Abstract
DNA polymerases are responsible for ensuring stability of the genome and avoiding genotoxicity caused by a variety of factors during DNA replication. Consequently, these proteins have been associated with an increased cancer risk. DNA polymerase kappa (POLK) is a specialized DNA polymerase involved in translesion DNA synthesis (TLS) that allows DNA synthesis over the damaged DNA. Recently, some studies investigated relationships between POLK polymorphisms and cancer risk, but the role of POLK genetic variants in breast cancer (BC) remains to be defined. In this study, we aimed to evaluate the effects of POLK polymorphisms on BC risk.
We used the Sequenom MassARRAY method to genotype 3 single nucleotide polymorphisms (SNPs) in POLK (rs3213801, rs10077427, and rs5744533), in order to determine the genotypes of 560 BC patients and 583 controls. The association of genotypes and BC was assessed by computing the odds ratio (OR) and 95% confidence intervals (95% CIs) from logistic regression analyses.
We found a statistically significant difference between patient and control groups in the POLK rs10077427 genotypic groups, excluding the recessive model. A positive correlation was also found between positive progesterone receptor (PR) status, higher Ki67 index, and rs10077427 polymorphism. For rs5744533 polymorphism, the codominant, dominant, and allele models frequencies were significantly higher in BC patients compared to healthy controls. Furthermore, our results indicated that rs5744533 SNP has a protective role in the postmenopausal women. However, we failed to find any associations between rs3213801 polymorphism and susceptibility to BC.
Our results indicate that POLK polymorphisms may influence the risk of developing BC, and, because of this, may serve as a prognostic biomarker among Chinese women.
INTRODUCTION
Breast cancer (BC) is the 2nd most common type of cancer in the world and the leading cancer type among women, with an estimated 1.67 million new cases diagnosed in 2012. Multiple factors, both genetic and nongenetic, are involved in its pathogenesis. Approximately 5% to 10% of all BC cases are considered to be hereditary.1 Previous studies showed that impaired DNA repair plays an important role in genetic instability and cancer development, especially during breast tumorigenesis.2
DNA molecule is constantly subjected to a wide variety of DNA damaging agents, either environmental (UV light, ionizing radiation, chemical poisons, and drugs) or endogenous (reactive oxygen species by products of routine metabolic processes) ones.3 DNA double-strand breaks (DSBs) are considered to be one of the most lethal forms of DNA damage, because a single unrepaired DSB results in a lethal cell growth arrest and an inhibition of cell cycle progression.4 An efficient DNA damage response signaling network commonly includes DNA repair pathways and tolerance mechanisms that, with cell cycle checkpoints, work together to ensure the integrity of the genome.5 DNA repair pathways include DNA strand break repair, mismatch repair, nucleotide excision repair, base excision repair, and ribonucleotide excision repair mechanisms.6 Loss of 1 DNA repair pathway constituent may be compensated for by an increase in the activity of different components of the same or other pathways. However, following major DSB lesions, these repair mechanisms are not effective in repairing DNA damage, because DNA molecule cannot serve as a template, and progression of the replication fork is impaired. Translesion DNA synthesis (TLS) can bypass damaged nucleosides and continue DNA replication, and this process is carried out by specialized DNA polymerases (β, ι, κ).7 Several recent studies reported possible association of DNA polymerase κ (POLK) with different types of cancer, for example, prostate cancer8 and nonsmall cell lung cancer.9 Until now, the link between POLK polymorphisms and BC has not been established.
Single nucleotide polymorphisms (SNPs) are thought to play an important role in genetic susceptibility to cancer. Numerous SNPs have been identified in the human POLK gene using sequence databases. We chose to investigate 3 POLK polymorphisms (rs3213801, rs10077427, and rs5744533) that are annotated in NCBI databases, but their association with BC risk was not previously determined. Herein, we conducted a case–control study to investigate this association in the Chinese Han population.
METHODS
Study Subjects
BC case–control study was conducted in the Second Affiliated Hospital of Xi’an Jiaotong University, China, between January 2013 and October 2014. A total of 560 pathologically confirmed BC patients were enrolled without age restrictions and nonfamilial BC cases, while patients who had received chemotherapy or radiotherapy before surgery or had another type of cancer were excluded. Eligible controls (n = 583) were recruited from the same hospital who are taking part in routine examinations in the outpatient clinic and randomly matched to individuals in the patient group, based on race and age (±5 years). All subjects were unrelated Han Chinese individuals and residents of Northwest China. Study subjects who met the criteria and signed written informed consent were interviewed in order to obtain their medical histories and information about prescribed therapy and demographic factors, and to collect specimens.
