Summary
In the first part of this overview, we described the life cycle of the influenza virus and the pharmacological action of the currently available drugs. This second part provides an overview of the molecular mechanisms and targets of still-experimental drugs for the treatment and management of influenza.
Briefly, we can distinguish between compounds with anti-influenza activity that target influenza virus proteins or genes, and molecules that target host components that are essential for viral replication and propagation. These latter compounds have been developed quite recently. Among the first group, we will focus especially on hemagglutinin, M2 channel and neuraminidase inhibitors. The second group of compounds may pave the way for personalized treatment and influenza management. Combination therapies are also discussed.
In recent decades, few antiviral molecules against influenza virus infections have been available; this has conditioned their use during human and animal outbreaks. Indeed, during seasonal and pandemic outbreaks, antiviral drugs have usually been administered in mono-therapy and, sometimes, in an uncontrolled manner to farm animals. This has led to the emergence of viral strains displaying resistance, especially to compounds of the amantadane family. For this reason, it is particularly important to develop new antiviral drugs against influenza viruses. Indeed, although vaccination is the most powerful means of mitigating the effects of influenza epidemics, antiviral drugs can be very useful, particularly in delaying the spread of new pandemic viruses, thereby enabling manufacturers to prepare large quantities of pandemic vaccine. In addition, antiviral drugs are particularly valuable in complicated cases of influenza, especially in hospitalized patients.
To write this overview, we mined various databases, including Embase, PubChem, DrugBank and Chemical Abstracts Service, and patent repositories.
Key words: Influenza, Antivirals, Experimental drugs
In the first part of this overview [1], we described the life cycle of the influenza virus and the pharmacological action of the currently available drugs. In this second part, we will overview the molecular mechanisms and the targets of still-experimental drugs for the treatment and management of influenza. Figure 1 shows the attack points of several potential antiviral drugs.
Fig. 1.
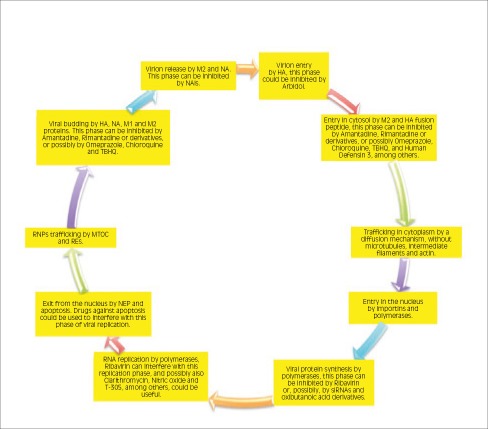
The attack points of several antiviral drugs are shown, with a particular focus on future potential compounds and strategies against influenza virus. Abbreviations: HA: hemagglutinin; M: Matrix protein; M1: Matrix type 1 protein; M2: Matrix type 2 protein; MTOC: microtubule- organizing center; NA: neuraminidase; NAIs: neuraminidase inhibitors; NEP: nuclear export protein; REs: recycling endosomes; RNA: ribonucleic acid; RNP: ribonucleoprotein; siRNA: short interfering RNA; TBHQ: Tert-butyl-hydroquinone.
Antiviral drug research is a particularly active field and new approaches have been developed. Briefly, we can distinguish between compounds with anti-influenza activity that directly target influenza virus proteins or genes, and molecules that target host components that are essential to viral replication and propagation. Among the former group, we will focus especially on hemagglutinin (HA), Matrix protein 2 (M2) and neuraminidase (NA) inhibitors (HAIs, NAIs). The latter molecules have been implemented quite recently and may pave the way for personalized treatment and management of influenza. Moreover, it is expected that the inhibition of host factors (such as single molecules) and/or complex mechanisms (such as intracellular signaling cascades and pathways) may act against different influenza virus strains and may be less prone to the emergence of drug resistance than the inhibition of viral components [2, 3]. Therapies that combine two or more compounds belonging to the same group or different groups are also discussed.
To write this overview, we mined various chemical databases, including Embase [4], PubChem [5, 6], Drug- Bank [7] and Chemical Abstracts Service (CAS) [8], as well as patent repositories and clinical trials registries [9]. We also scanned extant reviews and consulted the gray literature (books, proceedings, conference abstracts, posters and congress communications) in order to increase coverage of the anti-influenza drugs included in the present article. With regard to the search strategy, we used a mining approach similar to that described in Eyer and Hruska [10]. No time or language filters were applied.
To the best of our knowledge, this article constitutes the most comprehensive and up-to-date overview of antiinfluenza compounds in the literature. It can be used also as a working bibliography and a mapping review for scholars doing research in the field.
Along with this paper, a database is currently being designed and developed and will be accessible at the CIRIIT institutional website [11].
Entry and Attachment Inhibitors
Effective antiviral compounds that interfere with the attachment and entry of the influenza virus into the host cell include triterpenoids [12] such as glycyrrhizic acid (GA) [13], glycyrrhizin (GR) [14], glycyrrhetinic acid [15] and further derivatives extracted from licorice and present in some Chinese medicaments. GR is the most active of these molecules and can repress the replication of H3N2 and H5N1, as well as of several viruses [16]. It can be delivered as an approved parenteral GR preparation (Stronger Neo-Minophafen C, SNMC), and glutamyl-tryptophan can be added in order to increase its activity [17, 18]. GR is able to inhibit entry of the virus into the host cell, and reduces the level of pro-inflammatory molecules such as chemokine (C-X-C motif) ligand type 10 (CXCL10), interleukin 6 (IL6), CC chemokine ligand type 2 (CCL2), and CC chemokine ligand type 5 (CCL5) [19, 20]. It also exerts an anti-apoptotic action. In addition, GR hinders monocyte recruitment and has anti-oxidant activities, inhibiting the formation of influenza virus-induced reactive oxygen species (ROS) [21]. It extensively modulates gene expression, activating interferon- gamma (IFN-gamma) and reducing the expression of Nuclear factor kappa B (NFκB), c-Jun N-terminal kinase (JNK), and p38. Furthermore, GR reduces high-mobility-group box type 1 (HMGB1) [22]. Promising glycyrrhizin derivatives include spacer-linked 1-thioglucuronide analogues [23]. GA inhibits influenza virus growth and replication in embryonated eggs [24]. Moreover, it can be used as an adjuvant in the preparation of anti-influenza vaccines [25].
Other triterpenoids [26], such as the saponins and uralsaponins M-Y from the roots of Glycyrrhiza uralensis [27], exhibit anti-influenza and anti-HIV activities. Moreover, saponins can be used as vaccine adjuvants [28-31] and modulate the expression of cytokines and chemokines [32, 33]. Further triterpenoid derivatives share broad antiviral actions [34-38].
Dextran sulphate (DS) is a negatively charged sulphated polysaccharide. Besides inhibiting virus entry and attachment, it represses HA-dependent fusion activity [39-41] and NA-dependent activity [42]. However, mutations conferring resistance to DS are described in the literature [43]. Oxidized dextran can be administered as a prevention [44-46].
Other sulphated molecules include the sulphated syalil lipid NMS03, which is effective against IAV, Human Metapneumovirus (HMPV) and picoRNAvirus. It is assumed that it interferes with fusion, but the precise nature of its mechanism is still unknown [47].
Another potential fusion inhibitor is BTA9881, which has shown promising activity against RSV [48, 49].
Lysosomotropic agents, such as concanamycin A [50-53], the macrolide antibiotic bafilomycin A1 [54, 55], saliphenylhalamide [56], N,N'-Dicyclohexylcarbodiimide [52], and chloroquine [57-64], inhibit vacuolar ATPase (VATPase) and reduce endosome acidification and lysosome number. They act on the CME pathway, but are unable to block clathrin caveolae-independent endocytosis. It should be stressed that the anti-influenzal activity of these compounds strongly depends on the pH of the cellular environment and that some scholars have reported conflicting findings about their in vivo effectiveness [65].
Extract from milk thistle seeds, known as silymarin, a complex mixture of flavonolignans, and its main component silibinin are active against influenza [66]. Also silybin and its derivative can block virus entry and regulate autophagy, repressing the formation of oxidative stress species and triggering activation of the extracellular signal-regulated kinase (ERK)/p38 mitogenactivated protein kinase (MAPK) and IκB kinase (IKK) cascades [67]. Other silybin derivatives include silybin fatty acid conjugates, which have strong anti-oxidant properties [68].
Compounds from Melaleuca alternifolia (tea tree) oil (TTO) concentrate (MAC) [69, 70] have a broad antimicrobial activity. In silico simulations have shown that these compounds can interfere with virus entry and fusion of the influenza virus [71, 72].
Other potential compounds include Amaryllidaceae alkaloids from Lycoris radiate, such as lycorine, hippeastrine, hemanthamine and 11-hydroxy vittatine, which can also inhibit the nuclear-to-cytoplasmic export of the ribonucleoprotein (RNP) complex [73].
Curcumin is able to inhibit virus entry and HA [74]. It also has antioxidant, anti-inflammatory, anticancer, antiviral, antibacterial and antidiabetic properties, among others [75]. Curcumin acts against a large array of targets [76]. Curcumin is also active against other viruses [75, 77]. Rajput and collaborators showed that animals on a diet enriched in curcumin displayed an improved immune response [78]. Surprisingly, curcumin derivatives do not exhibit anti-influenza activity [79].
LADANIA067, extracted from the leaves of the wild blackcurrant (Ribes nigrum folium) [80, 81], has shown antiviral activities both in vivo and in vitro, without having any effect on influenza virus metabolism or growth/ proliferation.
Fattiviracin A1 is a recently discovered antiviral [82]. Besides inhibiting both IAV and IBV, it is active against HIV, HSV and VZV [83].
Lignans exert a good anti-influenza activity [84, 85] Germacrone is a molecule purified from Rhizoma curcuma. It can be effectively combined with oseltamivir [86].
Akt inhibitors are also effective entry inhibitors. These include peptide "Akt in", which may be TCL1- or TCL1b-based, MK2206 [87, 88] and Ma-xing-shi-gantang (MXSGT), a traditional Chinese herbal decoction [89]. Everolimus, an inhibitor of the PI3K-AktmTOR pathway, is also a valuable tool against influenza [90].
Among anti-attachment drugs, Fludase (DAS181) has potential anti-influenza virus properties [91-103]. This medication, which has proved capable of inhibiting human and avian influenza viruses in pre-clinical studies, acts by mimicking NA and destroying the molecules of sialic acid receptors on the host cell surface. It is also effective against NA-resistant influenza strains [92, 93, 103].
HA Inhibitors
An effective class of HAIs is that of the amide derivatives [104-107].
Gossypol is a natural phenolic aldehyde extracted from the cotton plant and blocks the dehydrogenase family enzymes [108, 109]. Its antiviral properties emerged during a 1970 study, in which an experimental model of influenza pneumonia was used [108]. In particular, chiral (+)-gossypol is more active than (–)-gossypol [110, 111].
Another antiviral against HA is Entry Block-peptide (EB-peptide), a peptide derived from fibroblast growth factor 4 (FGF4) [112]. EB-peptide can inhibit virus entry and attachment, being effective even when administered post-infection. Besides repressing influenza viruses, EBpeptide is also active against other viruses [113]. It can also be used as an adjuvant in the formalin-inactivated influenza whole-virus vaccine, triggering phagocytosis of influenza virions. Other peptides similar to EB-peptide are the FluPep (FP) peptides, such as FP1 (Tkip) and FP2-FP9 [114]. Tkip was designed as a mimetic of the suppressor of the cytokine signaling (SOCS) protein, which is involved in mediating the immune response to influenza. Furthermore, peptide NDFRSKT has strong antiviral properties, but with unknown therapeutic characteristics [115, 116].
Other molecules which bind to HA are collectins (CLs) [117]. Human CLs and bovine conglutinin, CL-43 and CL-46 confer protection against influenza infection [118-122].
A related group of molecules is the ficolins (such as H-ficolin and L-ficolin), present at high concentrations in serum and in bronchoalveolar secretions [123]. They bind not only to HA but also to NA in vitro models [124]. These proteins can be engineered in such a way as to become more active against influenza virus; for example, Chang and collaborators designed recombinant chimeric lectins consisting of mannose-binding lectin (MBL) and L-ficolin [125]. However, because of their role in the inflammatory response, their potential use in humans requires more complete analysis. Recently, agglutinins such as NICTABA, UDA [126] and protectins like protectin D1 [127-130] have been found to have anti-influenza propriety [131].
An interesting compound, which binds to specific high-mannose oligosaccharides of HA is Cyanovirin-N (CVN) [132]. In 2003, O'Keefe et al. demonstrated its potent in vitro antiviral activity against a wide range of IAVs and IBVs, including NA-resistant strains, though resistance induced by mutations that affect the glycosilation site of HA seems to arise quite naturally [133].
Clarithromycin (CAM), able to inhibit influenza virus replication in vitro and in cell cultures, appears to have 3 mechanisms of action against type A seasonal Influenza virus. It was recently showed that CAM reduces the expression of human influenza virus receptors on the mucosal surface of the airways, reduces the production of nuclear factor-kB (NF-kB), and increases pH inside the endosomes [134, 135].
Norakin (Triperiden) is an anticholinergic drug that interacts with HA [136, 137]. This interaction may be indirect, being mediated by an increase in the internal pH in the pre-lysosomal compartment [138-140]. However, strains resistant to Norakin have been described [141-144]. Also Norakin derivatives seem to be effective antiviral compounds [145].
Another interesting compound is nitazoxanide [146-151], useful for the treatment of protozoal and bacterial infections and is active against hepatitis and influenza viruses or rotaviruses. Further thiazolides act at the post-translational level by selectively blocking the maturation of viral HA at a stage preceding that of resistance to endoglycosidase H digestion, thus interfering with HA intracellular trafficking and insertion into the host plasma membrane, which is a key step in the correct assembly and exit of the virus from the host cell.
Bacillus intermedius ribonuclease (BINASE) shows a good anti-influenza activity. BINASE and HA interact with sialic acid on the cell surface and penetrate into the host cell. Subsequently, viral RNA is released and cleaved by BINASE [152, 153].
High mannose-binding lectins (HMBL) are powerful influenza and HIV inhibitors [154].
Rutin, quercetin, and related compounds, extracted from elderberry fruit (Sambucus nigra L.) [155-161] are other HA inhibitors. Xylopine and rosmaricin have an amine group that interacts with HA [162, 163].
Theaflavins (TFs) from black tea have a strong anti-influenza activity, inhibiting HA and reducing the level of IL6, thus exerting an anti-inflammatory and anti-apoptotic action [164-166].
M2 Inhibitors
M2 inhibitors can be basically divided into 2 groups. The first includes compounds derived from the leads of amantadine and rimantadine and its hydroxylated derivatives [167-172]. The second includes non-adamantane derivatives, which are promising drugs against influenza viruses [173]. Some of these compounds have been specifically designed for some important mutants of the M2 ion channel of IAV [174-177].
Regarding molecules putatively capable of blocking the ion pump, Gasparini and coworkers recently conducted a field investigation into the effect of omeprazole family compounds (OFC) [178] on Influenza-like Illness (ILIs). The results showed that subjects treated with omeprazole family compounds displayed a lower risk of catching ILI (ORadj = 0.29, 95% CI: 0.15-0.52) than non-treated subjects. Molecular docking and molecular dynamics (MD) simulations, which are a common method of searching for new potential drugs, seem to confirm these findings [179]. The M2 Protein – Protein Data Bank (PDB) code 3C9J [180] – was simulated as being embedded in a dipalmitoylphosphatidylcholine (DPPC) membrane in complex, with its ligands amantadine and rimantadine being used as positive controls and omeprazole as a putative ligand. The thermodynamic integration method was used in order to estimate binding free energies of the ligands. Free-energy calculations imply omeprazole as a potent anti-viral drug. Also another study has suggested the antiviral properties of omeprazole against Ebolavirus [181].
Polyamines such as spermine [182, 183], spermidine and putrescine have recently been identified as intrinsic rectifiers of potassium channels. Indeed, the M2 protein has a binding site for polyamines, which is different from the amantadine binding site [184]. Polyamines have quite recently been exploited in designing anti-influenza vaccines [185, 186].
Spiropiperidine M2 inhibitor and its derivatives appear promising in acting against amantadine-resistant viruses; in particular, spiropiperidine-9 seems to be the most active [187].
Among natural products, pinanamine derivatives [188] and 24-E-ferulate [188] have a good influenza activity.
Endosomal and lysosomal inhibitors
Substituted salicylanilides appear promising antiviral agents [190-193]. In particular, Niclosamide [192], which is approved for human use against helminthic infections, besides being active against influenza viruses, has also shown anti-neoplastic and broad antiviral effects, being active against SARS-related coronavirus and Human Rhinovirus (HRV).
Lysosomotropic agents [50-64] have also been already discussed. Further compounds include molecules obtained from TTO [69-72], which have already been mentioned.
Protease inhibitors
The cleavage of HA can be blocked not only by anti- M2 protein compounds, but also by inhibition of the necessary proteases [194]. Given the great importance of the proteases in the viral replication cycle, many authors [195, 196] have directed their research towards anti- protease medications that could block, or at least mitigate, the consequences of HA cleavage. HA can also be blocked by natural products such as Hepatocyte growth factor activator inhibitor 2 (HAI-2) [197]. Several anti- protease drugs have been studied in in vitro models, animals and humans, such as Camostat mesilate [198], epsilon-aminocapronic acid [199], leupeptin [200] and Aprotinin [201], which has been approved for topical use in a small-particle aerosol formulation in Russia. A theoretical advantage of antiviral activity against enzymatic activities of the host is that these molecules would not lead to the selection of resistant viral variants.
