Abstract
To systematically assess the relationship between smoking and glioma risk.
A dose–response meta-analysis of case–control and cohort studies was performed. Pertinent studies were identified by searching database and reference lists. Random-effects model was employed to pool the estimates of the relative risks (RRs) with corresponding 95% confidence intervals (CIs).
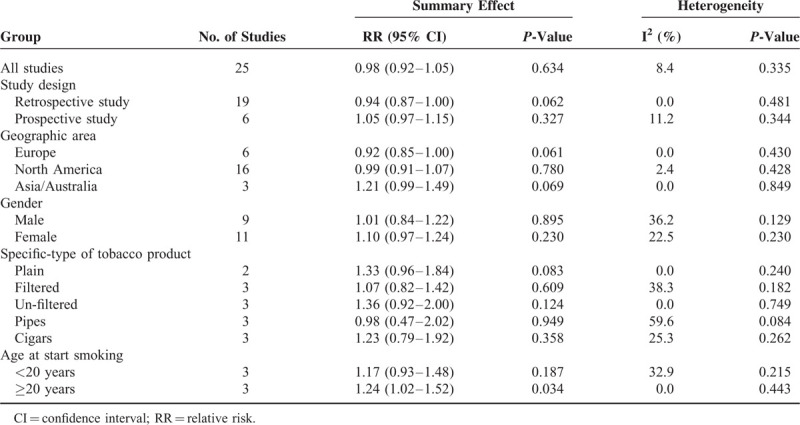
A total of 19 case–control and 6 cohort studies were included. Overall, compared with those who never smoked, the pooled RR and 95% CI was 0.98 (0.92–1.05) for ever smoker. The subgroups were not significantly different regarding risk of glioma except the group of age at start smoking (RR = 1.17, 95% CI: 0.93–1.48 for age < 20; RR = 1.25, 95% CI: 1.02–1.52 for age ≥ 20). Dose–response analysis also suggested no significant association between smoking and the risk of glioma, although some evidence for a linear relationship between smoking and glioma risk was observed.
In conclusion, this meta-analysis provides little support for a causal relationship between smoking and risk of glioma.
INTRODUCTION
Gliomas are the most frequently type of primary brain tumors with high invasiveness and poor prognosis, which are categorized into 4 groups (Grade I to IV) according to World Health Organization (WHO) guideline.1,2 Generally, gliomas are more often with increasing age, male gender, white race, and non-Hispanic ethnicity.3,4 Currently, besides the genetic syndromes (neurofibromatosis type I, Li Fraumeni syndrome), knowledge concerning glioma etiology remains limited to high dose therapeutic ionizing radiation, which was considered to be only well-documented environmental risk factor for gliomas.5,6
Tobacco products provide a major source of exogenous N-nitroso compounds and its neurocarcinogens effect has been shown to induce glioma in animal experiment.7–9 Therefore, cigarette smoking is a plausible and important behavior exposure that might influence the development of glioma and confirming the causal relationship between glioma risk and smoking can provide more effective strategies for the prevention of cancer. Previous epidemiological studies reported conflicting results regarding to smoking and glioma/total brain tumors.10–49 Thus, we systematically reviewed the available literature and performed a meta-analysis of case–control and cohort studies to provide a quantitative assessment of the relationship between smoking and glioma risk.
METHODS
Literature Search
Pertinent studies were identified through a literature search of PubMed and Embase databases. The search used a combination of the following keywords: smoking, smoke, cigarette, tobacco, glioma, brain cancer, brain tumors, and brain neoplasm. No restriction on language was set. Reference lists of eligible studies were also scrutinized to identify other publications of interest that were missed in our literature search. Ethical approval was not required, as our study is a meta-analysis of published studies.
Inclusion Criteria
Case–control or cohort studies of the relationship between smoking and glioma published before June 2015 were considered in this study. To be included in further analyses, estimates of the relative risk (RR) (such as odds ratio, hazard ratio, or risk ratio) with corresponding 95% confidence intervals (CIs) or standard errors (SEs), or raw data should be presented in original studies. When several publications from the same subjects were published, we selected the most informative one. We excluded those studies involving total brain tumors in their subjects, as total brain tumors included both benign tumors and malignant tumors.
Data Extraction
We extracted the following information: first author's last name, year of publication, country where study was undertaken, type of study design, study period for case–control/cohort studies and years of follow-up for cohorts, number of cases and controls/size of cohort, exposure-specific RR estimates with 95% CIs (when more than 1 RR and 95% CI were reported in 1 study, we included the RR that reflected the greatest degree of control for potential confounders), and variables matched between cases and controls/adjusted. Data were extracted and cross-checked independently by 2 reviewers and any disagreements were resolved by discussion.
