Abstract
Approximately 50% of patients with hepatocellular carcinoma (HCC) reside in China. HCC is associated with very high mortality compared with other cancers. Although numerous factors influence the survival of patients with HCC who undergo liver resection, the role of the tumor biomarker histone methyltransferase (G9a) is unknown.
We enrolled 350 patients with HCC who underwent liver resection and followed them for 40 months. Patients’ clinicopathologic characteristics were acquired from medical records, and overall survival was determined using multiple methods. We conducted an immunohistochemical analysis of study G9a expression in HCC tissues. We used χ2 test to evaluate the significance of the relationships between G9a and other factors and Cox proportional hazards regression to estimate the hazard ratios and 95% confidence intervals.
The levels of alpha-fetoprotein were significantly higher in patients with G9a-positive tumors. TNM stage, elevated alpha-fetoprotein level, and G9a overexpression were associated with worse outcomes.
High expression of G9a was associated with worse outcomes, indicating that G9a may serve as a prognostic biomarker for patients with HCC who undergo surgical resection. Because of its role in cell proliferation, G9a represents a potential therapeutic target.
INTRODUCTION
Hepatocellular carcinoma (HCC) is associated with very high mortality compared with other malignancies and is the third leading cause of cancer deaths worldwide.1 The incidence of HCC is significantly higher in males and residents of East Asia, Southeast Asia, and North Africa and is relatively low in citizens of Oceania and Europe. The high prevalence of HCC can be attributed, in part, to the high infection rate of patients with hepatitis B and C viruses, as well as the consumption of food containing aflatoxins.2 Approximately 50% of patients with HCC live in China, which is attributed to the 7% rate of HBV infection of the general population as well as excessive consumption of alcohol. HCC is therefore a pressing public health problem that places a heavy burden on the country's healthcare system and on patients’ families.
Many factors may alter the survival of patients with HCC, including residual liver function, tumor size, disease stage, and the efficacy of treatment.3 Moreover, treatments such as liver resection and transcatheter arterial chemoembolization, and particularly the latter, provide options for the patients with unresectable tumors.4 Liver resection is considered the first-line treatment for patients with resectable HCC and is first line therapy.5 However, liver resection incurs a high risk of undesirable outcomes for patients with coexisting liver cirrhosis and large tumors.
Certain biomarkers can facilitate the management of patients with diverse tumors. For example, euchromatic histone lysine N-methyltransferase 2 (EHMT2) (also known as histone methyltransferase G9a, referred to here as G9a) is associated with risk and progression of cancers of the breast cancer,6 lung,7 and ovary.8 However, there are few reports focused on the role of G9a in disease progression or the survival of patients with HCC.9 Therefore, to investigate the significance of G9a expression in patients with HCC after liver resection, we conducted an immunohistochemical and clinicopathologic study of the association between G9a expression and patient survival. Moreover, this is the first study, to our knowledge, to follow this population of patients for as long as 40 months.
METHODS
Patients
We enrolled 350 consecutive patients with pathologically confirmed primary HCC who underwent liver resection at Fuzhou General Hospital (a Class A, tertiary care hospital). Because the hospital is located in the provincial capital, the patients represented all 9 cities of Fujian province. The first and last patients underwent liver resection in January 2004 and December 2012, respectively. Subjects’ clinicopathological characteristics including age, sex, tumor histological grade, tumor size, TNM stage, and preoperative serum alpha-fetoprotein (AFP) levels were acquired from the hospital's database. Information regarding survival was obtained from medical records, telephone interviews, and the Index System of Social Security Death. Duration of survival was calculated from the day of liver resection to the termination date of follow-up (May, 2015). Follow-up ranged from 93 to 3038 days (median, 1278 days). The TNM and histological-grade stages were determined in accordance with the TNM Classification of Malignant Tumors and the World Health Organization Classification of Tumors, respectively. This study conforms to the ethical principles contained in the Declaration of Helsinki and the policy of the Ethics Committee of Fuzhou General Hospital.