Other molecules can interfere with the mechanism of fusion of the endosomal and viral membranes [202]. Indeed, numerous small molecules that block virus infectivity by inhibiting the conformational changes required for HA-mediated membrane fusion have been identified. Russell et al. [194] have demonstrated that TBHQ (Tertbutyl- hydroquinone) stabilizes the neutral pH structure and, in this way, presumably, inhibits the conformational rearrangements required for membrane fusion. Furthermore, Leikina et al. [203] have demonstrated that human β-defensin 3, a lectin, can inhibit HA-mediated influenza viral fusion.
Regarding the compounds targeted against the transcription and replication of vRNA, one of the first drugs developed is Ribavirin (RIB). RIB, also known by the trade name "Virazole", is a nucleoside analog [204]. Its mechanism of action is not completely known. However, Inosine 5'-monoposphate dehydrogenase (IMPDH) appears to be the principal target of the molecule. This inhibition diminishes the intracellular concentration of GTP (Guanosine-5'-triphosphate), and this is thought to stop viral protein synthesis and limit vRNA replication. Crotty et al. also demonstrated that RIB is a lethal vRNA mutagen [205]. However, the need for high doses of the drug in order to have obtain good clinical results has limited the use of RIB as an anti-influenza drug, and a recent revision of the literature by Chan-Tack et al. suggests that there are no conclusive results on the beneficial use of Virazole for the treatment of influenza [206]. RIB can also be delivered as a liposome encapsulated with muramyl tripeptide (MTP-PE) [207].
α(1)-antitrypsin (AAT) [208] is a serine protease inhibitor of elastase and proteinase-3 (PR-3). This protein is produced by the liver and its expression increases particularly during the acute-phase response. It also has immunomodulatory, anti-inflammatory and tissue-protective properties, reducing influenza-related complications and morbidities. As an immunomodulator, AAT mediates the maturation and differentiation of dendritic cells (DCs) and T regulatory cells (Tregs), activating the IL1 receptor antagonist (IL1RA) and inducing IL10 release. Moreover, it exerts an anti-apoptotic effect, inhibiting caspases-1 and -3. The role of AAT in inhibiting influenza viruses is consistent with the clinical observations that subjects with AAT deficiency are exposed to the risk of severe influenza-related complications and should therefore be vaccinated [209, 210].
Stachyflin, acetylstachyflin and its phosphate esther or oxo derivatives [211, 212] exert their inhibitory activity on a variety of HA subtypes of IAV (H1, H2, H5 and H6, among others) but have no activity on H3 subtype IAV or on IBV [213-217]. The metabolites of stachyflin and its derivatives include compounds such as cis-fused decalin [214]. Stachyflin compounds can be delivered intranasally or orally, using PEG 400 as vehicle [211]. However, some amino acid substitutions confer resistance to stachyflin [212].
BMY-27709, a salicylamide derivative, and its analogues are other useful compounds [218, 219].
Thiobenzamide derivatives have a good activity profile. In particular, the axial disposition of the thioamide moiety has proved to be crucial to inhibitory activity [220].
Ulinastatin [221] is a protease inhibitor, which also protects lysosome integrity. Its use has been suggested for the treatment of avian influenza [221] and severe influenzarelated complications, such as encephalopathy [222] and acute respiratory distress syndrome (ARDS) [223, 224]. Indeed, a recently published meta-analysis has shown that this drug is effective in managing acute lung injury (ALI) and ARDS [225].
The ubiquitin-specific peptidase type 18 (USP18) protease inhibitor ISG15 is another promising molecule [226]. ISG15 is part of the interferon-regulated cellular cascade. USP18 was found to be one of seven genes which predict a response to influenza virus [227]. This finding was reproduced by Liu and collaborators [228].
Polymerase inhibitors
Other antiviral strategies have been directed against the viral RNA polymerase [229, 230]. The trimeric polymerase complex has multiple enzymatic activities and can thus be targeted at different sites of action. For instance, nucleoside/nucleotide compounds have been developed against other viruses, namely HIV, HBV, etc.
A historical compound is moroxydine [231-233]. It is also active against HSV and VZV.
The most thoroughly studied of these molecules is Favipiravir (T-705). In vitro studies have demonstrated the high antiviral potency of the drug and mouse studies have demonstrated its protective efficacy against a wide range of influenza viruses A and B. This molecule also seems to be effective against other viruses [234-238].
More recently, other compounds directed towards antinucleasic activities have been studied, such as the series of hydroxypyridinone, which appears to have antiviral activity in cells [230].
On studying 33 different kinds of phytochemicals, other scholars have identified a family of drugs called marchantines, which appear to interact with the PA subunit of the endonuclease [239].
An attractive strategy for developing anti-polymerase compounds appears to be that of interfering with the subunit binding interfaces of PB1 and PA, which are very well conserved in different Influenza virus strains [240]. Thus, these compounds would reduce the transcriptional activity of the viral RNA polymerase. One such promising compound is AL18, which is also active against human cytomegalovirus [241].
Furthermore, the recent definition of the PB1/PB2 binding interface by means of crystallography [242] has prompted researchers to study synthetic peptides, such as peptide 1-37 and peptide 731-757, which seem to inhibit the interaction between PB1 and PB2 [243-247].
Azaindole VX-787, an inhibitor of PB2 [248-251], is able to interfere with the cap-snatching activity of the polymerase complex of the influenza virus. The small GTPase Rac1 inhibitor NSC23766 exhibits a similar activity profile [252].
Nuclear pathway inhibitors
Leptomycin B (LMB) inhibits nuclear export signal (NES)-mediated vRNP export, as well as NES-receptor CRM1/exportin-1 (XPO-1); however, it is somewhat toxic [253].
Verdinexor (KPT-335) [254] is a new-generation XPO- 1 antagonist that is well tolerated in animal models and seems to be effective against both IAV and IBV. It is a selective inhibitor of nuclear export (SINE).
NP inhibitors
Given the fundamental importance of the NP in modulating the replication cycle of the virus, many authors have investigated strategies for preventing its production. Moreover, molecules that prevent the functional polymerization of the NP monomers have also been studied, such as, for example, Nucleozin (NCZ) [255]. It also blocks viral RNA and protein synthesis and targets vRNP nuclear export and its cytoplasmatic trafficking. As a final result, fewer and smaller influenza viral particles are released. NCZ derivatives include a quite effective compound, namely 3061 (FA-2), which has been shown to inhibit the replication of the influenza A/ WSN/33 (H1N1) virus, though NP-mutant strains have displayed resistance to this drug [256].
Jiang and collaborators screened a peptide library and discovered that the NP-binding proline-rich peptide was particularly effective against influenza viruses [257].
Another interesting molecule is the interferon-inducible Mx1 protein [258, 259].
Cycloheximide (CHX), which is also active against enterovirus- 71 (EV-71), coxsackievirus B, and actinomycin D, are quite effective chemicals [260-262].
Intriguingly, clinically licensed anti-cyclooxygenase-2 (COX-2) Naproxen also appears to inhibit the functional polymerization of NP monomers. Its derivatives, such as naproxen A and C0, also appear quite promising [263].
Another drug directed against the NP is Ingavirin, which has been licensed in Russia. Indeed, Ingavirin interacts with the transport of newly synthesized NPs from the cytoplasm to the nucleus [264-272]. It is also active against parainfluenza virus, adenoviruses and human metapneumovirus [273].
NA inhibitors
NAIs include peramivir and lanimamivir derivatives [274-289].
Baicalin induces autophagy and acts against both NA [290] and NS1 [291-293].
Isoscutellarein is another compound that inhibits influenza virus sialidase. Its derivative is also active against influenza [294, 295].
NS1 inhibitors
Another potential strategy against influenza is to block the NS1 protein, a non-structural protein that is very important during the viral replication cycle. Indeed, the NS1 protein down-regulates the cellular production of IFN α/β. Furthermore, it has been demonstrated that NS1 also modulates other crucial aspects of influenza virus replication, namely viral RNA replication, viral protein synthesis, and general host-cell physiology [1, 296]. Finally, NS1 probably has an anti-apoptotic function in the early phases of replication. The meaning of apoptosis during influenza A virus replication is ambiguous, although it is usually considered to be a cellular antiviral defense that limits virus replication. Therefore, influenza viruses have acquired different ways of procrastinating this seeming host strategy [1]. Nonetheless, cellular pro-apoptotic factors favor the effective replication of influenza viruses, and some viral proteins, such as NA and PB1-F2, carry out pro-apoptotic tasks [1, 297]. Furthermore, some compounds that act against the NS1 protein have been studied. In this perspective, peptide-mediated inhibition of NS1 – CPSF30 has been proposed as a strategy for mitigating viral replication [298, 299]. Unfortunately, this virus-specific approach leads to viral mutation and the occurrence of drug resistance.
More recently, Jablonski et al. studied a class of molecules derived from the NSC125044 compound, which displayed NS1 protein inhibition in viral replication assays [300].
Regulated in development and DNA damage responses- 1 (REDD1) is a molecule that has recently emerged from comprehensive biochemical screening. Moreover, REDD1 inhibits the mTOR pathway [301].
Other RNA synthesis inhibitors
Cordycepins extracted from Cordyceps, a genus of ascomycete fungi, are used for diverse medicinal purposes because of their different pharmacological actions with hypothetical anti-viral activity [302].
Caspase inhibitors
Apoptosis plays a major role in the influenza virus life cycle [303-307]. Indeed, in order to replicate, the virus activates the mechanism of apoptosis through the activation of caspase 3. Cellular inhibitors of apoptosis proteins (cIAPs) are essential regulators of cell death and immunity. Nucleotide-binding oligomerization domain- like receptor type 1 (NLRX1) [308] binds to viral protein PB1-F2, preventing IAV-induced macrophage apoptosis and promoting both macrophage survival and type I IFN signaling. Interestingly, compounds that inhibit this enzymatic activity could be useful as anti-influenza antivirals. Indeed, Wurzer et al. have shown that apoptotic activation by caspase 3 is required for efficient virus production [306]. Furman and collaborators have demonstrated that the apoptotic index is a predictive biomarker of influenza vaccine responsiveness [309]. However, the question of whether apoptosis is beneficial to the viral reproductive cycle or to host cells is still under debate. Moreover, Hinshaw et al. [307] demonstrated that, on inhibiting apoptosis during viral infection, influenza virus RNP complexes were retained in the nucleus. Therefore, the use of caspase 3 inhibitors could have good potential as anti-influenza drugs [310].
Autophagy
Autophagy (or autophagocytosis) is a catabolic mechanism that involves cellular breakdown of dysfunctional cell components through the involvement of lysosomes. Procyanidin has an anti-IAV activity [311].
Glucosidase, mannosidase and glycosilation inhibitors
L-fructose and L-xylulose can inhibit influenza virus replication [312]. Glucosidase I and glucosidase II inhibitors include iminosugars, which alter glycan processing of influenza HA and NA [313].
Pathway inhibitors
Raf/MEK/ERK pathway inhibitors include compounds, which act as an inhibitor of MEK1 and MEK2 [3]. NFKB inhibitors include Bortezomib [3], among others. These proteasome inhibitors are also effective against paramyxoviruses, HRV, poliovirus, coxsackievirus, HSV and HIV.
Phospholipase inhibitors
Lipid metabolism plays a fundamental role during influenza virus replication: membranes and their components, such as sphingolipids, are crucial to all steps of the viral life cycle, from attachment and membrane fusion, to intracellular transport, replication, protein sorting and budding. Infection by influenza virus stimulates phospholipase D (PLD) activity [314].
Release inhibitors
HDAC6 is an anti-IAV host factor that negatively regulates the trafficking of viral components to the host cell plasma membrane via its substrate, acetylated microtubules [315].
As an anti-influenza chemical, cyclosporin A does not act through its classical targets, namely cyclophilin A (CypA), cyclophilin B (CypB) and P-glycoprotein (Pgp) [316], but by inhibiting influenza virus release. Ching-fang-pai-tu-san (CFPTS) has a similar action [317].
Anti-oxidants, anti-inflammatory compounds and immunomodulators
Oxidation plays a major role in influenza virus life cycle and replication [318]. With regard to anti-influenza drugs that act subsequently to the various stages of viral replication, after the formation of vRNPs, it is worth considering that Resveratrol may be useful as an antiinfluenza drug. Indeed, this compound could interfere with the translocation of RNPs from the nucleus to the cytoplasm [319-321]. Dehydroascorbic acid also has antiviral properties [322, 323].
Calcitriol prior to/or post-H1N1 exposure does not affect viral clearance but significantly reduces autophagy and restores the increased apoptosis seen on H1N1 infection to its constitutive level. However, it significantly reduces the levels of H1N1-induced TNF-α (tumor necrosis factor- alpha), RANTES, IL8, IFN-β (interferon-beta) and IFN-stimulated gene-15 (ISG15). 1,25[OH]2 D3 treatment prior to/or post-H1N1 infection significantly downregulates both IL-8 and IL-6 RNA levels [324, 325].
Publications on antiviral drugs are often devoted to the use of statins as anti-flu drugs [326-328]. In particular, Fedson has suggested treating patients affected by H5N1 with statins [326, 327]. Studies in vitro, in animals and in the field seem to support this strategy. Statins are held to act through various mechanisms: through immunomodulatory and anti-inflammatory activity, by interfering with the proteins of the cytoskeleton and the interaction between these and the lipid rafts, and by reducing the availability of intracellular cholesterol. The balanced content of cholesterol in the cell is critical to the replication of IAV. Indeed, a reduction in cholesterol could impair the infectivity of progeny influenza viruses, probably by reducing the cholesterol content of the viral envelope [328]. However, some studies have found statins to be ineffective against influenza viruses [329, 330].
Extracts from Epimedium koreanum Nakai have immunomodulatory properties [331], also against HSV, VSV and Newcastle Disease Virus (NDV). Carrageenan [332] extracted from edible red seaweeds can be administered as a nasal spray [333]. In particular, iota-carrageenan appears to be the most effective against influenza.
Cycloferon [334-336], amixin, Larifan, Kagocel and Ragosin stimulate B cells and macrophages to produce IFN-alpha [337]. They are widely used in Russia.
Apocynin, a NADPH oxidase type 2 (NOX2) inhibitor, stimulates cell superoxide production. However, in certain conditions, it can also act as a ROS production stimulator in non-phagocyte cells [338]. By contrast, NADPH oxidase type 1 (NOX1) has anti-inflammatory activity and inhibits ROS production [339, 340].
Rolipram, a selective phosphodiesterase-4 (PDE-4) inhibitor with antidepressant properties, and sertraline, a selective serotonin reuptake inhibitor (SSRI), exhibit strong antiviral activities if combined with oseltamivir [341]. The rationale for using PDE-4 is that it belongs to a family of enzymes that metabolize cyclic adenosin monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), which are commonly found during inflammatory and immune responses. By reducing bronchospasm and bronchoconstriction, it reduces mortality and morbidity in a mouse model. SSRI downregulates the expression of interferon-alpha, TNF-alpha, IL-6, IL-10 and T helper 1 (Th1) cells, and modulates immune responses from the Th1 toward the Th2 phenotype.
Sphingosine mimetics are able to finely modulate the release of cytokines and chemokines. In one study [342], neutralizing antibody and cytotoxic T cell responses were seen to be reduced, though still protective. As a result, the infiltration of PML and macrophages into the lung was markedly reduced, and thus also pulmonary tissue injury. DC maturation was suppressed, which limited the proliferation of specific antiviral T cells in the lung and draining lymph nodes. Furthermore, they were effective in controlling CD8(+) T cell accumulation in the lungs even when given 4 days after the onset of influenza virus infection.
Leucomycin A3 (LMA3), a macrolide antibiotic, inhibits neutrophil myeloperoxidase (MPO), which contributes to the pathogenesis and progression of severe influenza- induced pneumonia, and mediates the production of hypochlorous acid, a potent tissue injury factor [343].
BG-777, derived from leukotriene B4, exerts both antiviral and stimulatory activities on the host defence system. It is also active against HIV, RSV and Coronaviruses. It recruits leukocytes and fosters the release of chemokines such as MIP1-beta and defensins [344].
QS-21 is a molecule with immunomodulatory properties, and is currently being investigated as an adjuvant for vaccines against influenza [345]. Thymalfasin (Zadaxin), which is derived from thymosin alpha-1, is another powerful adjuvant [346-348]. Canakinumab (Ilaris), an IL1-beta blocking antibody, is also a promising compound in immunotherapy [349].
Some observations should be made on influenza therapy with non-steroidal anti-inflammatory drugs. Seasonal flu is normally treated with over-the-counter (OTC) drugs, which are designed to relieve symptoms. The most common are paracetamol, acetylsalicylic acid (which, however, is contraindicated in individuals under 18 years of age) and ibuprofen or other NSAIDs. Coughing is usually mitigated by means of drugs that use dextromethorphan or acetylcistein as their active ingredient [350-357].
The inflammation driven by innate immunity is usually sufficient to cure the disease. However, especially when the virus is particularly virulent or during pandemics, immunity may be dysregulated (cytokine-storm), which may give rise to very severe forms of influenza. The treatment of both seasonal and pandemic influenza therefore utilises appropriate and timely anti-inflammatory therapy. Some of the above-mentioned drugs, such as statins and naproxen, have anti-inflammatory properties; however, they are probably also able to exert a real antiviral activity.
In the light of the human cases of infection by the H5N1 strain and the lethal cases caused by the H1N1pdm virus, the need for modulators of innate immunity is of particular importance. Indeed, patients with severe or fatal human infections due to the H1N1pdm virus, for instance, have high pro-inflammatory responses early in the illness.
For the above-mentioned reasons, the literature often reports in vitro and animals studies which demonstrate the therapeutic utility of anti-inflammatory and immunemodulatory compounds, such as fibrates, against influenza.