Data Synthesis and Analysis
In current meta-analysis, odds ratio, hazard ratio, and risk ratio were deemed equivalent to RRs. This combining step is based on the assumption that the prevalence of glioma was rare.50 We estimated the pooled RR with corresponding 95% CI using the random-effects model that accounts for heterogeneity between studies.51 When studies provided results for females and males separately, the risk estimates for females and males were considered to be 2 separate reports.52 When multiple exposure categories in a study fell in the exposure level representing ever smoking, we combined the corresponding estimates with the method proposed by Hamling et al53; otherwise we used random-effects models. Statistical heterogeneity among studies was measured by Cochran Q (significance level at P < 0.10) and I2 tests (values of the I2 test ranges from 0% to 100%).54,55 We performed a sensitivity analysis in which 1 study at a time was omitted and the rest of studies were analyzed to assess whether the results could have been influenced significantly by a single study. An estimation of potential publication bias was performed through funnel plots and Egger test.56,57
We first performed a comparison of ever versus never smoking. Subset analyses were performed according to study design (Retrospective study vs. Prospective study), geographic region (North America vs. Europe vs. Asia/Australia), gender (Male vs. Female), specific-type of tobacco product (Plain vs. Filtered vs. Un-Filtered vs. Pipes vs. Cigars), age at start smoking (<20 vs. ≥20). In further analysis, a dose–response analysis of smoking duration, smoking intensity, and pack-years of smoking was undertaken using the method described by Greenland and Longnecker58 and Orsini et al.59 We assessed a potential curve linear association between smoking and glioma using restricted cubic splines with 3 knots at percentiles 25%, 50%, and 75% of the distribution.60 A P-value for linearity or nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to 0.61 This analysis requires that the distributions of cases and person-years or controls for at least 3 quantitative categories are presented. Also, the RRs with 95% CIs for each category have to be available in original studies. The median or mean smoking duration, smoking intensity, and pack-years of smoking in each category was used as the corresponding dose of consumption. When studies reported the ranges of smoking duration, smoking intensity, and pack-years of smoking, the value assigned to each category was the midpoint for closed categories. If the highest category was open ended, the corresponding category was set at 1.2 times the lower boundary.
All statistical analyses were performed with STATA 12.0 software (StataCorp, College Station, TX).
RESULTS
Literature Search
Our search flow is presented in Figure 1. The search terms initially yielded a total of 723 records from PubMed and Embase databases. The duplicates were omitted first, and then records were further excluded after screening the title and abstract. Thus, 38 records were chosen for full-text assessment. Reasons for exclusion were that studies were meta-analyses or conference abstracts,35–39 investigated the same population,40,41 used reference group improperly,42,43 and involved the total brain tumors.44–49 Three studies of interest were found in reference lists. Ultimately, there are altogether 25 studies included for our statistical analyses.10–34
FIGURE 1.
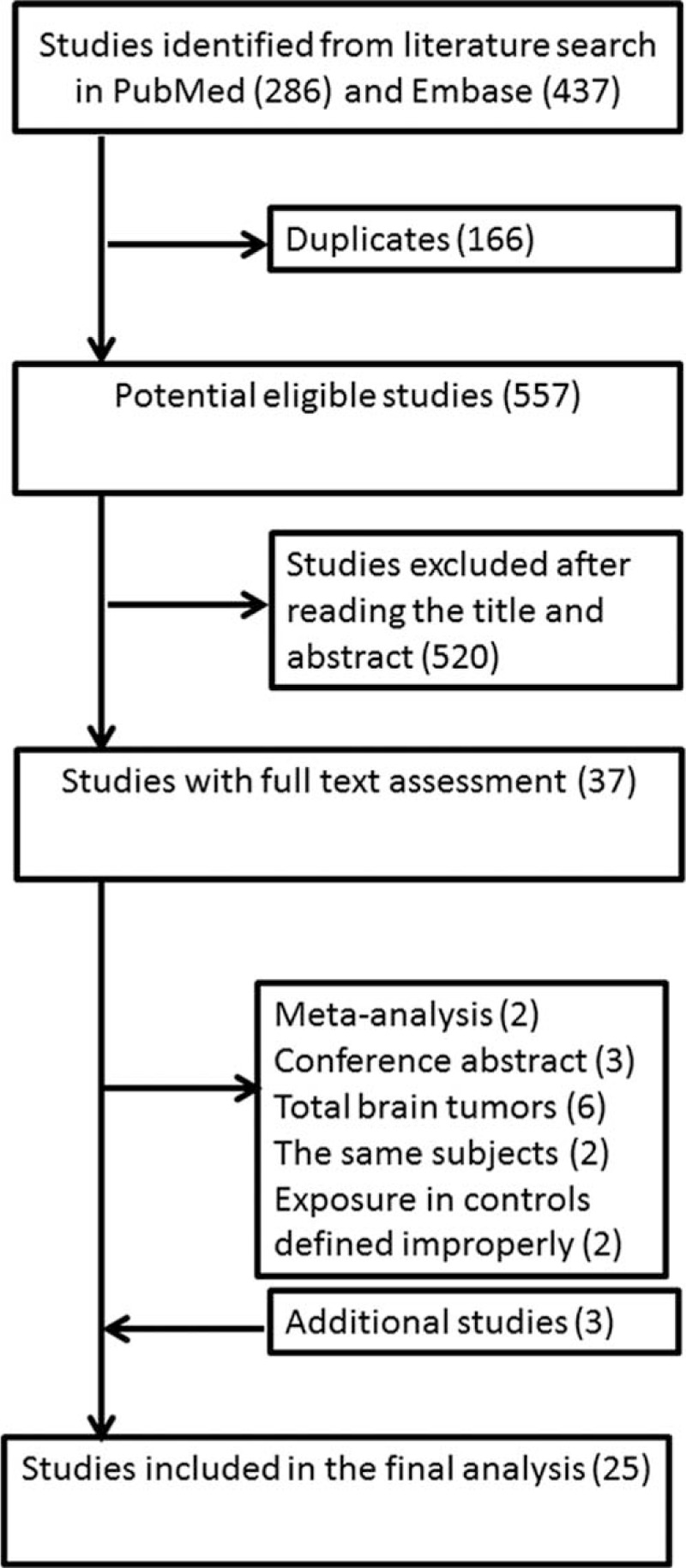
Flow diagram of studies selection for inclusion.