Determination of G9a Expression by Immunohistochemical Assay
The immunohistochemistry (IHC) was employed to evaluate G9a expression in the primary hepatocellular carcinoma tissue samples acquired from 350 subjects. The samples were fixed in 4% paraformaldehyde and paraffin embedded by standard methods. Antigen retrieval was performed with citrate buffer (10 mM citric acid-0.05% Tween 20, pH 6.0), and sections were stained by standard immunohistochemistry techniques, using antibodies as indicated and 1 g/L hematoxylin solution for counterstaining. Primary antibodies used were rabbit monoclonal G9a antibody (1:100 dilution; ab133482, Abcam, Cambridge, MA, USA), ll goat anti-rabbit biotinylated IgG in phosphate-buffered saline containing 1% bovine serum albumin for 30 min at ambient temperature, and then incubated with ABC reagent for 30 min. Immunostaining was visualized using 3,30-diaminobenzidine. Slides were dehydrated through graded alcohol to xylene and mounted with coverslips. Haematoxylin was used for nuclear counterstaining.
The expression of G9a was determined by 2 pathologists without any previous knowledge of subjects’ information. The positive G9a expression cells exhibited to have brownish-yellow granules in their nucleus. G9a expression was graded on the basis of the following standards: cancer cells were unstained or stained <10% were classified as negative, and cancer cells with stained ≥10% were identified as positive.
Statistical Analysis
We analyzed the association of patient survival with demographic and clinical characteristics such as age and sex, G9a expression, tumor size, AFP level, and TNM stage. These variables were included in the survival analysis, and we used the Cox proportional hazard regression model to calculate hazard ratios and 95% confidence intervals. Factors identified as statistically significant (P < 0.05) were further subjected to multivariate analysis. All statistical analyses were 2-sided and were performed using SPSS version 19.0 (SPSS Inc, Chicago, IL).
RESULTS
Patients’ Demographic and Clinical Characteristics
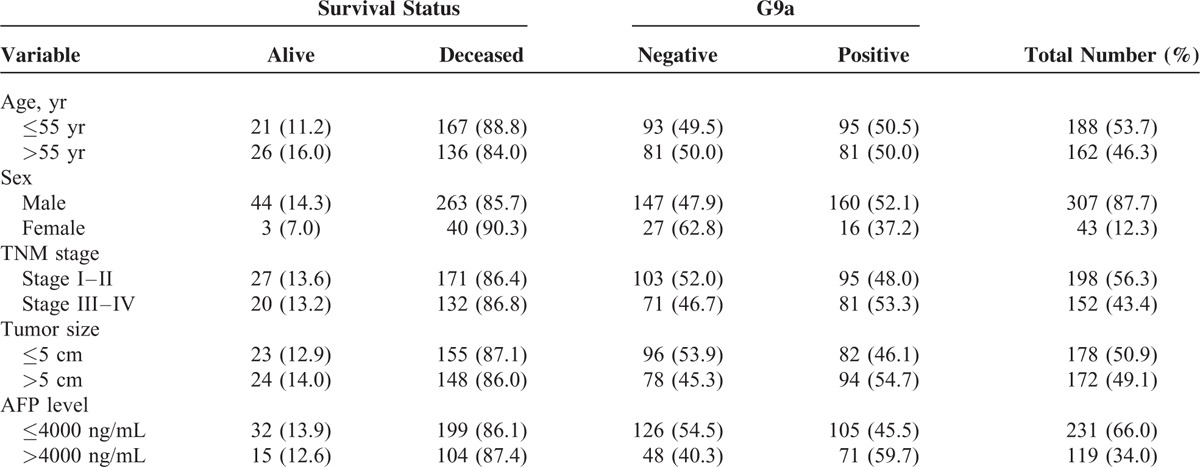
Of the 350 patients with HCC who underwent liver resection, 53.7% and 46.3% were ≤55 years or ≥55 years of age, respectively; 87.7% and 12.3% were men or women, respectively; 56.6% and 43.4% were classified with stage I–II or stage III–IV disease, respectively; and approximately 50.9% and 49.1% had tumors ≥5 cm or ≤5 cm, respectively. After liver surgery, the AFP levels of 66.0% and 34.0% of patients were <4000 or >4000 ng/mL, respectively. Immunohistochemical analysis revealed that G9a was expressed in approximately 50.3% of liver samples and was undetectable in 49.7% (Figure 1). The frequency of G9a-positive tumors was significantly higher when AFP levels were >4000 ng/mL. Otherwise, there were no significance differences between the other variables. At the end of follow-up, 303 subjects were deceased because of their tumors (Table 1).
