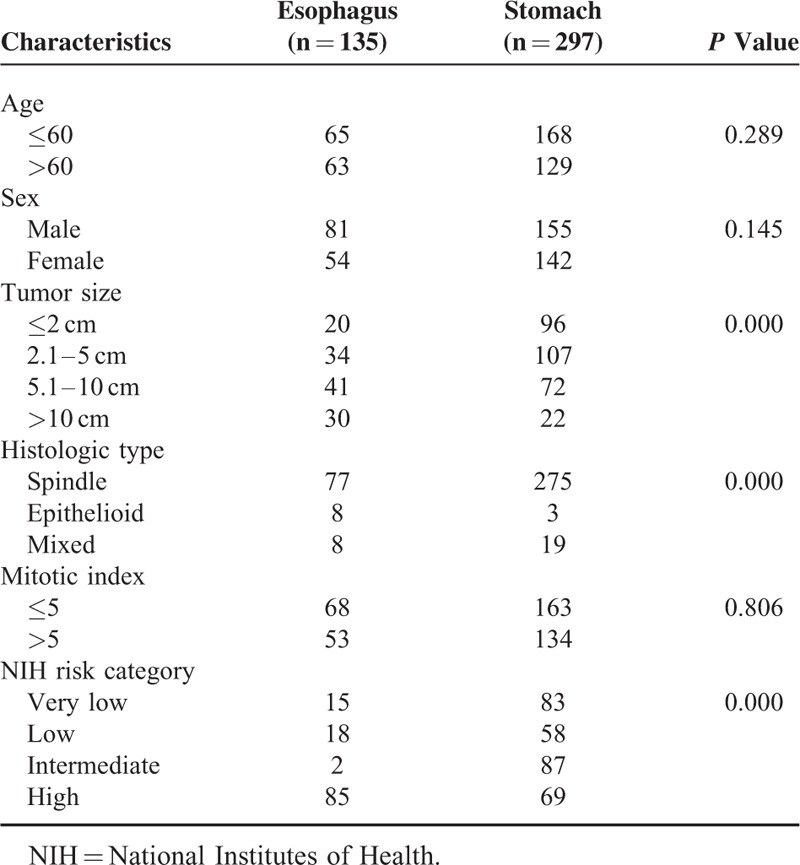
The clinicopathologic features were summarized in Table 1. There were 81 men (60%) and 54 women (40%). The patient age ranged from 12 to 87 years (median, 60 years; mean, 58.6 years). Four patients accompanied with GISTs in other locations (4.6%), including 2 patients of liver metastasis, 1 patient of liver and pleural metastasis, and 1 patient of bone and lung metastasis. Eleven patients accompanied with other malignant tumors (12.64%), including 7 patients of esophageal squamous cell carcinoma, 2 patients of Barrett carcinoma, 1 case of cardia adenocarcinoma, and 1 case of bladder carcinoma. The most common symptom was dysphagia (50/129, 38.76%), followed by chest pain (16/109, 14.68%), bleeding (9/109, 8.26%), and other symptoms including fatigue, cough, and dyspnea (10/109, 9.17%). The most common location was lower esophagus (99/114, 86.84%), followed by middle esophagus (13/114, 11.4%), and upper esophagus (2/114, 1.76%). A total of 121 patients underwent complete surgical resection (121/135, 89.63%), 4 patients underwent palliative surgical resection (4/135, 2.96%), and 10 patients did not receive surgical resection (10/135, 7.41%).
TABLE 1.
Clinicopathologic Characteristics of 135 Patients of Esophageal Gastrointestinal Stromal Tumors
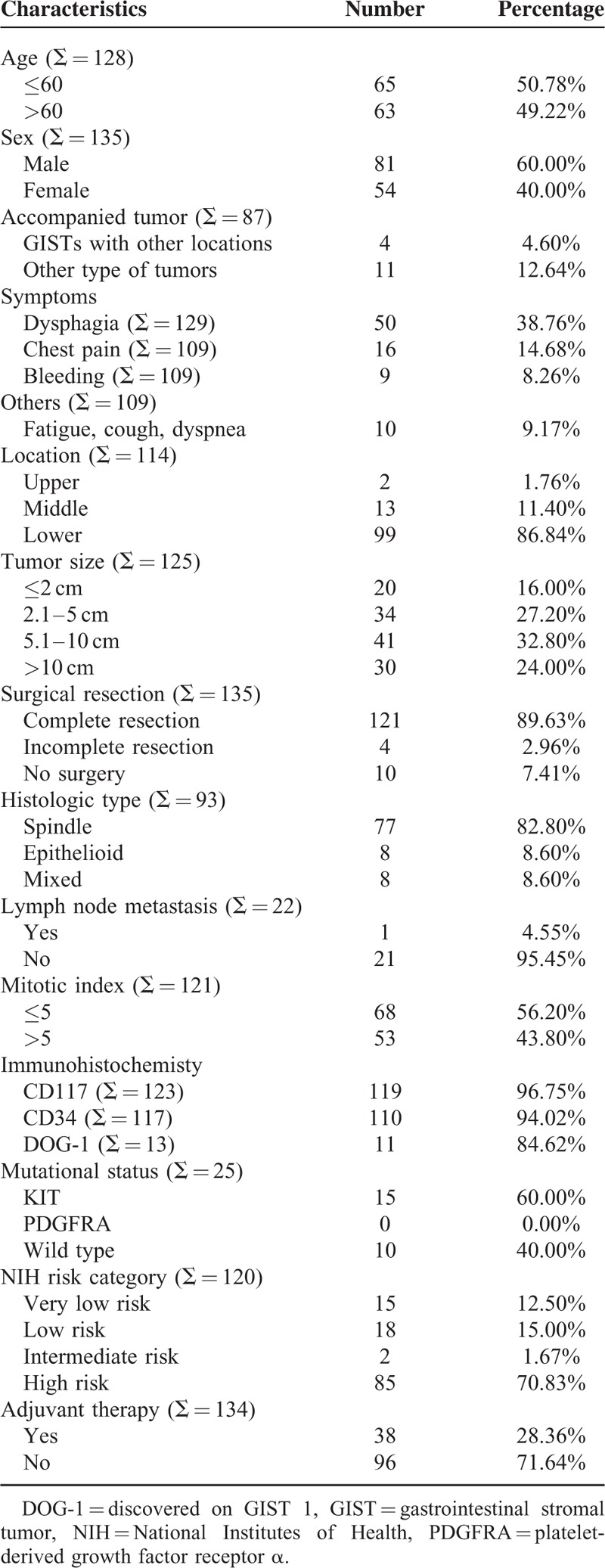
The tumors ranged from 0.2 to 30 cm in maximum diameter (median, 6 cm; mean, 7.3 cm). The mitotic index of 53 patients exceeded 5/50 HPF (53/121, 43.8%). Seventy-seven patients display spindle cell morphology (77/93, 82.8%), 8 patients display epithelioid morphology (8/93, 8.6%), and 8 patients display mixed morphology (8/93, 8.6%). Among the 22 patients with lymph node dissection, only 1 patient had lymph node metastasis (1/22, 4.55%). CD117 positivity was detected in 119 patients (119/123, 96.75%), CD34 positivity was detected in 110 patients (110/117, 94.02%), and discovered on GIST 1 positivity was detected in 11 patients (11/13, 84.62%). Twenty-five patients were analyzed for gene mutation status. Fifteen patients carried a mutation in exon 11 of KIT (15/25, 60%). The remaining 10 patients were