Abstract
The proposed bidirectional relationship between acute pancreatitis (AP) and diabetes has never been examined with the same source of data. Furthermore, the effects of disease severity on this relationship have not been fully evaluated. The present study employed the findings from a single database to measure the strength of the association between AP and diabetes.
Findings from 1 million National Health Insurance beneficiaries were utilized. Two cohort studies with this database were selected to evaluate the linkage between diabetes and AP. The first cohort analysis addressed the risk of AP among diabetic patients and was comprised of 42,080 diabetic patients and 672,146 unexposed subjects. The second cohort analysis considered the risk of diabetes among patients with AP and enrolled 3187 patients with AP and 709259 unexposed subjects. All adult beneficiaries were followed from January 1, 2005 to December 31, 2012 to identify outcomes of interest. Cox regression models were applied to compare hazards adjusted for potential confounders.
For the first cohort, the adjusted hazard ratio (HR) of AP was significantly increased by the presence of diabetes (1.72; 95% confidence interval [CI], 1.52–1.96). In diabetic patients with a history of hyperglycemic crisis episodes (HCEs), the HR was even higher (6.32; 95% CI, 4.54–8.81). For the second cohort, the adjusted HR of diabetes in patients with AP was increased compared to the general population (2.15; 95% CI, 1.92–2.41). For patients with severe AP, the HR was also higher (2.22; 95% CI, 1.50–3.29) but did not differ significantly from that for patients with nonsevere AP.
The 2 cohort studies provided evidence for the bidirectional relationship between diabetes and AP. Moreover, diabetic patients with history of HCEs may be associated with higher risk of AP.
INTRODUCTION
Acute pancreatitis (AP), a condition characterized by pancreatic inflammation, is often associated with increased morbidity and mortality.1,2 The etiology of AP is multifactorial, with factors such as alcohol consumption, gallstones, certain drugs, renal insufficiency, and hypertriglyceridemia reported to increase the risk of this condition.3–5 Additionally, recent findings of several large cohort studies reveal that patients with diabetes mellitus are at increased risk of AP.6–8 Despite this well-supported linkage, no study has yet been performed to examine the possibility that the risk of AP increases with the severity of diabetes.
Although hyperglycemia is commonly observed in patients with AP,9,10 findings of studies regarding newly diagnosed diabetes in subjects with AP are conflicting. Whereas a positive relationship between pre-existing AP and development of diabetes has been observed in some studies,11,12 no association was found in others.13 Authors of a recently published meta-analysis concluded that patients treated for AP may be at higher risk of developing diabetes after discharge; however, the severity of AP had a minimal effect on the studied outcomes in this analysis.14
To the knowledge of the authors of this report, no study investigating the bidirectional relationship between AP and diabetes has been performed with the same population and with the same source of data. Furthermore, the effects of disease severity on this relationship have not been fully evaluated. The present study employed a large administrative database from the Taiwan National Health Insurance (NHI) program, which provides healthcare coverage to Taiwan residents of all ages. The overall goal was to assist clinicians in the identification of individuals at risk of these frequently encountered diseases. Given the increasing incidence of both diseases,15,16 efforts to prevent their occurrences should result in a decrease in the population-attributable risk percentage.
METHODS
Ethics Statement
This study was initiated after approval from the Institutional Review Board of Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan. Since all personal identification was removed from the secondary files before any analysis was performed, the review board waived the requirement for written informed consent from the involved patients.
