Abstract
To elucidate clinical trial efficacy, safety, and dosing practices of AbobotulinumtoxinA (ABO) treatment in adult patients with lower limb spasticity.
A systematic literature review was performed to identify randomized controlled trials of ABO in the treatment of adult lower limb spasticity.
Of the 295 records identified, 6 primary publications evaluated ABO for the management of lower limb spasticity of various etiologies and were evaluated. Total ABO doses ranged between 500 and 2000 U for lower limb spasticity, depending on the muscles injected. All studies in lower limb spasticity showed statistically significant reduction in muscle tone based on Modified Ashworth Scale of ABO versus placebo. Significant effects on active movement and pain were demonstrated albeit less consistently. ABO was generally well tolerated across the individual studies; most adverse events reported were considered unrelated to treatment. Treatment-related adverse events included but not limited to fatigue, local pain at injection site, hypertonia, dry mouth, weakness of the noninjected muscle, abnormal gait, and urinary tract infection.
These data from 6 randomized clinical studies provide the beginnings of an evidence base for the use of ABO to reduce lower limb spasticity. Ongoing studies in this area will add to this evidence base.
INTRODUCTION
Adult lower limb spasticity (ALLS) is 1 of the disabling complications of multiple-neurological disorders such as stroke, multiple sclerosis, spinal cord injury, and even some central neurodegenerative disorders. People with ALLS can present with a variety of abnormal postures; the most common lower limb postures being spastic drop foot with hyper plantar flexion, equinovarus, knee flexion or hyperextension, and toe flexion.1 The increased tone due to spasticity can cause significant discomfort, and patients describe it as a leg spasm, cramp or dull pain. In many cases, ALLS can be more disabling than adult upper limb spasticity (AULS) because even mild spasticity can significantly affect patients’ stride, gait, and balance. If left unmanaged, patients with ALLS are often predisposed to secondary complications of reduced mobility such as tendon shortening, joint deformity, and eventually immobilization.2 These secondary complications can themselves cause more systemic complications including deep vein thrombosis3 and pressure ulcers.4
The integral role of botulinum neurotoxin (BoNT) in the management of focal spasticity is recognized by guidelines from around the world.5–7 At present, there is no FDA approved botulinum toxin for the treatment of lower limb spasticity in the United States. On the other hand, there is an accumulating body of evidence to support efficacy and safety of BoNT in managing ALLS. Although most literature reviews examine the effectiveness of all BoNT products as a class, the differences in dosing units and recommended schemes provide a clear rationale for reviewing the efficacy, safety, and dosing information for each product separately. We have previously reported on a systematic review evaluating the effectiveness of AbobotulinumtoxinA (ABO) in the management of AULS8; this report focuses on the results of a parallel systematic review of clinical studies of ABO in ALLS.
METHODS
The systematic literature review presented here is 1 part of a larger systematic review of all potential indications for ABO, the results of which will be presented separately per each relevant indication. The literature search strategy and methods for this systematic review were specified in advance and documented in a protocol.8 Components of the protocol include the literature search strategy, screening criteria, data extraction methods, and risk of bias appraisal used to assess studies selected for inclusion.
Screening Criteria
Specific study characteristics of interest were defined in the protocol. They include: study type—randomized controlled trials and other comparative clinical studies; patient population—adult patients with LLS; treatment—ABO; and outcomes—primary and secondary efficacy, safety, and dosing.
Literature Search Strategy and Data Sources
The literature search strategy was developed using a combination of Medical Subject Heading (MeSH) terms and keywords. Keywords of relevance to the review of ALLS were: AbobotulinumtoxinA (alternative spellings included: Abobotulinumtoxin A and Abobotulinum toxin A), Dysport, spasticity, and clinical trial. Language (English only) and date limits (January 1991 to January 2013) were also applied.8 Subsequently, the search was updated to include ALLS papers published between January 2013 and April 2015. The search was performed in 3 foundational and comprehensive electronic medical literature databases (PubMed, Cochrane Library, and Embase). Bibliographic reference lists of systematic reviews identified during screening were searched to identify any relevant studies that were not identified through the electronic database searches.
Study Selection
At level 1 screening, all publications reporting preclinical, Phase 1, prognostic/biomarker, genetic retrospective, registry, case report, and/or noncomparative studies were excluded, as were letters, consensus reports, editorials, and nonsystematic reviews. Although, systematic reviews and meta-analyses were not included in their own right, they were used for identification of additional primary studies. At level 2 screening, all publications that reported only biochemical or immunologic endpoints were excluded. Also at this stage, nonrandomized, controlled Phase 2 or 3 clinical trials, comparative long-term follow-up studies (eg, open-label follow-up of randomized, controlled clinical trials) and comparative prospective Phase 4 postmarketing trials were excluded, provided that adequate information from randomized phase 2 and phase 3 trials had been identified. The systematic literature review process of study selection was depicted in a PRISMA flow diagram.
Data Extraction
Study methodology, patient, and treatment-level data were extracted from the full-text publications under predefined headings (eg, efficacy, safety, dosing). Each included study underwent quality assessment for risk of bias based on Cochrane metrics. The quality assessment for RCTs systematically addresses 6 types of bias: selection, performance, detection, attrition, reporting, and other sources of bias not covered by other domains. If non-RCTs or other study types were deemed relevant for data extraction, quality assessment was performed using Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) appraisal criteria for non-RCTs.9
Role of the Funding Source
The study was partially funded by Ipsen for data collection and editorial support. KD developed the protocol and data collection was coordinated by RTI Health Solutions and designates. Aside from procuring the data collection and editorial support, Ipsen did not contribute to the study conduct or reporting of results. All authors had full access to all data, contributed to manuscript revisions, and had final approval for submission. KD wrote the initial draft and had final responsibility for the decision to submit the paper for publication. JJC wrote the revision draft.
RESULTS
Publications Identified
A total of 295 records were identified from the medical literature databases. Of these, 6 primary publications that evaluated ABO for the management of ALLS in adult patients were included in the final data report (Table 1).10–15Figure 1 shows the PRISMA diagram for the full systematic review of all randomized controlled trials of ABO. With the exception of the study by Burbaud et al,10 that was judged high risk, most of studies included in this report fulfilled criteria for low-risk selective reporting bias (Supplementary Table). Studies used a wide range of outcome measures including measures of spasticity (usually assessed with the Modified Ashworth Scale; MAS), range of movement (passive and active), walking parameters, pain, global clinical impression, activities of daily living, and goal attainment.
TABLE 1.