Genotyping Assay
Blood samples from patients and healthy controls were collected in EDTA-containing tubes. DNA was extracted from peripheral blood samples following standard phenol–chloroform extraction procedure. The extracted DNA was quantitated spectrophotometrically and stored at −80 °C until further use. Three tag SNPs (rs3213801, rs10077427, and rs5744533) were selected for our study; according to the Chinese population data available through HapMap project (http://www.hapmap.org), these SNPs represented the majority of known common variants of POLK. Sequenom MassARRAY Assay Design 3.0 Software (Sequenom, Inc., San Diego, CA) was used to design a Multiplexed SNP MassEXTEND assay. SNP genotyping was performed with a Sequenom MassARRAY RS1000 (Sequenom, Inc.) following the standard protocol recommended by the manufacturer. The corresponding primers used for each SNP in our study are listed in Table 1. Data analyses were performed using Sequenom Typer 3.0 Software (Sequenom, Inc.).
TABLE 1.
Primers Used for This Study

Statistical Analyses
Statistical analyses were performed using the statistical software package SPSS 18. The polymorphisms were tested for Hardy–Weinberg Equilibrium. Differences between the patient and healthy control groups in the distributions of demographic characteristics, selected variables, and genotype frequencies of the 3 investigated SNPs were evaluated using the Student's t-test or χ2-test. Associations between POLK polymorphisms, BC risk, and the patients’ clinical characteristics were assessed by computing odds ratios (ORs) and 95% confidence intervals (CIs) from unconditional logistic