wild type. Platelet-derived growth factor receptor α variants were not detected in these 25 patients. According to NIH risk classification, 15 patients were classified as very low risk (15/120, 12.5%), 18 patients were classified as low risk (18/120, 15%), 2 patients were classified as intermediate risk (2/120, 1.67%), and 85 patients were classified as high risk (85/120, 70.83%). Information of adjuvant imatinib therapy was recorded in 134 patients, and 38 patients (28.36%) received imatinib therapy. Among them, 6 patients received imatinib therapy before and after surgery, 2 patients only received imatinib therapy before surgery, 22 patients received imatinib therapy after surgery, and the remaining 8 patients received imatinib therapy only.
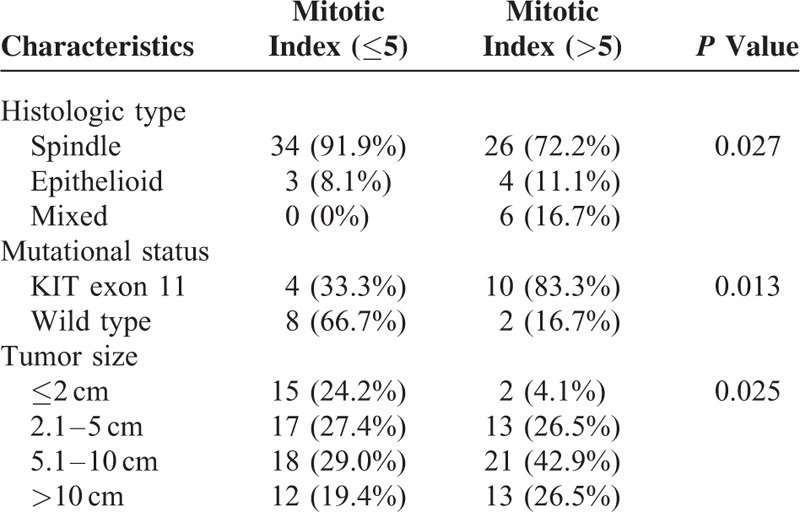
The relationship between clinicopathologic characteristics were analyzed and summarized in Table 2. The mitotic index was correlated with histologic type, mutational status, and tumor size. The mitotic index of all the mixed histologic type exceeded 5/50 HPF (P = 0.027). The mitotic index exceeded 5/50 HPF for the majority of KIT exon 11 mutation but only for the minority of wild-type GISTs (P = 0.013). The mitotic index was positively correlated with tumor size (P = 0.025).
TABLE 2.
The Relationship Between Clinicopathologic Characteristics
Survival data of esophageal GISTs were analyzed and summarized in Table 3. Survival data of 97 patients were eventually selected for analysis using exclusion criteria described in the materials and methods. The follow-up time ranged from 1 to 202 months (mean, 40.70 months; median, 28 months). Twenty-two patients showed recurrence or metastasis, 17 patients suffered from GISTs-related deaths. The 1-, 3-, and 5-year survival rate of DSS was 100%, 88.1%, and 65.9%, respectively. The 1-, 3- and 5-year survival rate of DFS was 93.3%, 78.3%, and 65.1%, respectively. The DFS and DSS of esophageal GISTs were analyzed using Kaplan–Meier survival analyses and shown in Figure 2.
TABLE 3.
Survival Data of 97 Cases of Esophageal Gastrointestinal Stromal Tumors
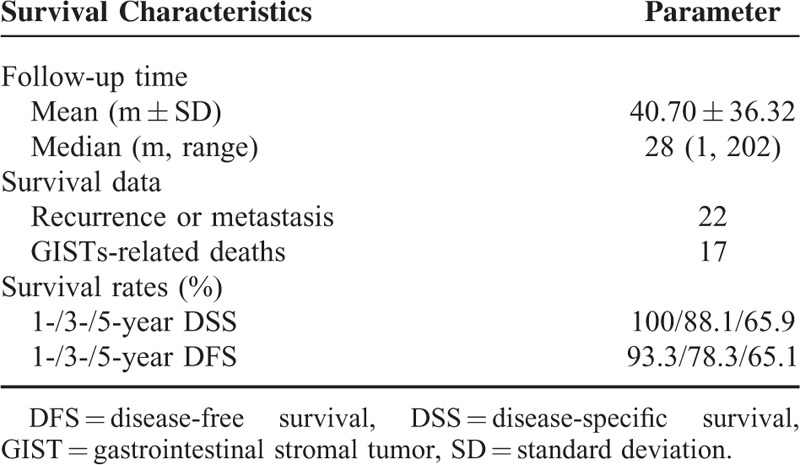
FIGURE 2.