Etiology of Lower Limb Spasticity
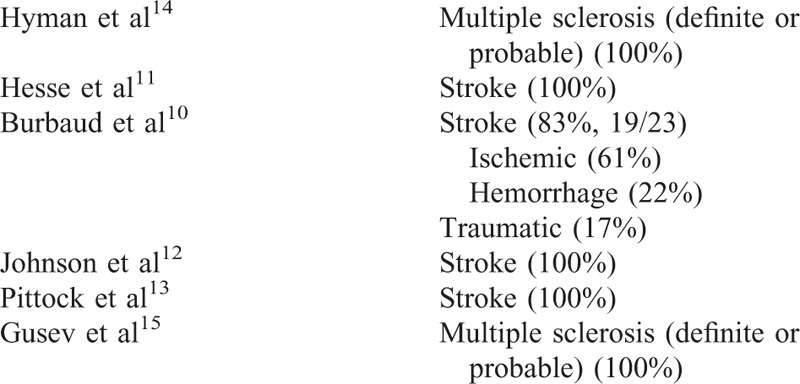
FIGURE 1.
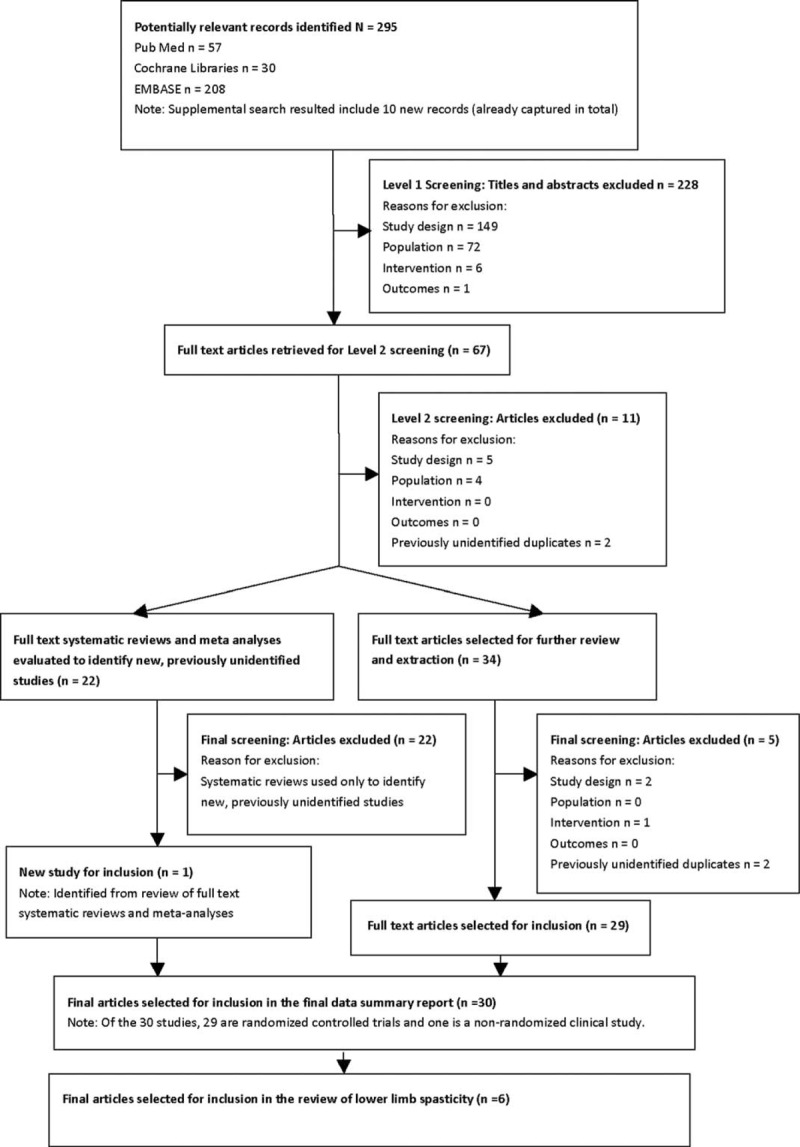
PRISMA flow diagram reporting the results of the systematic literature search.
Efficacy in ALLS
Statistically significant reductions in muscle tone versus baseline were reached for the majority of evaluations using MAS. When assessed, ABO treatment was consistently associated with significant effects on pain. Effects on walking parameters were less consistent but generally favored ABO injections. Table 2 provides an overview of efficacy and safety outcomes from each of the studies.
TABLE 2.
Evidence Table of Completed Trials for Lower Limb Spasticity
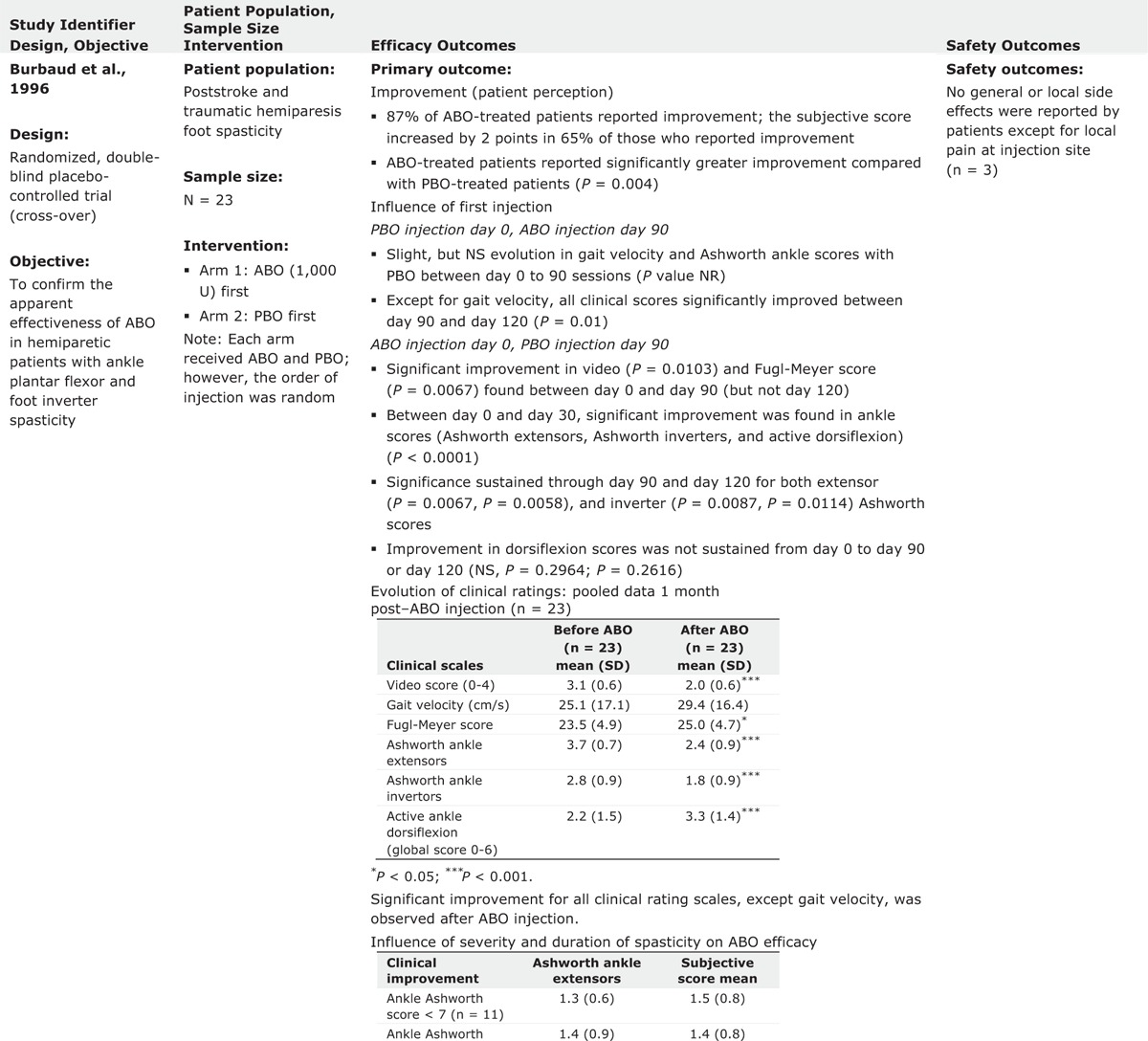
TABLE 2 (Continued).