FIGURE 1.
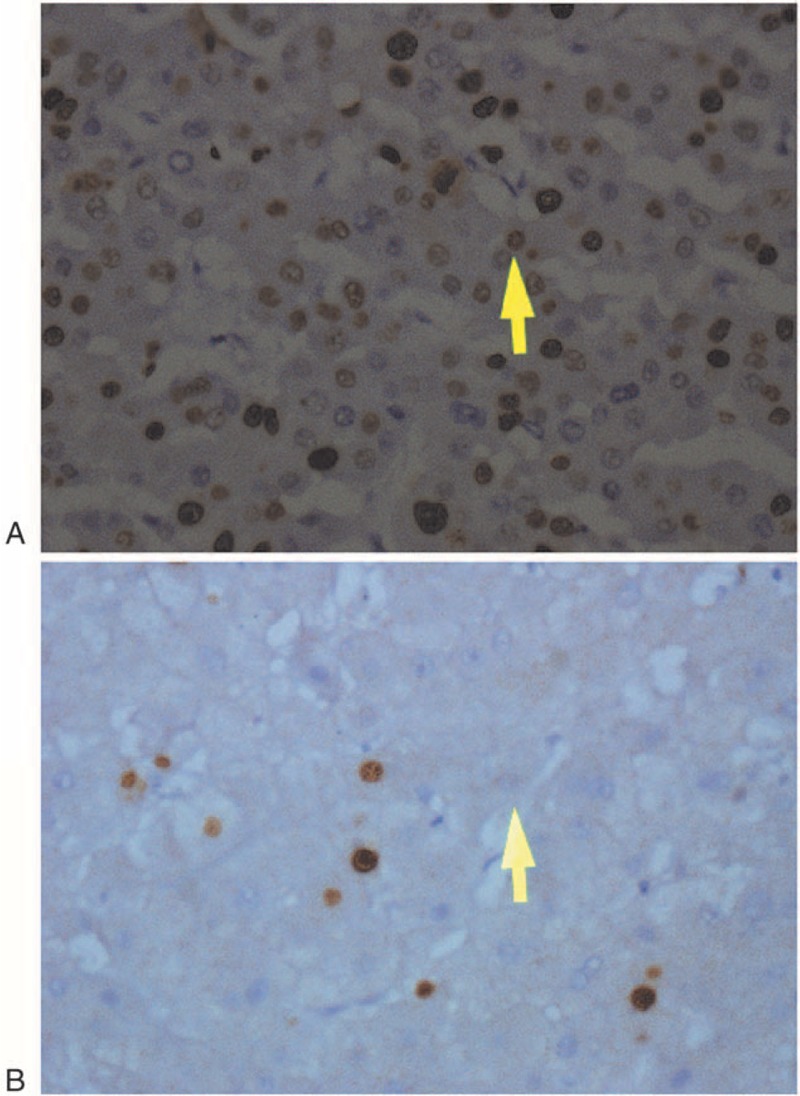
Immunohistochemical analysis of G9a expression. A, G9a-positive. B, G9a-negative. ×100 magnification.
TABLE 1.