Gene therapies
Gene therapy consists of modulating (up-regulating or down-regulating) genes and/or their products involved in the response to influenza [358].
microRNAs (miRNAs) are small non-coding RNA molecules (containing about 22 nucleotides) which function in RNA silencing or RNA interference (RNAi) and in the post-transcriptional regulation of gene expression. Host miRNAs are able to down-regulate the expression of viral genes. Therefore, miRNA modulation could be a promising approach in influenza treatment, despite the difficulties of delivering miRNAs to cells efficiently [359-363].
Small interfering RNAs (siRNAs) are also mediators of RNAi. They are short (19-26 nucleotides) and induce sequence-specific degradation of homologous mRNA [364-366].
Long non-coding RNAs (lncRNAs) modulate various biological processes [367]. One lncRNA, in particular, plays a major role; it acts as a negative regulator of antiviral response (NRAV) and is down-regulated during influenza infection. NRAV negatively regulates the transcription of multiple critical interferon-stimulated genes (ISGs), by remodeling chromatin [368].
Compounds with unknown mechanisms
In the case of some compounds, the precise nature of their pharmacological activity against influenza is still unknown and requires further research.
Nanoparticles are a promising nanobiotechnological tool that can act as carriers of non-conjugated nanoparticles. Silver nanoparticles [369, 370] modulate SP-A and SP-D [371], and can be used to deliver RNAi [372]. Poly(gamma-glutamic) acid [373], fullerenes [374], chitosan or N-trimethyl chitosan (TMC) [375] and polymeric nanoparticles have also been investigated as vaccine adjuvants [376, 377]. However, single-walled carbon nanotubes (SWCNTs) seem to increase influenza virus pathogenicity and infectivity [378].
Combination therapies
Combination therapies (CTs) can be divided into associations of two or more drugs directly targeting viral components, and associations of a direct-acting viral compound and a molecule targeting host components. CTs may improve clinical outcomes, reduce the risk of respiratory complications, mortality and morbidity, reduce the risks of using single drugs (such as resistance, dose-related toxicity or other side-effects) and may potentiate and enhance antiviral activity [379, 380]. CTs can, in turn, be further divided into early combination chemotherapy (ECC) and sequential multidrug chemotherapy (SMC). Furthermore, many studies have evaluated the efficacy of combining anti-inflammatory drugs with antiviral drugs in comparison with single-drug treatment. However, not all combination therapies, for instance the combination of oseltamivir with zanamivir or simvastatin with oseltamivir, are superior to monotherapy [102, 379, 380].
CTs can also exploit various chimeric monoclonal antibodies [381].
Conclusions
In the last few decades, few antiviral molecules against influenza virus infections have been available. This has conditioned their use during human and animal outbreaks. Indeed, during seasonal and pandemic outbreaks, antiviral drugs have usually been administered in mono-therapy and, sometimes, in an uncontrolled manner to farm animals. This has led to the emergence of viral strains displaying resistance, especially to compounds of the amantadane family. For this reason, it is particularly important to develop new antiviral drugs against influenza viruses. Indeed, although vaccination is the most powerful means of mitigating the effects of influenza epidemics, antiviral drugs can be very useful, particularly in delaying the spread of new pandemic viruses, thereby enabling manufacturers to prepare large quantities of pandemic vaccine. In addition, antiviral drugs are particularly valuable in complicated cases of influenza, especially in hospitalized patients. This latter are individuals at risk, such as the elderly or patients with chronic respiratory diseases. For these subjects, it would be particularly important to have more antivirals to be administered in appropriate manner.
In the light of the extensive experience gained through the use of anti-influenza drugs, and in the light of the considerable advances in the search for new effective molecules against influenza viruses, many important considerations can be made.
Firstly, the study of new compounds should be conducted in a more rational way. Indeed, the models and methods used by various scholars display marked differences. These studies often involve in vitro cell cultures and usually use Madin–Darby canine kidney (MDCK) cells and African green monkey kidney Vero cells. However, human tracheal epithelial cell cultures are sometimes used. While some authors have assessed the inhibition of viral growth by applying the haemagglutination test to the supernatant of the cell monolayer, others have used the inhibition of the virus-induced Cytopathic Effect (CPE). Furthermore, more sophisticated tests have been used – for instance, qPCR with the aim of amplifying sequences of viral genes, such as the M2 gene, NP gene, etc., or RT-PCR with the aim of quantifying IAV RNA after in vitro antiviral treatment of cell cultures exposed to different influenza virus strains. In addition, the murine model is the most widely used to study influenza compounds, as influenza causes fatal pneumonia in the mouse. Obviously, the human is the best, but results in humans are available only if clinical trials have been performed or if the drug has been licensed. However, as it is very costly to develop a new compound for commercialization, preliminary evaluations in vitro and in animal models are very important. In some cases, it is also useful to carry out epidemiological studies on drugs used for other purposes, in order to investigate their possible therapeutic efficacy against influenza.
To optimise the development of influenza antivirals, it is very important to define standardized methods for the evaluation of the molecules that have been hypothesized to have a potential antiviral effect. In in vitro studies, for instance, it is important to define the cell line that should be used (MDCK, or VERO, or THE cell line), the standard virus that should be tested (PR8 and/or High pathogenic virus, such as H5N1) and the antiviral assay that should be performed (Haemagglutination, CPE inhibition, RT-PCR). Likewise, in in vivo tests, the choice of which animal to utilize should be established, while in human studies it is important to determine the number and age of the subjects to be studied. Only if standardized methods are defined, will it be possible to correctly evaluate the antiviral potential of the compound under examination. In this perspective, it is also important to compare the antiviral activity of the hypothetical antiviral with that of reference drugs (amantadine, oseltamivir, etc) in order to ascertain the influenza antiviral index of the new molecule. In in vitro studies, it is also advisable to evaluate the capacity of the antiviral under study to induce viral resistance.
In the field of medicinal chemistry, the discovery and development of a completely New Molecular Entity (NME) or compound is particularly expensive in terms of time and costs. Research could therefore be carried out along two different lines: designing/optimising new derivatives from an existing lead (such as the secondgeneration NAI laninamivir and peramivir); and repurposing/ repositioning existing drugs for new potential clinical applications [382, 383]. The latter approach, also termed drug retasking or reprofiling, has already yielded promising results. While drug retargeting was initially serendipitous, it was later more systematically developed and exploited, not least by combining advanced biochemical, biophysical and bioinformatics/ cheminformatics techniques. Viroinformatics [384] and computational systems biology [385] can suggest rational inhibitors of viral transcription, replication, protein synthesis, nuclear export and assembly/release. Other strategies may emerge from gene data mining. In this regard, Bao and collaborators used a prioritizing gene approach in order to find the most important genes involved in host resistance to influenza virus [386]. They found that the response was controlled by two TNF-mediated pathways: apoptosis and TNF receptor-2 signaling pathways. In addition, systems pharmacometrics and systems pharmacology [387] could identify valuable CTs by studying drug synergy.
Secondly, the available anti-influenza drugs should be used in an appropriate manner, in order to impede or to mitigate the phenomenon of viral resistance. In this regard, the first question is: what anti-inflammatory drug should be chosen? The answer should take into account the age of the patient, the toxicity and tolerability of the drug and its efficacy in alleviating the patient's symptoms. Obviously, therapy should be initiated as soon as possible, and an NSAID (aspirin only for subjects over 18 years, ibuprofen, naproxen or paracetamol [acetaminophen]) should be chosen. These compounds not only relieve the symptoms, but also equilibrate the patient's innate immunity and sometimes have a direct or indirect antiviral effect. For instance, it is interesting that reducing pro-inflammatory cytokines diminishes the activity of proteases involved in HA cleavage. In addition, the administration of acetylcysteine is useful not only because of its mucolytic action, but also on account of its antioxidant activity.
The choice of the antiviral should take into account the broad resistance of influenza viruses to amantadane drugs and also the fact that mono-therapy can easily lead to the emergence of novel viral resistance. In this perspective, topic drugs, such as zanamivir, have proved to generate less resistant viral strains than drugs administered orally. In addition, other antivirals, such as antiprotease drugs, could be useful in influenza therapy. These compounds could have advantages in that, being inhibitors of cellular proteins, they should be less prone to selecting resistant viral strains. However, it should be borne in mind that disturbing the cellular environment in order to disrupt viral functions could have adverse side effects. Furthermore, it has been proposed that therapeutic protocols involving a combination of two or more antivirals should be drawn up in order to reduce the development of drug-resistant viral strains and, at the same time, administer lower drug doses. Another hypothesis could be to administer two or more different antivirals alternately.
Finally, the use of antivirals in the veterinary field (for example, chicken flocks) should be carefully controlled, and in this case the combined or alternated administration of at least two antiviral drugs should be the rule. It is important to realise that this implies a one world, one health, one medicine, one science approach [382, 383], in which human and veterinary medicine cooperate in the interest of global health in an increasingly interconnected world.
Glossary
Abbreviations
- AAT
alpha-1-antitrypsin;
- ALI
acute lung injury;
- ARDS
acute respiratory distress syndrome;
- Asp
aspartic acid;
- BINASE
Bacillus intermedius Ribonuclease;
- CAM
Clarihtromycin;
- cAMP
cyclic adenosin monophosphate;
- CAS
Chemical Abstract Service;
- CBP
CREB binding protein;
- CCL
CC chemokine ligand;
- CCL2
CCL type2;
- CCL5
CCL type 5;
- CFPTS
Ching-fang-pai-tu-san;
- cGMP
cyclic guanosine monophosphate;
- CHX
cycloheximide;
- CI
confidence interval;
- cIAPs
cellular inhibitors of apoptosis;
- CL
collectin;
- CL-43
CL type 43;
- CL-46
CL type 46;
- CME
Clathrin-Mediated Endocytosis;
- COX
cyclooxigenase;
- COX-2
COX type 2;
- CPE
cytopathic effect;
- CRM
chromosomal maintenance;
- CRM1
CRM type 1;
- CTs
combination therapies;
- CVN
Cyanovirin-N;
- CXCL
chemokine (C-X-C motif) ligand;
- CXCL10
CXCL type 10;
- CypA
cyclophilin A;
- CypB
cyclophilin B;
- DC
dendritic cell;
- DNA
deoxyribonucleic acid;
- DPPC
dipalmitoylphosphatidylcholine;
- DS
dextran sulphate;
- EB-peptide
entry block peptide;
- ECC
early combination chemotherapy;
- ERK
extracellular signal-regulated kinase;
- EV
enterovirus;
- EV71
EV type 71;
- FGF
fibroblast growth factor;
- FGF4
FGF type 4;
- FP
FluPep;
- FP1
FP type 1, also known as Tkip;
- GA
glycyrrhizic acid;
- GR
glycyrrhizin;
- GTP
guanosine-5'-triphosphate;
- GTPase
GTP hydrolase;
- HA
hemagglutinin;
- HAI-2
Hepatocyte growth factor activator inhibitor 2;
- HAIs
HA inhibitors;
- HBV
hepatitis B virus;
- HCV
hepatitis C virus;
- HGF
hepatocyte growth factor;
- HIV
Human Immunodeficiency Virus;
- HMBL
High mannose-binding lectin;
- HMG
3-hydroxy-3-methylglutaryl-coenzyme A;
- HMGB
high-mobility-group;
- HMGB1
HMGB type 1;
- HMPV
Human Metapneumovirus;
- HPV
Human Papillomavirus;
- HRV
Human Rhinovirus;
- HSV
Herpes Simplex Virus;
- HSV-1
HSV type 1;
- IAV
influenza A virus;
- IBV
influenza B virus;
- IFN
interferon;
- IFN-α
alpha IFN;
- IFN-β
beta IFN;
- IKK
IκB kinase;
- IL
interleukin;
- IL6
IL type 6;
- IL8
IL type 8;
- IL10
IL type 10;
- ILI
influenza-like illness;
- IL1RA
IL type 1 receptor antagonist;
- IMPDH
Inosine 5'-monoposphate dehydrogenase;
- IRF
interferon-regulatory factor;
- IRF3
IRF type 3;
- ISG
interferon-stimulated gene;
- ISG15
ISG type 15;
- JNK
c-Jun N-termninal kinase;
- LMA3
Leucomycin A3;
- LMB
Leptomycin B;
- lncRNA
long non-coding RNA;
- M protein
matrix protein;
- M1
Matrix type 1 protein;
- M2 protein
Matrix type 2 protein;
- MAC
Melaleuca alternifolia concentrate;
- MAPK
mitogen-activated protein kinase;
- MBL
mannose-binding lectin;
- MBP
mannose-binding protein;
- MD
molecular dynamics;
- MDCK
Madin Darby Canine Kidney cell line;
- MIP1-beta
macrophage inflammatory protein type 1 beta;
- miRNA
microRNA;
- MPO
myeloperoxidase;
- mRNA
messenger RNA;
- MTOC
microtubule organizing center;
- mTOR
mammalian target of rapamycin;
- MTP-PE
muramyl tripeptide;
- MXSGT
Ma-xing-shi-gan-tang;
- NA
neuraminidase;
- NAIs
NA inhibitors;
- NADPH
nicotinamide adenine dinucleotide phosphate reduced;
- NB-DNJ
N-butyl-deoxynojirimycin;
- NCZ
nucleozin;
- NDV
Newcastle Disease Virus;
- NEP
nuclear export protein;
- NES
nuclear- export signal;
- Neu5Ac-S-CH2-Lev
α-2-S-[m-(N-levulinyl)aminobenzyl]-5-N-acetylneuraminic acid;
- NFKB
nuclear factor kappa B;
- NOX1
NADPH oxidase type 1;
- NOX2
NADPH oxidase type 2;
- NLRX1
Nucleotide-binding oligomerization domain-like receptor type 1;
- NRAV
negative regulator of antiviral response;
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2, also known as NFE2L2;
- NS
Non-Structural protein;
- NS1
NS type 1;
- NS1A
NS type 1A;
- NS1ABP
NS1A binding protein;
- NSAIDs
non-steroidal anti-inflammatory drugs;
- OFCs
omeprazole family compounds;
- ORadj
adjusted odds ratio;
- OTC
over the counter;
- PA
polymerase acidic protein;
- PB
polymerase basic protein;
- PB1
PB type 1;
- PB1-F2
PB1 frame 2;
- PB2
PB type 2;
- PCR
polymerase chain reaction;
- PDB
Protein Data Bank;
- PDTC
pyrrolidine dithiocarbamate;
- Pet
petasiphenol;
- PGE2
prostaglandin E2;
- Pgp
P-glycoprotein;
- PI3K
phosphatidylinositol 3-kinase;
- PLD
phospholipase D;
- PR-3
proteinase 3;
- qPCR
quantitative PCR;
- RE
recycling endosome;
- REDD1
regulated in development and DNA damage responses-1;
- RIB
ribavirin;
- RNA
ribonucleic acid;
- RNAi
RNA interference;
- RNP
ribonucleoprotein;
- ROS
reactive oxygen species;
- RSV
Respiratory Syncytial Virus;
- RT-PCR
- SA
sialic acid;
- SARS
Severe Acute Respiratory Syndrome;
- SINE
selective inhibitor of nuclear export;
- siRNA
short interfering RNA;
- SMC
sequential multidrug chemotherapy;
- SOCS
Suppressor of cytokine signaling;
- SOCS1
SOCS type 1;
- SP-A
surfactant protein A;
- SP-D
surfactant protein D;
- SREBP-1
sterol regulatory element-binding protein 1;
- SNMC
Stronger Neo-Minophafen C;
- SWCNTs
single-walled carbon nano-tubes;
- TBHQ
Tert-butyl-hydroquinone;
- TFs
theaflavins;
- Th1
T helper 1 cell;
- THC
tetrahydrocurcumin;
- TLR
Toll-like receptor;
- TLR2
TLR type 2;
- TLR7
TLR type 7;
- TMC
N-trimethyl chitosan;
- TNF
tumor necrosis factor;
- TNF-α
TNF type α;
- Treg
T regulatory cell;
- TTO
tea-tree oil;
- TZV
triazavirine;
- US
United States of America;
- USP
ubiquitin-specific peptidase;
- USP18
USP type 18;
- Val
valine;
- vATPase VEGF
vascular endothelial growth factor;
- vRNA
viral RNA;
- vRNP
viral RNP;
- VZV
Varicella Zoster Virus;
- XPO-1
exportin-1.
References
- 1.Gasparini R, Amicizia D, Lai PL, et al. Compounds with antiinfluenza activity: present and future of strategies for the optimal treatment and management of influenza. Part I: influenza life-cycle and currently available drugs. J Prev Med Hyg. 2014;55:69–85. [PMC free article] [PubMed] [Google Scholar]
- 2.Müller KH, Kakkola L, Nagaraj AS, et al. Emerging cellular targets for influenza antiviral agents. Trends Pharmacol Sci. 2012;33:89–99. doi: 10.1016/j.tips.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Planz O. Development of cellular signaling pathway inhibitors as new antivirals against influenza. Antiviral Res. 2013;98:457–468. doi: 10.1016/j.antiviral.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 4. Embase. Accessible at http://www.elsevier.com/online-tools/ embase (last accessed: 16/07/2014).
- 5. PubChem. Accessible at http://www.ncbi.nlm.nih.gov/pcsubstance/ (last accessed: 16/07/2014).
- 6. PubChem. Accessible at http://www.ncbi.nlm.nih.gov/pccompound/ (last accessed: 16/09/2014).
- 7. DrugBank. Accessible at http://www.drugbank.ca (last accessed: 16/07/2014).
- 8. Chemical Abstracts Service. Accessible at https://scifinder.cas. org/ (last accessed: 16/07/2014).
- 9. Clinical trials registries. Accessible at https://clinicaltrials.gov/ct2/home (last accessed: 16/07/2014).