Evidence Table of Completed Trials for Lower Limb Spasticity
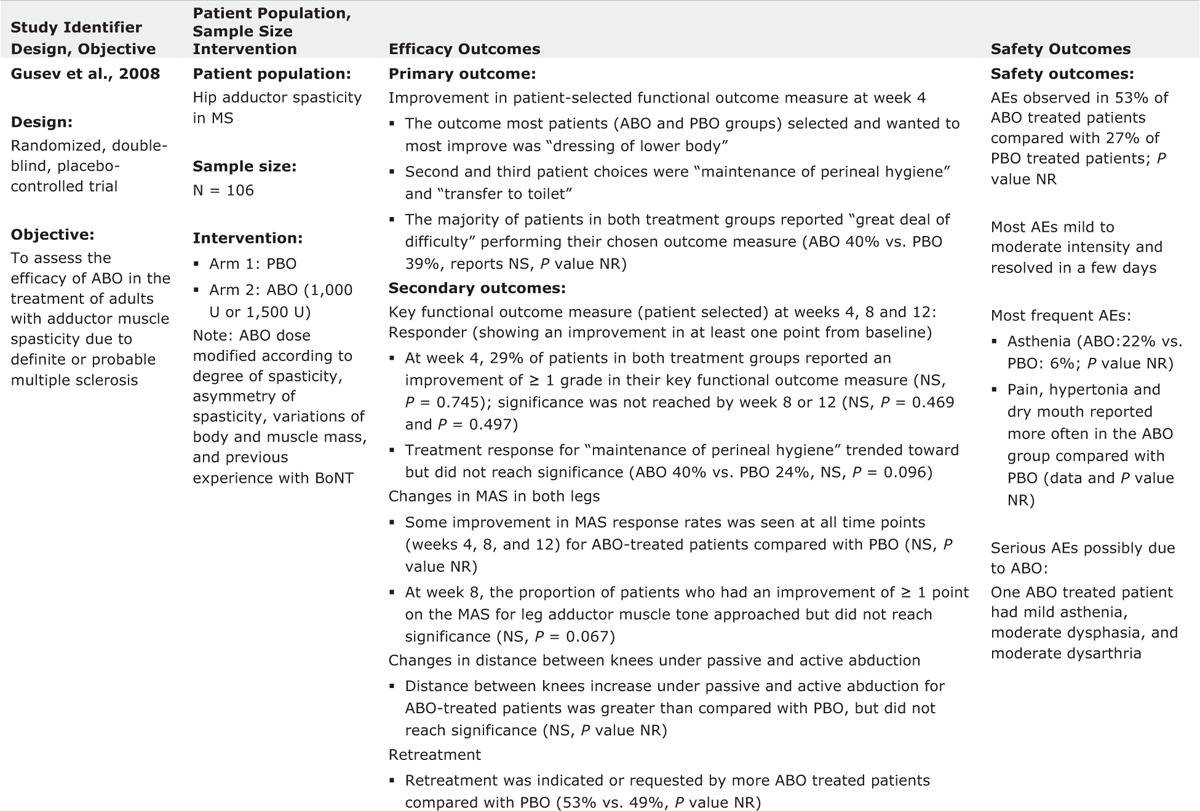
TABLE 2 (Continued).
Evidence Table of Completed Trials for Lower Limb Spasticity
Burbaud et al10 evaluated the efficacy and safety of ABO in 23 hemiparetic patients with spasticity of the ankle plantar flexors and foot invertors due to stroke or traumatic hemiparesis in a randomized double blind, placebo controlled crossover study.10 Patients received 1 injection of ABO and 1 of placebo in random order at day 0 and day 90. Injections were performed with electromyographic (EMG) guidance. Treatment efficacy was subjectively assessed by patients (on a scale of 0–3) and objectively assessed using clinical rating scales (assessing active dorsiflexion in the supine, sitting and standing position, and gait). The MAS and the Fugl–Meyer scale for the inferior limb were also applied. Treatment was with 1000 U ABO (diluted with 5 mL saline) and distributed among the various muscles according to their involvement in spasticity judged by the injector physician. The range of ABO dosages for individual muscles were as follows: triceps surae (500–1000 U), soleus (200–400 U), tibialis posterior (200–350 U), and flexor digitorum longus (150–300 U). Only 3 of the 23 patients reported no improvement after ABO injection. There was a clear difference (P = 0.0014) in patients’ subjective scores between ABO and placebo. Significant changes were noted in MAS values for ankle extensors (P < 0.0001) and invertors (P = 0.0002) and for active ankle dorsiflexion (P = 0.0001) and significant improvements were also noted in Fugl–Meyer scores (P = 0.0028). During active ankle dorsiflexion, 18/22 patients showed ≥1 point improvement in dorsiflexion score. Gait velocity was slightly but not significantly improved after ABO injections. The authors concluded that the efficacy of ABO injection in the treatment of spastic foot suggests that ABO may be particularly useful during the first year after a stroke.10
In an open-label study, Hesse et al11 examined the effect of ABO with or without electrical stimulation (ES) treatment postinjection on ALLS in 10 hemiparetic subjects with the history of stroke. Five subjects were randomized into each treatment group: 2000 U ABO followed by ES treatment (30 min, 6 times/d during the 3 days following the injection), 2000 units ABO without ES. Injections were performed into the following muscles using EMG guidance: soleus, tibialis posterior, and both heads of gastrocnemius muscles. Study results indicated that there were no significant differences in MAS across groups. However, the group receiving ABO + ES treatment demonstrated the greatest reduction in MAS (P = 0.011). The result of other measures, including limb position at rest and ability to perform 3 identified ADLs, were variable.