Study Characteristics
The main characteristics of included studies are shown in Table 1 and Table S1 (Supplemental Digital Content 1). The study publish period spanned from 1970 to 2015. All studies were in English. Studies were carried out in different countries, including USA, Italy, Sweden, Canada, Germany, Australia, China, the UK, and France. Nineteen of the included studies were retrospective case–control studies. Correspondingly, the rest of studies were prospective cohort studies. An overwhelming majority of the cases were histologically confirmed, but some cases were radiographic methods, clinical history, or cancer registries. In case–control studies, the controls were recruited from general population, hospitals, neighborhood, and friends. Data for smoking habits were collected by phone interview, face to face interview, or self-reported questionnaire, or reviewing medical records.
TABLE 1.
Characteristics of the Studies Included in This Meta-Analysis of Smoking and Glioma

Overall Association of Smoking and Risk of Glioma
Risk estimates for ever versus never smoking were reported in 25 studies and range from 0.64 to 1.8.10–34Figure 2 shows the forest plots of glioma with smoking. The pooled RR with 95% CI was 0.98 (0.92–1.05).
FIGURE 2.
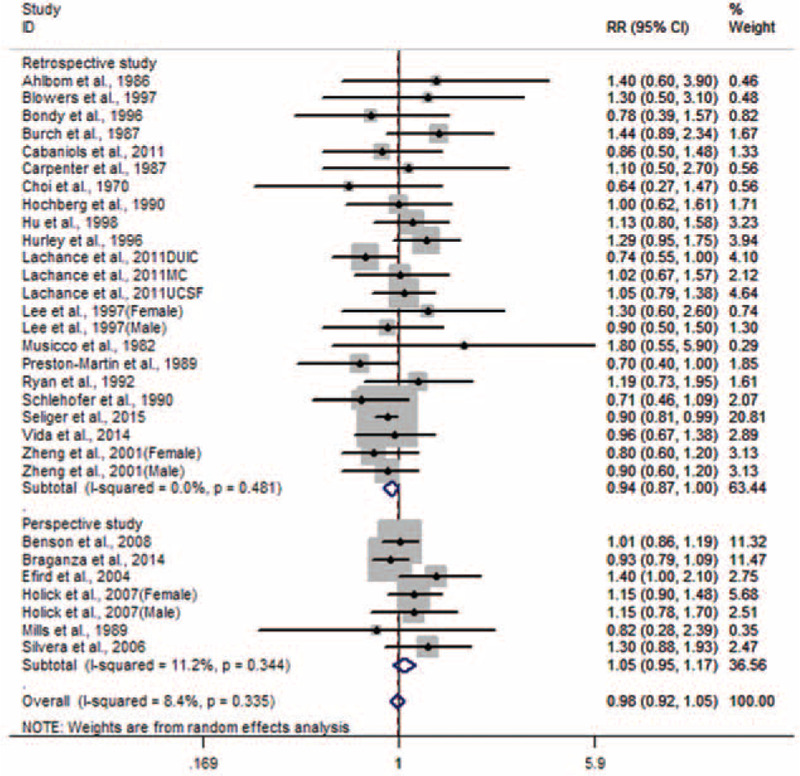
Forest plot for smoking and glioma.
Subset Analyses
Study Type
Associations of glioma with smoking were assessed in 19 retrospective studies and 6 prospective studies. On the basis of prospective studies,15,26–29,32 the pooled RR with 95% CI was 1.05 (0.97–1.15). Correspondingly, the pooled RR with 95% CI was 0.94 (0.87–1.00) for retrospective studies.10–14,16–25,30,31,33,34
Geographic Area
Of the 25 studies, 16 originated from North America,10,13–17,20,22,23,25,28,31–33 6 from Europe,11,12,18,29,30,34 and 3 from Asia/Australia.19,21,24 The pooled RRs with 95% CIs were 0.92 (0.85–1.00), 0.99 (0.91–1.07), and 1.21 (0.99–1.49) for Europe, North America, and Asia/Australia, respectively.
Sex
Twelve studies provided information for females and males separately.16,19,21–23,25–29,32,33 The pooled RR with 95% CI was 1.01 (0.84–1.22) for males, whereas for females group, the pooled RR with 95% CI was 1.10 (0.97–1.24).
Type of Tobacco Product Smoking
The correlation between risk of glioma and specific-types of tobacco product smoking was addressed in 5 studies.13,22,23,26,32 The pooled RRs with 95% CIs were 1.33 (0.96–1.84) for plain, 1.07 (0.82–1.42) for filtered, 1.36 (0.92–2.00) for un-filtered, 0.98 (0.47–2.02) for pipes, and 1.23 (0.79–1.92) for cigars.
Age at Start Smoking
Three studies investigated the relationship between glioma risk and age at start smoking.21,27,28 The pooled RR with 95% CI was 1.17 (0.93–1.48) for smokers who were younger than age 20 at start smoking, while in smokers who were older than 20, the pooled RR with 95% CI was 1.25 (1.02–1.52).