regression analysis, adjusting for age and body mass index. We evaluated the risk in the codominant model (Aa vs aa and AA vs aa; A represents the major allele, a the minor allele), dominant model (AA + Aa vs aa), recessive model (aa vs Aa + AA), overdominant model (aa + AA vs Aa), and the allele model (a vs A). A P value <0.05 was considered statistically significant. All statistical tests were 2 sided.
RESULTS
Patients and Healthy Controls Demographic Characteristics
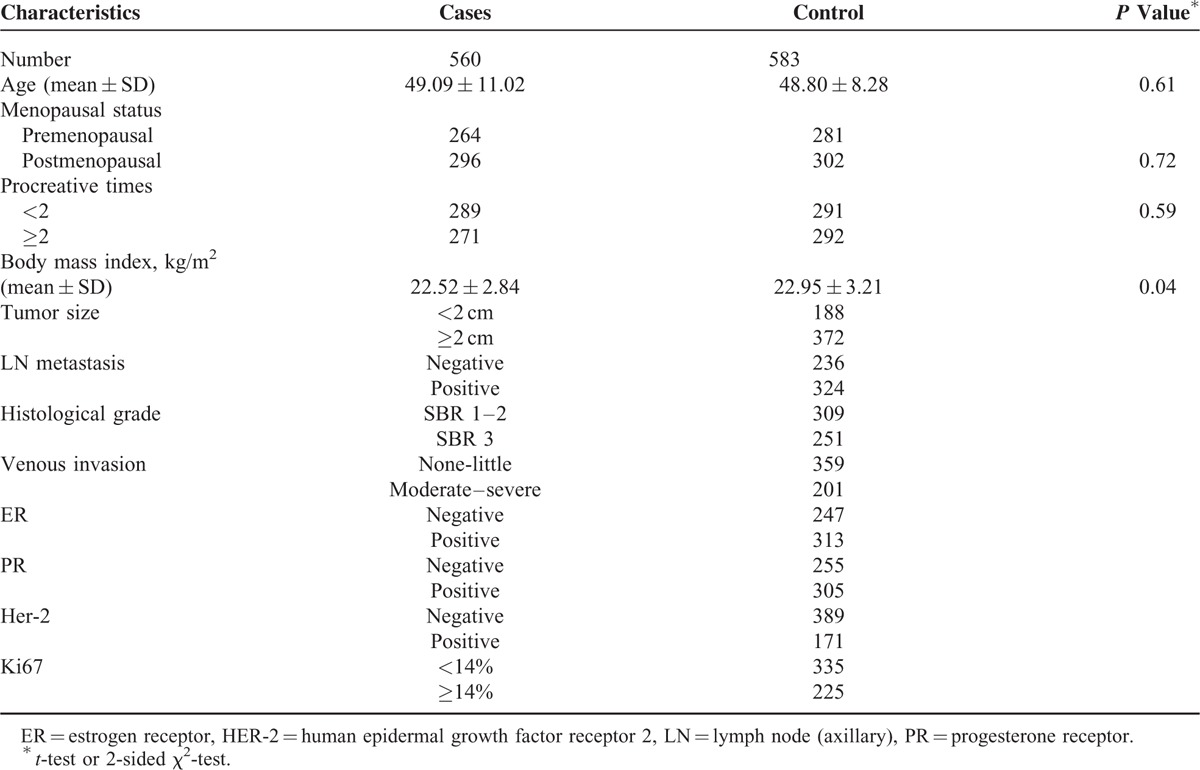
Baseline characteristics of the BC patient and control groups are described in Table 2. The distributions of age, menopausal status, and procreative times were similar between BC patients and healthy controls. However, body mass index (BMI) values were lower than those of healthy subjects (P = 0.04). The proportions of patients with larger tumor size (≥2 cm), patients that were human epidermal growth factor receptor 2 negative, and patients with lower venous invasion were 66.4%, 69.5%, and 64.1%, respectively. The percentages of patients with positive lymph node metastasis, Scarff–Bloom–Richardson grade 1 to 2, positive estrogen receptor, positive progesterone receptor (PR), and higher Ki67 index (≥14%) were 57.9%, 55.2%, 55.9%, 54.5%, and 40.2%, respectively.
TABLE 2.
Distributions of Select Variables in Breast Cancer Patients and Cancer-Free Controls
Association Between POLK Polymorphisms and BC Risk
The genotype and allele frequencies of the POLK polymorphisms (rs3213801, rs10077427, and rs5744533) are shown in Table 3. All polymorphisms conformed to Hardy–Weinberg Equilibrium (rs3213801: P = 0.86, rs10077427: P = 0.66, and rs5744533: P = 0.98).
