Summary
Objective.
Precise identification of various morphotypes of Pseduomonas aeruginosa which developed during cystic fibrosis (CF) is of prime importance. We aimed to identify the isolates of P. aeruginosa recovered from CF patients at the genus and species level through primers targeting oprI and oprL genes via PCR.
Methods.
Sputum samples or throat swabs were taken from 100 CF patients and plated on cetrimide agar. All suspected colonies were primarily screened for P. aeruginosa by a combination of phenotypic tests. Molecular identification of colonies was performed using specific primers for oprI and oprL genes.
Results.
Based on phenotypic tests, P. aeruginosa isolates were recovered from 40% of CF patients. Forty isolates yielded amplicon of oprI gene using genus-specific primers confirming the identity of fluorescent pseudomonads. However, 37 of 40 isolates yielded amplicon of oprL gene using species-specific primers, verifying the identity of P. aeruginosa.
Conclusion.
This study showed that the species-specific PCR targeting oprL gene can be used as accurate test for identification of highly adaptable P. aeruginosa in CF patients. This procedure may provide a simple and reliable method for identification of various morphotypes.
Key words: Pseudomonas aeruginosa, cystic fibrosis, oprI, oprL
Introduction
Pseduomonas aeruginosa is considered as the most common recovered bacterium from respiratory infections in cystic fibrosis (CF) patients [1]. Cystic fibrosis is a genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) encoding gene. CFTR primarily acts as a chloride ion channel and mutation in this gene affects the chloride transport and sodium absorption, leading to thickness of mucus in lungs [2]. CFTR is also recognized as a cellular receptor for binding and clearance of P. aeruginosa from the lungs [3], therefore malfunction of CFTR may lead to persistence of this species and ultimately severe pulmonary disease. CF patients are more prone to colonization by a variety of bacteria during their life [4] but the majority of CF patients endure P. aeruginosa chronic lung infections [5]. The early colonization of patients may occur by susceptible and non-mucoid strains of P. aeruginosa, but as early as the colonized bacteria are covered by thickened and dehydrated mucosa in lung, the bacterial microenvironment will be established. This microenvironment creates a way for evasion of bacteria and a barrier to antibiotics and consequently selection of variants of P. aeruginosa [6]. Despite the prolonged antibiotic therapy, P. aeruginosa is not eradicated in such patients and the formation of various morphotypes of bacteria during chronic and sustained infection is reported worldwide. The major morphologic alterations of P. aeruginosa in CF patients are conversion to mucoid variants, loss of pigments, overproduction of alginate, formation of small-colony variants, and evolution of mutator strains [7-10].
The emergence of mucoid variants P. aeruginosa is usually considered as a poor prognostic indicator in CF patients [11]. One of the critical control measures in CF patients is accurate species identification. The broad phenotypic variation of P. aeruginosa may lead to misindentification of these strains in CF patients. Therefore, precise identification of various morphotypes which developed during chronic infections and differentiation of P. areugionsa from other non-fermentative strains involving in the pathogensis of CF is of prime importance. Due to drawbacks of phenotypic methods for identification of P. areuginosa morphotypes, we aimed to identify P. aeruginosa at the genus and species level through primers targeting oprI and oprL genes via PCR.
Materials and methods
In this cross-sectional study, the cases that had a sweat chloride equal to or greater than 60 mmol/L and were diagnosed as CF patients were included. The patients who treated with antibiotics within the previous two weeks were excluded. One hundred patients suffering from CF, aged 1 to 23 years were studied from three health centers in Tehran during 2011 to 2012. Demographic characteristics and medical histories were collected from medical records. The study was approved by the local ethical committees.
Sputum samples or throat swabs were taken and cultured on cetrimide agar, blood agar and MacConkey agar and the plates were incubated for 72 h at 37 °C. Regardless of morphology of colonies, all suspected colonies primarily were screened for P. aeruginosa by a combination of tests including growth on cetrimide agar, growth at 42 °C, and biochemical tests such as oxidase, citrate, OF glucose, and arginine dihydrolase.
Genomic DNA extraction was performed using the standard phenol-chloroform extraction method [12]. Specific primers targeting the genes oprI and oprL were used to amplify 249 base pair (bp) and 504 bp products [13], respectively (Tab. I). Amplification of oprI and oprL was carried out in a total reaction volume of 20 μl containing 2 μl 10x PCR buffer, 1.5 mM MgCl2, 0.2 mM deoxynucleotide, 0.25 mM each primer (Bioneer, Seoul, South Korea), 0.5 U Taq polymerase, and 10 ng DNA. The Thermocycler (Peqlab, Germany) was set with the following conditions: Initial denaturation for 5 min at 95 °C and 30 cycles consisted of denaturation for 30s at 95°C, annealing for 30s at 57 °C, extension for 1 min at 72 °C, and final extension for 10 min at 72 °C. Electrophoresis was performed in a 1.5% agarose gel along with GeneRuler 100 bp DNA Ladder (Fermentas, Lithuania) and was stained with 0.5 μg/ml ethidium bromide. Pseudomonas aeruginosa ATCC 27853 was used as a positive control in all experiments.