Test Heterogeneity
Evaluation of heterogeneity suggested there was no significant heterogeneity observed (Table 2) except in subgroups of pipes (I2 = 59.6%, P = 0.084).
TABLE 2.
Summary RRs for Smoking and Glioma
Sensitivity Analysis and Publication Bias
The results were robust to the exclusion of any individual study (Figure 3). No significant publication bias was detected by Egger test (P for Egger test = 0.157) and Begg funnel plot (Figure 4).
FIGURE 3.

Sensitivity analyses for smoking and glioma.
FIGURE 4.

Begg funnel plot for smoking and glioma.
Dose–Response
Duration of Smoking
Five studies were eligible for the dose–response analysis of duration of smoking with glioma risk.21,25,27,28,33 Some evidence of a linear relationship between smoking and glioma risk was observed (P = 0.236), but the results were not statistically significant and the risk did not increase sharply (Figure 5).
FIGURE 5.
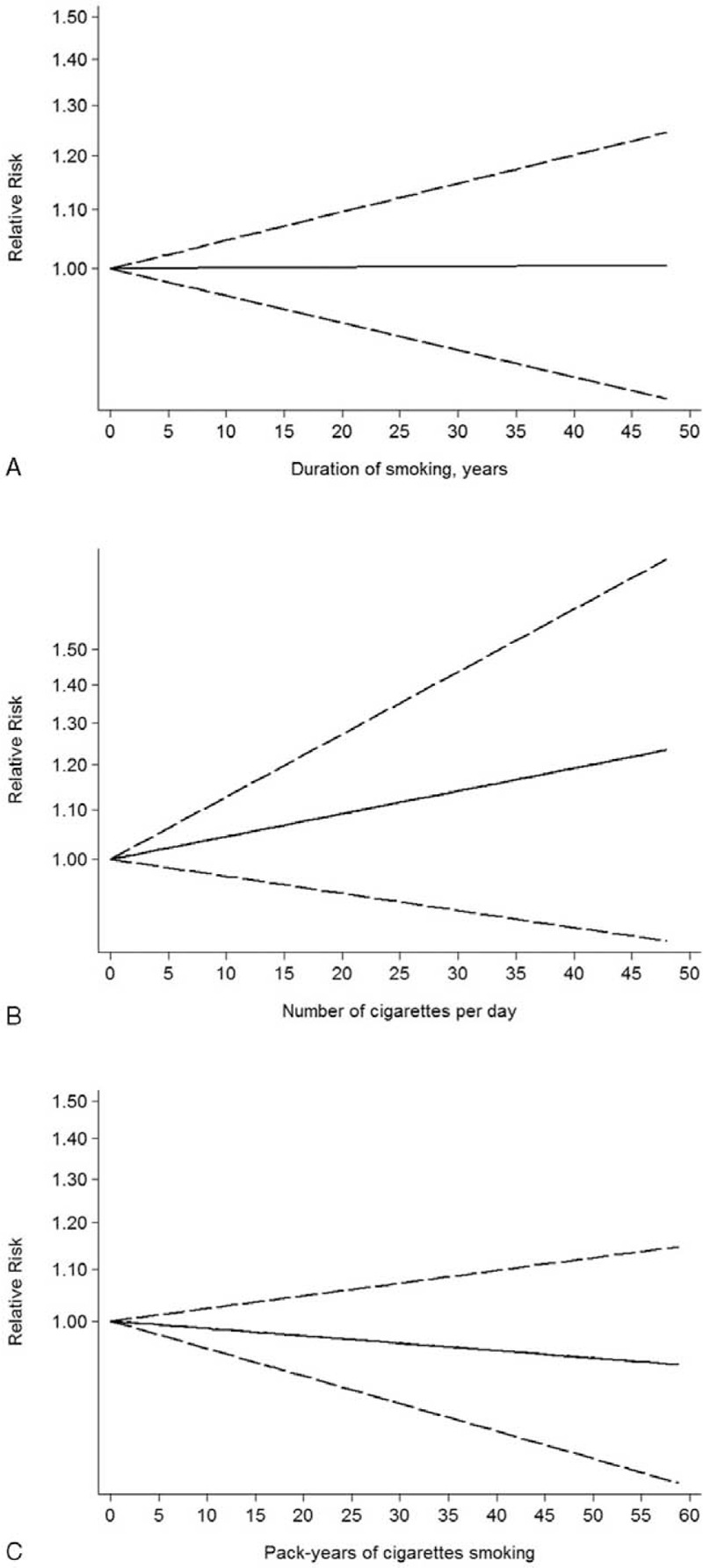
Dose–response relationship between glioma risk and smoking duration (A), smoking intensity (B), and pack-years of smoking (C). Solid line represents the estimated relative risk and the dashed lines represent the 95% confidence intervals.
Smoking Intensity
There are 4 studies providing the sufficient data required for dose effect of smoking intensity.17,25–27 The linear dose–response trend (P = 0.568) showed a nonstatistically significant increased risk of glioma with increasing number of cigarettes per day (Figure 5).
Number of Pack-Years
Five studies investigated a dose–risk relationship between number of pack-years smoking and glioma.21,25,27,28,33 Similarly, no evidence of statistically significant departure from linearity (P = 0.201). As shown in Figure 5, a linear trend of nonstatistically significant decreased risk of glioma risk with larger pack-years of smoking.