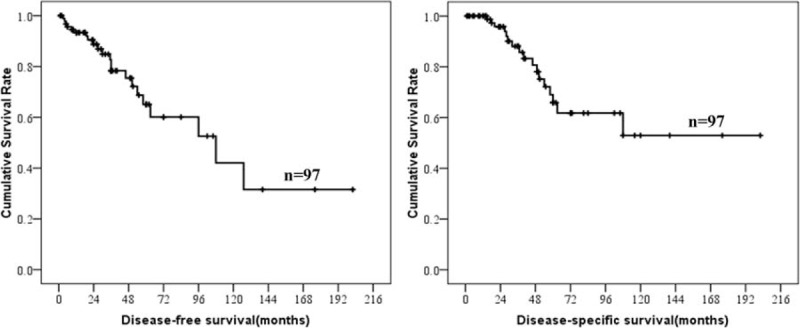
Disease-free-survival and disease-specific survival of esophageal gastrointestinal stromal tumors.
Prognostic factors for DFS and DSS in patients with esophageal GISTs according to univariate and multivariate analysis were summarized in Table 4. The results showed that tumor size, mitotic index, and NIH risk classification were associated with prognosis of esophageal GISTs. Only tumor size, however, was the independent risk factor for the prognosis of esophageal GISTs. The DFS and DSS of esophageal GISTs according to tumor size, mitotic index, and NIH risk classification were shown in Figures 3 to 5. National Institutes of Health risk classification could not be included in the logistic regression analysis, although it showed significant correlation with prognosis, because no patients suffered from recurrence, metastasis, or death in NIH risk category 1 and 2. When calculating the log of the odds, this null frequency caused a computational error because of the presence of logarithm of zero.
TABLE 4.
Prognostic Factors for Disease-specific Survival and Disease-free Survival in Patients With Esophageal Gastrointestinal Stromal Tumors According to Univariate and Multivariate Analysis (n = 97)
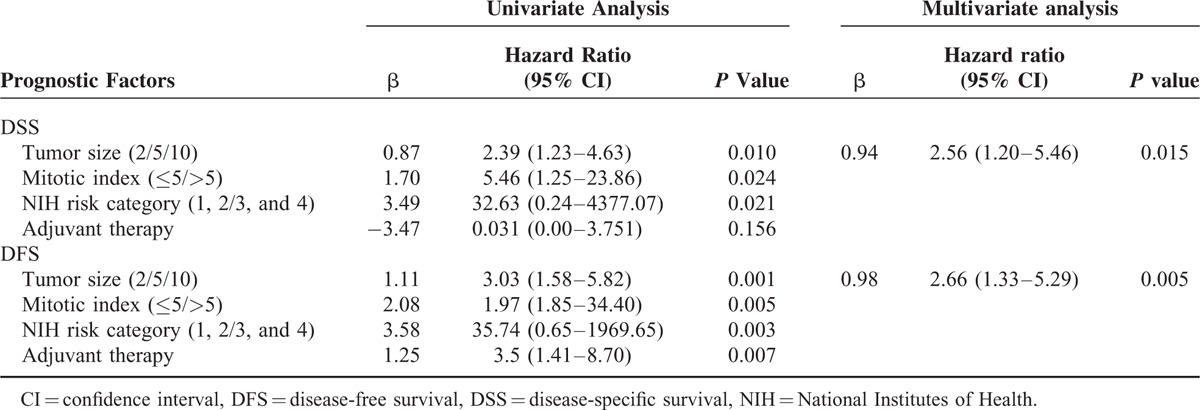
FIGURE 3.
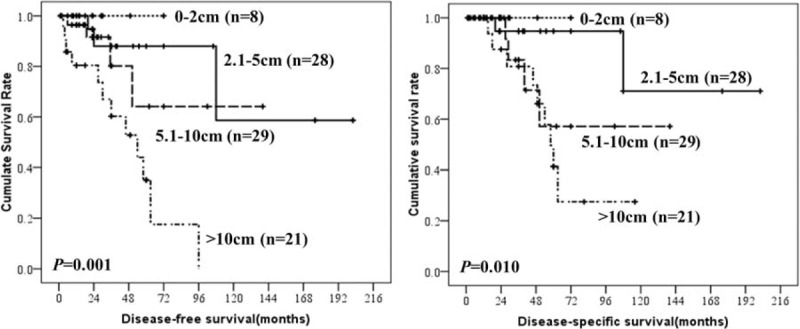
Disease-free-survival and disease-specific survival of esophageal gastrointestinal stromal tumors by tumor size.
FIGURE 5.
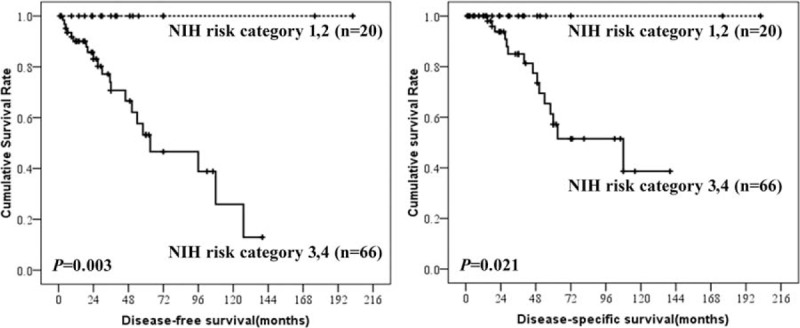
Disease-free-survival and disease-specific survival of esophageal gastrointestinal stromal tumors by National Institutes of Health risk category.
FIGURE 4.