Demographic and Clinical Characteristics of Study Subjects by Survival Status and G9a Results
Association of TNM Stage and the Levels of AFP and G9a With Shorter Survival
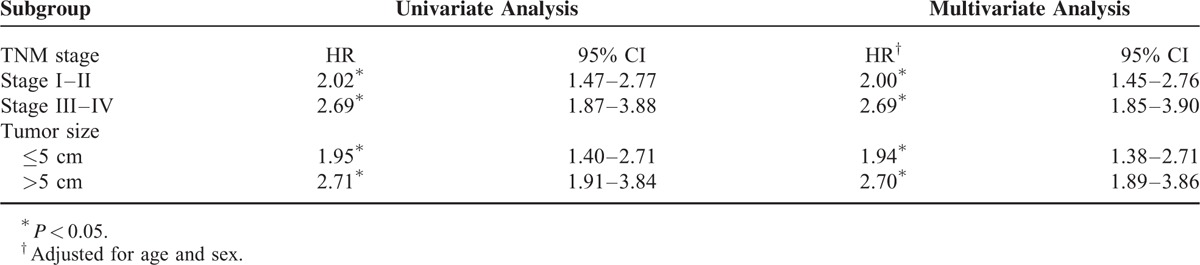
Univariate analysis revealed that subjects who with TNM stage III–IV, tumors >5 cm, AFP levels >4000 ng/mL, and G9a-positive experienced relatively poor overall survival. Further, age and sex were not significantly associated with survival. Multivariate analysis identified TNM stage, AFP levels, as factors significantly associated with overall survival, in contrast to tumor size (Table 2, Figure 2).
TABLE 2.
Overall Survival Analysis on the HCC Cases After Liver Resection
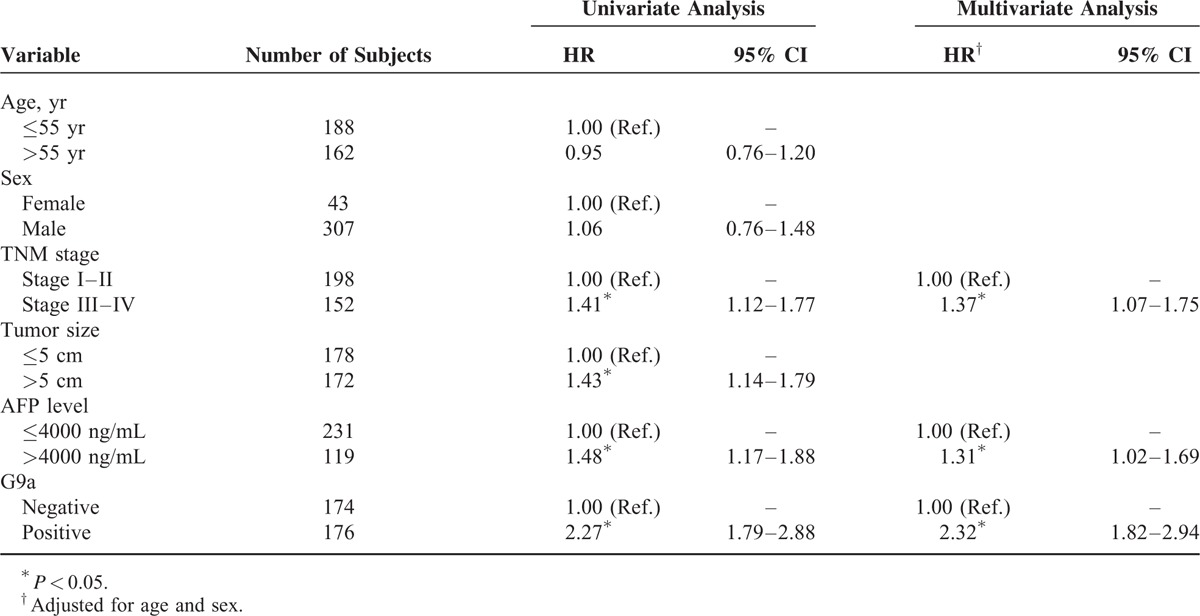
FIGURE 2.