Database
The NHI program was implemented in 1995 and provides compulsory universal health insurance, which enrolls up to 99% of the Taiwanese population and has contracts with 97% of all medical providers.17 The database analyzed in this study included 1 million beneficiaries randomly selected from all beneficiaries insured in 2005. Statistically significant differences were not observed for this group as compared to the larger cohort with respect to age, sex, or healthcare costs according to the Taiwan National Health Research Institute.18,19 The database contains comprehensive information on all insured individuals, including gender, date of birth, residential location, dates of clinical visits, the International Classification of Diseases (Ninth Revision) Clinical Modification (ICD-9-CM) diagnostic codes, details regarding prescribed medications, and outcomes at hospital discharge.20,21
Analysis of the Association Between Diabetes and Risk of Acute Pancreatitis
The sampled population was followed from January 1, 2003 to December 31, 2012. Individuals were initially identified for the study cohort who were still alive in 2005 and who were older than 18 years. Patients diagnosed with diabetes (ICD-9-CM code: 250) were then identified from records of their ambulatory care claims during the follow-up period.22 To avoid coding error, an individual could be classified as a diabetic patient only if she/he received a diagnosis of diabetes and then experienced another one or more diagnoses within the subsequent 12 months. Moreover, the first and last visits during the 10-year period had to be separated by at least 30 days to avoid accidental inclusion of patients with miscoded diagnoses. AP was defined by the presence of ICD-9-CM code 577.0 in any position of the diagnoses. In order to maximize case ascertainment, only patients hospitalized for AP were included. These selection processes have been well-validated with high positive predictive values.4,23
After excluding patients with diabetes and AP before January 1, 2005, 42,080 patients were included in the diabetic group and 672,146 in the unexposed group. Diabetes with poor compliance was defined as a hospitalization with the diagnosis of a hyperglycemic crisis episode (HCE) (ICD-9-CM codes: diabetic ketoacidosis, 250.1 or hyperosmolar hyperglycemic state, 250.2).24 The index date for each diabetic patient was the date of his or her first diabetes diagnosis. The index date for subjects in the unexposed group was set as January 1, 2005. Subjects in the diabetic and unexposed groups subjects were then followed through December 31, 2012 for possible episodes of AP. Cases were censored for patients who were no longer beneficiaries of the NHI Program (ie, death or transfer out) or who were still robust at the end of the follow-up period (Figure 1A).
FIGURE 1.

Flow diagram of the population-based study. (A) Diabetes and the risk of acute pancreatitis. (B) Pancreatitis and the risk of diabetes.
Analysis of the Association Between Acute Pancreatitis and Risk of Diabetes
The second cohort analysis assessed the association of AP with an increased risk of diabetes. The diagnostic criteria for AP and diabetes were the same as those for the first cohort. In total, 3187 subjects with AP were enrolled in the AP group and 709,259 were enrolled in the unexposed group after excluding patients with diabetes and AP before January 1, 2005. Severe AP was defined as a hospitalization in an intensive care unit setting.2 The index date for each AP patient was the date of his or her first admission. The index date for subjects in the unexposed group was set as January 1, 2005. Subjects in the AP and unexposed groups were then followed until December 31, 2012 for possible diagnosis of diabetes. Cases were censored for patients who were no longer beneficiaries of the NHI Program (ie, death or transfer out) or who were still robust at the end of the follow-up period (Figure 1B).
Covariates
To better characterize the relationships between diabetes and AP, several covariates were used, including patient demographics such as age, gender, urbanization level, and socioeconomic status (SES). The age of each patient was defined by the difference between the index date and the date of birth. Income-related insurance payment amounts were used as a proxy measure of individual SES at follow-up.20
Additionally, the prevalence of specific comorbid conditions (chronic liver disease, hypertension, coronary artery disease, hypertriglyceridemia, malignancies, smoking, obesity, biliary tract disease, chronic renal insufficiency, and a history of alcohol intoxication) and the Charlson Comorbidity Index (CCI) score were determined according to discharge diagnoses following either outpatient clinic visits or hospitalizations before January 1, 2005. The detailed ICD-9-CM codes for comorbidities are described elsewhere, and the processes for selecting comorbidities are widely used and accepted.4,18,23,25
Statistical Analysis
All covariates were taken as categorical variables excepting age, which was treated as a continuous variable. To reveal the baseline heterogeneity in the 2 groups, categorical variables were compared with the Pearson Chi-square test and continuous variables were compared with the t test. The Nelson–Aalen cumulative hazard estimates were plotted initially to reveal the trends. Cox proportional hazard regression models were then used to calculate the hazard ratios (HRs) of AP for individuals with diabetes and of diabetes for individuals with AP; calculations were performed after adjustments for age, gender, urbanization level, SES, chronic liver disease, hypertension, coronary artery disease, hypertriglyceridemia, history of alcohol intoxication, malignancies, smoking, obesity, biliary tract disease, chronic renal insufficiency, and the CCI. The SAS statistical package, version 9.4 (SAS Institute, Inc., Cary, NC), and STATA version 11.2 (StataCorp, College Station, TX) were used for data analysis. A 2-tailed P-value of <0.05 was considered significant.