Tab. I.
Specific primers sequences targeting oprI and oprL.
| Amplicon size (bp) |
Primer sequence (5'-3') | Target region |
|---|---|---|
| 249 | 5'- ATGAACAACGTTCTGAAATTCTCT -3' 5'- CTTGCGGCTGGCTTTTTCCAG -3' |
oprI |
| 504 | 5'- ATGGAAATGCTGAAATTCGGC -3' 5'- CTTCTTCAGCTCGACGCGACG -3' |
oprL |
Statistical analysis
The descriptive analysis was performed to calculate the frequency and percentage of variable using SPSS version 11.5.
Results
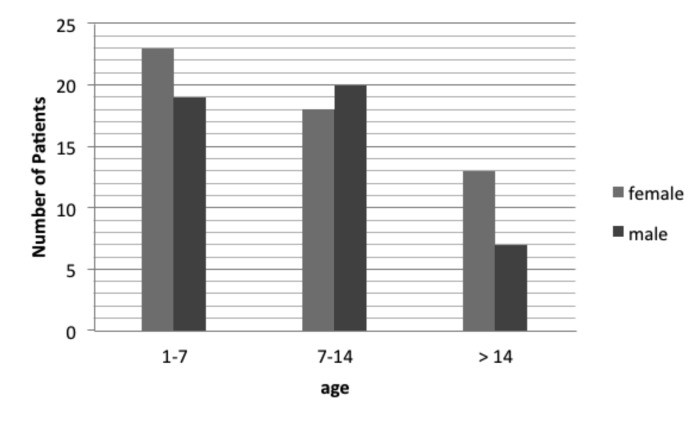
Among 100 patients with CF, 54 were females and 46 were males. Forty two patients were younger than 7 years old, 38 aged 7 to 14 years, and 20 aged more than 14 years. Demographic characteristics of CF patients including age and gender is shown in Fig. 1.
Fig. 1.
Distribution of patients with cystic fibrosis in relation of age and gender.
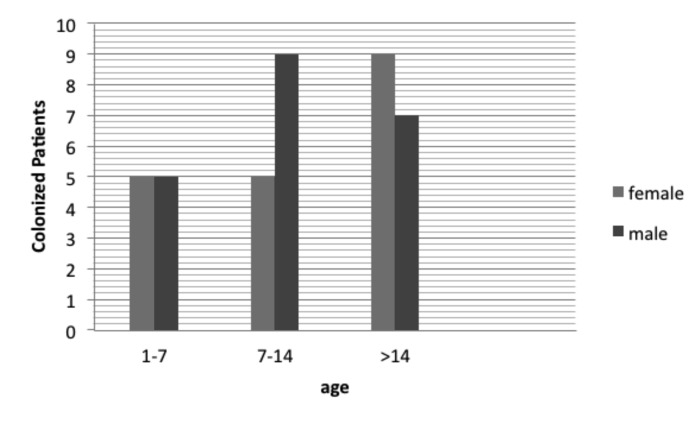
Based on phenotypic tests, P. aeruginosa isolates were recovered from 40% of CF patients. The distribution of CF patients colonized with P. aeruginosa in relation to demographic characteristics is shown in Fig. 2. P. aeruginosa were recovered from females (n = 19, 47.5%) and males (n = 21, 52.5%) in approximately similar frequency and no statistically significant difference was found. The colonization rate by P. aeruginosa among various age groups was different. An increasing trend of P. aeruginosa colonization was seen ranging from 23.8% in the 1-7 years age group to 36.8% in the 7-14 years age group, and 80% in cases older than 14 years.
Fig. 2.
Distribution of P. aeruginosa colonized patients suffering from cystic fibrosis in relation of age and gender.