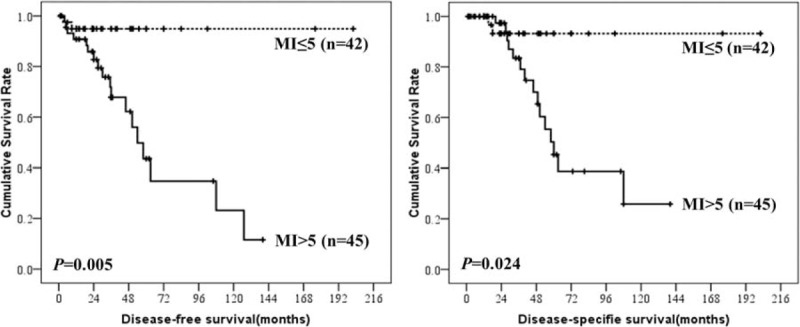
Disease-free-survival and disease-specific survival of esophageal gastrointestinal stromal tumors by mitotic index.
The clinicophathologic features of 135 esophageal GISTs, including age, sex, tumor size, histologic type, mitotic index, and NIH risk category were compared with 297 gastric GISTs in our center (Table 5). The results showed that the distribution of tumor size, histologic type, and NIH risk classification were significantly different between esophageal GISTs and gastric GISTs (both P = 0.000).
TABLE 5.
Comparison of Selected Clinicopathologic Parameters Between Esophageal and Gastric Gastrointestinal Stromal Tumors
To compare the prognosis of esophageal GISTs with gastric GISTs, patients were selected using the exclusion criteria described above. Then the 2 groups were matched according to tumor size, mitotic index, and adjuvant imatinib therapy described above. The entire process was shown in Figure 6. Finally, 73 patients of esophageal GISTs and 73 patients of gastric GISTs were selected. There were no intergroup differences in age, sex, tumor size, mitotic index, and adjuvant imatinib therapy (Table 6). The survival analysis showed in Figure 7 indicated that the DFS (P = 0.026) and DSS (P = 0.041) in patients with esophageal GISTs were significantly lower than that of gastric GISTs (58.3% versus 94.7%, 71.8% versus 95.2%).
FIGURE 6.
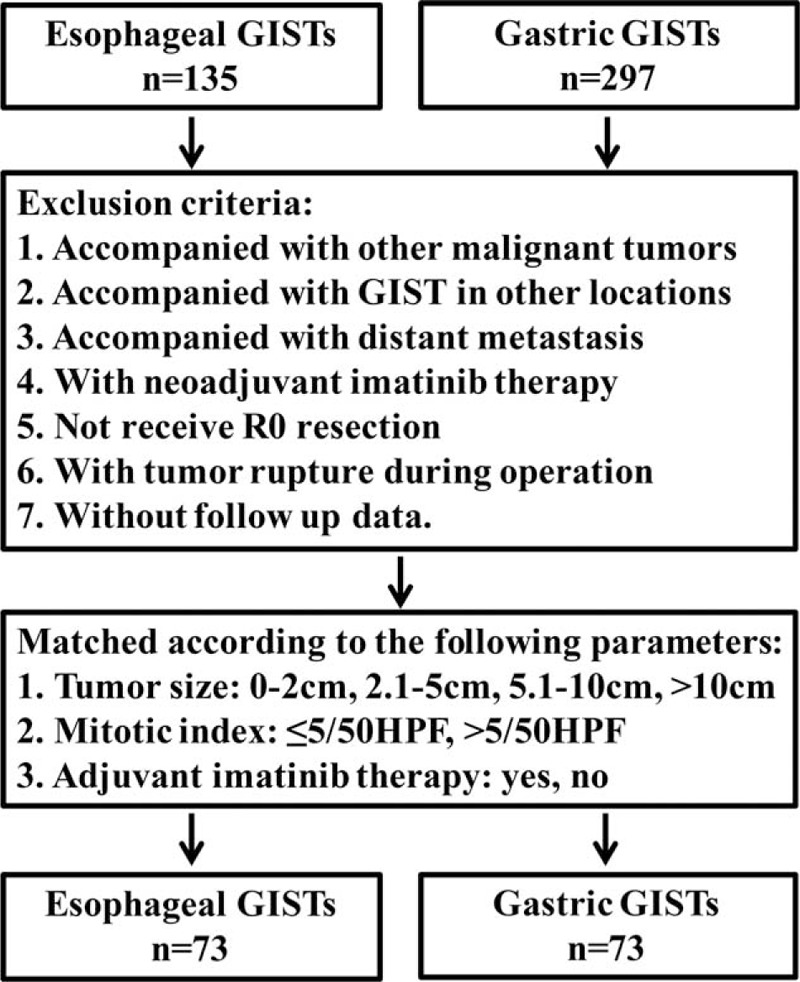
Flow chart of match strategy between esophageal and gastric gastrointestinal stromal tumors.
TABLE 6.
Comparison of Predefined Variables Between Esophageal and Gastric Gastrointestinal Stromal Tumors
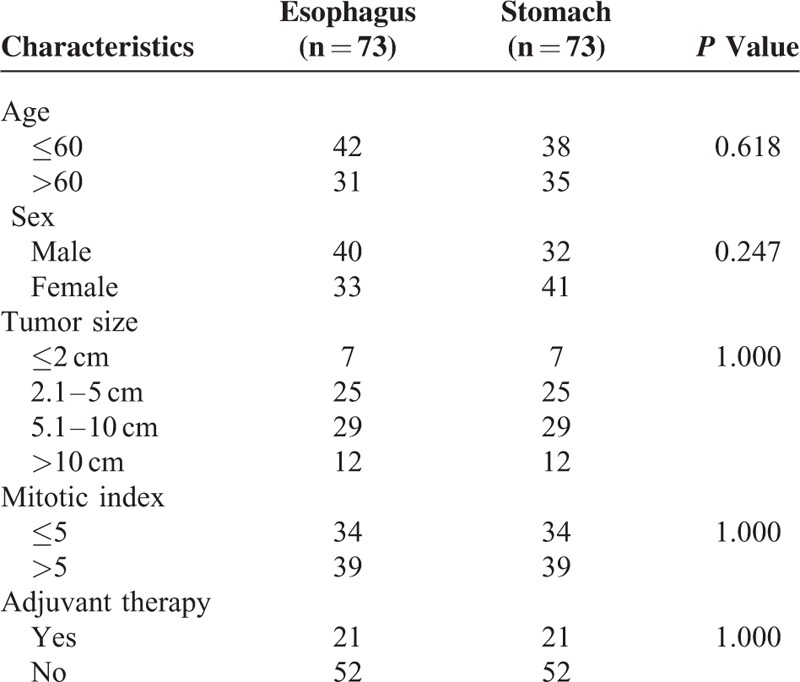
FIGURE 7.