- 10.Eyer L, Hruska K. Antiviral agents targeting the influenza virus: a review and publication analysis. Veterinarni Medicina. 2013;58:113–185. [Google Scholar]
- 11. CIRI-IT. Accessible at http://www.cirinet.it/jm/ (last accessed: 16/09/2014).
- 12.Pu JY, He L, Wu SY, et al. Anti-virus research of triterpenoids in licorice. Bing Du Xue Bao. 2013;29:673–679. [PubMed] [Google Scholar]
- 13.Jia W, Wang C, Wang Y, et al. Qualitative and quantitative analysis of the major constituents in chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. Scientific World Journal. 2015;2015:731–765. doi: 10.1155/2015/731765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utsunomiya T, Kobayashi M, Pollard RB, et al. Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob Agents Chemother. 1997;41:551–556. doi: 10.1128/aac.41.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiore C, Eisenhut M, Krausse R, et al. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem J. 2005;392:191–199. doi: 10.1042/BJ20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smirnov VS, Garshinina AV, Guseva VM, et al. The anti-viral activity of the complex glycyrrhizic acid-alpha-glutamyl-tryptophan against experimental lethal influenza infection in white mice caused by oseltamivir-resistant strain of the virus. Vopr Virusol. 2013;58:19–26. [PubMed] [Google Scholar]
- 18.Smirnov VS, Zarubaev VV, Anfimov PM, et al. Effect of a combination of glutamyl-tryptophan and glycyrrhizic acid on the course of acute infection caused by influenza (H3H2) virus in mice. Vopr Virusol. 2012;57:23–27. [PubMed] [Google Scholar]
- 19.Wolkerstorfer A, Kurz H, Bachhofner N, et al. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Res. 2009;83:171–178. doi: 10.1016/j.antiviral.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaelis M, Geiler J, Naczk P, et al. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med Microbiol Immunol. 2010;199:291–297. doi: 10.1007/s00430-010-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaelis M, Geiler J, Naczk P, et al. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS One. 2011;6:e19705–e19705. doi: 10.1371/journal.pone.0019705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moisy D, Avilov SV, Jacob Y, et al. HMGB1 protein binds to influenza virus nucleoprotein and promotes viral replication. J Virol. 2012;86:9122–9133. doi: 10.1128/JVI.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanetty C, Wolkerstorfer A, Amer H, et al. Synthesis and antiviral activities of spacer-linked 1-thioglucuronide analogues of glycyrrhizin. Beilstein J Org Chem. 2012;8:705–711. doi: 10.3762/bjoc.8.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pompei R, Paghi L, Ingianni A, et al. Glycyrrhizic acid inhibits influenza virus growth in embryonated eggs. Microbiologica. 1983;6:247–250. [PubMed] [Google Scholar]
- 25.Scherließ R, Ajmera A, Dennis M, et al. Induction of protective immunity against H1N1 influenza A(H1N1)pdm09 with spraydried and electron-beam sterilised vaccines in non-human primates. Vaccine. 2014;32:2231–2240. doi: 10.1016/j.vaccine.2014.01.077. [DOI] [PubMed] [Google Scholar]
- 26.Song G, Yang S, Zhang W, et al. Discovery of the first series of small molecule H5N1 entry inhibitors. J Med Chem. 2009;52:7368–7371. doi: 10.1021/jm900275m. [DOI] [PubMed] [Google Scholar]
- 27.Song W, Si L, Ji S, et al. Uralsaponins M-Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J Nat Prod. 2014;77:1632–1643. doi: 10.1021/np500253m. [DOI] [PubMed] [Google Scholar]
- 28.Song X, Chen J, Sakwiwatkul K, et al. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int Immunopharmacol. 2010;10:351–356. doi: 10.1016/j.intimp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Barr IG, Mitchell GF. ISCOMs (immunostimulating complexes): the first decade. Immunol Cell Biol. 1996;74:8–25. doi: 10.1038/icb.1996.2. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Patil HP, Vries-Idema J, et al. Enhancement of the immunogenicity and protective efficacy of a mucosal influenza subunit vaccine by the saponin adjuvant GPI-0100. PLoS One. 2012;7:e52135–e52135. doi: 10.1371/journal.pone.0052135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Vries-Idema J, Ter Veer W, et al. Influenza virosomes supplemented with GPI-0100 adjuvant: a potent vaccine formulation for antigen dose sparing. Med Microbiol Immunol. 2014;203:47–55. doi: 10.1007/s00430-013-0313-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhai L, Li Y, Wang W, et al. Enhancement of humoral immune responses to inactivated Newcastle disease and avian influenza vaccines by oral administration of ginseng stem-and-leaf saponins in chickens. Poult Sci. 2011;90:1955–1959. doi: 10.3382/ps.2011-01433. [DOI] [PubMed] [Google Scholar]
- 33.Sun H, He S, Shi M. Adjuvant-active fraction from Albizia julibrissin saponins improves immune responses by inducing cytokine and chemokine at the site of injection. Int Immunopharmacol. 2014;22:346–355. doi: 10.1016/j.intimp.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Kazakova OB, Giniyatullina GV, Yamansarov EY, et al. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg Med Chem Lett. 2010;20:4088–4090. doi: 10.1016/j.bmcl.2010.05.083. [DOI] [PubMed] [Google Scholar]
- 35.Kazakova OB, Medvedeva NI, Baĭkova IP, et al. Synthesis of triterpenoid acylates - an effective reproduction inhibitors of influenza A (H1N1) and papilloma viruses. Bioorg Khim. 2010;36:841–848. [PubMed] [Google Scholar]
- 36.Flekhter OB, Medvedeva NI, Kukovinets OS, et al. Synthesis and antiviral activity of lupane triterpenoids with modified cycle E. Bioorg Khim. 2007;33:629–634. doi: 10.1134/s1068162007060088. [DOI] [PubMed] [Google Scholar]
- 37.Baltina LA, Flekhter OB, Nigmatullina LR, et al. Lupane triterpenes and derivatives with antiviral activity. Bioorg Med Chem Lett. 2003;13:3549–3552. doi: 10.1016/s0960-894x(03)00714-5. [DOI] [PubMed] [Google Scholar]
- 38.Grishko VV, Galaiko NV, Tolmacheva IA, et al. Functionalization, cyclization and antiviral activity of A-secotriterpenoids. Eur J Med Chem. 2014;83:601–608. doi: 10.1016/j.ejmech.2013.12.058. [DOI] [PubMed] [Google Scholar]
- 39.Krumbiegel M, Dimitrov DS, Puri A, et al. Dextran sulfate inhibits fusion of influenza virus and cells expressing influenza hemagglutinin with red blood cells. Biochim Biophys Acta. 1992;1110:158–164. doi: 10.1016/0005-2736(92)90353-n. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann A, Korte T, Arnold K, et al. The influence of dextran sulfate on influenza A virus fusion with erythrocyte membranes. Antiviral Res. 1992;19:295–311. doi: 10.1016/0166-3542(92)90011-s. [DOI] [PubMed] [Google Scholar]
- 41.Lüscher-Mattli M, Glück R, Kempf C, et al. A comparative study of the effect of dextran sulfate on the fusion and the in vitro replication of influenza A and B, Semliki Forest, vesicular stomatitis, rabies, Sendai, and mumps virus. Arch Virol. 1993;130:317–326. doi: 10.1007/BF01309663. [DOI] [PubMed] [Google Scholar]
- 42.Yamada H, Moriishi E, Haredy AM, et al. Influenza virus neuraminidase contributes to the dextran sulfate-dependent suppressive replication of some influenza A virus strains. Antiviral Res. 2012;96:344–352. doi: 10.1016/j.antiviral.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Yamada H, Nagao C, Haredy AM, et al. Dextran sulfate-resistant A/Puerto Rico/8/34 influenza virus is associated with the emergence of specific mutations in the neuraminidase glycoprotein. Antiviral Res. 2014;111:69–77. doi: 10.1016/j.antiviral.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Shkurupy VA, Potapova OV, Sharkova TV, et al. Experimental study of the efficiency of oxidized dextran for prevention of influenza A/H5N1. Bull Exp Biol Med. 2014;158:112–114. doi: 10.1007/s10517-014-2704-9. [DOI] [PubMed] [Google Scholar]
- 45.Shkurupy VA, Potapova OV, Sharkova TV, et al. Effects of Preventive Administration of Oxidized Dextran on Liver Injury and Reparative Regeneration in Mice Infected with Influenza A/ H5N1 Virus. Bull Exp Biol Med. 2015;158:483–488. doi: 10.1007/s10517-015-2790-3. [DOI] [PubMed] [Google Scholar]
- 46.Potapova OV, Shkurupiy VA, Sharkova TV, et al. Preventive efficacy of oxidized dextran and pathomorphological processes in mouse lungs in avian influenza A/H5N1. Bull Exp Biol Med. 2011;150:707–710. doi: 10.1007/s10517-011-1229-8. [DOI] [PubMed] [Google Scholar]
- 47.Clercq E. Highlights in the development of new antiviral agents. Mini Rev Med Chem. 2002;2:163–175. doi: 10.2174/1389557024605474. [DOI] [PubMed] [Google Scholar]
- 48.Bond S, Draffan AG, Fenner JE, et al. 1,2,3,9b-Tetrahydro-5Himidazo, 1-a]isoindol-5-ones as a new class of respiratory syncytial virus (RSV) fusion inhibitors. Part 2: identification of BTA9881 as a preclinical candidate. Bioorg Med Chem Lett. 2015;25:976–981. doi: 10.1016/j.bmcl.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 49.Weisman LE. Respiratory syncytial virus (RSV) prevention and treatment: past, present, and future. Cardiovasc Hematol Agents Med Chem. 2009;7:223–233. doi: 10.2174/187152509789105471. [DOI] [PubMed] [Google Scholar]
- 50.Guinea R, Carrasco L. Concanamycin A blocks influenza virus entry into cells under acidic conditions. FEBS Lett. 1994;349:327–330. doi: 10.1016/0014-5793(94)00695-4. [DOI] [PubMed] [Google Scholar]
- 51.Guinea R, Carrasco L. Concanamycin A: a powerful inhibitor of enveloped animal-virus entry into cells. Biochem Biophys Res Commun. 1994;201:1270–1278. doi: 10.1006/bbrc.1994.1842. [DOI] [PubMed] [Google Scholar]
- 52.Guinea R, Carrasco L. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J Virol. 1995;69:2306–2312. doi: 10.1128/jvi.69.4.2306-2312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller KH, Kainov DE, El Bakkouri K, et al. The proton translocation domain of cellular vacuolar ATPase provides a target for the treatment of influenza A virus infections. Br J Pharmacol. 2011;164:344–357. doi: 10.1111/j.1476-5381.2011.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeganeh B, Ghavami S, Kroeker AL, et al. Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1. Am J Physiol Lung Cell Mol Physiol. 2015;308:L270–L286. doi: 10.1152/ajplung.00011.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ochiai H, Sakai S, Hirabayashi T, et al. Inhibitory effect of bafilomycin A1, a specific inhibitor of vacuolar-type proton pump, on the growth of influenza A and B viruses in MDCK cells. Antiviral Res. 1995;27:425–430. doi: 10.1016/0166-3542(95)00040-s. [DOI] [PubMed] [Google Scholar]
- 56.Bimbo LM, Denisova OV, Mäkilä E, et al. Inhibition of influenza A virus infection in vitro by saliphenylhalamide-loaded porous silicon nanoparticles. ACS Nano. 2013;7:6884–6893. doi: 10.1021/nn402062f. [DOI] [PubMed] [Google Scholar]
- 57.Paton NI, Lee L, Xu Y, et al. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis. 2011;11:677–683. doi: 10.1016/S1473-3099(11)70065-2. [DOI] [PubMed] [Google Scholar]
- 58.Savarino A. Use of chloroquine in viral diseases. Lancet Infect Dis. 2011;11:653–654. doi: 10.1016/S1473-3099(11)70092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ooi EE, Chew JS, Loh JP, et al. In vitro inhibition of human influenza A virus replication by chloroquine. Virol J. 2006;3:39–39. doi: 10.1186/1743-422X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trani L, Savarino A, Campitelli L, et al. Different pH requirements are associated with divergent inhibitory effects of chloroquine on human and avian influenza A viruses. Virol J. 2007;4:39–39. doi: 10.1186/1743-422X-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vigerust DJ, McCullers JA. Chloroquine is effective against influenza A virus in vitro but not in vivo. Influenza Other Respir Viruses. 2007;1:189–192. doi: 10.1111/j.1750-2659.2007.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan Y, Zou Z, Sun Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garulli B, Mario G, Sciaraffia E, et al. Enhancement of T cell-mediated immune responses to whole inactivated influenza virus by chloroquine treatment in vivo. Vaccine. 2013;31:1717–1724. doi: 10.1016/j.vaccine.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 64.Wu L, Dai J, Zhao X, et al. Chloroquine enhances replication of influenza A virus A/WSN/33 (H1N1) in dose-, time-, and MOIdependent manners in human lung epithelial cells A549. J Med Virol. 2015 doi: 10.1002/jmv.24135. in press. [DOI] [PubMed] [Google Scholar]
- 65.Clercq E. A Cutting-edge view on the current state of antiviral drug development. Med Res Rev. 2013;33:1249–1277. doi: 10.1002/med.21281. [DOI] [PubMed] [Google Scholar]
- 66.Blaising J, Lévy PL, Gondeau C, et al. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell Microbiol. 2013;15:1866–1882. doi: 10.1111/cmi.12155. [DOI] [PubMed] [Google Scholar]
- 67.Dai JP, Wu LQ, Li R, et al. Identification of 23-(s)-2-amino- 3-phenylpropanoyl-silybin as an antiviral agent for influenza A virus infection in vitro and in vivo. Antimicrob Agents Chemother. 2013;57:4433–4443. doi: 10.1128/AAC.00759-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gazák R, Purchartová K, Marhol P, et al. Antioxidant and antiviral activities of silybin fatty acid conjugates. Eur J Med Chem. 2010;45:1059–1067. doi: 10.1016/j.ejmech.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 69.Garozzo A, Timpanaro R, Bisignano B, et al. In vitro antiviral activity of Melaleuca alternifolia essential oil. Lett Appl Microbiol. 2009;49:806–808. doi: 10.1111/j.1472-765X.2009.02740.x. [DOI] [PubMed] [Google Scholar]
- 70.Garozzo A, Timpanaro R, Stivala A, et al. Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: study on the mechanism of action. Antiviral Res. 2011;89:83–88. doi: 10.1016/j.antiviral.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Duan S, Chu C, et al. Melaleuca alternifolia concentrate inhibits in vitro entry of influenza virus into host cells. Molecules. 2013;18:9550–9566. doi: 10.3390/molecules18089550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mantil E, Daly G, Avis TJ. Effect of tea tree (Melaleuca alternifolia) oil as a natural antimicrobial agent in lipophilic formulations. Can J Microbiol. 2015;61:82–88. doi: 10.1139/cjm-2014-0667. [DOI] [PubMed] [Google Scholar]
- 73.He J, Qi WB, Wang L, et al. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir Viruses. 2013;7:922–931. doi: 10.1111/irv.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aggarwal BB, Deb L, Prasad S. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2014;20:185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen TY, Chen DY, Wen HW, et al. Inhibition of enveloped viruses infectivity by curcumin. PLoS One. 2013;8:e62482–e62482. doi: 10.1371/journal.pone.0062482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shuto T. Regulation of expression, function, and inflammatory responses of innate immune receptor Toll-like receptor-2 (TLR2) during inflammatory responses against infection. Yakugaku Zasshi. 2013;133:1401–1409. doi: 10.1248/yakushi.13-00208. [DOI] [PubMed] [Google Scholar]
- 77.Kim K, Kim KH, Kim HY, et al. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010;584:707–712. doi: 10.1016/j.febslet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 78.Rajput N, Naeem M, Ali S, et al. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on broiler pigmentation and immunity. Poult Sci. 2013;92:1177–1185. doi: 10.3382/ps.2012-02853. [DOI] [PubMed] [Google Scholar]
- 79.Ou JL, Mizushina Y, Wang SY, et al. Structure-activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013;280:5829–5840. doi: 10.1111/febs.12503. [DOI] [PubMed] [Google Scholar]
- 80.Haasbach E, Hartmayer C, Hettler A, et al. Antiviral activity of Ladania067, an extract from wild black currant leaves against influenza A virus in vitro and in vivo. Front Microbiol. 2014;5:171–171. doi: 10.3389/fmicb.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ehrhardt C, Dudek SE, Holzberg M, et al. A plant extract of Ribes nigrum folium possesses anti-influenza virus activity in vitro and in vivo by preventing virus entry to host cells. PLoS One. 2013;8:e63657–e63657. doi: 10.1371/journal.pone.0063657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yokomizo K, Miyamoto Y, Nagao K, et al. Fattiviracin A1, a novel antiviral agent produced by Streptomyces microflavus strain No. 2445. II. Biological properties. J Antibiot (Tokyo) 1998;51:1035–1039. doi: 10.7164/antibiotics.51.1035. [DOI] [PubMed] [Google Scholar]
- 83.Habib ES, Yokomizo K, Nagao K, et al. Antiviral activity of fattiviracin FV-8 against human immunodeficiency virus type 1 (HIV-1) Biosci Biotechnol Biochem. 2001;65:683–685. doi: 10.1271/bbb.65.683. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka T, Ikeda T, Kaku M, et al. A new lignan glycoside and phenylethanoid glycosides from Strobilanthes cusia BREMEK. Chem Pharm Bull (Tokyo) 2004;52:1242–1245. doi: 10.1248/cpb.52.1242. [DOI] [PubMed] [Google Scholar]
- 85.Uyeda M. Metabolites produced by actinomycetes-antiviral antibiotics and enzyme inhibitors. Yakugaku Zasshi. 2004;124:469–479. doi: 10.1248/yakushi.124.469. [DOI] [PubMed] [Google Scholar]
- 86.Liao Q, Qian Z, Liu R, et al. Germacrone inhibits early stages of influenza virus infection. Antiviral Res. 2013;100:578–588. doi: 10.1016/j.antiviral.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 87.Denisova OV, Söderholm S, Virtanen S, et al. Akt inhibitor MK2206 prevents influenza pH1N1 virus infection in vitro. Antimicrob Agents Chemother. 2014;58:3689–3696. doi: 10.1128/AAC.02798-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirata N, Suizu F, Matsuda-Lennikov M, et al. Inhibition of Akt kinase activity suppresses entryand replication of influenza virus. Biochem Biophys Res Commun. 2014;450:891–898. doi: 10.1016/j.bbrc.2014.06.077. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh CF, Lo CW, Liu CH, et al. Mechanism by which ma-xingshi- gan-tang inhibits the entry of influenza virus. J Ethnopharmacol. 2012;143:57–67. doi: 10.1016/j.jep.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 90.Murray JL, McDonald NJ, Sheng J, et al. Inhibition of influenza A virus replication by antagonism of a PI3K-AKT-mTOR pathway member identified by gene-trap insertional mutagenesis. Antivir Chem Chemother. 2012;22:205–215. doi: 10.3851/IMP2080. [DOI] [PubMed] [Google Scholar]