RESULTS
Cohort Analysis of the Association Between Diabetes and Risk of Acute Pancreatitis
The distributions of demographic characteristics and selected comorbidities for the study subjects are shown in Table 1. There were 42,080 patients in the diabetes group (982 with HCE) and 672,146 in the unexposed group. The average follow-up durations for the diabetes and exposed groups were 3.98 and 7.60 years, respectively. Patients with diabetes were predominantly male and significantly older. These patients were also more likely to have lower SES values, higher CCI scores, chronic liver disease, hypertension, coronary artery disease, hypertriglyceridemia, malignancies, chronic renal insufficiency, and biliary tract disease, and were more likely to be smokers, to be obese, to reside in nonrural areas, and to have a history of alcohol intoxication.
TABLE 1.
Baseline Characteristics of the Diabetes Group and the Unexposed Group

At the end of follow-up, 3149 patients had been admitted for AP; this group included 311 diabetic patients (36 with HCE) and 2838 nondiabetics. The average time from diabetes to AP was 3.80 years. The incidence rate of AP per 1000 person-years was 1.7 for patients with diabetes, 8.2 for diabetic patients with HCE, and 0.6 for nondiabetics. As compared to the general population, the crude HRs of AP for diabetic patients and for diabetics with HCE were 3.03 (95% confidence interval [CI], 2.67–3.43) and 14.75 (95% CI, 10.62–20.50), respectively. The Nelson–Aalen plot showed higher cumulative risk of AP for both diabetes groups (Figure 2).
FIGURE 2.
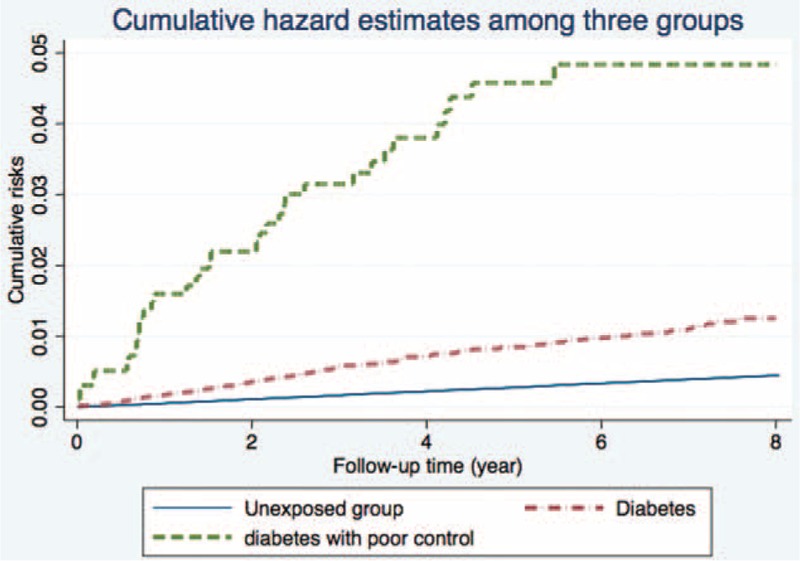
Nelson–Aalen curves showing a higher cumulative risk of acute pancreatitis in the diabetes group.
The multivariate Cox regression model was then employed to determine the adjusted HRs of AP. After controlling for the above-mentioned covariates, an increased HR was still observed for diabetic patients (1.72; 95% CI, 1.52–1.92). For the subgroup of diabetic patients with HCE, the HR was also significantly higher and greater than that for diabetics with no history of HCE (6.32; 95% CI, 4.54–8.81). Other independent risk factors for AP included male gender, older age, lower SES, living outside of an urban area, higher CCI, chronic liver disease, hypertension, history of alcohol intoxication, chronic renal insufficiency, and biliary tract disease (Figure 3). Findings with relevant statistics are summarized in Table 2.
FIGURE 3.

Forest plots of adjusted hazard ratios for acute pancreatitis.
TABLE 2.