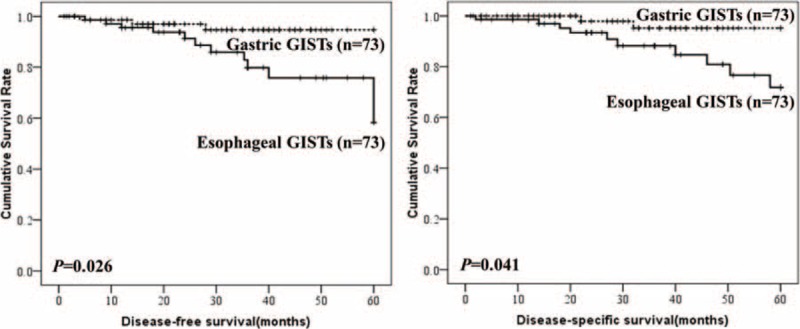
Comparison of disease-free-survival and disease-specific survival between esophageal and gastric gastrointestinal stromal tumors.
DISCUSSION
Gastrointestinal stromal tumors located in the esophagus constitute a very rare subset of GISTs with limited data on the clinicopathologic features and clinical outcomes. Therefore, we evaluated data of 135 cases of esophageal GISTs from our center and from literatures in MEDLINE. The current study represents the largest analysis of esophageal GISTs and indicates some features significantly associated with esophageal GISTs. We found that the most common location for esophageal GISTs was lower esophagus, and most of the esophageal GISTs are high-risk category. Tumor size was the independent risk factor for the prognosis of esophageal GISTs. Esophageal GISTs differ significantly from gastric GISTs in respect to clinicopathologic features. The prognosis of esophageal GISTs was worse than that of gastric GISTs.
There is only 1 clinical study containing a relatively larger number of esophageal GISTs reported by Lott et al.63 Clinicopathologic features of 55 esophageal GISTs were analyzed in the study. In their series, the most common location of esophageal GISTs was lower esophagus, followed by middle esophagus. No esophageal GISTs were found in the upper esophagus. In our current study, the most common location of esophageal GISTs was also lower esophagus, followed by middle esophagus, and upper esophagus. This was consistent with the above study. It is well known that GISTs are considered to arise from the ICCs. Thus, the distribution of esophageal GISTs may be attributed to the distribution of ICCs in the esophagus. Radenkovic et al64 investigated the distribution of ICC populations in human embryonal and fetal esophagus. They found that ICC were abundant in the lower portion, less numerous in the middle region, and rare in the upper part. The reported distribution of ICC was completely in accordance with the distribution of esophageal GISTs in our study. This partially interpreted the distribution of esophageal GISTs.
It was reported that KIT gene mutation occurred in approximately 70% to 80% of GISTs.65 Among them, most are exon 11 mutations,66 followed by exon 9, 13, and 17 mutations.67 Only 20% to 25% of gastric GISTs were associated with platelet-derived growth factor receptor α mutations, including exon 18 and exon 12 mutations.68 B-type Raf kinase kinase mutation occurred rarely according to the previous report.69 In our current study, 25 esophageal GISTs received mutational analysis. Among them, 15 patients (60%) harbor KIT mutations in exon 11, the remaining 10 patients (40%) were KIT wild type. Interestingly, exon 11 mutation was associated with mitotic index in our current study. We found that the mitotic index exceeded 5/50 HPF in the majority of esophageal GISTs with exon 11 mutation, but only in the minority of esophageal GISTs with KIT wild type. The association between mitotic index and KIT mutation needed further investigation in future.
Even with early and R0 resection, there is a high risk of recurrence and metastasis. Distant metastases are the more frequent treatment failure for GISTs and are associated with poor prognosis. No mention of esophageal GISTs-specific recurrence, however, was made. Metastases have a predilection to the liver, omentum, peritoneum, and other intra-abdominal sites, whereas metastases outside the abdomen are uncommon.70 In our current study, the most common site of distant metastasis in esophageal GISTs is liver, followed by lung, thoracic cavity, pleura, peritoneal, and subcutaneous. It is reported that the venous plexus of esophagus in the thorax drain through the hemiazygos and azygos veins in to the superior vena cava and also drain into the portal venous systems.71 Thus, the difference between esophageal and other GISTs in respect to the site of metastasis may attribute to the different venous drainage and specific anatomic site of esophagus.
Approximately 10% to 30% of GISTs are regarded as clinically malignant.72 The majority reports from the literature support a higher malignant potential of esophageal GISTs with an unfavorable outcome,58 and it was considered that the poor outcome is related to the significant higher rate of large tumor size and higher mitotic rate.63 In our current study, the clinicopathologic features of esophageal GISTs were compared with gastric GISTs in our center. The results showed that the distribution of tumor size, histologic type, and NIH risk classification were significantly different between esophageal and gastric GISTs. The esophageal GISTs showed larger tumor size and higher risk classification than gastric GISTs. The distribution of mitotic index between esophageal and gastric GISTs, however, was comparable in our current study.