The effect of combined use of ABO and functional electric stimulation (FES) in the treatment of spastic drop foot following stroke was also investigated by Johnson et al.12 Twenty-one subjects participated in this nonblinded randomized controlled study and 18 of them completed the study. Every subject received ABO injection into the medial and lateral heads of the gastrocnemius and tibialis posterior. Injections were performed with EMG guidance. Walking speed increased in both injection and control groups over 12 weeks study with statistically significant difference in favor of the group with FES. Authors concluded that the combined treatment improved walking and function.
Pittock et al evaluated the effect of ABO injections on calf muscle hypertonicity following stroke in a prospective, multicenter, double-blind, placebo-controlled, and dose-ranging study. Two-hundred and thirty-four stroke patients were randomized into the ABO group and were dosed with 500, 1000, or 1500 U.13 Within the calf, upper, and lower injection sites were determined by palpation of the femoral and calcaneal insertions of the gastrocnemius muscle; 1/4 and 1/3 of the total length from the femoral insertions, respectively, and EMG guidance was not used. The primary outcome measures, 2-min walking distance and stepping rate, increased significantly in each group with no significant difference between groups. There were also small, but statistically significant improvements in calf spasticity, limb pain, and reduction in the use of walking aids compared with placebo. Authors concluded that ABO injection resulted in a significant reduction in muscle tone, limb pain, and dependence on walking aids. The best benefit was achieved with 1500 U of ABO.
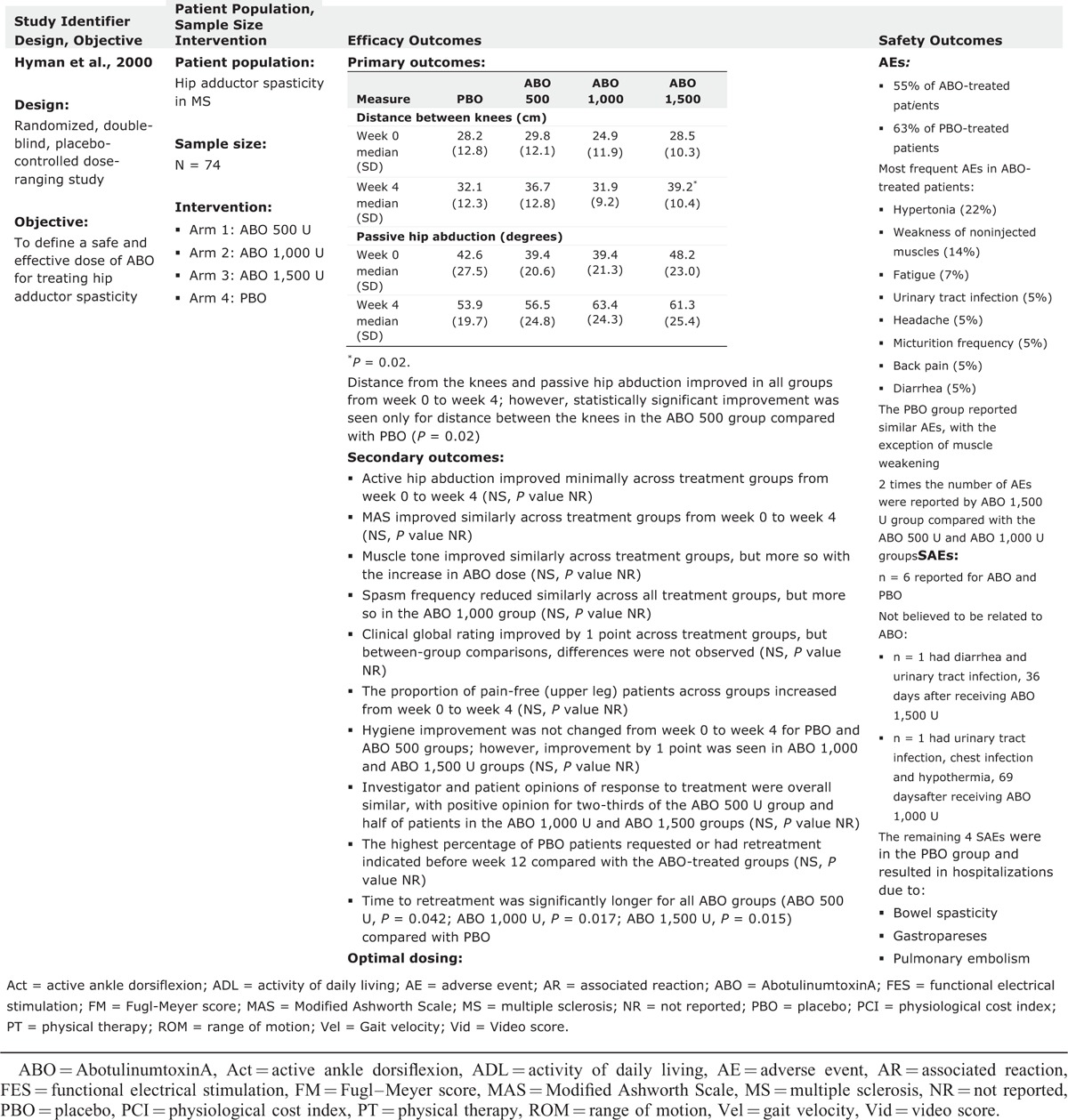