- 91.Chan RW, Chan MC, Wong AC, et al. DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother. 2009;53:3935–3941. doi: 10.1128/AAC.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Triana-Baltzer GB, Gubareva LV, Nicholls JM, et al. Novel pandemic influenza A(H1N1) viruses are potently inhibited by DAS181, a sialidase fusion protein. PLoS One. 2009;4:e7788–e7788. doi: 10.1371/journal.pone.0007788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Triana-Baltzer GB, Gubareva LV, Klimov AI, et al. Inhibition of neuraminidase inhibitor-resistant influenza virus by DAS181, a novel sialidase fusion protein. PLoS One. 2009;4:e7838–e7838. doi: 10.1371/journal.pone.0007838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Triana-Baltzer GB, Babizki M, Chan MC, et al. DAS181, a sialidase fusion protein, protects human airway epithelium against influenza virus infection: an in vitro pharmacodynamic analysis. J Antimicrob Chemother. 2010;65:275–284. doi: 10.1093/jac/dkp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moss RB, Hansen C, Sanders RL, et al. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J Infect Dis. 2012;206:1844–1851. doi: 10.1093/infdis/jis622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ison MG. Expanding the armamentarium against respiratory viral infections: DAS181. J Infect Dis. 2012;206:1806–1808. doi: 10.1093/infdis/jis623. [DOI] [PubMed] [Google Scholar]
- 97.Zhang H. DAS181 and H5N1 virus infection. J Infect Dis. 2009;199:1250–1250. doi: 10.1086/597479. author reply 1250-1. [DOI] [PubMed] [Google Scholar]
- 98.Moscona A, Porotto M, Palmer S, et al. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo. J Infect Dis. 2010;202:234–241. doi: 10.1086/653621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones BG, Hayden RT, Hurwitz JL. Inhibition of primary clinical isolates of human parainfluenza virus by DAS181 in cell culture and in a cotton rat model. Antiviral Res. 2013;100:562–566. doi: 10.1016/j.antiviral.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006;50:1470–1479. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis. 2009;48(Suppl 1):S3–S13. doi: 10.1086/591851. [DOI] [PubMed] [Google Scholar]
- 102.Belser JA, Lu X, Szretter KJ, et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis. 2007;196:1493–1499. doi: 10.1086/522609. [DOI] [PubMed] [Google Scholar]
- 103.Marjuki H, Mishin VP, Chesnokov AP, et al. An investigational antiviral drug, DAS181, effectively inhibits replication of zoonotic influenza A virus subtype H7N9 and protects mice from lethality. J Infect Dis. 2014;210:435–440. doi: 10.1093/infdis/jiu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu L, Li Y, Li S, et al. Inhibition of influenza A virus (H1N1) fusion by benzenesulfonamide derivatives targeting viral hemagglutinin. PLoS One. 2011;6:e29120–e29120. doi: 10.1371/journal.pone.0029120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsieh HP, Hsu JT. Strategies of development of antiviral agents directed against influenza virus replication. Curr Pharm Des. 2007;13:3531–3542. doi: 10.2174/138161207782794248. [DOI] [PubMed] [Google Scholar]
- 106.Shigeta S. Current status of research and development for anti-influenza virus drugs--chemotherapy for influenza. Nihon Rinsho. 1997;55:2758–2764. [PubMed] [Google Scholar]
- 107.Luo G, Torri A, Harte WE, et al. Molecular mechanism underlying the action of a novel fusion inhibitor of influenza A virus. J Virol. 1997;71:4062–4070. doi: 10.1128/jvi.71.5.4062-4070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vichkanova SA, Oĭfa AI, Goriunova LV. Antiviral properties of gossypol in experimental influenza pneumonia. Antibiotiki. 1970;15:1071–1073. [PubMed] [Google Scholar]
- 109.Krylov VF. Treatment of patients with influenza. Ter Arkh. 1975;47:49–55. [PubMed] [Google Scholar]
- 110.Yang J, Zhang F, Li J, et al. Synthesis and antiviral activities of novel gossypol derivatives. Bioorg Med Chem Lett. 2012;22:1415–1420. doi: 10.1016/j.bmcl.2011.12.076. [DOI] [PubMed] [Google Scholar]
- 111.Yang J, Chen G, Li LL, et al. Synthesis and anti-H5N1 activity of chiral gossypol derivatives and its analogs implicated by a viral entry blocking mechanism. Bioorg Med Chem Lett. 2013;23:2619–2623. doi: 10.1016/j.bmcl.2013.02.101. [DOI] [PubMed] [Google Scholar]
- 112.Jones JC, Turpin EA, Bultmann H, et al. Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. J Virol. 2006;80:11960–11967. doi: 10.1128/JVI.01678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Altmann SE, Brandt CR, Jahrling PB, et al. Antiviral activity of the EB peptide against zoonotic poxviruses. Virol J. 2012;9:6–6. doi: 10.1186/1743-422X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nicol MQ, Ligertwood Y, Bacon MN, et al. A novel family of peptides with potent activity against influenza A viruses. J Gen Virol. 2012;93:980–986. doi: 10.1099/vir.0.038679-0. [DOI] [PubMed] [Google Scholar]
- 115.Rajik M, Jahanshiri F, Omar AR, et al. Identification and characterisation of a novel anti-viral peptide against avian influenza virus H9N2. Virol J. 2009;6:74–74. doi: 10.1186/1743-422X-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matsubara T. Potential of peptides as inhibitors and mimotopes: selection of carbohydrate-mimetic peptides from phage display libraries. J Nucleic Acids. 2012;2012:740982–740982. doi: 10.1155/2012/740982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Selman L, Hansen S. Structure and function of collectin liver 1 (CL-L1) and collectin 11 (CL-11, CL-K1) Immunobiology. 2012;217:851–863. doi: 10.1016/j.imbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 118.Ling MT, Tu W, Han Y, et al. Mannose-binding lectin contributes to deleterious inflammatory response in pandemic H1N1 and avian H9N2 infection. J Infect Dis. 2012;205:44–53. doi: 10.1093/infdis/jir691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dec M, Wernicki A. Conglutinin, CL-43 and CL-46-three bovine collectins. Pol J Vet Sci. 2006;9:265–275. [PubMed] [Google Scholar]
- 120.Kawai T, Kase T, Suzuki Y, et al. Anti-influenza A virus activities of mannan-binding lectins and bovine conglutinin. J Vet Med Sci. 2007;69:221–224. doi: 10.1292/jvms.69.221. [DOI] [PubMed] [Google Scholar]
- 121.Malhotra R, Haurum JS, Thiel S, et al. Binding of human collectins (SP-A and MBP) to influenza virus. Biochem J. 1994;304:455–461. doi: 10.1042/bj3040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hartshorn KL, Sastry K, Brown D, et al. Conglutinin acts as an opsonin for influenza A viruses. J Immunol. 1993;151:6265–6273. [PubMed] [Google Scholar]
- 123.Verma A, White M, Vathipadiekal V, et al. Human H-ficolin inhibits replication of seasonal and pandemic influenza A viruses. J Immunol. 2012;189:2478–2487. doi: 10.4049/jimmunol.1103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan Q, Chen H, Wang F, et al. L-ficolin binds to the glycoproteins hemagglutinin and neuraminidase and inhibits influenza A virus infection both in vitro and in vivo. J Innate Immun. 2012;4:312–324. doi: 10.1159/000335670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang WC, Hartshorn KL, White MR, et al. Recombinant chimeric lectins consisting of mannose-binding lectin and L-ficolin are potent inhibitors of influenza A virus compared with mannose- binding lectin. Biochem Pharmacol. 2011;81:388–395. doi: 10.1016/j.bcp.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gordts SC, Renders M, Férir G, et al. NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J Antimicrob Chemother. 2015 doi: 10.1093/jac/dkv034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pustylnikov S, Sagar D, Jain P, et al. Targeting the C-type lectins- mediated host-pathogen interactions with dextran. J Pharm Pharm Sci. 2014;17:371–392. doi: 10.18433/j3n590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morita M, Kuba K, Ichikawa A, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 129.Zhang C, Xu Y, Jia L, et al. A new therapeutic strategy for lung tissue injury induced by influenza with CR2 targeting complement inhibitor. Virol J. 2010;7:30–30. doi: 10.1186/1743-422X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Imai Y. Role of omega-3 PUFA-derived mediators, the protectins, in influenza virus infection. Biochim Biophys Acta. 2015;1851:496–502. doi: 10.1016/j.bbalip.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 131.Takahashi M, Mori S, Shigeta S, et al. Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. Adv Exp Med Biol. 2007;598:93–104. doi: 10.1007/978-0-387-71767-8_8. [DOI] [PubMed] [Google Scholar]
- 132.Smee DF, Bailey KW, Wong MH, et al. Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin- N. Antiviral Res. 2008;80:266–271. doi: 10.1016/j.antiviral.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O'Keefe BR, Smee DF, Turpin JA, et al. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miyamoto D, Hasegawa S, Sriwilaijaroen N, et al. Clarithromycin inhibits progeny virus production from human influenza virus-infected host cells. Biol Pharm Bull. 2008;31:217–222. doi: 10.1248/bpb.31.217. [DOI] [PubMed] [Google Scholar]
- 135.Yamaya M, Shinya K, Hatachi Y, et al. Clarithromycin inhibits type a seasonal influenza virus infection in human airway epithelial cells. J Pharmacol Exp Ther. 2010;333:81–90. doi: 10.1124/jpet.109.162149. [DOI] [PubMed] [Google Scholar]
- 136.Ghendon Y, Markushin S, Heider H, et al. Haemagglutinin of influenza A virus is a target for the antiviral effect of Norakin. J Gen Virol. 1986;67:1115–1122. doi: 10.1099/0022-1317-67-6-1115. [DOI] [PubMed] [Google Scholar]
- 137.Ott S, Wunderli-Allenspach H. Effect of the virostatic Norakin (triperiden) on influenza virus activities. Antiviral Res. 1994;24:37–42. doi: 10.1016/0166-3542(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 138.Presber HW, Schroeder C, Hegenscheid B, et al. Antiviral activity of Norakin (triperiden) and related anticholinergic antiparkinsonism drugs. Acta Virol. 1984;28:501–507. [PubMed] [Google Scholar]
- 139.Heider H, Markushin S, Schroeder C, Ghendon Y. The influence of Norakin on the reproduction of influenza A and B viruses. Arch Virol. 1985;86:283–290. doi: 10.1007/BF01309832. [DOI] [PubMed] [Google Scholar]
- 140.Schroeder C, Heider H, Hegenscheid B, et al. The anticholinergic anti-Parkinson drug Norakin selectively inhibits influenza virus replication. Antiviral Res. 1985;(Suppl 1):95–99. doi: 10.1016/s0166-3542(85)80014-0. [DOI] [PubMed] [Google Scholar]
- 141.Prösch S, Heider H, Schroeder C, et al. Mutations in the hemagglutinin gene associated with influenza virus resistance to norakin. Arch Virol. 1988;102:125–129. doi: 10.1007/BF01315569. [DOI] [PubMed] [Google Scholar]
- 142.Prösch S, Heider H, Schroeder C, et al. Mapping mutations in influenza A virus resistant to norakin. FEBS Lett. 1990;267:19–21. doi: 10.1016/0014-5793(90)80277-p. [DOI] [PubMed] [Google Scholar]
- 143.Klimov AI, Markushin SG, Prösch S, et al. Relation between drug resistance and antigenicity among norakin-resistant mutants of influenza A (fowl plague) virus. Arch Virol. 1992;124:147–155. doi: 10.1007/BF01314632. [DOI] [PubMed] [Google Scholar]
- 144.Markushin SG, Ginzburg VP, Khaĭder AM, et al. Factors that cause a change in the antigenic structure of of the influenza virus hemagglutinin. Vopr Virusol. 1992;37:196–199. [PubMed] [Google Scholar]
- 145.Oka M, Ishiwata Y, Iwata N, et al. Synthesis and anti-influenza virus activity of tricyclic compounds with a unique amine moiety. Chem Pharm Bull (Tokyo) 2001;49:379–383. doi: 10.1248/cpb.49.379. [DOI] [PubMed] [Google Scholar]
- 146.Rossignol JF. Nitazoxanide. A first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014 doi: 10.1016/j.antiviral.2014.07.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ashiru O, Howe JD, Butters TD. Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca(2+) stores. Virology. 2014;462-463:135–148. doi: 10.1016/j.virol.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 148.Belardo G, Cenciarelli O, La Frazia S, et al. Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza A viruses in vitro. Antimicrob Agents Chemother. 2015;59:1061–1069. doi: 10.1128/AAC.03947-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Täubel J, Lorch U, Rossignol JF, et al. Analyzing the relationship of QT interval and exposure to nitazoxanide, a prospective candidate for influenza antiviral therapy – A formal TQT study. J Clin Pharmacol. 2014;54:987–994. doi: 10.1002/jcph.300. [DOI] [PubMed] [Google Scholar]
- 150.Haffizulla J, Hartman A, Hoppers M, et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14:609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ashton LV, Callan RL, Rao S, et al. In vitro susceptibility of canine influenza A (H3N8) virus to nitazoxanide and tizoxanide. Vet Med Int. 2010;2010 doi: 10.4061/2010/891010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ulyanova V, Vershinina V, Ilinskaya O. Barnase and binase: twins with distinct fates. FEBS J. 2011;278:3633–3643. doi: 10.1111/j.1742-4658.2011.08294.x. [DOI] [PubMed] [Google Scholar]
- 153.Shah Mahmud R, Ilinskaya ON. Antiviral Activity of Binase against the Pandemic Influenza A (H1N1) Virus. Acta Naturae. 2013;5:44–51. [PMC free article] [PubMed] [Google Scholar]
- 154.Sato Y, Hirayama M, Morimoto K, et al. High mannose-binding lectin with preference for the cluster of alpha1-2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J Biol Chem. 2011;286:19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Savov VM, Galabov AS, Tantcheva LP, et al. Effects of rutin and quercetin on monooxygenase activities in experimental influenza virus infection. Exp Toxicol Pathol. 2006;58:59–64. doi: 10.1016/j.etp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 156.Davis JM, Murphy EA, McClellan JL, et al. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol Regul Integr Comp Physiol. 2008;295:R505–R509. doi: 10.1152/ajpregu.90319.2008. [DOI] [PubMed] [Google Scholar]
- 157.Choi HJ, Song JH, Park KS, et al. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur J Pharm Sci. 2009;37:329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 158.Kumar P, Khanna M, Srivastava V, et al. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp Lung Res. 2005;31:449–459. doi: 10.1080/019021490927088. [DOI] [PubMed] [Google Scholar]
- 159.Raju TA, Lakshmi AN, Anand T, et al. Protective effects of quercetin during influenza virus-induced oxidative stress. Asia Pac J Clin Nutr. 2000;9:314–317. doi: 10.1046/j.1440-6047.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- 160.Friel H, Lederman H. A nutritional supplement formula for influenza A (H5N1) infection in humans. Med Hypotheses. 2006;67:578–587. doi: 10.1016/j.mehy.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 161.Eşanu V, Prahoveanu E, Crişan I, et al. The effect of an aqueous propolis extract, of rutin and of a rutin-quercetin mixture on experimental influenza virus infection in mice. Virologie. 1981;32:213–215. [PubMed] [Google Scholar]
- 162.Chang SS, Huang HJ, Chen CY. Two birds with one stone? Possible dual-targeting H1N1 inhibitors from traditional Chinese medicine. PLoS Comput Biol. 2011;7:e1002315–e1002315. doi: 10.1371/journal.pcbi.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chang TT, Sun MF, Chen HY, et al. Screening from the world�s largest TCM database against H1N1 virus. J Biomol Struct Dyn. 2011;28:773–786. doi: 10.1080/07391102.2011.10508605. [DOI] [PubMed] [Google Scholar]
- 164.Nakayama M, Suzuki K, Toda M, et al. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 1993;21:289–299. doi: 10.1016/0166-3542(93)90008-7. [DOI] [PubMed] [Google Scholar]
- 165.Yang ZF, Bai LP, Huang WB, et al. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure-activity relationship analysis. Fitoterapia. 2014;93:47–53. doi: 10.1016/j.fitote.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 166.Zu M, Yang F, Zhou W, et al. In vitro anti-influenza virus and anti-inflammatory activities of theaflavin derivatives. Antiviral Res. 2012;94:217–224. doi: 10.1016/j.antiviral.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 167.Hayden FG, Aoki FY. Amantadine, rimatadine, and related agents. In: Barriere SL, editor. Antimicrobial Therapy and Vaccines. Baltimore: Williams & Williams; 1999. pp. 1344–1365. [Google Scholar]
- 168.Wang C, Takeuchi K, Pinto LH, et al. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993:675585–675594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ruigrok RW, Hirst EM, Hay AJ. The specific inhibition of influenza A virus maturation by amantadine: an electron microscopic examination. J Gen Virol. 1991;72:191–194. doi: 10.1099/0022-1317-72-1-191. [DOI] [PubMed] [Google Scholar]
- 170.Sheu TG, Fry AM, Garten RJ, et al. Dual resistance to adamantanes and oseltamivir among seasonal influenza A(H1N1) viruses: 2008-2010. J Infect Dis. 2011;203:13–17. doi: 10.1093/infdis/jiq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smirnova TD, Danilenko DM, Eropkin MIu, et al. Influence of rimantadine, ribavirine and triazavirine on influenza A virus replication in human monolayer and lymphoblastoid cell lines. Antibiot Khimioter. 2011;56:11–16. [PubMed] [Google Scholar]
- 172.Karpenko I, Deev S, Kiselev O, et al. Antiviral properties, metabolism, and pharmacokinetics of a novel azolo-1,2,4-triazinederived inhibitor of influenza A and B virus replication. Antimicrob Agents Chemother. 2010;54:2017–2022. doi: 10.1128/AAC.01186-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tanner JA, Zheng BJ, Zhou J, et al. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem Biol. 2005;12:303–311. doi: 10.1016/j.chembiol.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moorthy NS, Poongavanam V, Pratheepa V. Viral M2 ion channel protein: a promising target for anti-influenza drug discovery. Mini Rev Med Chem. 2014;14:819–830. [PubMed] [Google Scholar]
- 175.Rey-Carrizo M, Torres E, Ma C, et al. 3-Azatetracyclo. 2.1.1(5,8).0(1,5)]undecane derivatives: from wild-type inhibitors of the M2 ion channel of influenza A virus to derivatives with potent activity against the V27A mutant. J Med Chem. 2013;56:9265–9274. doi: 10.1021/jm401340p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang J, Wu Y, Ma C, et al. Structure and inhibition of the drugresistant S31N mutant of the M2 ion channel of influenza A virus. Proc Natl Acad Sci U S A. 2013;110:1315–1320. doi: 10.1073/pnas.1216526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang J, Ma C, Wang J, et al. Discovery of novel dual inhibitors of the wild-type and the most prevalent drug-resistant mutant, S31N, of the M2 proton channel from influenza A virus. J Med Chem. 2013;56:2804–2812. doi: 10.1021/jm301538e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gasparini R, Lai PL, Casabona F, et al. Do the omeprazole family compounds exert a protective effect against influenza-like illness? BMC Infect Dis. 2014;14:297–297. doi: 10.1186/1471-2334-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bozdaganyan M, Orekhov P, Bragazzi NL, et al. Docking and molecular dynamics (MD) simulations in potential drugs discovery: an application to influenza virus M2 protein. Am J Biochem Biotechnol. 2014;10:180–188. [Google Scholar]
- 180. 3C9J. The Crystal structure of Transmembrane domain of M2 protein and Amantadine complex. Accesible at http://www.rcsb.org/pdb/explore.do?structureId=3c9j.