It is reported that tumor size and mitotic index are the best prognostic indicators for determining the malignant potential of GISTs.73 In our current study, larger tumor size, mitotic index more than 5/50 HPF, and high-risk category were associated with poorer prognosis. Tumor size, however, was the only independent risk factor for prognosis of esophageal GISTs. Rutkowski et al74 reported that primary tumor location was an independent prognostic factor for the prognosis of GISTs. The prognostic features of esophageal GISTs, however, still remain unknown. Considering the significantly different distribution of tumor size and NIH risk category between esophageal and gastric GISTs, patients in the 2 groups were matched by tumor size, mitotic index, and adjuvant imatinib therapy to compare the prognosis between esophageal and gastric GISTs. The survival analysis showed that the DFS and DSS of esophageal GISTs were significantly lower than that of gastric GISTs.
There are some limitations of the current study. First, it is retrospective analysis and lacks systematic prospective data. Therefore, completeness of the data is limited. Second, the sample size of esophageal GISTs was not large enough, which will result in sampling error. Third, because of the limited sample size of duodenal, small intestinal and rectal GISTs in our center, we could not compare the clinicopathologic features and prognosis of esophageal GISTs with nongastric GISTs.
CONCLUSIONS
The most common location for esophageal GISTs was lower esophagus, and most of the esophageal GISTs are high-risk category. Tumor size was the independent risk factor for the prognosis of esophageal GISTs. Esophageal GISTs differ significantly from gastric GISTs in respect to clinicopathologic features. The prognosis of esophageal GISTs was worse than that of gastric GISTs.
Footnotes
Abbreviations: DFS = disease-free survival; DSS = disease-specific survival; GIST = gastrointestinal stromal tumor; HPF = high power field; ICC = interstitial cells of Cajal; NIH = National Institutes of Health; PDGFRA = platelet-derived growth factor receptor α.
FF, YT, and ZL contributed equally to this work.
This study was supported in part by grants from the National Natural Scientific Foundation of China [NO. 31100643, 31570907, 81572306, 81502403, XJZT12Z03].
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Grignol VP, Termuhlen PM. Gastrointestinal stromal tumor surgery and adjuvant therapy. Surg Clin North Am 2011; 91:1079–1087. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Feng F, Li M, et al. Surgical resection should be taken into consideration for the treatment of small gastric gastrointestinal stromal tumors. World J Surg Oncol 2013; 11:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agaimy A, Wang LM, Eck M, et al. Loss of DOG-1 expression associated with shift from spindled to epithelioid morphology in gastric gastrointestinal stromal tumors with KIT and platelet-derived growth factor receptor alpha mutations. Ann Diagn Pathol 2013; 17:187–191. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006; 130:1466–1478. [DOI] [PubMed] [Google Scholar]
- 5.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279:577–580. [DOI] [PubMed] [Google Scholar]
- 6.De Vogelaere K, Van Loo I, Peters O, et al. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg Endosc 2012; 26:2339–2345. [DOI] [PubMed] [Google Scholar]
- 7.Monges G, Bisot-Locard S, Blay JY, et al. The estimated incidence of gastrointestinal stromal tumors in France. Results of PROGIST study conducted among pathologists. Bull Cancer 2010; 97:E16–E22. [DOI] [PubMed] [Google Scholar]
- 8.Constantinoiu S, Gheorghe M, Popa L, et al. Giant esophageal GIST: diagnostic and therapeutic challenge: case report. Chirurgia (Burcur) 2015; 110:300–307. [PubMed] [Google Scholar]
- 9.Neofytou K, Costa Neves M, Giakoustidis A, et al. Effective downsizing of a large oesophageal gastrointestinal stromal tumour with neoadjuvant imatinib enabling an uncomplicated and without tumour rupture laparoscopic-assisted Ivor-Lewis oesophagectomy. Case Rep Oncol Med 2015; 2015:165736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomos P, Damaskos C, Dimitroulis D, et al. Giant esophageal gastrointestinal stromal tumor mimicking mediastinal tumor treated by thoracic approach. Ann Gastroenterol 2015; 28:295–296. [PMC free article] [PubMed] [Google Scholar]
- 11.Nemeth K, Williams C, Rashid M, et al. Oesophageal GIST: a rare breed case report and review of the literature. Int J Surg Case Rep 2015; 10:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaka T, Kanzaki M, Onuki T. Long-term survival after thoracoscopic enucleation of a gastrointestinal stromal tumor arising from the esophagus. J Surg Case Rep 2015; 2015: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Jiffry BO, Allam HM, Hatem M. Single-center experience of surgically resected gastrointestinal stromal tumors: a report of six cases, including a rare case involving the lower esophagus. Oncol Lett 2015; 9:745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mu ZM, Xie YC, Peng XX, et al. Long-term survival after enucleation of a giant esophageal gastrointestinal stromal tumor. World J Gastroenterol 2014; 20:13632–13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massironi S, Rossi RE, Ferrero S, et al. An esophageal gastrointestinal stromal tumor in a patient with MEN1-related pancreatic gastrinoma: an unusual association and review of the literature. J Cancer Res Ther 2014; 10:443–445. [DOI] [PubMed] [Google Scholar]