Hyman et al14 investigated the effectiveness and safety of ABO for treating hip adductor spasticity. In this double-blind study, 74 patients with multiple sclerosis and spasticity affecting the hip adductor muscles of both lower limbs were recruited and randomized to 4 groups of ABO 500, 1000, and 1500 U or placebo. The primary efficacy variables—passive hip abduction and distance between the knees—improved for all groups, and the distance between the knees for the 1500 U group was significantly greater than placebo (P = 0.02). Spasm frequency was reduced in all groups, but muscle tone was reduced in the ABO groups only. Pain was reduced in all groups, but improvements in hygiene scores were evident only in the 1000 and 1500 U groups.14
Gusev et al15 also assessed the effectiveness of ABO in the treatment of adults with adductor muscle spasticity due to definite or probable multiple sclerosis. In this double-blind study, 106 subjects were recruited, and randomized to receive injections with placebo, or ABO: 1000–1500 U in a 1:1 ratio. The subjects received injection into adductor muscles of each lower limb (500–750 U/leg). ABO was shown to provide effective pain relief in patients with severe adductor spasticity. Other predefined outcome measures, including functional performance (primary outcome), did not reach statistical significance. However, there was a significant improvement in favor of ABO for maintenance of perineal hygiene (P = 0.0096) and there were trends to significance for MAS response rates and in the distance between knees under passive abduction.15
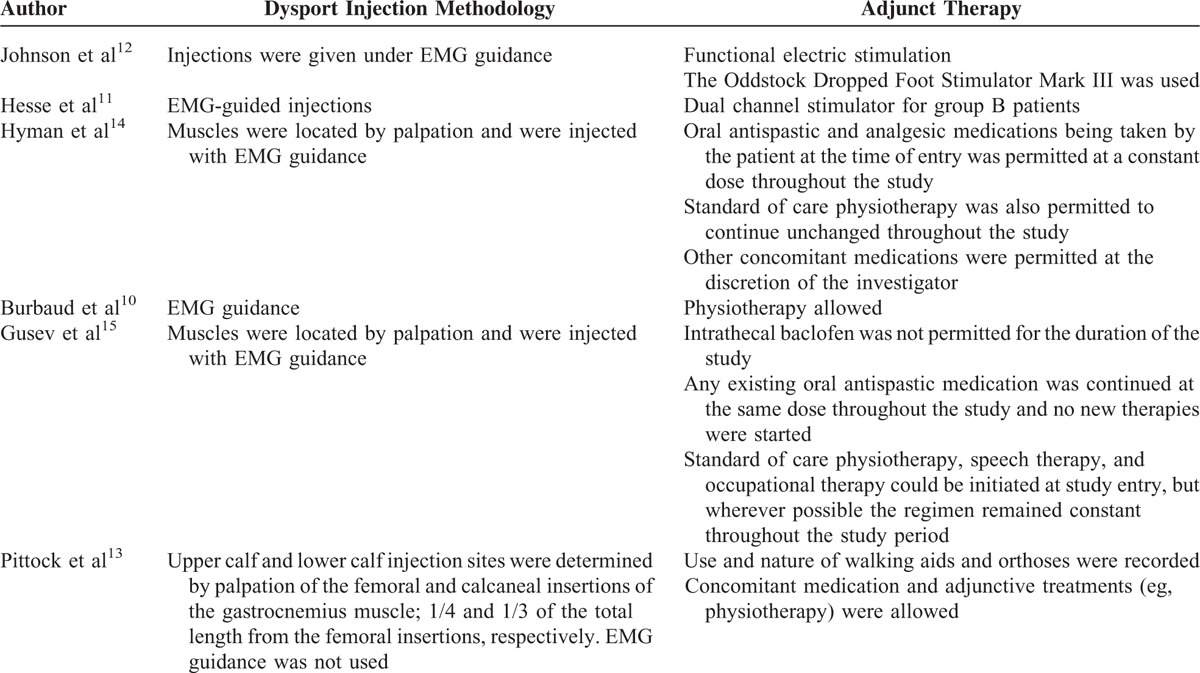
Table 3 shows the injection methodology and adjunct therapies that were used in each study.
TABLE 2 (Continued).
Evidence Table of Completed Trials for Lower Limb Spasticity
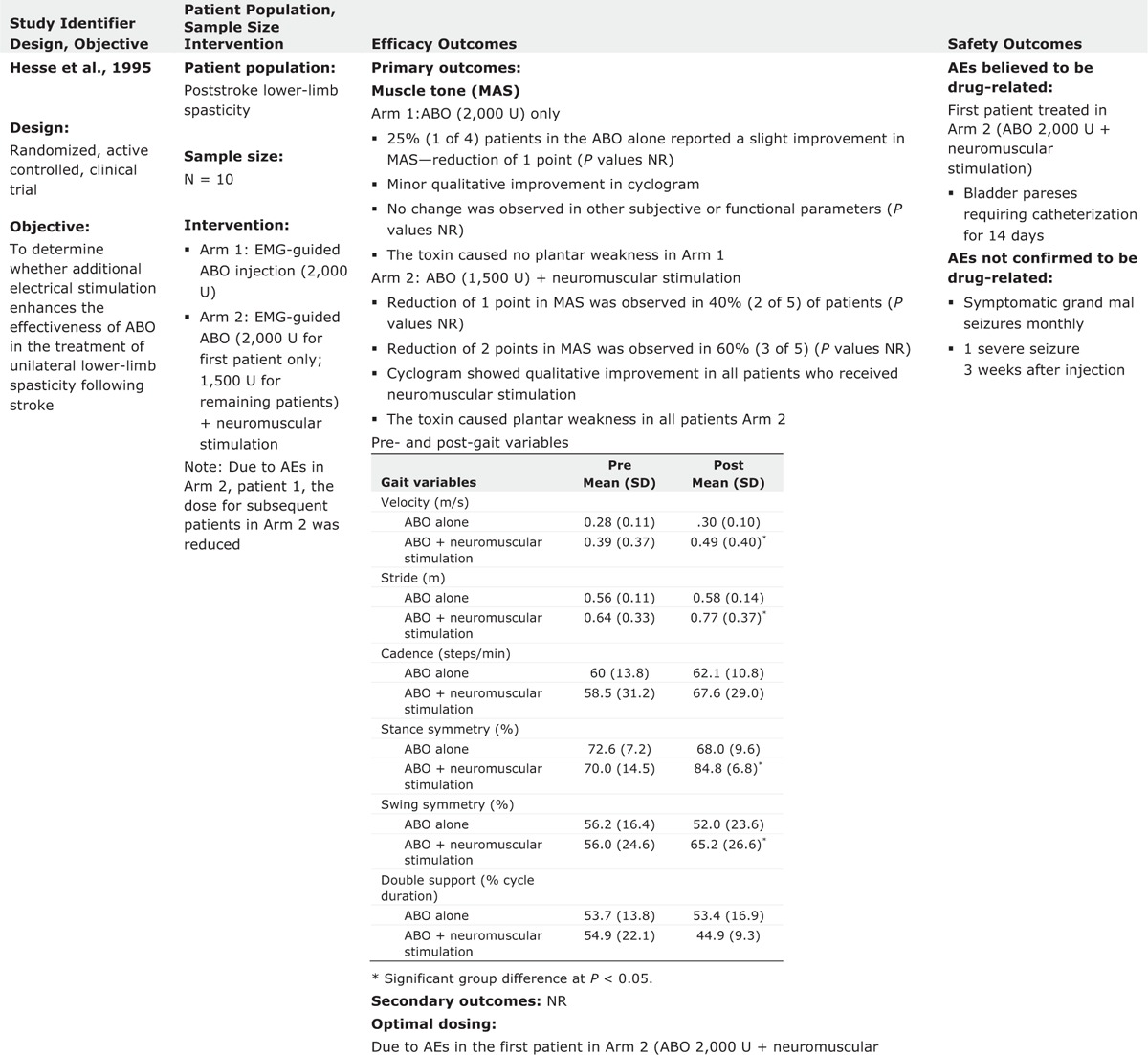
TABLE 3.
Injection Methodology and Adjunctive Therapies
Safety in ALLS
ABO was well tolerated across the individual studies.10–15 Most adverse events reported were considered unrelated to treatment. Adverse events considered associated with ABO treatment included: local pain at injection site, fatigue, asthenia, somnolence, hypertonia, dry mouth, bladder paresis, urinary tract infection, urinary frequency, diarrhea, weakness of noninjected muscles, headache, abnormal gait, and dysphagia. Most of the AEs were more common at higher doses of ABO.