Adjusted HRs of AP in Patients With Diabetes

Cohort Analysis of the Association Between Acute Pancreatitis and Risk of Diabetes
The distributions of demographic characteristics and selected comorbidities for the study subjects are shown in Table 3. There were 3187 patients in the AP group and 709,259 in the unexposed group. Of those in the AP group, 255 were further defined as having severe AP. The average follow-up durations for the AP and unexposed groups were 3.21 and 7.39 years, respectively. Patients with AP were predominantly male and significantly older. They were also more likely to have lower SES values, higher CCI scores, chronic liver disease, hypertension, coronary artery disease, hypertriglyceridemia, malignancies, chronic renal insufficiency, and biliary tract disease, and were more likely to be smokers, to be obese, to reside in nonrural areas, and to have a history of alcohol intoxication.
TABLE 3.
Baseline Characteristics of the Acute Pancreatitis Group and the Unexposed Group

At the end of follow-up, 41681 patients had been diagnosed with diabetes; this group included 324 patients with AP (25 with severe AP) and 41,357 without AP. The average time from AP to diabetes was 3.78 years. The incidence rates of diabetes per 100 person-years were 3.1 for patients with AP, 4.5 for patients with severe AP, and 0.8 for patients without AP. The crude HRs of diabetes for patients with AP and patients with severe AP were 3.81 (95% CI, 3.40–4.27) and 5.55 (95% CI, 3.75–8.21), respectively. The Nelson–Aalen plot also revealed higher cumulative risk of diabetes in both AP groups (Figure 4).
FIGURE 4.

Nelson–Aalen curves showing a higher cumulative risk of diabetes in the acute pancreatitis group.
The multivariate Cox regression model was then applied to determine the adjusted HRs of diabetes. After controlling for the covariates, an increased HR was still observed for patients with AP (2.15; 95% CI, 1.92–2.41). For patients with severe AP, the HR was also higher but did not differ significantly from that for patients without severe disease (2.22; 95% CI, 1.50–3.29). Other independent risk factors for diabetes included male gender, older age, lower SES, higher CCI, chronic liver disease, hypertension, hypertriglyceridemia, obesity, and a history of alcohol intoxication (Figure 5). Findings with relevant statistics are summarized in Table 4.
FIGURE 5.
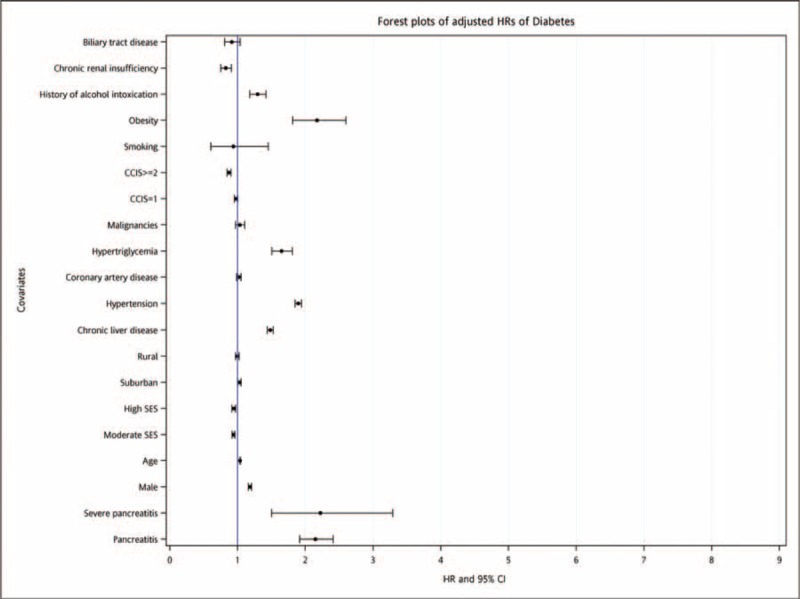
Forest plots of adjusted hazard ratios for diabetes.
TABLE 4.
Adjusted HRs of Diabetes in Patients With AP

For subjects in the AP group who were diagnosed with diabetes, the diagnosis frequently occurred shortly after hospitalization for AP, with 45.1% of diagnoses occurring within 1 year of an AP episode. Further validation of an increased risk of diabetes to patients with AP was therefore sought. To this end, 324 patients diagnosed with diabetes after an episode of AP were followed for the presence of diabetes for a median period of 1.7 years. Findings revealed that the diagnosis of diabetes was not simply the result of a miscoded transient hyperglycemia; rather, a persistent diabetic state was confirmed for these AP patients.