- 16.Nakano A, Akutsu Y, Shuto K, et al. Giant esophageal gastrointestinal stromal tumor: report of a case. Surg Today 2015; 45:247–252. [DOI] [PubMed] [Google Scholar]
- 17.Qian T, Gao F, Chen MZ, et al. Collision tumor of the esophagus: report of a case with mixed squamous cell carcinoma and gastrointestinal stromal tumor. Int J Clin Exp Pathol 2014; 7:1206–1211. [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagawa S, Tanabe K, Suzuki T, et al. A large esophageal gastrointestinal stromal tumor that was successfully resected after neoadjuvant imatinib treatment: case report. World J Surg Oncol 2014; 12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnamurthy A. A targeted approach to a giant gastrointestinal stromal tumor of the esophagus. Indian J Surg Oncol 2013; 4:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kafeel M, Cheedella NK, Wang JC. Esophageal gastrointestinal stromal tumors presenting as mediastinal mass. Case Rep Oncol 2013; 6:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeno S, Kamei M, Takahashi Y, et al. Long-term survival after excision of a giant esophageal gastrointestinal stromal tumor with imatinib mesylate resistance: report of a case. Surg Today 2014; 44:1764–1767. [DOI] [PubMed] [Google Scholar]
- 22.Nawara C, Augscholl C, Hutter J, et al. Oesophageal GIST at the left tracheobronchial angle: resection with right-sided VATS. Zentralbl Chir 2013; 138:499–501. [DOI] [PubMed] [Google Scholar]
- 23.Markakis CG, Spartalis ED, Liarmakopoulos E, et al. Esophageal gastrointestinal stromal tumor: diagnostic complexity and management pitfalls. Case Rep Surg 2013; 2013:968394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjogren PP, Banerji N, Batts KP, et al. Rare presentation of a gastrointestinal stromal tumor with spontaneous esophageal perforation: a case report. Int J Surg Case Rep 2013; 4:636–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho J, Kang GH, Kim KM, et al. Aggressive gastrointestinal stromal tumour of the oesophagus with homozygous KIT exon 11 deletion mutation. Pathology 2012; 44:260–261. [DOI] [PubMed] [Google Scholar]
- 26.Fardoun T, Peyronnet B, Pery C, et al. Gastrointestinal stromal tumor of the esophagus. Dis Esophagus 2013; 26:336–337. [DOI] [PubMed] [Google Scholar]
- 27.Yamada H, Shinohara T, Yokoyama K, et al. Thoracoscopic enucleation of esophageal gastrointestinal stromal tumor using prone positioning in a patient with severe chronic obstructive lung disease. J Laparoendosc Adv Surg Tech A 2011; 21:635–639. [DOI] [PubMed] [Google Scholar]
- 28.Iannicelli E, Sapori A, Panzuto F, et al. Oesophageal GIST: MDCT findings of two cases and review of the literature. J Gastrointest Cancer 2012; 43:481–485. [DOI] [PubMed] [Google Scholar]
- 29.Wang BY, Liu CC, Shih CS. Thoracoscopic enucleation of a gastrointestinal stromal tumor of the esophagus. Thorac Cardiovasc Surg 2011; 59:190–192. [DOI] [PubMed] [Google Scholar]
- 30.Yilmaz R, Caliskan C, Icoz G. Giant gastrointestinal stromal tumour of the oesophagus presenting with atypic symptom. Aust N Z J Surg 2010; 80:293–294. [DOI] [PubMed] [Google Scholar]
- 31.Imai K, Saito H, Minamiya Y, et al. Pleural dissemination of esophageal gastrointestinal stromal tumors after an eight-year interval following the primary surgery. Gen Thorac Cardiovasc Surg 2010; 58:302–305. [DOI] [PubMed] [Google Scholar]
- 32.Hamada S, Itami A, Watanabe G, et al. Intracranial metastasis from an esophageal gastrointestinal stromal tumor. Int Med (Tokyo, Japan) 2010; 49:781–785. [DOI] [PubMed] [Google Scholar]
- 33.Ozan E, Oztekin O, Alacacioglu A, et al. Esophageal gastrointestinal stromal tumor with pulmonary and bone metastases. Diagn Interv Radiol (Ankara, Turkey) 2010; 16:217–220. [DOI] [PubMed] [Google Scholar]
- 34.Miyata K, Igaki H. A case of gastrointestinal stromal tumor of the esophagus. Jpn J Clin Oncol 2009; 39:695. [DOI] [PubMed] [Google Scholar]
- 35.Dan D, Seetahal S, Persad R. Gastrointestinal stromal tumor of the esophagus. J Natl Med Assoc 2009; 101:462–465. [DOI] [PubMed] [Google Scholar]
- 36.Milman S, Kim AW, Farlow E, et al. Enucleation of a giant esophageal gastrointestinal stromal tumor. Ann Thorac Surg 2009; 87:1603–1605. [DOI] [PubMed] [Google Scholar]
- 37.Spinelli GP, Miele E, Tomao F, et al. The synchronous occurrence of squamous cell carcinoma and gastrointestinal stromal tumor (GIST) at esophageal site. World J Surg Oncol 2008; 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang FC, Tzao C, Cheng YL, et al. Surgical treatment of gastrointestinal stromal tumor in the esophagus: report of three cases. Z Gastroenterol 2007; 45:1252–1256. [DOI] [PubMed] [Google Scholar]
- 39.Blum MG, Bilimoria KY, Wayne JD, et al. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2007; 84:1717–1723. [DOI] [PubMed] [Google Scholar]
- 40.Portale G, Zaninotto G, Costantini M, et al. Esophageal GIST: case report of surgical enucleation and update on current diagnostic and therapeutic options. Int J Surg Pathol 2007; 15:393–396. [DOI] [PubMed] [Google Scholar]
- 41.Masuda T, Toh Y, Kabashima A, et al. Overt lymph node metastases from a gastrointestinal stromal tumor of the esophagus. J Thorac Cardiovasc Surg 2007; 134:810–811. [DOI] [PubMed] [Google Scholar]
- 42.Sakurai N, Yamauchi J, Shibuma H, et al. A case of recurrent GIST of the esophagus which completely responded to imatinib mesilate. Gan To Kagaku Ryoho 2007; 34:237–240. [PubMed] [Google Scholar]
- 43.Al-Salam S, El-Teraifi HA, Taha MS. Could imatinib replace surgery in esophageal gastrointestinal stromal tumor. Saudi Med J 2006; 27:1236–1239. [PubMed] [Google Scholar]