Dosing Across Indications
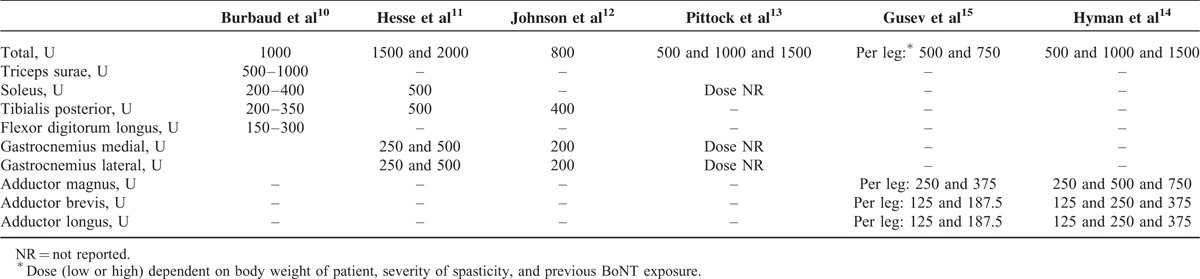
Total ABO doses ranged between 500 and 2000 U for ALLS. The most commonly injected muscles were the triceps surae, soleus, tibialis posterior, flexor digitrom longus, adductor magnus, adductor longus, adductor brevis, and gastrocnemius. Dose ranges for different muscles are summarized overall in Table 4 and by each individual study in Table 5.
TABLE 2 (Continued).
Evidence Table of Completed Trials for Lower Limb Spasticity
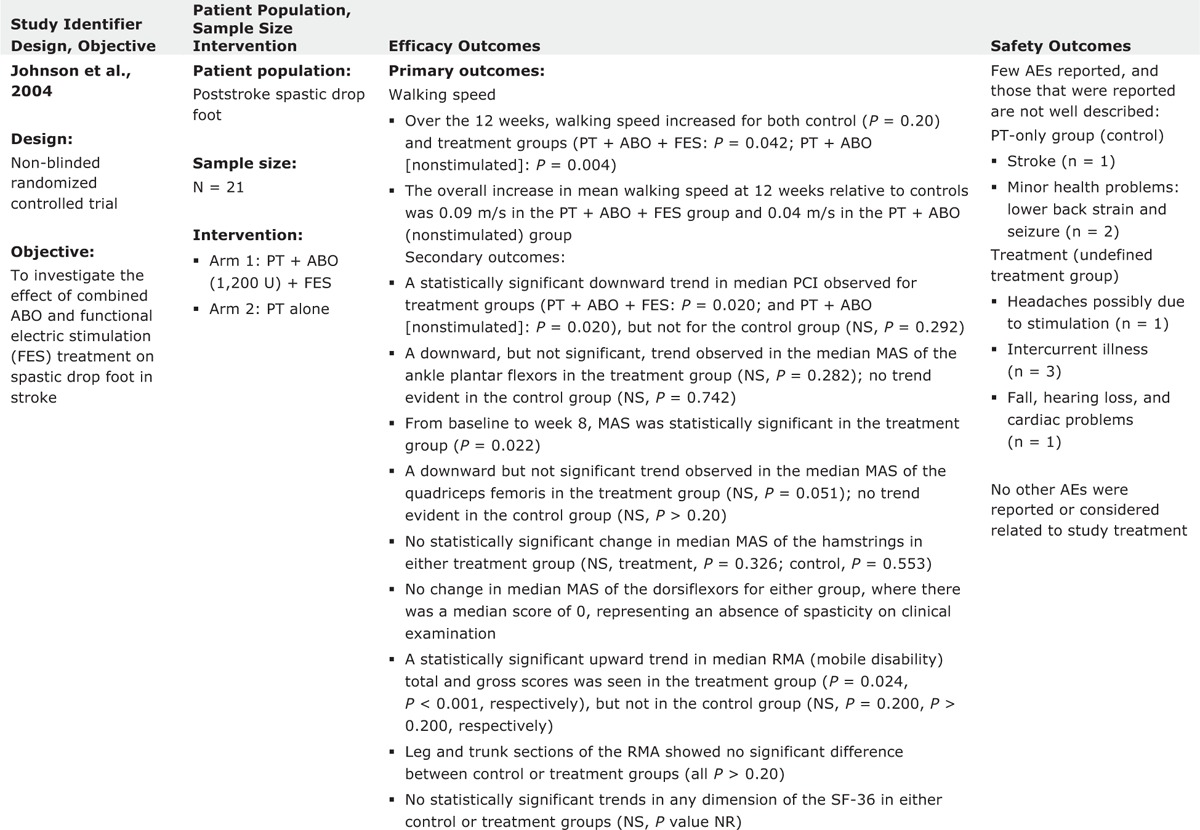
TABLE 2 (Continued).
Evidence Table of Completed Trials for Lower Limb Spasticity
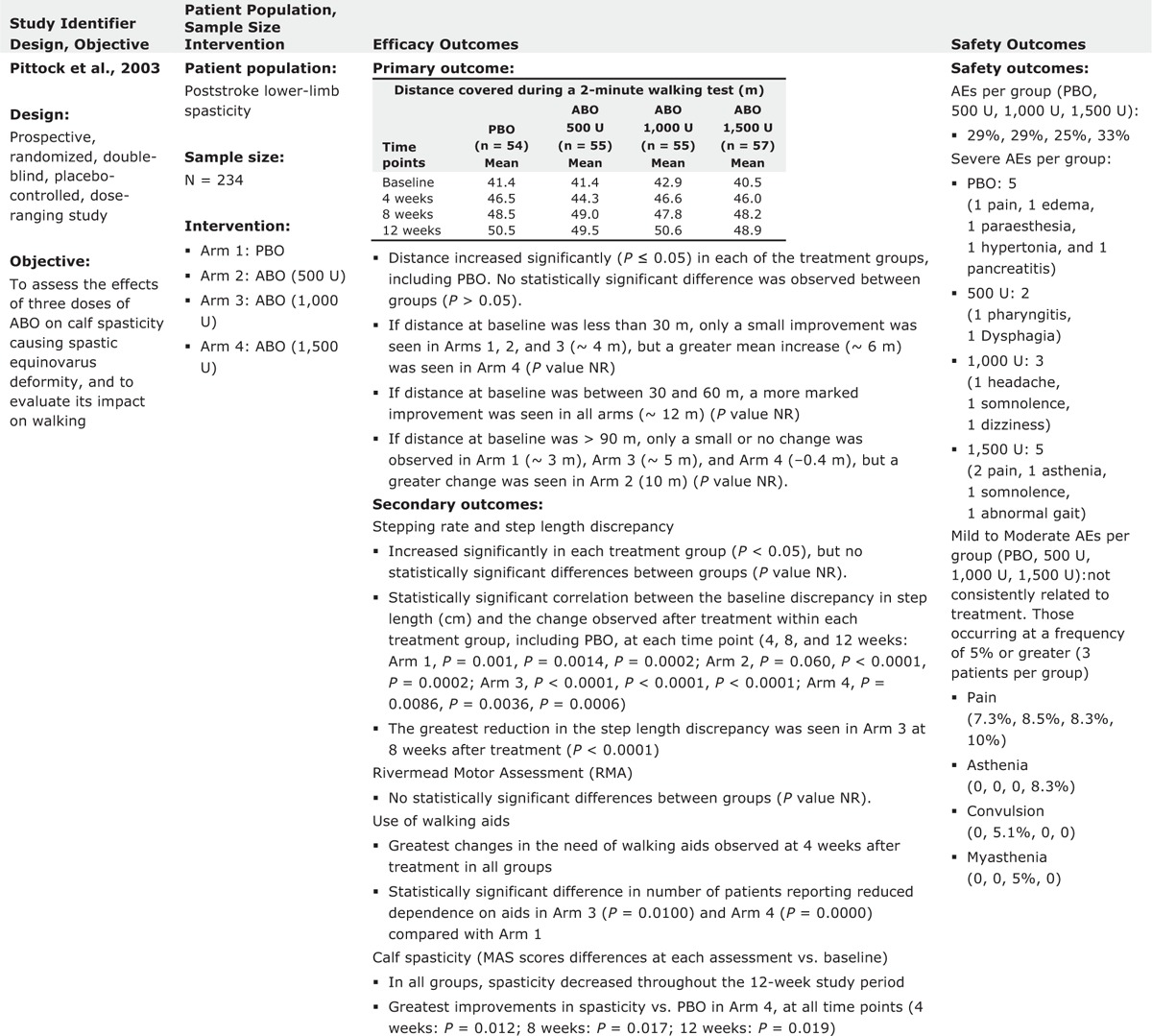
TABLE 4.