DISCUSSION
A significant number of case–control and cohort studies investigated the relationship between smoking and risk of glioma, but inconsistent results were shown. To settle disputes, a meta-analysis of 17 case–control and cohort studies which covered the studies published up to the end of 2008 was performed, and Mandelzweig et al36 concluded that smoking was not associated with risk of glioma. Since then, several studies with large simple size were published. Therefore, an updated meta-analysis was conducted to better understand the association between smoking exposure and glioma. Compared with previous meta-analysis, in our study, we excluded the studies of total brain tumors associated with smoking,44–49 included studies published to date; group-analyzed by study design (Retrospective study vs. Prospective study), geographic region (North America vs. Europe vs. Asia), gender (Male vs. Female), and specific-type of tobacco product (Plain vs. Filtered vs. Un-Filtered vs. Pipes vs. Cigars), age at start smoking (<20 vs. ≥20), and investigated the possible dose–response analysis of duration of smoking, smoking intensity, and number of pack-years smoking with glioma risk. Our meta-analysis included 19 case–control and 6 cohort studies with more than 7000 patients. Finding from current meta-analysis shows that when compared with people who have never smoked, ever smoking was not is significantly associated with glioma risk. These results were consisted between retrospective studies and prospective studies. In subgroup analysis by geographic area, an increased risk of borderline significance was observed for Asia/Australia, but a decreased risk of marginal significance for Europe. The significance of these findings is unclear, and thus this is a field of ongoing investigation. Besides the issue of geographic area, several other points disclosed in our study were also worth of paying attention.
Involvement of the neuroprotective effect of estrogens in the development of glioma has been shown in experimental and animal studies.62 In observational studies, glioma occurs 1.5 to 2 times more frequently in men than in women.62 Among the included studies, 12 studies provided data for females and males separately.16,19,21–23,25–29,32,33 However, the issue of risk modification by gender was not addressed previous meta-analyses.35,36 In present study, further stratification by sex revealed no sex-based differences were observed between smoking and glioma risk.
Changes in cigarette design and composition have gradually occurred since 1950.63,64 The changes included lower tar and nicotine and increasing use of tobacco additives, some of which were thought to be carcinogens or lead to an increase of carcinogenic substances during combustion. These changes were mainly achieved through the introduction of filter tips, selection of tobacco types and varieties, use of highly porous cigarette paper. Hence, having a good understand of the type of tobacco smoking on tumor risk is important. Specific-type of tobacco smoking (filtered, unfiltered, regular (85 mm,), king-sized (100 mm), long, mentholated, plain, pipes, and cigars) with glioma risk was assessed in 5 studies.13,22,23,26,32 Overall, no significant association between specific-type of tobacco smoking and the risk of glioma, with one exception of 133,811 subjects, which showed a small and positive risk of marginal significance associated with filtered, regular (85 mm), and plain.26 Our study revealed that there was no difference in the risk of glioma by type of tobacco smoking.
Mixed results have been reported between age at start smoking and the risk of glioma.21,22,27,28 The observation of a 67% increase in glioma risk among smokers who started smoking before the age of 20 was observed in a prospective cohort study of 89,709 Canadian women.27 However, the opposite was reported only for men aged ≥20 when the subjects started smoking (RR = 2.72; 95% CI: 1.48–5.02).21 Two other investigation reported no significant association between age at start smoking and glioma risk.22,28 Since the age groups matched perfectly in previous studies, we performed a subset analyses of age at smoking initiation (<20 vs. ≥20). The pooled results showed that a significant increased risk of glioma was found in smokers who were older than 20 years, but not in smokers who were younger than age 20 at start smoking. Early age at the start of smoking usually implies a longer period of tobacco exposure and thus those smokers may bear a larger risk of glioma. Thus, interpretation of this finding should be with caution.
Estimation of dose–response association in observational studies provides more evidence for establishing a causal association between lifelong exposure and disease. We performed a dose–response analysis of smoking duration, smoking intensity, and pack-years of smoking using the method described by Greenland and Longnecker and Orsini and colleagues. These analyses showed a linear trend between smoking and glioma risk, although all results were not statistically significant. Interestedly, a linear trend of decreased risk of glioma risk with larger pack-years of smoking was observed, whereas a linear relationship of increased risk of glioma with increasing number of cigarettes per day. Considering few studies were included in dose–response analysis, the finding, of 2 opposite trends, is a chance finding. Therefore, the issue of how glioma risk changed with the dose effect of smoking duration, smoking intensity, and pack-years of smoking deserves open discussion.