- 44.Basoglu A, Kaya E, Celik B, et al. Giant gastrointestinal stromal tumor of the esophagus presenting with dyspnea. J Thorac Cardiovasc Surg 2006; 131:1198–1199. [DOI] [PubMed] [Google Scholar]
- 45.Huang CS, Hsu WH, Wu YC, et al. Enucleation of an advanced esophageal gastrointestinal stromal tumor with liver metastasis. J Gastroenterol Hepatol 2006; 21:482–483. [DOI] [PubMed] [Google Scholar]
- 46.Axel J, Weickert U, Dancygier H. Gastrointestinal tumor (GIST) of the esophagus in a 34-year-old man: clubbed fingers and alopecia arealis as an early paraneoplastic phenomenon. Dtsch Med Wochenschr 2005; 130:2380–2383. [DOI] [PubMed] [Google Scholar]
- 47.Manu N, Richard P, Howard S. Bleeding esophageal GIST. Dis Esophagus 2005; 18:281–282. [DOI] [PubMed] [Google Scholar]
- 48.Feakins RM, Mears L, Atkinson P, et al. Oesophageal gastrointestinal stromal tumour masquerading as neuroendocrine carcinoma. Histopathology 2005; 47:327–329. [DOI] [PubMed] [Google Scholar]
- 49.Chang WC, Tzao C, Shen DH, et al. Gastrointestinal stromal tumor (GIST) of the esophagus detected by positron emission tomography/computed tomography. Dig Dis Sci 2005; 50:1315–1318. [DOI] [PubMed] [Google Scholar]
- 50.Gouveia AM, Pimenta AP, Lopes JM, et al. Esophageal GIST: therapeutic implications of an uncommon presentation of a rare tumor. Dis Esophagus 2005; 18:70–73. [DOI] [PubMed] [Google Scholar]
- 51.Padula A, Chin NW, Azeez S, et al. Primary gastrointestinal stromal tumor of the esophagus in an HIV-positive patient. Ann Diagn Pathol 2005; 9:49–53. [DOI] [PubMed] [Google Scholar]
- 52.Wada Y, Kadokura M, Kamio Y, et al. Esophageal gastrointestinal stromal tumor surrounding the middle esophagus with dysphagia for 8 years; report of a case. Kyobu Geka 2004; 57:1250–1253. [PubMed] [Google Scholar]
- 53.Iijima S, Maesawa C, Sato N, et al. Gastrointestinal stromal tumour of the oesophagus: significance of immunohistochemical and genetic analyses of the c-kit gene. Eur J Gastroenterol Hepatol 2002; 14:445–448. [DOI] [PubMed] [Google Scholar]
- 54.Zhang FB, Shi HC, Shu YS, et al. Diagnosis and surgical treatment of esophageal gastrointestinal stromal tumors. World J Gastroenterol 2015; 21:5630–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robb WB, Bruyere E, Amielh D, et al. Esophageal gastrointestinal stromal tumor: is tumoral enucleation a viable therapeutic option? Ann Surg 2015; 261:117–124. [DOI] [PubMed] [Google Scholar]
- 56.Winant AJ, Gollub MJ, Shia J, et al. Imaging and clinicopathologic features of esophageal gastrointestinal stromal tumors. AJR Am J Roentgenol 2014; 203:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shinagare AB, Zukotynski KA, Krajewski KM, et al. Esophageal gastrointestinal stromal tumor: report of 7 patients. Cancer Imaging 2012; 12:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang P, Jiao Z, Han B, et al. Clinical characteristics and surgical treatment of oesophageal gastrointestinal stromal tumours. Eur J Cardiothorac Surg 2010; 38:223–227. [DOI] [PubMed] [Google Scholar]
- 59.Lee HJ, Park SI, Kim DK, et al. Surgical resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2009; 87:1569–1571. [DOI] [PubMed] [Google Scholar]
- 60.Agaimy A, Wunsch PH, Dirnhofer S, et al. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: a clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am J Surg Pathol 2008; 32:867–873. [DOI] [PubMed] [Google Scholar]
- 61.Miettinen M, Sarlomo-Rikala M, Sobin LH, et al. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol 2000; 24:211–222. [DOI] [PubMed] [Google Scholar]
- 62.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008; 39:1411–1419. [DOI] [PubMed] [Google Scholar]
- 63.Lott S, Schmieder M, Mayer B, et al. Gastrointestinal stromal tumors of the esophagus: evaluation of a pooled case series regarding clinicopathological features and clinical outcome. Am J Cancer Res 2015; 5:333–343. [PMC free article] [PubMed] [Google Scholar]
- 64.Radenkovic G, Ilic I, Zivanovic D, et al. C-kit-immunopositive interstitial cells of Cajal in human embryonal and fetal oesophagus. Cell Tissue Res 2010; 340:427–436. [DOI] [PubMed] [Google Scholar]
- 65.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 2011; 11:865–878. [DOI] [PubMed] [Google Scholar]
- 66.Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol 2011; 104:865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roggin KK, Posner MC. Modern treatment of gastric gastrointestinal stromal tumors. World J Gastroenterol 2012; 18:6720–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008; 53:245–266. [DOI] [PubMed] [Google Scholar]
- 69.Hostein I, Faur N, Primois C, et al. BRAF mutation status in gastrointestinal stromal tumors. Am J Clin Pathol 2010; 133:141–148. [DOI] [PubMed] [Google Scholar]
- 70.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet (London, England) 2013; 382:973–983. [DOI] [PubMed] [Google Scholar]
- 71.Gavaghan M. Anatomy and physiology of the esophagus. AORN J 1999; 69:372–386.quiz 387-379, 392, 393-374. [DOI] [PubMed] [Google Scholar]
- 72.Huang RX, Xiang P, Huang C. Gastrointestinal stromal tumors: current translational research and management modalities. Eur Rev Med Pharmacol Sci 2014; 18:3076–3085. [PubMed] [Google Scholar]
- 73.Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008; 112:608–615. [DOI] [PubMed] [Google Scholar]
- 74.Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol 2007; 14:2018–2027. [DOI] [PubMed] [Google Scholar]