TABLE 3.
Genotype and Allele Frequencies of DNA Polymerase Kappa Polymorphisms Among the Cases and Controls and the Associations With Breast Cancer Risk
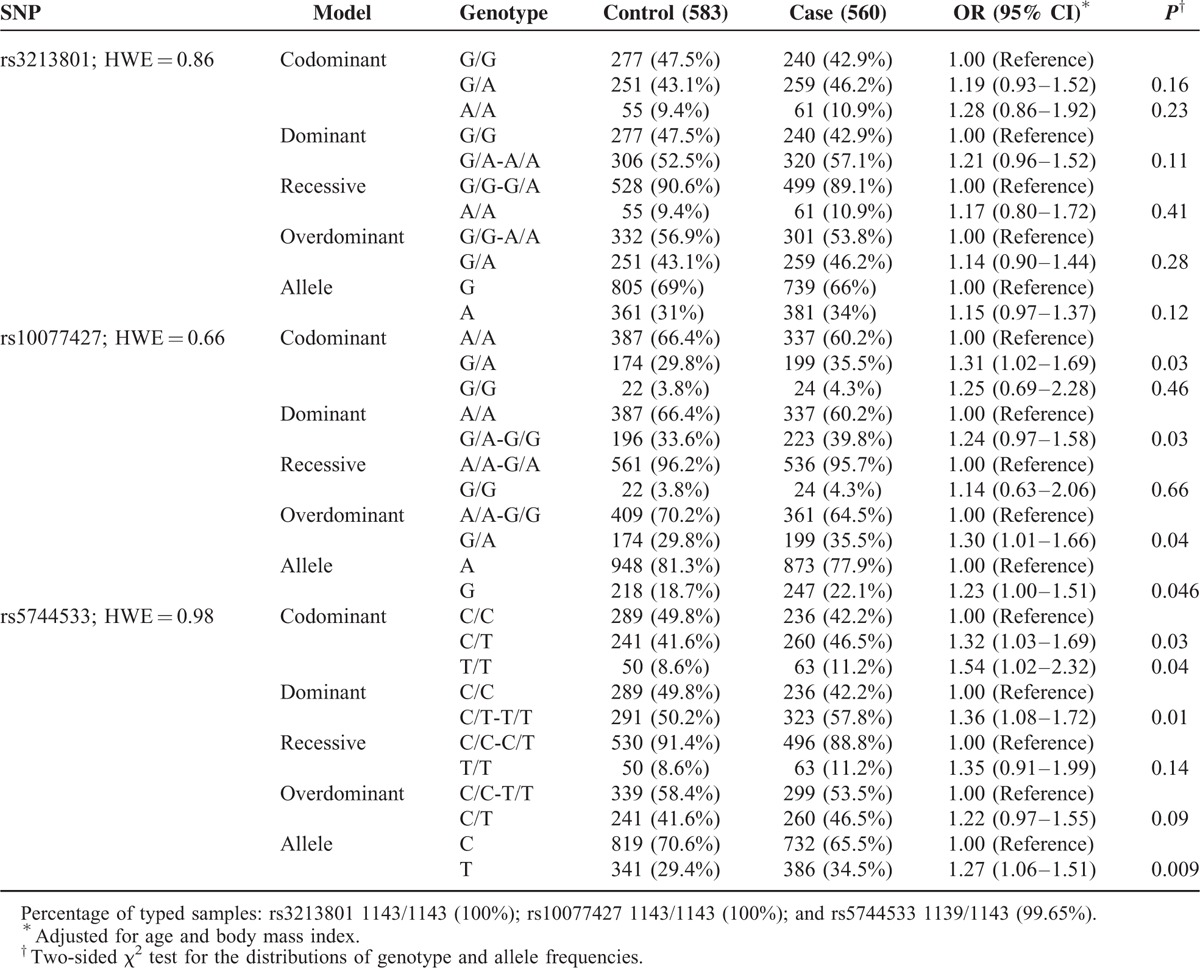
Rs3213801 did not show any meaningful association with the increase or decrease in BC risk. The rs10077427 genotype frequency distribution in BC patients was: AA, 60.2%; GA, 35.5%; and GG 4.3%, while for in the controls these values are: AA, 66.4%; GA, 29.8%; and GG 3.8%. The GA genotype increased the risk of BC compared AA (GA vs AA: OR = 1.31, 95% CI = 1.02–1.69, P = 0.03). In a similar fashion, GA + GG genotype, and G allele were positively associated with BC risk. Rs5744533 showed a statistically significant association with an increased ORs under most models except recessive and overdominant model (CT vs CC: OR = 1.32, 95% CI = 1.03–1.69, P = 0.03; TT vs CC: OR = 1.54, 95% CI = 1.02–2.32, P = 0.04; CT + TT vs CC: OR = 1.36, 95% CI = 1.08–1.72, P = 0.01; T vs C: OR = 1.27, 95% CI = 1.06–1.51, P = 0.009). These results suggested that POLK rs10077427 and rs5744533 polymorphisms may contribute to BC risk.
Genotype Associations With BC Risk by Menopausal Status
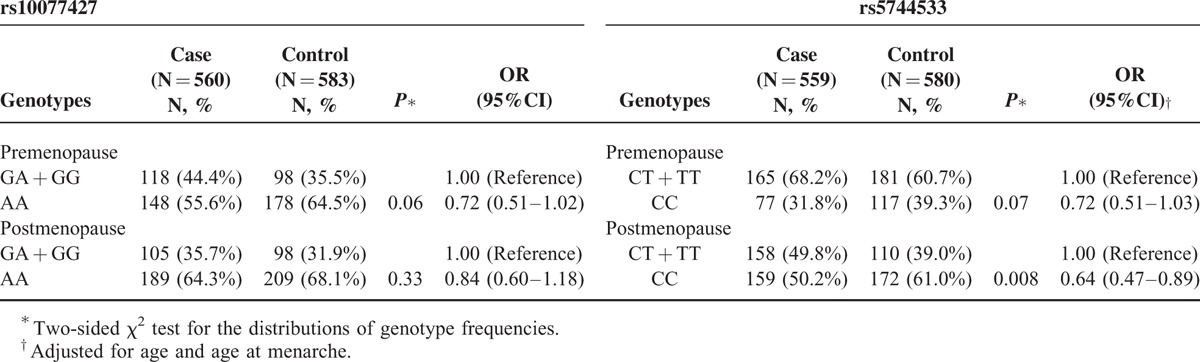
Stratified analysis of the effects of rs10077427 and rs5744533 polymorphisms on BC risk by menopausal status is displayed in Table 4. The results indicate that rs5744533 is associated with decreased BC susceptibility in postmenopausal women (OR = 0.64, 95% CI = 0.47–0.89, P = 0.008). However, there was no association between rs10077427 and BC risk in either premenopausal or postmenopausal patients.