Two problematic smoking exposure of interest were “current smoking” and “past smoking,” which were evaluated in 9 studies.11,18,19,25–29,32 Mandelzweig et al36 found that current smoking was not significantly associated with glioma risk, while past smokers seemed to have an increased risk of glioma (RR = 1.10, 95% CI: 0.99–1.22). In subset analyses by study design, this trend was much stronger in case–control studies (RR = 1.16, 95% CI: 1.04–1.29) and disappeared in cohort studies (RR = 0.90, 95% CI: 0.73–1.11). It is important to note that different exposure time before reference date in retrospective versus prospective studies was taken into account between current and past smoking. Therefore, a uniform definition of current and past smoking was not established in original studies and thus a subgroup analysis by smoking statue associated with glioma risk was not performed in our study. Moreover, 4 studies raised the question whether there was a threshold after quitting smoking when glioma decreased.17,22,27,32 No significant association (inverse or positive) between time since smoking cessation and glioma risk was shown in 2 case–control and one cohort studies.17,22,32 However, an inverse association between past smokers who stopped >10 years before baseline in comparison to those who stopped within the 10 years before baseline (RR = 0.39, 95% CI: 0.19–0.82) in a prospective cohort study with a mean of 16.4 years of follow-up, which included 89,709 Canadian women aged 40 to 59 years.27
The relationship between smoking and other brain tumors have been investigated by a multitude of case–control or cohort studies, with conflicted finding reported. Two meta-analyses of the association between smoking and meningioma had been published.65,66 A meta-analysis of 6 studies found that females who had ever smoking were at significantly decreased risk of meningioma relative to never smokers (OR = 0.82, 95% CI: 0.68–0.98).65 However, for males, ever smokers were associated with a significantly increased risk of meningioma, compared with never smokers (OR = 1.39, 95% CI: 1.08–1.79).65 In another meta-analysis of 7 case–control and 2 cohort studies by Fan et al,66 no association between ever smoking and the risk of meningioma was observed and no significant differences in subgroup results of study design, type of exposure assessment, and gender. For pituitary tumors and acoustic neuroma, 4 studies investigated the relationship with smoking.67–70 Schoemaker et al67 first performed a population-based case–control study of 563 acoustic neuroma cases and 2703 controls in the UK and Nordic countries. Acoustic neuroma tumor risk was significantly reduced in subjects who had ever regularly smoked cigarettes (OR = 0.7, 95% CI: 0.6–0.9), but the reduction did not apply to ex-smokers (OR = 1.0, 95% CI: 0.8–1.3).67 Evidence from a case–control study of 299 cases and 630 controls aged 18 to 59 years shows no association was found between smoking and incidence of pituitary tumor.68 In a prospective study of 1.2 million middle-aged women in the United Kingdom with an average of 8.2 follow-up years, Benson et al69 confirmed that current smokers were at a decreased risk of acoustic neuroma (RR = 0.41, 95% CI:0.24–0.70) and past smokers did not have significantly different risk of acoustic neuroma than never smokers (RR = 0.87, 95% CI:0.62–1.22). Similarly, females smokers were not at a significantly reduced risk of pituitary tumors relative to never smokers (RR in current vs. never smokers = 0.9, 95% CI:0.60–1.40)69 and the risk of acoustic neuroma was reduced in current smokers (OR = 0.54, 95% CI:0.36–0.81).70
Three major limitations involved in our study should be raised. First, because of the observational design of the included studies, the effect of potential confounding is a well-known problem of concern. A close relationship between smoking and alcohol, education, income, and social class is usually to be considered. Thus, residual confounding or even interaction between these variables should be clarified in future. Moreover, various biases (ie, select bias, recall bias, information bias) in observational studies may also result in an overestimation or underestimation of the true association. For example, 2 opposite trends were observed between case–control studies and cohort studies, although the results did not reach significance (Table 2). Second, since our analysis was based on the published studies, potential publication bias could have reduced the reliability of our finding. Third, we could not perform a comprehensive analysis according to different grades of glioma defined by WHO guideline to investigate the true relationship, as almost all of include studies did not report results separately for subtype of gliomas. Gliomas include astrocytomas, oligodendrogliomas, ependymomas, glioblastoma, and other tumors arising from glial cells, which show different biological behaviors, such as invasion and prognosis. Thus, they may share little in the field of etiology, which would likely reflect different responses to neurocarcinogens from smoking. Further assessment of the relationship between smoking and gliomas should pay more attention to the subtype of tumors.
In conclusion, this updated dose–response meta-analysis suggests smoking is not significantly associated with risk of glioma. Further large sample size, prospective design, and long follow-up studies with particular attention to the effect of gender, type of tobacco product smoking, smoking status, dosage, duration, intensity, age at smoking initiation, and years since quitting on glioma risk are warranted to confirmed these findings.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals; RR = relative risk; UK = United Kingdom; WHO = World Health Organization.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article.