Dose Range of ABO for Each Individual Muscle

TABLE 5.
Doses of ABO Used in Individual Muscles by Study
DISCUSSION
The main aim of this systematic review was to provide guidance for physicians who manage ALLS using ABO. Other reviews are available regarding assessment of BoNT for treatment of ALLS with no emphasis on the dosing per muscles for ABO. This is essential since the dosing units of each BoNT-A product are not interchangeable with another toxin. In this review, all studies showed the efficacy of ABO in spasticity but using different outcome measures such as MAS, pain, or gait velocity. All of the studies showed reductions in spasticity using MAS, but not all of them reached statistical significance versus placebo. This may be due to the relatively small sample sizes employed and other study design issues. For example, most patients in the study of ABO for hip adductor spasticity reported by Gusev et al15 received doses that were lower than the 1500 U dose that Hyman et al14 had previously reported to be effective.
All 6 studies included in this review used different range of dosing that was shown to be safe and effective for the variety of postures in ALLS. Five of the 6 studies utilized EMG guidance as technique to optimize injection site localization and it is unknown if the use of other localization techniques (eg, ultrasonagraphy) would provide substantively different results. The dosing table provided here is based on the published studies and does not suggest that other doses should not be applied; physicians should always use clinical judgment on dosing schedules based upon on the severity of impairment. A recent international survey of routine therapeutic usage revealed that experienced European ABO injectors report injecting doses between 100 and 3000 U for ALLS (mean total dose ranged from 600 to 832 U), depending on the patient's needs.16 Likewise, all 6 studies preselected only a certain number of muscles for injection. Due to complexity of ALLS, it is vital that the injector has a proper understanding of the patients’ individual clinical needs. Clinical trials often prespecify muscles in an attempt to standardize findings, but it is likely that this restricted approach may have affected the outcome of the studies. When deciding on dosing strategies, the injector must consider multiple patient factors such as the muscles affected, patient functionality, size of the patient, residual deficit, and the etiology of the spasticity. For example, whereas some patients with poststroke spasticity may gradually improve their function with proper rehabilitation, patients with spasticity secondary to central neurodegenerative disorders will usually worsen. In patients with multiple sclerosis, the degree of deficit is subject to remission and exacerbation. Each of these different disease states requires a different dosing approach. Overall, additional research is needed in order to better define the optimal ABO dose and dilution parameters for individual muscles, the number and location of injection sites, and the most suitable technique for injection localization (eg, surface anatomy, EMG guidance, ES, and ultrasonography).
The degree of functional impairment is 1 of the most important factors to consider for managing spasticity. Unfortunately, spasticity research is hampered by the lack of outcome measures that are able to properly assess functional impairment before and after toxin injection,17 and this methodological limitation was evident in these 6 studies as well. For many patients, the impact of ALLS on their walking ability and gait significantly impacts their safety, comfort, social integration, and quality of life.18 Speed of gait was included in all 4 of the poststroke studies included in this review; however, statistical differences between ABO and placebo were difficult to show despite the fact that tone was consistently decreased after ABO injection.10–13 As Burbaud and colleagues note, there are many factors that can affect speed of gait—for example, a poststroke patient who no longer requires a walking stick or ankle orthosis to help them walk may walk more slowly because they are cautious of their regained ability.10 Further, it is worth noting that significant effects on speed of gait seemed to rely on the presence of concomitant physiotherapy. Whereas all patients in the Hesse study (which found a positive effect on speed of gait) had concomitant physiotherapy,11 only 38% of patients in the trial reported by Pittock et al received any physiotherapy (and, of these, most only received 1 session).13 It is important to note that many factors affect can impact functional outcomes. For example, as Burbaud and colleagues note, a lack of improvement in gait velocity could also reflect a detrimental effect of the toxin, which might produce too much weakness in plantar flexion.10 Gusev and colleagues note that if toxin spreads and weakens muscles adjacent to leg adductors, it is conceivable that the weakness could have negative influenced the ability to perform functional tasks with the legs.15 Such observations confirm the need for a highly individualized and multidisciplinary approach to manage ALLS, where functional outcomes and physical intervention are vital for achieving patient-specific treatment goals. Future research should also consider measurement of activity-based outcomes, such as total step count profiles, which have been found to be useful when assessing for meaningful changes in ambulatory performance in patients with spinal cord injury, spinocerebellar ataxias, and chronic stroke.19–21
This systematic literature review is part of a larger review where the use of ABO in other indications such as AULS has also been evaluated.8 When comparing the present results with the strength of the literature for AULS, it is apparent that more high-quality studies are required to inform practice. This need for more research is not limited to the use of ABO in ALLS, but there is also a clear and urgent need to better understand the burden of the condition2 and also the effectiveness of other treatments (including other BoNT formulations).22 Such work is ongoing and over 10 clinical trials of interventions for ALLS are listed on clinicaltrials.gov (including a double-blind study with ABO NCT01249404 and its open label extension study NCT01251367).