TABLE 4.
Genotype Association by Menopause Status Between DNA Polymerase Kappa Polymorphisms and Risk of Breast Cancer
Relationship Between POLK Polymorphisms and Clinical Parameters of BC Patients
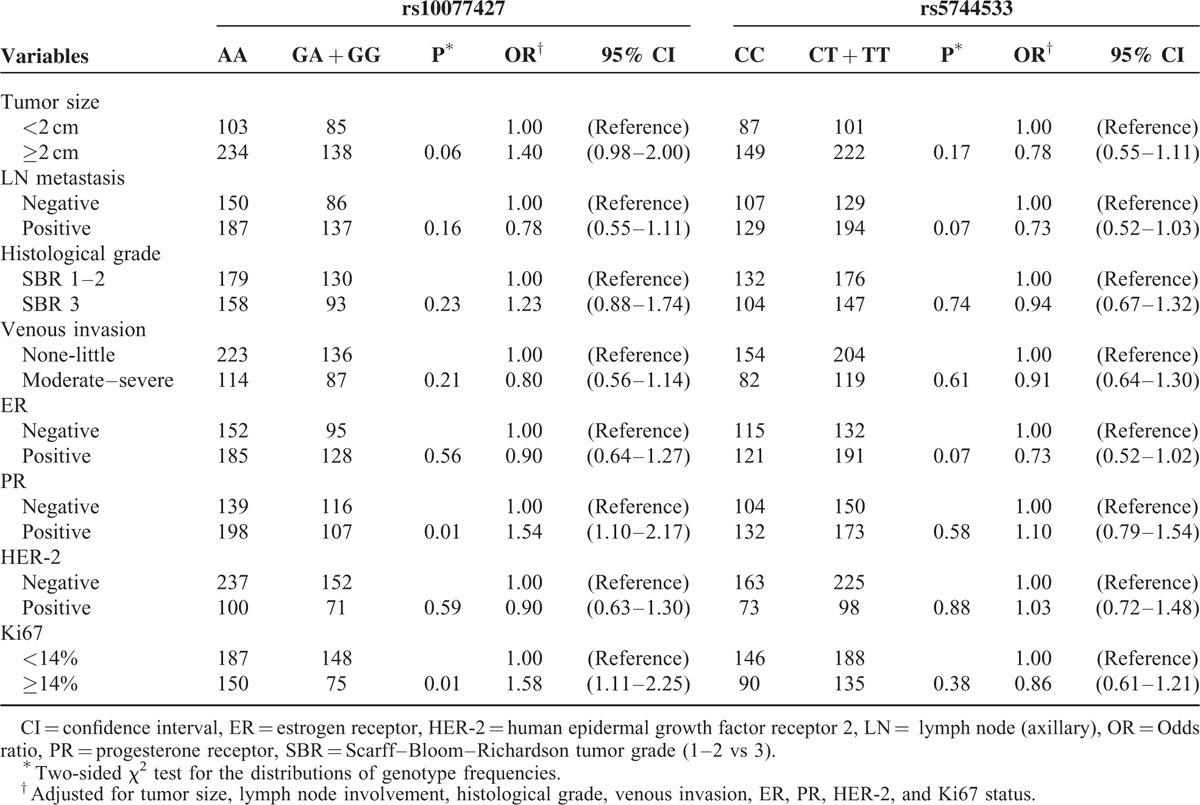
POLK gene polymorphisms were also analyzed in order to establish their associations with clinicopathological features, including tumor size, lymph node metastasis, histological grade, venous invasion and the statuses of estrogen receptor, PR, human epidermal growth factor receptor 2, and Ki67. As shown in Table 5, individuals with rs10077437 TC + CC genotype are more likely to have tumors that are PR-positive (OR = 1.54, 95% CI = 1.10–2.17, P = 0.01) and Ki67 ≥14% (OR = 1.58, 95% CI = 1.11–2.25, P = 0.01) compared with the TT genotype subjects. We found no relationship between any clinical parameters of BC patients and rs5744533.