- 181.Long J, Wright E, Molesti E, et al. Antiviral therapies against Ebola and other emerging viral diseases using existing medicines that block virus entry. F1000Research. 2015;4:30–30. doi: 10.12688/f1000research.6085.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bachrach U, Don S. Inactivation of influenza and Newcastle disease viruses by oxidized spermine. Isr J Med Sci. 1970;6:435–437. [PubMed] [Google Scholar]
- 183.Bachrach U. Antiviral activity of oxidized polyamines. Amino Acids. 2007;33:267–272. doi: 10.1007/s00726-007-0535-y. [DOI] [PubMed] [Google Scholar]
- 184.Lin TI, Heider H, Schroeder C. Different modes of inhibition by adamantane amine derivatives and natural polyamines of the functionally reconstituted influenza virus M2 proton channel protein. J Gen Virol. 1997;78:767–774. doi: 10.1099/0022-1317-78-4-767. [DOI] [PubMed] [Google Scholar]
- 185.Even-Or O, Samira S, Rochlin E, et al. Immunogenicity, protective efficacy and mechanism of novel CCS adjuvanted influenza vaccine. Vaccine. 2010;28:6527–6541. doi: 10.1016/j.vaccine.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 186.Even-Or O, Joseph A, Itskovitz-Cooper N, et al. A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine. 2011;29:2474–2486. doi: 10.1016/j.vaccine.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 187.Fytas C, Kolocouris A, Fytas G, et al. Influence of an additional amino group on the potency of aminoadamantanes against influenza virus A. II - Synthesis of spiropiperazines and in vitro activity against influenza A H3N2 virus. Bioorg Chem. 2010;38:247–251. doi: 10.1016/j.bioorg.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao X, Jie Y, Rosenberg MR, et al. Design and synthesis of pinanamine derivatives as anti-influenza A M2 ion channel inhibitors. Antiviral Res. 2012;96:91–99. doi: 10.1016/j.antiviral.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 189.Serkedjieva J, Manolova N, Bankova V. Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids) J Nat Prod. 1992;55:294–302. doi: 10.1021/np50081a003. [DOI] [PubMed] [Google Scholar]
- 190.Kesel AJ. Synthesis of novel test compounds for antiviral chemotherapy of severe acute respiratory syndrome (SARS) Curr Med Chem. 2005;12:2095–2162. doi: 10.2174/0929867054637644. [DOI] [PubMed] [Google Scholar]
- 191.Iwai A, Shiozaki T, Miyazaki T. Relevance of signaling molecules for apoptosis induction on influenza A virus replication. Biochem Biophys Res Commun. 2013;441:531–537. doi: 10.1016/j.bbrc.2013.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jurgeit A, McDowell R, Moese S, et al. Niclosamide is a proton carrier and targets acidic endosomes with broad antiviral effects. PLoS Pathog. 2012;8:e1002976–e1002976. doi: 10.1371/journal.ppat.1002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Krátký M, Vinšová J. Antiviral activity of substituted salicylanilides – a review. Mini Rev Med Chem. 2011;11:956–967. doi: 10.2174/138955711797068382. [DOI] [PubMed] [Google Scholar]
- 194.Russell RJ, Kerry PS, Stevens DJ, et al. Structure of influenza hemagglutinin in complex with an inhibitor of membrane fusion. Proc Natl Acad Sci U S A. 2008;105:17736–17741. doi: 10.1073/pnas.0807142105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hosoya M, Matsuyama S, Baba M, et al. Effects of protease inhibitors on replication of various myxoviruses. Antimicrob Agents Chemother. 1992;36:1432–1436. doi: 10.1128/aac.36.7.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bahgat MM, Blazejewska P, Schughart K. Inhibition of lung serine proteases in mice: a potentially new approach to control influenza infection. Virol J. 2011;8:27–27. doi: 10.1186/1743-422X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hamilton BS, Chung C, Cyphers SY, et al. Inhibition of influenza virus infection and hemagglutinin cleavage by the protease inhibitor HAI-2. Biochem Biophys Res Commun. 2014;450:1070–1075. doi: 10.1016/j.bbrc.2014.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee MG, Kim KH, Park KY, et al. Evaluation of anti-influenza effects of camostat in mice infected with non-adapted human influenza viruses. Arch Virol. 1996;141:1979–1989. doi: 10.1007/BF01718208. [DOI] [PubMed] [Google Scholar]
- 199.Puzis LE, Lozitsky VP. Action of epsilon-aminocaproic acid on the proteolysis system during experimental influenza in mice. Acta Virol. 1988;32:515–521. [PubMed] [Google Scholar]
- 200.Tashiro M, Klenk HD, Rott R. Inhibitory effect of a protease inhibitor, leupeptin, on the development of influenza pneumonia, mediated by concomitant bacteria. J Gen Virol. 1987;68:2039–2041. doi: 10.1099/0022-1317-68-7-2039. [DOI] [PubMed] [Google Scholar]
- 201.Zhirnov OP, Klenk HD, Wright PF. Aprotinin and similar protease inhibitors as drugs against influenza. Antiviral Res. 2011;92:27–36. doi: 10.1016/j.antiviral.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 202.Zhou Y, Wu C, Zhao L, et al. Exploring the early stages of the pH-induced conformational change of influenza hemagglutinin. Proteins. 2014;82:2412–2428. doi: 10.1002/prot.24606. [DOI] [PubMed] [Google Scholar]
- 203.Leikina E, Delanoe-Ayari H, Melikov K, et al. Carbohydratebinding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol. 2005;6:995–1001. doi: 10.1038/ni1248. [DOI] [PubMed] [Google Scholar]
- 204.Streeter DG, Witkowski JT, Khare GP, et al. Mechanism of action of 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci U S A. 1973;70:1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Crotty S, Cameron C, Andino R. Ribavirin's antiviral mechanism of action: lethal mutagenesis? J Mol Med (Berl) 2002;80:86–95. doi: 10.1007/s00109-001-0308-0. [DOI] [PubMed] [Google Scholar]
- 206.Chan-Tack KM, Murray JS, Birnkrant DB. Use of ribavirin to treat influenza. N Engl J Med. 2009;361:1713–1714. doi: 10.1056/NEJMc0905290. [DOI] [PubMed] [Google Scholar]
- 207.Gangemi JD, Nachtigal M, Barnhart D, et al. Therapeutic efficacy of liposome-encapsulated ribavirin and muramyl tripeptide in experimental infection with influenza or herpes simplex virus. J Infect Dis. 1987;155:510–517. doi: 10.1093/infdis/155.3.510. [DOI] [PubMed] [Google Scholar]
- 208.Smee DF, Hurst BL, Day CW, et al. Influenza Virus H1N1 inhibition by serine protease inhibitor (serpin) antithrombin III. Int Trends Immun. 2014;2:83–86. [PMC free article] [PubMed] [Google Scholar]
- 209.Stoller JK, Lacbawan FL, Aboussouan LS. Alpha-1 Antitrypsin Deficiency. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews ® [Internet] Seattle: University of Washington; 1993-2014. 2006 Oct 27 [updated 2014 May 01] [PubMed] [Google Scholar]
- 210.Campos MA, Alazemi S, Zhang G, et al. Influenza vaccination in subjects with alpha1-antitrypsin deficiency. Chest. 2008;133:49–55. doi: 10.1378/chest.07-1482. [DOI] [PubMed] [Google Scholar]
- 211.Yagi S, Ono J, Yoshimoto J, et al. Development of anti-influenza virus drugs I: improvement of oral absorption and in vivo anti-influenza activity of Stachyflin and its derivatives. Pharm Res. 1999;16:1041–1046. doi: 10.1023/a:1018983715982. [DOI] [PubMed] [Google Scholar]
- 212.Yoshimoto J, Yagi S, Ono J, et al. Development of anti-influenza drugs: II. Improvement of oral and intranasal absorption and the anti-influenza activity of stachyflin derivatives. J Pharm Pharmacol. 2000;52:1247–1255. doi: 10.1211/0022357001777225. [DOI] [PubMed] [Google Scholar]
- 213.Minagawa K, Kouzuki S, Yoshimoto J, et al. Stachyflin and acetylstachyflin, novel anti-influenza A virus substances, produced by Stachybotrys sp. RF-7260. I. Isolation, structure elucidation and biological activities. J Antibiot (Tokyo) 2002;55:155–164. doi: 10.7164/antibiotics.55.155. [DOI] [PubMed] [Google Scholar]
- 214.Minagawa K, Kouzuki S, Kamigauchi T. Stachyflin and acetylstachyflin, novel anti-influenza A virus substances, produced by Stachybotrys sp. RF-7260. II. Synthesis and preliminary structure- activity relationships of stachyflin derivatives. J Antibiot (Tokyo) 2002;55:165–171. doi: 10.7164/antibiotics.55.165. [DOI] [PubMed] [Google Scholar]
- 215.Motohashi Y, Igarashi M, Okamatsu M, et al. Antiviral activity of stachyflin on influenza A viruses of different hemagglutinin subtypes. Virol J. 2013;10:118–118. doi: 10.1186/1743-422X-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Watanabe K, Sakurai J, Abe H, et al. Total synthesis of (+)-stachyflin: a potential anti-influenza A virus agent. Chem Commun (Camb) 2010;46:4055–4057. doi: 10.1039/c000193g. [DOI] [PubMed] [Google Scholar]
- 217.Nakatani M, Nakamura M, Suzuki A, et al. A new strategy toward the total synthesis of stachyflin, a potent anti-influenza A virus agent: concise route to the tetracyclic core structure. Org Lett. 2002;4:4483–4486. doi: 10.1021/ol0271032. [DOI] [PubMed] [Google Scholar]
- 218.Combrink KD, Gulgeze HB, Yu KL, et al. Salicylamide inhibitors of influenza virus fusion. Bioorg Med Chem Lett. 2000;10:1649–1652. doi: 10.1016/s0960-894x(00)00335-8. [DOI] [PubMed] [Google Scholar]
- 219.Zhu L, Li Y, Li S, et al. Inhibition of influenza A virus (H1N1) fusion by benzenesulfonamide derivatives targeting viral hemagglutinin. PLoS One. 2011;6:e29120–e29120. doi: 10.1371/journal.pone.0029120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yu KL, Torri AF, Luo G, et al. Structure-activity relationships for a series of thiobenzamide influenza fusion inhibitors derived from 1,3,3-trimethyl-5-hydroxy-cyclohexylmethylamine. Bioorg Med Chem Lett. 2002;12:3379–3382. doi: 10.1016/s0960-894x(02)00761-8. [DOI] [PubMed] [Google Scholar]
- 221.Yuan S. Drugs to cure avian influenza infection-multiple ways to prevent cell death. Cell Death Dis. 2013;4:e835–e835. doi: 10.1038/cddis.2013.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ye M, Zheng JB, Yu KJ, et al. Effects of high dose ulinastatin treatment in patients with severe pneumonia complicating influenza A H1N1 infection. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23:48–49. [PubMed] [Google Scholar]
- 223.Naganuma A, Mizuma H, Doi I, et al. A case of acute respiratory distress syndrome induced by fulminant influenza A (H3 N2) pneumonia. Nihon Kokyuki Gakkai Zasshi. 2000;38:783–787. [PubMed] [Google Scholar]
- 224.Munakata M, Kato R, Yokoyama H, et al. Combined therapy with hypothermia and anticytokine agents in influenza A encephalopathy. Brain Dev. 2000;22:373–377. doi: 10.1016/s0387-7604(00)00169-8. [DOI] [PubMed] [Google Scholar]
- 225.Leng YX, Yang SG, Song YH, et al. Ulinastatin for acute lung injury and acute respiratory distress syndrome: A systematic review and meta-analysis. World J Crit Care Med. 2014;3:34–41. doi: 10.5492/wjccm.v3.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ketscher L, Hannß R, Morales DJ, et al. Selective inactivation of USP18 isopeptidase activity in vivo enhances ISG15 conjugation and viral resistance. Proc Natl Acad Sci U S A. 2015;112:1577–1582. doi: 10.1073/pnas.1412881112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Uchida Y, Watanabe C, Takemae N, et al. Identification of host genes linked with the survivability of chickens infected with recombinant viruses possessing H5N1 surface antigens from a highly pathogenic avian influenza virus. J Virol. 2012;86:2686–2695. doi: 10.1128/JVI.06374-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu AL, Li YF, Qi W, et al. Comparative analysis of selected innate immune-related genes following infection of immortal DF-1 cells with highly pathogenic (H5N1) and low pathogenic (H9N2) avian influenza viruses. Virus Genes. 2015 doi: 10.1007/s11262-014-1151-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Loregian A, Mercorelli B, Nannetti G, et al. Antiviral strategies against influenza virus: towards new therapeutic approaches. Cell Mol Life Sci. 2014 doi: 10.1007/s00018-014-1615-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bauman JD, Patel D, Baker SF, et al. Crystallographic fragment screening and structure-based optimization yields a new class of influenza endonuclease inhibitors. ACS Chem Biol. 2013;8:2501–2508. doi: 10.1021/cb400400j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sheppard S. Moroxydine: the story of a mislaid antiviral. Acta Derm Venereol Suppl (Stockh) 1994;183:1–9. [PubMed] [Google Scholar]
- 232.Mertens T, Eggers HJ. Moroxydine. Dtsch Med Wochenschr. 1980;105:184–184. [PubMed] [Google Scholar]
- 233.Dreyfus P. Treatment of influenzal infections by a moroxydine derivative. Sem Ther. 1966;42:51–52. [PubMed] [Google Scholar]
- 234.Furuta Y, Takahashi K, Fukuda Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Caroline AL, Powell DS, Bethel LM, et al. Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever. PLoS Negl Trop Dis. 2014;8:e2790–e2790. doi: 10.1371/journal.pntd.0002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Oestereich L, Rieger T, Neumann M, et al. Evaluation of antiviral efficacy of ribavirin, arbidol, and T-705 (favipiravir) in a mouse model for Crimean-Congo hemorrhagic fever. PLoS Negl Trop Dis. 2014;8:e2804–e2804. doi: 10.1371/journal.pntd.0002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Smither SJ, Eastaugh LS, Steward JA, et al. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res. 2014;104:153–155. doi: 10.1016/j.antiviral.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 238.Safronetz D, Falzarano D, Scott DP, et al. Antiviral efficacy of favipiravir against two prominent etiological agents of hantavirus pulmonary syndrome. Antimicrob Agents Chemother. 2013;57:4673–4680. doi: 10.1128/AAC.00886-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Iwai Y, Murakami K, Gomi Y, et al. Anti-influenza activity of marchantins, macrocyclic bisbibenzyls contained in liverworts. PLoS One. 2011;6:e19825–e19825. doi: 10.1371/journal.pone.0019825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shaw ML, Klumpp K. Successes and challenges in the antiviral field. Curr Opin Virol. 2013;3:483–486. doi: 10.1016/j.coviro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 241.Loregian A, Coen DM. Selective anti-cytomegalovirus compounds discovered by screening for inhibitors of subunit interactions of the viral polymerase. Chem Biol. 2006;13:191–200. doi: 10.1016/j.chembiol.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 242.Fukuoka M, Minakuchi M, Kawaguchi A, et al. Structure-based discovery of anti-influenza virus A compounds among medicines. Biochim Biophys Acta. 2012;1820:90–95. doi: 10.1016/j.bbagen.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 243.Sugiyama K, Obayashi E, Kawaguchi A, et al. Structural insight into the essential PB1-PB2 subunit contact of the influenza virus RNA polymerase. EMBO J. 2009;28:1803–1811. doi: 10.1038/emboj.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chase G, Wunderlich K, Reuther P, et al. Identification of influenza virus inhibitors which disrupt of viral polymerase proteinprotein interactions. Methods. 2011;55:188–191. doi: 10.1016/j.ymeth.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 245.Li C, Ba Q, Wu A, et al. A peptide derived from the C-terminus of PB1 inhibits influenza virus replication by interfering with viral polymerase assembly. FEBS J. 2013;280:1139–1149. doi: 10.1111/febs.12107. [DOI] [PubMed] [Google Scholar]
- 246.Nasser EH, Judd AK, Sanchez A, et al. Antiviral activity of influenza virus M1 zinc finger peptides. J Virol. 1996;70:8639–8644. doi: 10.1128/jvi.70.12.8639-8644.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Li L, Chang S, Xiang J, et al. Screen anti-influenza lead compounds that target the PA(C) subunit of H5N1 viral RNA polymerase. PLoS One. 2012;7:e35234–e35234. doi: 10.1371/journal.pone.0035234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Clark MP, Ledeboer MW, Davies I, et al. Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem. 2014;57:6668–6678. doi: 10.1021/jm5007275. [DOI] [PubMed] [Google Scholar]