DISCUSSION
To the knowledge of the authors, the study described in the present report is the first to assess the strength of the bidirectional relationship between diabetes and AP using the same subject population. The database selected for this study was representative of the entire Taiwanese population; therefore, losses to follow-up and selection bias are not concerns.
In patients with diabetes, the risk of AP was significantly increased (HR, 1.72; 95% CI, 1.52–1.96); these findings are compatible with those of other reports.6–8 Moreover, diabetics with a history of HCE were found to have an even higher risk (HR, 6.32; 95% CI, 4.54–8.81) as compared to diabetics without HCE. The latter observation is consistent with a “severity-response” relationship between diabetes and risk of AP and represents a novel finding. In this regard, it is of interest that the increased production of reactive oxygen species and increased lipid peroxidation associated with chronic hyperglycemia may be key events in the pathogenesis of AP.26,27 Furthermore, diabetes is also associated with comorbidities, such as obesity, hyperlipidemia, or gallstones, with the ability to accelerate the development of AP. Additionally, ryanodine receptor-related alterations in cellular calcium homeostasis may be involved in the mechanisms though which diabetes facilitates the development of AP. In accord with this proposal, enhanced ryanodine receptor function, which has been reported for several disorders,28,29 is observed in both pancreatitis and diabetes.30–32 Further studies are required to delineate the biological mechanism(s) responsible for the development of AP in diabetic patients. However, studies that focus on methods to achieve better glycemic control and thereby avoid occurrence of AP in diabetics are particularly warranted.
A higher HR of development of diabetes was found in the present study for patients admitted with AP (HR, 2.15; 95% CI 1.92–2.41). This finding, which is in agreement with those of other studies,11,12,14 was confirmed by the observation that the “diabetes” diagnosed in these AP patients was not attributable to a transient hyperglycemia but rather to a persistent disease. In patients with severe as compared to mild AP, a slightly but not significantly increased HR of diabetes was observed (2.22; 95% CI, 1.50–3.29). This finding is compatible with findings from a recent comprehensive systemic review.14 Although loss of pancreatic β cells due to necrosis is generally considered responsible for development of diabetes in patients with AP,33,34 the minimal effect of AP severity on the risk of diabetes as described in the present report is consistent with existence of another mechanism. For example, it is possible that AP itself triggers an event in patients specifically at risk of developing diabetes35–37; however, studies focusing on the mechanism(s) whereby diabetes develops in some patients with AP are needed to confirm this hypothesis.
LIMITATIONS
Five limitations of the present study are acknowledged. First, findings were derived from administrative data. The exposures and outcomes were collected using ICD-9-CM diagnosis codes, and the validities of diagnoses (ie, sensitivity, specificity, and accuracy) cannot be fully assessed. However, the definitions of exposures and outcomes in this study are well-accepted in the administrative database, and previous studies focusing on either AP or diabetes and using similar enrollment criteria from the same database revealed good validities.4,23,38,39
Second, laboratory findings relevant to the severity of diabetes, including HbA1c and blood glucose values, were unavailable in the database. Alternatively, since uncontrolled diabetes is the most common factor precipitating HCE,24 diabetes with poor compliance was defined as diabetes with at least one episode of HCE. Because diabetic ketoacidosis and the hyperosmolar hyperglycemic state are extremely severe states, they may not serve as perfect substitutes for poor control. However, if the definition of diabetes with poor compliance as “diabetes with at least one episode of HCE” failed to differentiate these patients from average diabetic patients, a higher HR would not have been observed.
Third, because of the limitation of administrative database, we believe the diagnostic coding could not perfectly differentiate type I diabetes from type II. As a result, we decide not to perform subgroup analyses regarding the different effects of AP among different types of diabetes. For the same reason, we did not perform analyses regarding the risks of AP on different types of HCE. Individualized studies are better options to solve the study question.