REFERENCES
- 1.Adel Fahmideh M, Schwartzbaum J, Frumento P, et al. Association between DNA repair gene polymorphisms and risk of glioma: a systematic review and meta-analysis. Neuro Oncol 2014; 16:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing WK, Shao C, Qi ZY, et al. The role of Gliadel wafers in the treatment of newly diagnosed GBM: a meta-analysis. Drug Des Devel Ther 2015; 9:3341–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 2013; 15(Suppl. 2):ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol 2002; 4:278–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrom QT, Barnholtz-Sloan JS. Current state of our knowledge on brain tumor epidemiology. Curr Neurol Neurosci Rep 2011; 11:329–335. [DOI] [PubMed] [Google Scholar]
- 6.Qi ZY, Shao C, Yang C, et al. Alcohol consumption and risk of glioma: a meta-analysis of 19 observational studies. Nutrients 2014; 6:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleihues P, Lantos PL, Magee PN. Chemical carcinogenesis in the nervous system. Int Rev Exp Pathol 1976; 15:153–232. [PubMed] [Google Scholar]
- 8.Bogovski P, Bogovski S. Animal Species in which N-nitroso compounds induce cancer. Int J Cancer 1981; 27:471–474. [DOI] [PubMed] [Google Scholar]
- 9.Maekawa A, Mitsumori K. Spontaneous occurrence and chemical induction of neurogenic tumors in rats—influence of host factors and specificity of chemical structure. Crit Rev Toxicol 1990; 20:287–310. [DOI] [PubMed] [Google Scholar]
- 10.Choi NW, Schuman LM, Gullen WH. Epidemiology of primary central nervous system neoplasms. II. Case-control study. Am J Epidemiol 1970; 91:467–485. [DOI] [PubMed] [Google Scholar]
- 11.Musicco M, Filippini G, Bordo BM, et al. Gliomas and occupational exposure to carcinogens: case-control study. Am J Epidemiol 1982; 116:782–790. [DOI] [PubMed] [Google Scholar]
- 12.Ahlbom A, Navier IL, Norell S, et al. Nonoccupational risk indicators for astrocytomas in adults. Am J Epidemiol 1986; 124:334–337. [DOI] [PubMed] [Google Scholar]
- 13.Burch JD, Craib KJ, Choi BC, et al. An exploratory case-control study of brain tumors in adults. J Natl Cancer Inst 1987; 78:601–609. [PubMed] [Google Scholar]
- 14.Carpenter AV, Flanders WD, Frome EL, et al. Brain cancer and nonoccupational risk factors: a case-control study among workers at two nuclear facilities. Am J Public Health 1987; 77:1180–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills PK, Preston-Martin S, Annegers JF, et al. Risk factors for tumors of the brain and cranial meninges in Seventh-Day Adventists. Neuroepidemiology 1989; 8:266–275. [DOI] [PubMed] [Google Scholar]
- 16.Preston-Martin S, Mack W, Henderson BE. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res 1989; 49:6137–6143. [PubMed] [Google Scholar]
- 17.Hochberg F, Toniolo P, Cole P, et al. Nonoccupational risk indicators of glioblastoma in adults. J Neurooncol 1990; 8:55–60. [DOI] [PubMed] [Google Scholar]
- 18.Schlehofer B, Kunze S, Sachsenheimer W, et al. Occupational risk factors for brain tumors: results from a population-based case-control study in Germany. Cancer Causes Control 1990; 1:209–215. [DOI] [PubMed] [Google Scholar]
- 19.Ryan P, Lee MW, North B, et al. Risk factors for tumors of the brain and meninges: results from the Adelaide Adult Brain Tumor Study. Int J Cancer 1992; 51:20–27. [DOI] [PubMed] [Google Scholar]
- 20.Bondy ML, Kyritsis AP, Gu J, et al. Mutagen sensitivity and risk of gliomas: a case-control analysis. Cancer Res 1996; 56:1484–1486. [PubMed] [Google Scholar]
- 21.Hurley SF, McNeil JJ, Donnan GA, et al. Tobacco smoking and alcohol consumption as risk factors for glioma: a case-control study in Melbourne, Australia. J Epidemiol Community Health 1996; 50:442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blowers L, Preston-Martin S, Mack WJ. Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA). Cancer Causes Control 1997; 8:5–12. [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Wrensch M, Miike R. Dietary and tobacco risk factors for adult onset glioma in the San Francisco Bay Area (California, USA). Cancer Causes Control 1997; 8:13–24. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Johnson KC, Mao Y, et al. Risk factors for glioma in adults: a case-control study in northeast China. Cancer Detect Prev 1998; 22:100–108. [DOI] [PubMed] [Google Scholar]
- 25.Zheng T, Cantor KP, Zhang Y, et al. Risk of brain glioma not associated with cigarette smoking or use of other tobacco products in Iowa. Cancer Epidemiol Biomarkers Prev 2001; 10:413–414. [PubMed] [Google Scholar]
- 26.Efird JT, Friedman GD, Sidney S, et al. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neurooncol 2004; 68:57–69. [DOI] [PubMed] [Google Scholar]
- 27.Silvera SA, Miller AB, Rohan TE. Cigarette smoking and risk of glioma: a prospective cohort study. Int J Cancer 2006; 118:1848–1851. [DOI] [PubMed] [Google Scholar]
- 28.Holick CN, Giovannucci EL, Rosner B, et al. Prospective study of cigarette smoking and adult glioma: dosage, duration, and latency. Neuro Oncol 2007; 9:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benson VS, Pirie K, Green J, et al. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer 2008; 99:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabaniols C, Giorgi R, Chinot O, et al. Links between private habits, psychological stress and brain cancer: a case-control pilot study in France. J Neurooncol 2011; 103:307–316. [DOI] [PubMed] [Google Scholar]