- 249.Pagano M, Castagnolo D, Bernardini M, et al. The fight against the influenza A virus H1N1: synthesis, molecular modeling, and biological evaluation of benzofurazan derivatives as viral RNA polymerase inhibitors. Chem Med Chem. 2014;9:129–150. doi: 10.1002/cmdc.201300378. [DOI] [PubMed] [Google Scholar]
- 250.Lepri S, Nannetti G, Muratore G, et al. Optimization of smallmolecule inhibitors of influenza virus polymerase: from thiophene- 3-carboxamide to polyamido scaffolds. J Med Chem. 2014;57:4337–4350. doi: 10.1021/jm500300r. [DOI] [PubMed] [Google Scholar]
- 251.Gao J, Luo X, Li Y, et al. Synthesis and biological evaluation of 2-oxo-pyrazine-3-carboxamide-yl nucleoside analogues and their epimers as inhibitors of influenza A viruses. Chem Biol Drug Des. 2014 doi: 10.1111/cbdd.12382. in press. [DOI] [PubMed] [Google Scholar]
- 252.Dierkes R, Warnking K, Liedmann S, et al. The Rac1 inhibitor NSC23766 exerts anti-influenza virus properties by affecting the viral polymerase complex activity. PLoS One. 2014;9:e88520–e88520. doi: 10.1371/journal.pone.0088520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Elton D, Simpson-Holley M, Archer K, et al. Interaction of the influenza virus nucleoprotein with the cellular CRM1-mediated nuclear export pathway. J Virol. 2001;75:408–419. doi: 10.1128/JVI.75.1.408-419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Perwitasari O, Johnson S, Yan X, et al. Verdinexor, a novel selective inhibitor of nuclear export, reduces influenza a virus replication in vitro and in vivo. J Virol. 2014;88:10228–10243. doi: 10.1128/JVI.01774-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Amorim MJ, Kao RY, Digard P. Nucleozin targets cytoplasmic trafficking of viral ribonucleoprotein-Rab11 complexes in influenza A virus infection. J Virol. 2013;87:4694–4703. doi: 10.1128/JVI.03123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Su CY, Cheng TJ, Lin MI, et al. High-throughput identification of compounds targeting influenza RNA-dependent RNA polymerase activity. Proc Natl Acad Sci USA. 2010;107:19151–19156. doi: 10.1073/pnas.1013592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jiang H, Xu Y, Li L, et al. Inhibition of influenza virus replication by constrained peptides targeting nucleoprotein. Antivir Chem Chemother. 2011;22:119–130. doi: 10.3851/IMP1902. [DOI] [PubMed] [Google Scholar]
- 258.Verhelst J, Parthoens E, Schepens B, et al. Interferon-inducible protein Mx1 inhibits influenza virus by interfering with functional viral ribonucleoprotein complex assembly. J Virol. 2012;86:13445–13455. doi: 10.1128/JVI.01682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cianci C, Gerritz SW, Deminie C, et al. Influenza nucleoprotein: promising target for antiviral chemotherapy. Antivir Chem Chemother. 2012;23:77–91. doi: 10.3851/IMP2235. [DOI] [PubMed] [Google Scholar]
- 260.Pons M. Effect of actinomycin D on the replication of influenza virus and influenza virus RNA. Virology. 1967;33:150–154. doi: 10.1016/0042-6822(67)90104-3. [DOI] [PubMed] [Google Scholar]
- 261.Vogel U, Scholtissek C. Inhibition of the intracellular transport of influenza viral RNA by actinomycin D. Arch Virol. 1995;140:1715–1723. doi: 10.1007/BF01384336. [DOI] [PubMed] [Google Scholar]
- 262.Pons MW. The inhibition of influenza virus RNA synthesis by actinomycin D and cycloheximide. Virology. 1973;51:120–128. doi: 10.1016/0042-6822(73)90372-3. [DOI] [PubMed] [Google Scholar]
- 263.Lejal N, Tarus B, Bouguyon E, et al. Structure-based discovery of the novel antiviral properties of naproxen against the nucleoprotein of influenza A virus. Antimicrob Agents Chemother. 2013;57:2231–2242. doi: 10.1128/AAC.02335-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zarubaev VV, Beliaevskaia SV, Sirotkin AK, et al. In vitro and in vivo effects of ingavirin on the ultrastructure and infectivity of influenza virus. Vopr Virusol. 2011;56:21–25. [PubMed] [Google Scholar]
- 265.Zarubaev VV, Garshinina AV, Kalinina NA, et al. Activity of Ingavirin (6 -(1H-Imidazol-4-yl)ethylamino]-5-oxohexanoic acid) against human respiratory viruses in vivo experiments. Pharmaceuticals. 2011;4:1518–1534. doi: 10.3390/ph4121518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Semenova NP, Prokudina EN, Livov DK, et al. Effect of the antiviral drug Ingaviruin on intracellular transformations and import into the nucleus of influenza A virus nucleocapsid protein. Vopr Virusol. 2010;55:17–20. [PubMed] [Google Scholar]
- 267.Loginova SIa, Borisevich SV, Shkliaeva OM, et al. Prophylactic and therapeutic efficacies of Ingavirin, a novel Russian chemotherapeutic, with respect to influenza pathogen A (H5N1) Antibiot Khimioter. 2010;55:10–12. [PubMed] [Google Scholar]
- 268.Shishkina LN, Nebol'sin VE, Skarnovich MO, et al. In vivo efficacy of Ingavirin against pandemic A (H1N1/09)v influenza virus. Antibiot Khimioter. 2010;55:32–35. [PubMed] [Google Scholar]
- 269.Kolobukhina LV, Merkulova LN, Shchelkanov MI, et al. Efficacy of ingavirin in adults with influenza. Ter Arkh. 2009;81:51–54. [PubMed] [Google Scholar]
- 270.Galegov GA, Andronova VL, Nebol'sin VE. Antiviral effect of Ingavirin against seasonal influenza virus A/H1N1 in MDCK cell culture. Antibiot Khimioter. 2009;54:19–22. [PubMed] [Google Scholar]
- 271.Loginova SIa, Borisevich SV, Maksimov VA, et al. Investigation of prophylactic activity of Ingavirin, a new Russian drug, against grippe A virus (H3N2) Antibiot Khimioter. 2008;53:19–21. [PubMed] [Google Scholar]
- 272.Shul'diakov AA, Liapina EP, Kuznetsov VI, et al. Current principles in the chemoprophylaxis of acute respiratory viral infections. Ter Arkh. 2013;85:27–33. [PubMed] [Google Scholar]
- 273.Isaeva EI, Nebol'sin VE, Kozulina IS, et al. In vitro investigation of the antiviral activity of Ingavirin against human metapneumovirus. Vopr Virusol. 2012;57:34–38. [PubMed] [Google Scholar]
- 274.Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000;355:827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- 275.Feng E, Ye D, Li J, et al. Recent advances in neuraminidase inhibitor development as anti-influenza drugs. ChemMedChem. 2012;7:1527–1536. doi: 10.1002/cmdc.201200155. [DOI] [PubMed] [Google Scholar]
- 276.Verma RP, Hansch C. A QSAR study on influenza neuraminidase inhibitors. Bioorg Med Chem. 2006;14:982–996. doi: 10.1016/j.bmc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 277.Barroso L, Treanor J, Gubareva L, et al. Efficacy and tolerability of the oral neuraminidase inhibitor peramivir in experimental human influenza: randomized, controlled trials for prophylaxis and treatment. Antivir Ther. 2005;10:901–910. [PubMed] [Google Scholar]
- 278.Birnkrant D, Cox E. The Emergency Use Authorization of peramivir for treatment of 2009 H1N1 influenza. N Engl J Med. 2009;361:2204–2207. doi: 10.1056/NEJMp0910479. [DOI] [PubMed] [Google Scholar]
- 279.Koyama K, Ogura Y, Nakai D, et al. Identification of bioactivating enzymes involved in the hydrolysis of laninamivir octanoate, a long-acting neuraminidase inhibitor, in human pulmonary tissue. Drug Metab Dispos. 2014;42:1031–1038. doi: 10.1124/dmd.114.057620. [DOI] [PubMed] [Google Scholar]
- 280.Weight AK, Haldar J, Alvarez de Cienfuegos L, et al. Attaching zanamivir to a polymer markedly enhances its activity against drug-resistant strains of influenza a virus. J Pharm Sci. 2011;100:831–835. doi: 10.1002/jps.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hayden FG, Cote KM, Douglas RG., Jr Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob Agents Chemother. 1980;17:865–870. doi: 10.1128/aac.17.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jedrzejas MJ, Singh S, Brouillette WJ, et al. Structures of aromatic inhibitors of influenza virus neuraminidase. Biochemistry. 1995;34:3144–3151. doi: 10.1021/bi00010a003. [DOI] [PubMed] [Google Scholar]
- 283.Li Y, Silamkoti A, Kolavi G, et al. Pyrrolidinobenzoic acid inhibitors of influenza virus neuraminidase: the hydrophobic side chain influences type A subtype selectivity. Bioorg Med Chem. 2012;20:4582–4589. doi: 10.1016/j.bmc.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kim CU, Lew W, Williams MA, et al. Structure-activity relationship studies of novel carbocyclic influenza neuraminidase inhibitors. J Med Chem. 1998;41:2451–2460. doi: 10.1021/jm980162u. [DOI] [PubMed] [Google Scholar]
- 285.Kati WM, Saldivar AS, Mohamadi F, et al. GS4071 is a slowbinding inhibitor of influenza neuraminidase from both A and B strains. Biochem Biophys Res Commun. 1998;244:408–413. doi: 10.1006/bbrc.1998.8282. [DOI] [PubMed] [Google Scholar]
- 286.Kim CU, Lew W, Williams MA, et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 287.Jang YJ, Achary R, Lee HW, et al. Synthesis and anti-influenza virus activity of 4-oxo- or thioxo-4,5-dihydrofuro,4-c]pyridin- 3(1H)-ones. Antiviral Res. 2014;107:66–75. doi: 10.1016/j.antiviral.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lou J, Yang X, Rao Z, et al. Design and synthesis of 6-oxo- 1,4,5,6-tetrahydropyrimidine-5-carboxylate derivatives as neuraminidase inhibitors. Eur J Med Chem. 2014;83:466–473. doi: 10.1016/j.ejmech.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 289.Li J, Zhang D, Zhu X, et al. Studies on synthesis and structureactivity relationship (SAR) of derivatives of a new natural product from marine fungi as inhibitors of influenza virus neuraminidase. Mar Drugs. 2011;9:1887–1901. doi: 10.3390/md9101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ding Y, Dou J, Teng Z, et al. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch Virol. 2014;159:3269–3278. doi: 10.1007/s00705-014-2192-2. [DOI] [PubMed] [Google Scholar]
- 291.Nayak MK, Agrawal AS, Bose S, et al. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J Antimicrob Chemother. 2014;69:1298–1310. doi: 10.1093/jac/dkt534. [DOI] [PubMed] [Google Scholar]
- 292.Wan Q, Wang H, Han X, et al. Baicalin inhibits TLR7/MYD88 signaling pathway activation to suppress lung inflammation in mice infected with influenza A virus. Biomed Rep. 2014;2:437–441. doi: 10.3892/br.2014.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Xu G, Dou J, Zhang L, et al. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol Pharm Bull. 2010;33:238–243. doi: 10.1248/bpb.33.238. [DOI] [PubMed] [Google Scholar]
- 294.Nagai T, Suzuki Y, Tomimori T, et al. Antiviral activity of plant flavonoid, 5,7,4'-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis against influenza A (H3N2) and B viruses. Biol Pharm Bull. 1995;18:295–299. doi: 10.1248/bpb.18.295. [DOI] [PubMed] [Google Scholar]
- 295.Nagai T, Miyaichi Y, Tomimori T, et al. In vivo anti-influenza virus activity of plant flavonoids possessing inhibitory activity for influenza virus sialidase. Antiviral Res. 1992;19:207–217. doi: 10.1016/0166-3542(92)90080-o. [DOI] [PubMed] [Google Scholar]
- 296.Hale BG, Randall RE, Ortìn J, et al. The multifunctional NS1 protein of influenza A viruses. J Gen Virol. 2008;89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 297.Zhirnov OP, Konakova TE, Wolff T, et al. NS1 protein of influenza A virus down-regulates apoptosis. J Virol. 2002;76:1617–1625. doi: 10.1128/JVI.76.4.1617-1625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kong JQ, Shen JH, Huang Y, et al. Development of a yeast twohybrid screen for selection of A/H1N1 influenza NS1 non-structural protein and human CPSF30 protein interaction inhibitors. Yao Xue Xue Bao. 2010;45:388–394. [PubMed] [Google Scholar]
- 299.Twu KY, Noah DL, Rao P, et al. The CPSF30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J Virol. 2006;80:3957–3965. doi: 10.1128/JVI.80.8.3957-3965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jablonski JJ, Basu D, Engel DA, et al. Design, synthesis, and evaluation of novel small molecule inhibitors of the influenza virus protein NS1. Bioorg Med Chem. 2012;20:487–497. doi: 10.1016/j.bmc.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Mata MA, Satterly N, Versteeg GA, et al. Chemical inhibition of RNA viruses reveals REDD1 as a host defense factor. Nat Chem Biol. 2011;7:712–719. doi: 10.1038/nchembio.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Mahy BW, Cox NJ, Armstrong SJ, et al. Multiplication of influenza virus in the presence of cordycepin, an inhibitor of cellular RNA synthesis. Nat New Biol. 1973;243:172–174. doi: 10.1038/newbio243172a0. [DOI] [PubMed] [Google Scholar]
- 303.Kurokawa M, Koyama AH, Yasuoka S, et al. Influenza virus overcomes apoptosis by rapid multiplication. Int J Mol Med. 1999;3:527–530. doi: 10.3892/ijmm.3.5.527. [DOI] [PubMed] [Google Scholar]
- 304.Zhirnov OP, Klenk HD. Control of apoptosis in influenza virusinfected cells by up-regulation of Akt and p53 signaling. Apoptosis. 2007;12:1419–1432. doi: 10.1007/s10495-007-0071-y. [DOI] [PubMed] [Google Scholar]
- 305.Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Holey PM, editors. Fields Virology. 5th Edition. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1647–1689. [Google Scholar]
- 306.Wurzer WJ, Planz O, Ehrhardt C, et al. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003;22:2717–2728. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hinshaw VS, Olsen CW, Dybdahl-Sissoko N, et al. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Jaworska J, Coulombe F, Downey J, et al. NLRX1 prevents mitochondrial induced apoptosis and enhances macrophage antiviral immunity by interacting with influenza virus PB1-F2 protein. Proc Natl Acad Sci USA. 2014;111:E2110–E2119. doi: 10.1073/pnas.1322118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Furman D, Jojic V, Kidd B, et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol. 2014;10:750–750. doi: 10.1038/msb.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Feldman T, Kabaleeswaran V, Jang SB, et al. A class of allosteric caspase inhibitors identified by high-throughput screening. Mol Cell. 2012;47:585–595. doi: 10.1016/j.molcel.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Dai J, Wang G, Li W, et al. High-throughput screening for antiinfluenza A virus drugs and study of the mechanism of procyanidin on influenza A virus-induced autophagy. J Biomol Screen. 2012;17:605–617. doi: 10.1177/1087057111435236. [DOI] [PubMed] [Google Scholar]
- 312.Muniruzzaman S, Pan YT, Zeng Y, et al. Inhibition of glycoprotein processing by L-fructose and L-xylulose. Glycobiology. 1996;6:795–803. doi: 10.1093/glycob/6.8.795. [DOI] [PubMed] [Google Scholar]
- 313.Hussain S, Miller JL, Harvey DJ, et al. Strain-specific antiviral activity of iminosugars against human influenza A viruses. J Antimicrob Chemother. 2014 doi: 10.1093/jac/dku349. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Oguin TH, 3rd, Sharma S, Stuart AD, et al. Phospholipase D facilitates efficient entry of influenza virus, allowing escape from innate immune inhibition. J Biol Chem. 2014;289:25405–25417. doi: 10.1074/jbc.M114.558817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Husain M, Cheung CY. Histone deacetylase 6 inhibits influenza A virus release by downregulating the trafficking of viral components to the plasma membrane via its substrate, acetylated microtubules. J Virol. 2014;88:11229–11239. doi: 10.1128/JVI.00727-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hamamoto I, Harazaki K, Inase N, et al. Cyclosporin A inhibits the propagation of influenza virus by interfering with a late event in the virus life cycle. Jpn J Infect Dis. 2013;66:276–283. doi: 10.7883/yoken.66.276. [DOI] [PubMed] [Google Scholar]
- 317.Hsieh CF, Yen HR, Liu CH, et al. Ching-fang-pai-tu-san inhibits the release of influenza virus. J Ethnopharmacol. 2012;144:533–544. doi: 10.1016/j.jep.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 318.Buffinton GD, Christen S, Peterhans E, et al. Oxidative stress in lungs of mice infected with influenza A virus. Free Radic Res Commun. 1992;16:99–110. doi: 10.3109/10715769209049163. [DOI] [PubMed] [Google Scholar]
- 319.Drago L, Nicola L, Ossola F, et al. In vitro antiviral activity of resveratrol against respiratory viruses. J Chemother. 2008;20:393–394. doi: 10.1179/joc.2008.20.3.393. [DOI] [PubMed] [Google Scholar]