For each isolate, the distinct morphotypes either mucoid or non-mucoied colonies grown on cetrimide agar were primarily identified as P. aeruginosa which oxidase- and citrate-positive but were OF glucose nonfermenter. Subsequently, all morphotypes were distinguished from other members of fluorescent pseudomonads by growth at 42 °C. Among 40 isolates of P. aeruginosa, 19 (47.5%) and 17 (42.5%) were mucoid and non-mucoid, respectively. In remaining isolates (n = 4, 10%) both mucoid and non-mucoid colonies were observed. Four (10%) and 17 (42.5%) isolates were arginine dihydrolase negative and non-pigmented, respectively. According to the combination of phenotypic and biochemical tests, 40 CF isolates were identified as P. aeruginosa and were considered for further identification via PCR-based assays.
Forty isolates yielded 249 bp (Fig. 3) amplicon through amplification of oprI gene using genus specific primers confirming the identity of fluorescent pseudomonads. However, 37 of 40 isolates yielded 573 bp (Fig. 4) amplicon through amplification of oprL gene using species specific primers, verifying the identity of P. aeruginosa. Three isolates were identified as P. aeruginosa by phenotypic tests but did not confirmed via species specific PCR.
Fig. 3.
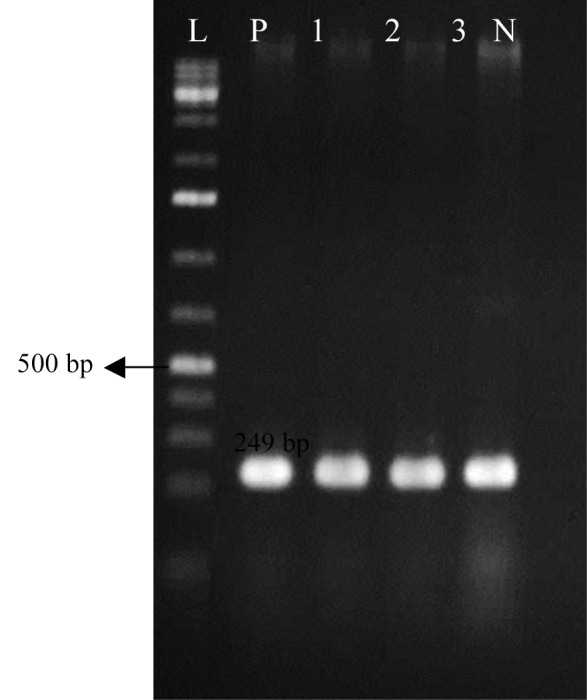
The oprI gene amplification using specific primers yielded a product of 249 bp typical to Speudomonas genus. L: 100 bp ladder (Fermentas, Lithuania); P: Positive control; 1-3: Pseudomonas isolates of CF patients; N: Negative control (distilled water).
Fig. 4.
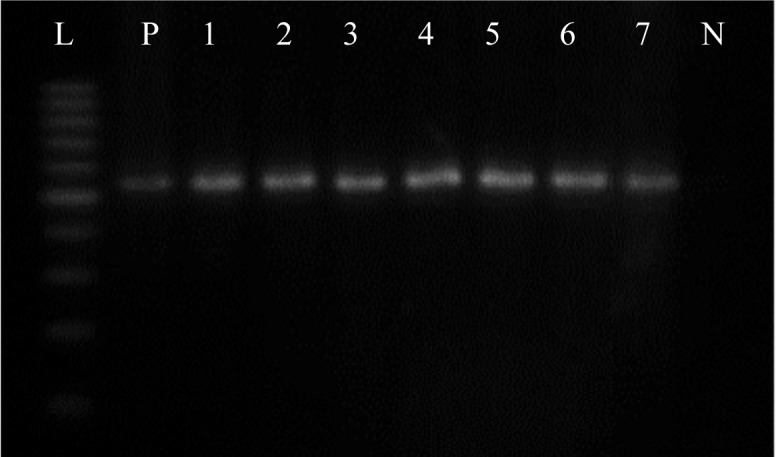
The oprI gene amplification using specific primers yielded a product of 504bp typical to P. aeruginosa. L: 100 bp ladder (Fermentas, Lithuania); P: Positive control; 1-7: P. aeruginosa isolates of CF patients; N: Negative control (distilled water).