- 31.Lachance DH, Yang P, Johnson DR, et al. Associations of high-grade glioma with glioma risk alleles and histories of allergy and smoking. Am J Epidemiol 2011; 174:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braganza MZ, Rajaraman P1, Park Y1, et al. Cigarette smoking, alcohol intake, and risk of glioma in the NIH-AARP Diet and Health Study. Br J Cancer 2014; 110:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vida S, Richardson L, Cardis E, et al. Brain tumours and cigarette smoking: analysis of the INTERPHONE Canada case-control study. Environ Health 2014; 13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seliger C, Ricci C, Meier CR, et al. Diabetes, use of antidiabetic drugs, and the risk of glioma. Neuro Oncol 2015; pii: nov100.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang HJ, Yang F, Gai Y. Meta-analysis of the association between smoking and glioma. Chinese J Cancer Prevent Treat 2012; 19:1848–1851. [Google Scholar]
- 36.Mandelzweig L, Novikov I, Sadetzki S. Smoking and risk of glioma: a meta-analysis. Cancer Causes Control 2009; 20:1927–1938. [DOI] [PubMed] [Google Scholar]
- 37.Braganza MZ, Rajaraman P, Park Y, et al. Are alcohol drinking and cigarette smoking related to risk of glioma? A large prospective U.S. cohort study. Cancer Res 2013; 73:2516. [Google Scholar]
- 38.Pardaz MZ, Jessri M, Shirazi MM, et al. Dietary risk factors of adult-onset glioma: a case-control study. Support Care Cancer 2012; 20:S208. [Google Scholar]
- 39.Choi NW, Schuman LM, Gullen WH. Epidemiology of central nervous system neoplasms: a case-control study. Neurology 1968; 18:S208. [PubMed] [Google Scholar]
- 40.Preston-Martin S, Mack W. Gliomas and meningiomas in men in Los Angeles County: investigation of exposures to N-nitroso compounds. IARC Sci Publ 1991; 105:197–203. [PubMed] [Google Scholar]
- 41.Giles GG, McNeil JJ, Donnan G, et al. Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne, Australia. Int J Cancer 1994; 59:357–362. [DOI] [PubMed] [Google Scholar]
- 42.Gousias K, Markou M, Voulgaris S, et al. Descriptive epidemiology of cerebral gliomas in northwest Greece and study of potential predisposing factors, 2005–2007. Neuroepidemiology 2009; 33:89–95. [DOI] [PubMed] [Google Scholar]
- 43.Zampieri P, Meneghini F, Grigoletto F, et al. Risk factors for cerebral glioma in adults: a case-control study in an Italian population. J Neurooncol 1994; 19:61–67. [DOI] [PubMed] [Google Scholar]
- 44.Brownson RC, Reif JS, Chang JC, et al. An analysis of occupational risks for brain cancer. Am J Public Health 1990; 80:169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLaughlin JK, Hrubec Z, Blot WJ, et al. Smoking and cancer mortality among U.S. veterans: a 26-year follow-up. Int J Cancer 1995; 60:190–193. [PubMed] [Google Scholar]
- 46.Batty GD, Kivimaki M, Gray L, et al. Cigarette smoking and site-specific cancer mortality: testing uncertain associations using extended follow-up of the original Whitehall study. Ann Oncol 2008; 19:996–1002. [DOI] [PubMed] [Google Scholar]
- 47.Hu J, La Vecchia C, Negri E, et al. Diet and brain cancer in adults: a case-control study in northeast China. Int J Cancer 1999; 81:20–23. [DOI] [PubMed] [Google Scholar]
- 48.Cerhan JR, Cantor KP, Williamson K, et al. Cancer mortality among Iowa farmers: recent results, time trends, and lifestyle factors (United States). Cancer Causes Control 1998; 9:311–319. [DOI] [PubMed] [Google Scholar]
- 49.Hinds MW, Kolonel LN, Lee J, et al. Associations between cancer incidence and alcohol/cigarette consumption among five ethnic groups in Hawaii. Br J Cancer 1980; 41:929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9:1–30. [DOI] [PubMed] [Google Scholar]
- 51.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 52.Rong Y, Chen L, Zhu T, et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ 2013; 346:e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008; 27:954–970. [DOI] [PubMed] [Google Scholar]
- 54.Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10:101–129. [Google Scholar]
- 55.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Begg CB1, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 57.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992; 135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 59.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006; 6:40–57. [Google Scholar]
- 60.Ma Y, Zhang P, Wang F, et al. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol 2011; 29:3775–3782. [DOI] [PubMed] [Google Scholar]
- 61.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 1988; 80:1198–1202. [DOI] [PubMed] [Google Scholar]
- 62.Qi ZY, Shao C, Zhang X, et al. Exogenous and endogenous hormones in relation to glioma in women: a meta-analysis of 11 case-control studies. PLoS ONE 2013; 8:e68695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holbrook JH. The changing cigarette. West J Med 1981; 134:306. [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health 1997; 50:307–364. [DOI] [PubMed] [Google Scholar]
- 65.Claus EB, Walsh KM, Calvocoressi L, et al. Cigarette smoking and risk of meningioma: the effect of gender. Cancer Epidemiol Biomarkers Prev 2012; 21:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan Z, Ji T, Wan S, et al. Smoking and risk of meningioma: a meta-analysis. Cancer Epidemiol 2013; 37:39–45. [DOI] [PubMed] [Google Scholar]
- 67.Schoemaker MJ, Swerdlow AJ, Auvinen A, et al. Medical history, cigarette smoking and risk of acoustic neuroma: an international case-control study. Int J Cancer 2007; 120:103–110. [DOI] [PubMed] [Google Scholar]
- 68.Schoemaker MJ, Swerdlow AJ. Risk factors for pituitary tumors: a case-control study. Cancer Epidemiol Biomarkers Prev 2009; 18:1492–1500. [DOI] [PubMed] [Google Scholar]
- 69.Benson VS, Green J, Pirie K, et al. Cigarette smoking and risk of acoustic neuromas and pituitary tumours in the Million Women Study. Br J Cancer 2010; 102:1654–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palmisano S, Schwartzbaum J, Prochazka M, et al. Role of tobacco use in the etiology of acoustic neuroma. Am J Epidemiol 2012; 175:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