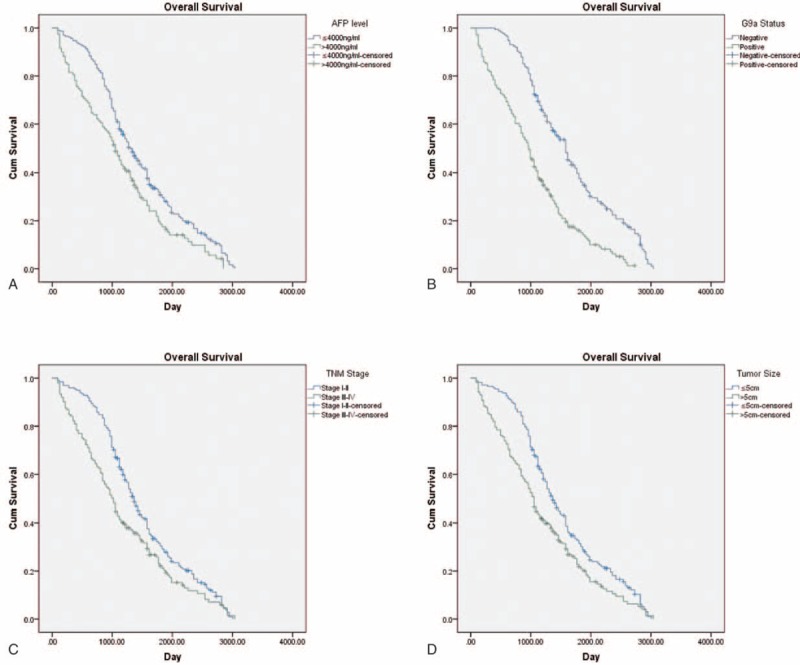
Kaplan–Meier analysis. A, AFP levels. B, G9a expression. C, TNM stage. D, Tumor size.
G9a Expression Is an Independent Predictor of Worse Survival
Cox regression analysis revealed an association between G9a-positive tumors and TNM stage and tumor size (Supplementary Material 1). When we performed the analysis after stratification according to these variables, we found that G9a-positive tumors correlated significantly with worse survival of patients with all TNM stages and tumor sizes. Independent of stratification, the hazard ratio for G9a-positive tumors was the highest for patients with stage III–IV disease. A similar association was detected for patients with larger tumors (see Table 3).
TABLE 3.
Stratification Analysis on G9a by TNM Stage and Tumor Size
DISCUSSION
Here, we show that advanced TNM stage, larger tumor size, serum AFP level, and detection of G9a expression were associated with worse survival of patients with HCC after liver resection. Although it is generally acknowledged that stage is the key prognostic factor for HCC, liver function and tumor size play an important role in survivals, and our study reveals that tumors larger than 5 cm were associated with worse outcomes, which is consistent with the findings of a previous report.10 A study conducted in Korea of 2558 patients with HCC indicates that there is a linear relationship between increased tumor size, shorter overall survival, and disease-free survival and that tumors larger than 5-cm serve as an independent prognostic factor for patients with HBV-related HCC.11
AFP is the most common HCC biomarker used in clinical practice, and a level of AFP >400 ng/mL in adults may indicate HCC. Moreover, high AFP levels are associated with reoccurrence and metastasis after treatment,12 which is consistent with the present findings. Furthermore, AFP levels serve as an independent prognostic factor of different treatment regimens.13 For example, the findings of a retrospective study of patients with HCC who received liver transplants suggest that patients with a high tumor burden survive if they have low AFP levels.14 Because serum AFP levels vary dramatically among HCC patients, a cutoff value is difficult to define, and different studies employ different cutoff values. For example, a study that used 400 ng/mL as the cutoff value to predict overall survival of 226 patients with HCC shows that preoperative AFP levels are associated with long-term outcomes.15 Furthermore, liver cirrhosis can lead to elevated AFP levels, and this confounding factor potentially influences the accuracy of AFP levels to predict survival. The results of a study that focused on HCC patients without cirrhosis suggest that preoperative AFP levels correlate with poorer survival as well as those with cirrhosis.16
We focused here on G9a, which catalyzes the dimethylation of lysine residue 9 of histone H317 because it mediates cell differentiation, proliferation, and gene expression as well as patients’ responses to drugs. Because of its vital role in cell proliferation, we reasoned that G9a is involved in tumorigenesis. Moreover, G9a is expressed at higher levels in cancer tissues compared with the corresponding normal tissue. For example, elevated transcription of EHMT2 is detected in colon, prostate, lung, and liver cancers,18 and knockdown of G9a expression inhibits cell growth and apoptosis of bladder and lung cancer cell lines.19 We show here that high expression of G9a is associated with poorer outcomes compared with patients with low expression, indicating that G9a may serve as a prognostic biomarker for HCC after surgical resection. Moreover, we found that detection of G9a expression is associated with AFP, indicating that G9a promotes metastasis and vascular invasion of HCC. For example, high expression of G9a is associated with shorter survival of 135 patients with esophageal squamous cell carcinoma,20 which is consistent with our findings. Moreover, the unique aspect of the present study is the identification of G9a as an independent risk factor of poor prognosis of patients with HCC who underwent liver resection.