TABLE 5.
The Associations Between POLK Polymorphisms and Clinical Characteristics of Breast Cancer Patients
DISCUSSION
Genomic DNA replication is a prerequisite for cell proliferation. High fidelity of DNA replication is important for maintaining genetic information over many generations and avoiding the accumulation of deleterious mutations. On the other hand, low fidelity of DNA replication is necessary for the evolution of species, for generating diversity that can lead to increased survival and adaptability of species in the changing environment.10,11 TLS, which largely depends on specialized DNA polymerases, repairs and replicates damage DNA with low fidelity.12 Y-family DNA polymerases are specialized polymerases involved in TLS, replicating damaged DNA, bypassing damaged nucleotides that block the normal progression of replication forks.13 Y-family is divided into 6 major groups based on amino acid sequence, which are represented by Escherichia coli Pol IV and Pol V, and 4 human enzymes, Pol η, ι, κ, and Rev1.14 These enzymes share a similar catalytic core in their N-terminal region and most of them have a noncatalytic C-terminus.15 The protein product of POLK gene is reported to be involved in checkpoint activation,16 rescue of methylnitrosourea-associated cytotoxicity,17 and microsatellite stability.18 POLK not only represents a DNA polymerase involved in the protection of cells from genotoxic DNA lesions, but is also included in genome integrity maintenance by acting as a noncatalytic protein against oxidative damage caused by hydrogen peroxide and menadione.19
Missense mutations of POLK (p.E29K, p.G154E, p.F155S, p.E430K, and p.L442F) were previously reported to be involved in prostate carcinogenesis, by diminishing the catalytic efficiencies of this polymerase.8 Overexpression of POLK was associated with advanced stages and poorer prognosis in glioma patients.20POLK gene plays an important role in DNA damage response and the upregulation of DNA damage response may cause cancer, as well as promote tumorigenesis.3 In our study, the role of POLK polymorphisms in BC was investigated for the first time, using a case–control approach. We observed that POLK genetic variants, rs10077427 and rs5744533, but not rs3213801, were positively associated with susceptibility to BC. Variants of these polymorphisms may affect the expression of POLK and eventually result in cancer. Overexpression of POLK results not only in increased spontaneous mutagenesis, but also in pleiotropic alterations such as DNA breaks, genetic exchanges, and aneuploidy.21 Furthermore, we showed that the rs5744533 polymorphism in POLK gene was inversely associated with positive PR status and Ki67 index. The rs10077427 genetic variant was more frequent in premenopausal women. Shao et al9 found that carriers with AA genotype of POLK rs5744533 and rs3213801 were more likely to respond to the platinum-based chemotherapy treatment and the patients carrying rs10077427 A allele had decreased progression-free survival (PFS). Our results indicate that these 2 polymorphisms in POLK gene may determine susceptibility to BC in individuals from the northwest of China.
The limitations of the present study were following:
BC is a disease caused by multiple factors, but we have not investigated the effect of wide array of factors, including family heredity history, endocrine factors, environmental influences, and style of life.
In this study, the 3 investigated polymorphisms were independently evaluated for susceptibility to BC, without considering the effect of and interactions with other variants.
In conclusion, our case–control study indicates that POLK polymorphisms have significant effects on the susceptibility and progression of BC among Han Chinese women. Further research on POLK polymorphisms is required to validate our findings. Because BC is a prevalent disease among the female population worldwide, identifying potential predictive and prognostic markers for this disease is of great significance for diagnosing and treating BC in general population.
Acknowledgments
The authors thank National Natural Science Foundation, China (No. 81472822; 81471670); China Postdoctoral Science Foundation (No. 2014M560791; 2015T81037); Shaanxi province science and technology plan project of innovation project, China (No. 2015KTCL03–06); and the Fundamental Research Funds for the Central Universities, China (No. 2014qngz-04) for the support.
Footnotes
Abbreviations: BC = breast cancer; CI = confidence interval; DSB = double strand break; OR = odds ratio; POLK = DNA polymerase kappa; PR = progesterone receptor; SNP = single nucleotide polymorphism; TLS = translesion DNA synthesis.
Z-D, X-L, and Y-FM contributed equally to this work and share joint first authorship.