Fourth, the presumed etiology of AP was not considered in the analyses. For example, no effort was made to determine whether either biliary pancreatitis or alcoholic pancreatitis contributed to the higher risk of developing diabetes. Diagnostic coding in the administrative database was considered a limitation to differentiation among possible etiologies of AP. Although the lack of information regarding etiology did not bias the results of this study, it is acknowledged that availability of this information will promote further understanding of the pathophysiology whereby diabetes develops in patients with AP. Future individual-based cohort studies will be needed to address this limitation.
Finally, although extensive adjustments were made for possible comorbidities, unmeasured cofounding remains an issue. Based on the nature of the dataset, certain important risk factors, such as body mass index, diet, and life style, could not be taken into account. However, the adjusted HRs were of high enough significance that residual confounding is not likely to fully explain the findings of a bidirectional relationship between diabetes and AP. Furthermore, the “severity-response” effect observed for development of AP in diabetic patients cannot be explained by unmeasured confounding.
CONCLUSIONS
The 2 cohort studies provided evidence for the bidirectional relationship between diabetes and AP. Moreover, diabetic patients with history of HCEs may be associated with higher risk of acute pancreatitis.
Footnotes
Abbreviations: AP = acute pancreatitis; CCI = Charlson Comorbidity Index; CI = confidence interval; HCE = hyperglycemic crisis episode; HR = hazard ratio; ICD-9-CM = International Classification of Diseases (Ninth Revision) Clinical Modification; NHI = National Health Insurance; SES = socioeconomic status.
Author contributions’: Dr. Yi-Kung Lee: study supervision and manuscript formation, Dr. Ming-Yuan Huang: data analysis and study supervision, Dr. Chen-Yang Hsu: data analysis, Dr. Yung-Cheng Su: analysis and interpretation.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep 2009; 11:97–103. [DOI] [PubMed] [Google Scholar]
- 2.Shen HN, Lu CL. Incidence, resource use, and outcome of acute pancreatitis with/without intensive care: a nationwide population-based study in Taiwan. Pancreas 2011; 40:10–15. [DOI] [PubMed] [Google Scholar]
- 3.Chang MC, Su CH, Sun MS, et al. Etiology of acute pancreatitis—a multi-center study in Taiwan. Hepatogastroenterology 2003; 50:1655–1657. [PubMed] [Google Scholar]
- 4.Hou SW, Lee YK, Hsu CY, et al. Increased risk of acute pancreatitis in patients with chronic hemodialysis: a 4-year follow-up study. PLoS ONE 2013; 8:e71801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omdal T, Dale J, Lie SA, et al. Time trends in incidence, etiology, and case fatality rate of the first attack of acute pancreatitis. Scand J Gastroenterol 2011; 46:1389–1398. [DOI] [PubMed] [Google Scholar]
- 6.Girman CJ, Kou TD, Cai B, et al. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab 2010; 12:766–771. [DOI] [PubMed] [Google Scholar]
- 7.Lai SW, Muo CH, Liao KF, et al. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol 2011; 106:1697–1704. [DOI] [PubMed] [Google Scholar]
- 8.Noel RA, Braun DK, Patterson RE, et al. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 2009; 32:834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair S, Yadav D, Pitchumoni CS. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol 2000; 95:2795–2800. [DOI] [PubMed] [Google Scholar]
- 10.Petrov MS, Zagainov VE. Influence of enteral versus parenteral nutrition on blood glucose control in acute pancreatitis: a systematic review. Clin Nutr 2007; 26:514–523. [DOI] [PubMed] [Google Scholar]
- 11.Boreham B, Ammori BJ. A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency. Pancreatology 2003; 3:303–308. [DOI] [PubMed] [Google Scholar]
- 12.Symersky T, van Hoorn B, Masclee AA. The outcome of a long-term follow-up of pancreatic function after recovery from acute pancreatitis. JOP 2006; 7:447–453. [PubMed] [Google Scholar]
- 13.Ibars EP, Sanchez de Rojas EA, Quereda LA, et al. Pancreatic function after acute biliary pancreatitis: does it change? World J Surg 2002; 26:479–486. [DOI] [PubMed] [Google Scholar]
- 14.Das SL, Singh PP, Phillips AR, et al. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut 2014; 63:818–831. [DOI] [PubMed] [Google Scholar]