- 320.Palamara AT, Nencioni L, Aquilano K, et al. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191:1719–1729. doi: 10.1086/429694. [DOI] [PubMed] [Google Scholar]
- 321.Li C, Fang JS, Lian WW, et al. In vitro antiviral effects and 3D QSAR Study of resveratrol derivatives as potent inhibitors of influenza H1N1 neuraminidase. Chem Biol Drug Des. 2015;85:427–438. doi: 10.1111/cbdd.12425. [DOI] [PubMed] [Google Scholar]
- 322.Furuya A, Uozaki M, Yamasaki H, et al. Antiviral effects of ascorbic and dehydroascorbic acids in vitro. Int J Mol Med. 2008;22:541–545. [PubMed] [Google Scholar]
- 323.Uozaki M, Ikeda K, Tsujimoto K, et al. Antiviral effects of dehydroascorbic acid. Exp Ther Med. 2010;1:983–986. doi: 10.3892/etm.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Khare D, Godbole NM, Pawar SD, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. 2013;52:1405–1415. doi: 10.1007/s00394-012-0449-7. [DOI] [PubMed] [Google Scholar]
- 325.Goldstein MR, Mascitelli L, Pezzetta F. Pandemic influenza A (H1N1): mandatory vitamin D supplementation? Med Hypotheses. 2010;74:756–756. doi: 10.1016/j.mehy.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 326.Fedson DS. Pandemic influenza: a potential role for statins in treatment and prophylaxis. Clin. Infect. Dis. 2006;43:199–205. doi: 10.1086/505116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–435. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 328.Mehrbod P, Hair-Bejo M, Tengku Ibrahim TA, et al. Simvastatin modulates cellular components in influenza A virus-infected cells. Int J Mol Med. 2014;34:61–73. doi: 10.3892/ijmm.2014.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Glück B, Schmidtke M, Walther M, et al. Simvastatin treatment showed no prophylactic effect in influenza virus-infected mice. J Med Virol. 2013;85:1978–1982. doi: 10.1002/jmv.23682. [DOI] [PubMed] [Google Scholar]
- 330.Magulick JP, Frei CR, Ali SK, et al. The effect of statin therapy on the incidence of infections: a retrospective cohort analysis. Am J Med Sci. 2014;347:211–216. doi: 10.1097/MAJ.0b013e31828318e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Cho WK, Weeratunga P, Lee BH, et al. Epimedium koreanum Nakai displays broad spectrum of antiviral activity in vitro and in vivo by inducing cellular antiviral state. Viruses. 2015;7:352–377. doi: 10.3390/v7010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Leibbrandt A, Meier C, König-Schuster M, et al. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS One. 2010;5:e14320–e14320. doi: 10.1371/journal.pone.0014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Koenighofer M, Lion T, Bodenteich A, et al. Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidiscip Respir Med. 2014;9:57–57. doi: 10.1186/2049-6958-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Romantsov MG, Golofeevskiĭ SV. Cycloferon efficacy in the treatment of acute respiratory tract viral infection and influenza during the morbidity outbreak in 2009-201. Antibiot Khimioter. 2010;55:30–35. [PubMed] [Google Scholar]
- 335.Romantsov MG, Ershov FI, Kovalenko AL, et al. The therapeutic efficacy of cycloferon and the pharmacological activity of interferon inducers. Ter Arkh. 2014;86:83–88. [PubMed] [Google Scholar]
- 336.Sukhinin VP, Zarubaev VV, Platonov VG, et al. Protective effect of cycloferon in experimental influenza. Vopr Virusol. 2000;45:26–30. [PubMed] [Google Scholar]
- 337.Tazulakhova EB, Parshina OV, Guseva TS, et al. Russian experience in screening, analysis, and clinical application of novel interferon inducers. J Interferon Cytokine Res. 2001;21:65–73. doi: 10.1089/107999001750069926. [DOI] [PubMed] [Google Scholar]
- 338.Ye S, Lowther S, Stambas J. Inhibition of Reactive Oxygen Species Production Ameliorates Inflammation Induced by Influenza A Viruses via Upregulation of SOCS1 and SOCS3. J Virol. 2015;89:2672–2683. doi: 10.1128/JVI.03529-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Selemidis S, Seow HJ, Broughton BR, et al. Nox1 oxidase suppresses influenza a virus-induced lung inflammation and oxidative stress. PLoS One. 2013;8:e60792–e60792. doi: 10.1371/journal.pone.0060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Vlahos R, Selemidis S. NADPH oxidases as novel pharmacologic targets against influenza A virus infection. Mol Pharmacol. 2014;86:747–759. doi: 10.1124/mol.114.095216. [DOI] [PubMed] [Google Scholar]
- 341.Sharma G, Sharma DC, Fen LH, et al. Reduction of influenza virus-induced lung inflammation and mortality in animals treated with a phosophodisestrase-4 inhibitor and a selective serotonin reuptake inhibitor. Emerging Microbes & Infections. 2013;2:e54–e54. doi: 10.1038/emi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Marsolais D, Hahm B, Walsh KB, et al. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci USA. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sugamata R, Sugawara A, Nagao T, et al. Leucomycin A3, a 16-membered macrolide antibiotic, inhibits influenza A virus infection and disease progression. J Antibiot (Tokyo) 2014;67:213–222. doi: 10.1038/ja.2013.132. [DOI] [PubMed] [Google Scholar]
- 344. BG-777. Accessible at http://www.drugbank.ca/drugs/DB05839.
- 345.Mbawuike I, Zang Y, Couch RB. Humoral and cell-mediated immune responses of humans to inactivated influenza vaccine with or without QS21 adjuvant. Vaccine. 2007;25:3263–3269. doi: 10.1016/j.vaccine.2007.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Panatto D, Amicizia D, Lai PL, et al. Utility of thymosin alpha-1 (Zadaxin) as a co-adjuvant in influenza vaccines: a review. J Prev Med Hyg. 2011;52:111–115. [PubMed] [Google Scholar]
- 347.Carraro G, Naso A, Montomoli E, et al. Thymosin-alpha 1 (Zadaxin) enhances the immunogenicity of an adjuvated pandemic H1N1v influenza vaccine (Focetria) in hemodialyzed patients: a pilot study. Vaccine. 2012;30:1170–1180. doi: 10.1016/j.vaccine.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 348.Gravenstein S, Duthie EH, Miller BA, et al. Augmentation of influenza antibody response in elderly men by thymosin alpha one. A double-blind placebo-controlled clinical study. J Am Geriatr Soc. 1989;37:1–8. doi: 10.1111/j.1532-5415.1989.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 349.Chioato A, Noseda E, Felix SD, et al. Influenza and meningococcal vaccinations are effective in healthy subjects treated with the interleukin-1 beta-blocking antibody canakinumab: results of an open-label, parallel group, randomized, singlecenter study. Clin Vaccine Immunol. 2010;17:1952–1957. doi: 10.1128/CVI.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;4:CD008965–CD008965. doi: 10.1002/14651858.CD008965.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. CDC , author. Influenza antiviral medications: summary for clinicians (current for the 2013-14 influenza season) Document available at: http://www.cdc.gov/flu/professionals/antivirals/summaryclinicians.htm. Accessed on 24th July 2014.
- 352. WHO , author. Global Alert and Response (GAR) antiviral drugs for pandemic (H1N1) 2009: definitions and use. Document available at: http://www.who.int/csr/disease/swineflu/frequently_asked_questions/antivirals/definitions_use/en/. Accessed on 24th July 2014).
- 353.Budd A, Alleva L, Alsharifi M, et al. Increased survival after gemfibrozil treatment of severe mouse influenza. Antimicrob Agents Chemother. 2007;51:2965–2968. doi: 10.1128/AAC.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Lin KL, Sweeney S, Kang BD, et al. CCR2-antagonist prophylaxis reduces pulmonary immune pathology and markedly improves survival during influenza infection. J Immunol. 2011;186:508–515. doi: 10.4049/jimmunol.1001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Zheng BJ, Chan KW, Lin YP, et al. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci USA. 2008;105:8091–8096. doi: 10.1073/pnas.0711942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ghezzi P, Ungheri D. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int J Immunopathol Pharmacol. 2004;17:99–102. doi: 10.1177/039463200401700114. [DOI] [PubMed] [Google Scholar]
- 357.Ottolini M, Blanco J, Porter D, et al. Combination anti-inflammatory and antiviral therapy of influenza in a cotton rat model. Pediatr Pulmonol. 2003;36:290–294. doi: 10.1002/ppul.10320. [DOI] [PubMed] [Google Scholar]
- 358.Khanna M, Saxena L, Rajput R, et al. Gene silencing: a therapeutic approach to combat influenza virus infections. Future Microbiol. 2015;10:131–140. doi: 10.2217/fmb.14.94. [DOI] [PubMed] [Google Scholar]
- 359.Wichadakul D, Mhuantong W, Jongkaewwattana A, et al. A computational tool for the design of live attenuated virus vaccine based on microRNA-mediated gene silencing. BMC Genomics. 2012;13(Suppl 7):S15–S15. doi: 10.1186/1471-2164-13-S7-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Buggele WA, Johnson KE, Horvath CM. Influenza A virus infection of human respiratory cells induces primary microRNA expression. J Biol Chem. 2012;287:31027–31040. doi: 10.1074/jbc.M112.387670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Langlois RA, Albrecht RA, Kimble B, et al. MicroRNA-based strategy to mitigate the risk of gain-of-function influenza studies. Nat Biotechnol. 2013;31:844–847. doi: 10.1038/nbt.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhang H, Li Z, Li Y, et al. A computational method for predicting regulation of human microRNAs on the influenza virus genome. BMC Syst Biol. 2013;7(Suppl 2):S3–S3. doi: 10.1186/1752-0509-7-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Li Y, Chan EY, Li J, et al. MicroRNA expression and virulence in pandemic influenza virus-infected mice. J Virol. 2010;84:3023–3032. doi: 10.1128/JVI.02203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Betáková T, Svančarová P. Role and application of RNA interference in replication of influenza viruses. Acta Virol. 2013;57:97–104. doi: 10.4149/av_2013_02_97. [DOI] [PubMed] [Google Scholar]
- 365.Seth S, Templin MV, Severson G, et al. A potential therapeutic for pandemic influenza using RNA interference. Methods Mol Biol. 2010;623:397–422. doi: 10.1007/978-1-60761-588-0_26. [DOI] [PubMed] [Google Scholar]
- 366.Ge Q, Filip L, Bai A, et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci USA. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Winterling C, Koch M, Koeppel M, et al. Evidence for a crucial role of a host non-coding RNA in influenza A virus replication. RNA Biol. 2014;11:66–75. doi: 10.4161/rna.27504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ouyang J, Zhu X, Chen Y, et al. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe. 2014;16:616–626. doi: 10.1016/j.chom.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Xiang DX, Chen Q, Pang L, et al. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J Virol Methods. 2011;178:137–142. doi: 10.1016/j.jviromet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 370.Xiang D, Zheng Y, Duan W, et al. Inhibition of A/Human/ Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int J Nanomedicine. 2013;8:4103–4113. doi: 10.2147/IJN.S53622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.McKenzie Z, Kendall M, Mackay RM, et al. Nanoparticles modulate surfactant protein A and D mediated protection against influenza A infection in vitro. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140049–20140049. doi: 10.1098/rstb.2014.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Torrecilla J, Rodríguez-Gascón A, Solinís MÁ, et al. Lipid nanoparticles as carriers for RNAi against viral infections: current status and future perspectives. Biomed Res Int. 2014;2014:161794–161794. doi: 10.1155/2014/161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Okamoto S, Yoshii H, Akagi T, et al. Influenza hemagglutinin vaccine with poly(gamma-glutamic acid) nanoparticles enhances the protection against influenza virus infection through both humoral and cell-mediated immunity. Vaccine. 2007;25:8270–8278. doi: 10.1016/j.vaccine.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 374.Shoji M, Takahashi E, Hatakeyama D, et al. Anti-influenza activity of c60 fullerene derivatives. PLoS One. 2013;8:e66337–e66337. doi: 10.1371/journal.pone.0066337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Amidi M, Romeijn SG, Verhoef JC, et al. N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine. 2007;25:144–153. doi: 10.1016/j.vaccine.2006.06.086. [DOI] [PubMed] [Google Scholar]
- 376.Sawaengsak C, Mori Y, Yamanishi K, et al. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech. 2014;15:317–325. doi: 10.1208/s12249-013-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Sawaengsak C, Mori Y, Yamanishi K, et al. Intranasal chitosan- DNA vaccines that protect across influenza virus subtypes. Int J Pharm. 2014;473:113–125. doi: 10.1016/j.ijpharm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 378.Sanpui P, Zheng X, Loeb JC, et al. Single-walled carbon nanotubes increase pandemic influenza A H1N1 virus infectivity of lung epithelial cells. Part Fibre Toxicol. 2014;11:66–66. doi: 10.1186/s12989-014-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Govorkova EA, McCullers JA. Therapeutics against influenza. Curr Top Microbiol Immunol. 2013;370:273–300. doi: 10.1007/82_2011_198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Govorkova EA, Webster RG. Combination chemotherapy for influenza. Viruses. 2010;2:1510–1529. doi: 10.3390/v2081510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Prabakaran M, Prabhu N, He F, et al. Combination therapy using chimeric monoclonal antibodies protects mice from lethal H5N1 infection and prevents formation of escape mutants. PLoS One. 2009;4:e5672–e5672. doi: 10.1371/journal.pone.0005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Bastos LF, Coelho MM. Drug repositioning: playing dirty to kill pain. CNS Drugs. 2014;28:45–61. doi: 10.1007/s40263-013-0128-0. [DOI] [PubMed] [Google Scholar]
- 383.Wilkinson GF, Pritchard K. In vitro screening for drug repositioning. J Biomol Screen. 2015;20:167–179. doi: 10.1177/1087057114563024. [DOI] [PubMed] [Google Scholar]
- 384.Heldt FS, Frensing T, Pflugmacher A, et al. Multiscale modeling of influenza A virus infection supports the development of direct-acting antivirals. PLoS Comput Biol. 2013;9:e1003372–e1003372. doi: 10.1371/journal.pcbi.1003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Smith SB, Dampier W, Tozeren A, et al. Identification of common biological pathways and drug targets across multiple respiratory viruses based on human host gene expression analysis. PLoS One. 2012;7:e33174–e33174. doi: 10.1371/journal.pone.0033174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Law GL, Tisoncik-Go J, Korth MJ, et al. Drug repurposing: a better approach for infectious disease drug discovery? Curr Opin Immunol. 2013;25:588–592. doi: 10.1016/j.coi.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Sharma D, Priyadarshini P, Vrati S. Unraveling the web of viroinformatics: computational tools and databases in virus research. J Virol. 2015;89:1489–1501. doi: 10.1128/JVI.02027-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Bao S, Zhou X, Zhang L, et al. Prioritizing genes responsible for host resistance to influenza using network approaches. BMC Genomics. 2013;14:816–816. doi: 10.1186/1471-2164-14-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Li Z, Zhou H, Lu Y, et al. A critical role for immune system response in mediating anti-influenza drug synergies assessed by mechanistic modeling. CPT Pharmacometrics Syst Pharmacol. 2014;3:e135–e135. doi: 10.1038/psp.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Frank D. One world, one health, one medicine. Can Vet J. 2008;49:1063–1065. [PMC free article] [PubMed] [Google Scholar]
- 391.Travis DA, Sriramarao P, Cardona C, et al. One medicine one science: a framework for exploring challenges at the intersection of animals, humans, and the environment. Ann N Y Acad Sci. 2014;1334:26–44. doi: 10.1111/nyas.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]