This study was supported by National Natural Science Foundation, China (No. 81472822; 81471670); China Postdoctoral Science Foundation (No. 2014M560791; 2015T81037); Shaanxi province science and technology plan project of innovation project, China (No. 2015KTCL03–06); and the Fundamental Research Funds for the Central Universities, China (No. 2014qngz-04).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hirotsu Y, Nakagomi H, Sakamoto I, et al. Multigene panel analysis identified germline mutations of DNA repair genes in breast and ovarian cancer. Mol Genet Genomic Med 2015; 3:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralhan R, Kaur J, Kreienberg R, et al. Links between DNA double strand break repair and breast cancer: accumulating evidence from both familial and nonfamilial cases. Cancer Lett 2007; 248:1–17. [DOI] [PubMed] [Google Scholar]
- 3.Broustas CG, Lieberman HB. DNA damage response genes and the development of cancer metastasis. Radiat Res 2014; 181:111–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett CB, Lewis AL, Baldwin KK, et al. Lethality induced by a single site-specific double-strand break in a dispensable yeast plasmid. Proc Natl Acad Sci U S A 1993; 90:5613–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer 2012; 12:801–817. [DOI] [PubMed] [Google Scholar]
- 6.Wyrick JJ, Roberts SA. Genomic approaches to DNA repair and mutagenesis. DNA repair 2015; 36:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol 2012; 13:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav S, Mukhopadhyay S, Anbalagan M, et al. Somatic mutations in catalytic core of POLK reported in prostate cancer alter translesion DNA synthesis. Hum Mutat 2015; 36:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao M, Jin B, Niu Y, et al. Association of POLK polymorphisms with platinum-based chemotherapy response and severe toxicity in non-small cell lung cancer patients. Cell Biochem Biophys 2014; 70:1227–1237. [DOI] [PubMed] [Google Scholar]
- 10.Fijalkowska IJ, Schaaper RM, Jonczyk P. DNA replication fidelity in Escherichia coli: a multi-DNA polymerase affair. FEMS Microbiol Rev 2012; 36:1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nevin P, Lu X, Zhang K, et al. Noncognate DNA damage prevents the formation of the active conformation of the Y-family DNA polymerases DinB and DNA polymerase kappa. FEBS J 2015; 282:2646–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertolin AP, Mansilla SF, Gottifredi V. The identification of translesion DNA synthesis regulators: Inhibitors in the spotlight. DNA Repair 2015; 32:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W. An overview of Y-Family DNA polymerases and a case study of human DNA polymerase eta. Biochemistry 2014; 53:2793–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohmori H, Friedberg EC, Fuchs RP, et al. The Y-family of DNA polymerases. Mol Cell 2001; 8:7–8. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Ortiz MV, Roldan-Arjona T, Ariza RR. The noncatalytic C-terminus of AtPOLK Y-family DNA polymerase affects synthesis fidelity, mismatch extension and translesion replication. FEBS J 2007; 274:3340–3350. [DOI] [PubMed] [Google Scholar]
- 16.Maiorano D, Hoffmann JS. Pol kappa in replication checkpoint. Cell Cycle (Georgetown, Tex) 2013; 12:3713–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupari E, Ventura I, Marcon F, et al. Pol kappa partially rescues MMR-dependent cytotoxicity of O6-methylguanine. DNA Repair 2012; 11:579–586. [DOI] [PubMed] [Google Scholar]
- 18.Baptiste BA, Eckert KA. DNA polymerase kappa microsatellite synthesis: two distinct mechanisms of slippage-mediated errors. Environ Mol Mutagen 2012; 53:787–796. [DOI] [PubMed] [Google Scholar]
- 19.Kanemaru Y, Suzuki T, Niimi N, et al. Catalytic and non-catalytic roles of DNA polymerase kappa in the protection of human cells against genotoxic stresses. Environ Mol Mutagen 2015. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Wu W, Wang HW, et al. Analysis of specialized DNA polymerases expression in human gliomas: association with prognostic significance. Neuro Oncol 2010; 12:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bavoux C, Hoffmann JS, Cazaux C. Adaptation to DNA damage and stimulation of genetic instability: the double-edged sword mammalian DNA polymerase kappa. Biochimie 2005; 87:637–646. [DOI] [PubMed] [Google Scholar]