- 15.Chen PC, Chan YT, Chen HF, et al. Population-based cohort analyses of the bidirectional relationship between type 2 diabetes and depression. Diabetes Care 2013; 36:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey CF, Zhou H, Harvey DJ, et al. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994–2001. Pancreas 2006; 33:336–344. [DOI] [PubMed] [Google Scholar]
- 17.National Health Insurance Database. 2014. [cited 2014 April 9]; Available from: http://nhird.nhri.org.tw/en/. [Google Scholar]
- 18.Lee YK, Hou SW, Lee CC, et al. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS ONE 2013; 8:e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YK, Lee CC, Chen CC, et al. High risk of ‘failure’ among emergency physicians compared with other specialists: a nationwide cohort study. Emerg Med J 2013; 30:620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng PL, Lin HY, Lee YK, et al. Higher mortality rates among the elderly with mild traumatic brain injury: a nationwide cohort study. Scand J Trauma Resusc Emerg Med 2014; 22:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YK, Lee CW, Huang MY, et al. Increased risk of ischemic stroke in patients with mild traumatic brain injury: a nationwide cohort study. Scand J Trauma Resusc Emerg Med 2014; 22:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang CJ, Wang SY, Lee MH, et al. Prevalence and incidence of mental illness in diabetes: a national population-based cohort study. Diabetes Res Clin Pract 2011; 93:106–114. [DOI] [PubMed] [Google Scholar]
- 23.Ng KJ, Lee YK, Huang MY, et al. Risks of venous thromboembolism in patients with liver cirrhosis: a nationwide cohort study in Taiwan. J Thromb Haemost 2015; 13:206–213. [DOI] [PubMed] [Google Scholar]
- 24.Huang CC, Weng SF, Tsai KT, et al. Long-term mortality risk after hyperglycemic crisis episodes in geriatric patients with diabetes: a national population-based cohort study. Diabetes Care 2015; 38:746–751. [DOI] [PubMed] [Google Scholar]
- 25.Lee WH, Hsu PC, Chu CY, et al. Cardiovascular events in patients with atherothrombotic disease: a population-based longitudinal study in Taiwan. PLoS ONE 2014; 9:e92577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halangk W, Lerch MM. Early events in acute pancreatitis. Gastroenterol Clin North Am 2004; 33:717–731. [DOI] [PubMed] [Google Scholar]
- 27.Kamboj SS, Sandhir R. Protective effect of N-acetylcysteine supplementation on mitochondrial oxidative stress and mitochondrial enzymes in cerebral cortex of streptozotocin-treated diabetic rats. Mitochondrion 2011; 11:214–222. [DOI] [PubMed] [Google Scholar]
- 28.Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol 2015; 8:206–222. [DOI] [PubMed] [Google Scholar]
- 29.Santulli G, Xie W, Reiken SR, et al. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A 2015; 112:11389–11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain SZ, Orabi AI, Muili KA, et al. Ryanodine receptors contribute to bile acid-induced pathological calcium signaling and pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol 2012; 302:G1423–G1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husain SZ, Prasad P, Grant WM, et al. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci U S A 2005; 102:14386–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santulli G, Pagano G, Sardu C, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 2015; 125:1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology 2011; 11:279–294. [DOI] [PubMed] [Google Scholar]
- 34.Hardt PD, Brendel MD, Kloer HU, et al. Is pancreatic diabetes (type 3c diabetes) underdiagnosed and misdiagnosed? Diabetes Care 2008; 31 (Suppl. 2):S165–S169. [DOI] [PubMed] [Google Scholar]
- 35.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010; 42:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365:1415–1428. [DOI] [PubMed] [Google Scholar]
- 37.Sadr-Azodi O, Orsini N, Andren-Sandberg A, et al. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol 2013; 108:133–139. [DOI] [PubMed] [Google Scholar]
- 38.Shen HN, Lu CL, Li CY. Effect of diabetes on severity and hospital mortality in patients with acute pancreatitis: a national population-based study. Diabetes Care 2012; 35:1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen HN, Lu CL, Li CY. Epidemiology of first-attack acute pancreatitis in Taiwan from 2000 through 2009: a nationwide population-based study. Pancreas 2012; 41:696–702. [DOI] [PubMed] [Google Scholar]