LIMITATIONS
This systematic review employed strict inclusion criteria as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.23 Based on our criteria a large number of uncontrolled, exploratory studies were excluded and affected our sample size. While this obviously eliminated some clinically relevant information, this established methodology is considered necessary to avoid bias by using explicit, systematic methods. A key aim of this systematic review was to produce a comprehensive, evidenced-based data report that provides information on the injection schema used and associated outcomes for ABO in ALLS. However, the substantial heterogeneity among patients included in these 6 studies regarding their geographical location, etiology and severity of spasticity significantly limits our ability to draw firm conclusions. The heterogeneity of outcome measures made it difficult to directly compare studies, and so we preferred to review the effectiveness of ABO in each study separately. Another limitation is due to lack of long follow up after ABO injection.
CONCLUSIONS
This systematic review provided current evidence regarding safety and efficacy of ABO injection for ALLS. Based on the evidence reviewed, it can be concluded that ABO injections consistently reduce tone and can be effectively employed in the management of ALLS of various etiologies. However, the review also revealed lack of large trials of ABO to manage ALLS, and highlighted the need for future trials to employ relevant outcome measures that properly assess patients’ functional ability.
Acknowledgments
Thanks to Lee-Ann Braun (RTI Health Solutions funded by Ipsen) for assistance with conducting the literature search and Anita Chadha-Patel, PhD (ACP Clinical Communications Ltd funded by Ipsen), for editorial support, including referencing.
Authors are also grateful to Professor Sherry Downie, PhD, who reviewed this manuscript before the final submission.
Footnotes
Abbreviations: ABO = AbobotulinumtoxinA; ALLS = adult lower limb spasticity; AULS = adult upper limb spasticity; BoNT = botulinum neurotoxin; MAS = Modified Ashworth Scale.
KD has received compensation/honoraria for services as a consultant or an advisory committee member or speaker from Allergan, Inc., Ipsen Biopharmaceuticals, Inc., Lundbeck Inc., Merz Pharmaceuticals, Teva Pharmaceutical Industries Ltd., Impax Pharmaceuticals, and US World Meds. JJC has received compensation/honoraria for services as a consultant or an advisory committee member from Ipsen Biopharmaceuticals, Inc. HWW has received compensation/honoraria for services as a consultant or an advisory committee member from Ipsen Biopharmaceuticals, Inc. and Merz Pharmaceuticals. MYL has received compensation/honoraria for services as a consultant or an advisory committee member from Ipsen Biopharmaceuticals, Inc. and Merz Pharmaceuticals.
Study partially funded by Ipsen for data collection and editorial support.
REFERENCES
- 1.Pathak M, Truong D. Brashear A, Elovic E. Anatomic correlation of common patterns of spasticity. Demos Medical, Spasticity Diagnosis and Management. New York:2011. [Google Scholar]
- 2.Martin A, Abogunrin S, Kurth H, et al. Epidemiological, humanistic, and economic burden of illness of lower limb spasticity in adults: a systematic review. Neuropsychiatr Dis Treat 2014; 10:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara Y. Deep venous thrombosis in stroke patients during rehabilitation phase. Keio J Med 2008; 57:196–204. [DOI] [PubMed] [Google Scholar]
- 4.Atiyeh BS, Hayek SN. Pressure sores with associated spasticity: a clinical challenge. Int Wound J 2005; 2:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royal College of Physicians, British Society of Rehabilitation Medicine, Chartered Society of Physiotherapy, Association of Chartered Physiotherapists Interested in Neurology. Spasticity in adults: management using botulinum toxin. National guidelines. London: RCP, 2009. [Google Scholar]
- 6.Simpson DM, Gracies JM, Graham K, et al. Assessment: botulinum neurotoxin for the treatment of spasticity (an evidence-based review). Neurology 2009; 73:736–737.author reply 737–738. [PubMed] [Google Scholar]
- 7.Wissel J, Ward AB, Erztgaard P, et al. European consensus table on the use of botulinum toxin type A in adult spasticity. J Rehabil Med 2009; 41:13–25. [DOI] [PubMed] [Google Scholar]
- 8.Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of AbobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil 2015; 94:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Des Jarlais DC, Lyles C, Crepaz N, et al. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 2004; 94:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbaud P, Wiart L, Dubos JL, et al. A randomised, double blind, placebo controlled trial of botulinum toxin in the treatment of spastic foot in hemiparetic patients. J Neurol Neurosurg Psychiatry 1996; 61:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesse S, Jahnke MT, Luecke D, et al. Short-term electrical stimulation enhances the effectiveness of botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett 1995; 201:37–40. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CA, Burridge JH, Strike PW, et al. The effect of combined use of botulinum toxin type A and functional electric stimulation in the treatment of spastic drop foot after stroke: a preliminary investigation. Arch Phys Med Rehabil 2004; 85:902–909. [DOI] [PubMed] [Google Scholar]
- 13.Pittock SJ, Moore AP, Hardiman O, et al. A double-blind randomised placebo-controlled evaluation of three doses of botulinum toxin type A (Dysport) in the treatment of spastic equinovarus deformity after stroke. Cerebrovasc Dis 2003; 15:289–300. [DOI] [PubMed] [Google Scholar]
- 14.Hyman N, Barnes M, Bhakta B, et al. Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomised, double blind, placebo controlled, dose ranging study. J Neurol Neurosurg Psychiatry 2000; 68:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gusev Y, Banach M, Simonow A, et al. Efficacy and safety of botulinum type A toxin in adductor spasticity due to multiple sclerosis. J Musc Pain 2008; 16:175–188. [Google Scholar]
- 16.Hubble J, Schwab J, Hubert C, et al. Dysport (botulinum toxin type A) in routine therapeutic usage: a telephone needs assessment survey of European physicians to evaluate current awareness and adherence to product labeling changes. Clin Neuropharmacol 2013; 36:122–127. [DOI] [PubMed] [Google Scholar]
- 17.Burridge JH, Wood DE, Hermens HJ, et al. Theoretical and methodological considerations in the measurement of spasticity. Disabil Rehabil 2005; 27:69–80. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre A, Lee T, Janzen S, et al. Systematic review of the effectiveness of pharmacological interventions in the treatment of spasticity of the hemiparetic lower extremity more than six months post stroke. Top Stroke Rehabil 2012; 19:479–490. [DOI] [PubMed] [Google Scholar]
- 19.Bowden MG, Behrman AL. “Step activity monitor” accuracy and test–retest reliability in patients with incomplete spinal cord injury. J Rehabil Res Dev. 2007;44:355–362. [DOI] [PubMed] [Google Scholar]
- 20.Mudge S, Stott N. Test–retest reliability of the StepWatch activity monitor outputs in individuals with chronic stroke. Clin Rehabl. 2008;22:871–877. [DOI] [PubMed] [Google Scholar]
- 21.Subramony SH, Kedar S, Murray E, et al. Objective home-based gait assessment in spinocerebellar ataxia. J Neurol Sci 2012;313:95–98. [DOI] [PubMed] [Google Scholar]
- 22.Esquenazi A, Albanese A, Chancellor MB, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon 2013; 67:115–128. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]