The underlying mechanism of promoting tumor invasion and metastasis may be linked to the suppression of the activity of epithelial adhesion molecule (EPCAM), because the expression of EPCAM in primary lung cancer tissues inversely correlates with that of G9a.7 Furthermore, RNAi-mediated knockdown of G9a in vitro inhibits the migration and invasiveness of a lung cancer cell line.7 These studies and ours suggest that G9a is associated with cancer progression and prognosis and may serve as a novel therapeutic target. However, the role of G9a in HCC is unknown and will therefore be the subject of future investigations.
Footnotes
Abbreviations: AFP = alpha-fetoprotein; G9a = G9aHistone methyltransferase; HCC = heptaocellular carcinoma; IHC = immunohistochemistry.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gomaa AI, Khan SA, Toledano MB, et al. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol 2008; 14:4300–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011; 15:223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010; 30:61–74. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Ngan H, Lo CM, et al. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol 2000; 73:109–114. [DOI] [PubMed] [Google Scholar]
- 5.Benson AB, Abrams TA, Ben-Josef E, 3rd, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009; 7:350–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CC, Wu MJ, Yang JY, et al. Leptin-STAT3-G9a signaling promotes obesity-mediated breast cancer progression. Cancer Res 2015; 75:2375–2386. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 7.Chen MW, Hua KT, Kao HJ, et al. H3K9 histone methyltransferase G9a promotes lung cancer invasion and metastasis by silencing the cell adhesion molecule Ep-CAM. Cancer Res 2010; 70:7830–7840. [DOI] [PubMed] [Google Scholar]
- 8.Hua KT, Wang MY, Chen MW, et al. The H3K9 methyltransferase G9a is a marker of aggressive ovarian cancer that promotes peritoneal metastasis. Mol Cancer 2014; 13:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung SY, Lin HH, Yeh KT, et al. Histone-modifying genes as biomarkers in hepatocellular carcinoma. Int J Clin Exp Pathol 2014; 7:2496–2507. [PMC free article] [PubMed] [Google Scholar]
- 10.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int 2009; 29:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang S, Lee YJ, Kim KH, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg 2015; 19:1281–1290. [DOI] [PubMed] [Google Scholar]
- 12.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003; 38:200–207. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Shen Z, Zhu Z, et al. Clinical values of AFP, GPC3 mRNA in peripheral blood for prediction of hepatocellular carcinoma recurrence following OLT: AFP, GPC3 mRNA for prediction of HCC. Hepat Mon 2011; 11:195–199. [PMC free article] [PubMed] [Google Scholar]
- 14.Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl 2013; 19:634–645. [DOI] [PubMed] [Google Scholar]
- 15.Wan P, Zhang J, Long X, et al. Serum levels of preoperative á-fetoprotein and CA19-9 predict survival of hepatic carcinoma patients after liver transplantation. Eur J Gastroenterol Hepatol 2014; 26:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witjes CD, Polak WG, Verhoef C, et al. Increased alpha-fetoprotein serum level is predictive for survival and recurrence of hepatocellular carcinoma in non-cirrhotic livers. Dig Surg 2012; 29:522–528. [DOI] [PubMed] [Google Scholar]
- 17.Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev 201; 25:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Dorsey J, Chuikov S, et al. G9a and Glp methylate lysine 373 in the tumor suppressor p53. J Biol Chem 2010; 285:9636–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho HS, Kelly JD, Hayami S, et al. Enhanced expression of EHMT2 is involved in the proliferation of cancer cells through negative regulation of SIAH1. Neoplasia 2011; 13:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong X, Chen X, Guan X, et al. Overexpression of G9a and MCM7 in oesophageal squamous cell carcinoma is associated with poor prognosis. Histopathology 2015; 66:192–200. [DOI] [PubMed] [Google Scholar]