Discussion
P. aeruginosa is not a fastidious organism and the identification of clinical isolates of P. aeruginosa is usually based on phenotypic methods. The phenotypic tests including macroscopic characteristics and biochemical tests are the most reliable tests for identification of typical isolates of P. aeruginosa [14]. However, extensive alterations in phenotype of P. aeruginosa may occur during chronic infection. For instance, the microenvironment of the CF lung may provide suitable conditions for mutation and selection of unique population of colonized bacteria [15]. Conversion to mucoid and non-pigmented colonies is common in P. aeruginosa recovered from CF patients. On the other hand, CF lungs may be colonized with other non-fermentative Gram-negative bacilli which are not easily differentiated from P. aeruginosa. These limitations may result in misidentification of P. aeruginosa as the most frequent pathogen in CF respiratory samples [16]. Inaccurate identification may affect the antibiotic susceptibility testing, administration of effective antipseudomonal antibiotics, and patient care. In the current study, we identified some of isolates with atypical phenotype; the isolates which non-pigmented and some isolates display a negative reaction for arginine dihydrolase. A possible explanation for these findings might be the plasticity and adaptation of P. aeruginosa in response to the unusual environment in CF lungs during chronic infection. As the isolates of P. aeruginosa with atypical phenotype were observed, we considered both mucoid and non-mucoid as well as pigmented and non-pigmented colonies for molecular identification. In this study, we determined the identity of multiple morphotypes of P. aeruginosa recovered from Iranian patients suffering from CF based on PCR assasys. To minimize the potential error of single-target assays, we used two targets for molecular identification of P. aeruginosa. We used PCR targeting two genes; oprI and oprL which are peptidoglycan associated outer membrane lipoproteins. oprI gene was previously identified as a conserved gene in members of fluorescent pseudomonads and in Pseudomonadaceae family. As reported previously, oprI gene sequence is highly conserved in P. aeruginosa isolates [18]. We found that amplicon of oprI gene were detected in all the phenotypically identified isolates including mucoid and non-pigmented Pseudomonas. This finding indicates that all isolates are more likely a member of fluorescent pseudomonads or Pseudomonas genus. These results are consistent with previous studies that applied oprI gene for identification of Pseudomonas genus [13, 19]. Moreover, we identified the P. aeruginosa at the species level using oprL gene specific primers. Our findings indicate that the majority of isolates were P. aeruginosa and three isolates belonged to Pseudomonas genus as amplified only through genus-specific primers and these isolates were misidentified via phenotypic methods. This study also demonstrated that non-aeruginosa species may isolated from CF patients. The discrepancy between the results of phenotypic assays and molecular tests was not statistically significant, but species-specific PCR is more reliable and sensitive method for identification of morphotypes of P. aeruginosa.
This study showed that the species specific PCR targeting oprL gene can be used as accurate test for identification of highly adaptable P. aeruginosa in CF patients. This procedure may provide a simple and reliable method for identification of various morphotypes which misidentified via phenotypic methods.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Ratjen F, McColley SA. Update in cystic fibrosis 2011. Am J Respir Crit Care Med. 2012;185:933–936. doi: 10.1164/rccm.201202-0306UP. [DOI] [PubMed] [Google Scholar]
- 3.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. PNAS. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart CA, Winstanley C. Persistent and aggressive bacteria in the lungs of cystic fibrosis children. Br Med Bull. 2002;61:81–96. doi: 10.1093/bmb/61.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Starner TD, McCray PB. Pathogenesis of early lung disease in cystic fibrosis: a window of opportunity to eradicate bacteria. Ann Intern Med. 2005;143:816–822. doi: 10.7326/0003-4819-143-11-200512060-00010. [DOI] [PubMed] [Google Scholar]
- 6.Hassett DJ, Sutton MD, Schurr MJ, Herr AB, et al. Pseudomonas aeruginosa hypoxic or anaerobic biofilm infections within cystic fibrosis airways. Trends Microbiol. 2009;17:130–138. doi: 10.1016/j.tim.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Mathee K, Ciofu O, Sternberg C, et al. Mucoid conversion of Pseudomonas aeruginos by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology. 1999;145:1349–1357. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 8.Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver A, Cantón R, Campo P, et al. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–1253. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 10.Ciofu O, Mandsberg LF, Bjarnsholt T, et al. Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants. Microbiology. 2010;156:1108–1119. doi: 10.1099/mic.0.033993-0. [DOI] [PubMed] [Google Scholar]
- 11.Emerson J, Rosenfeld M, McNamara S, et al. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 13.Vos D, Lim A, Pirnay JP, et al. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J Clin Microbiol. 1997;35:1295–1299. doi: 10.1128/jcm.35.6.1295-1299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MB, Gilligan PH. Laboratory aspects of management of chronic pulmonary infections in patients with cystic fibrosis. J Clin Microbiol. 2003;41:4009–4015. doi: 10.1128/JCM.41.9.4009-4015.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns JL, Emerson J, Stapp JR, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 16.Kidd TJ, Ramsay KA, Hu H, et al. Low rates of Pseudomonas aeruginosa misidentification in isolates from cystic fibrosis patients. J Clin Microbiol. 2009;47:1503–1509. doi: 10.1128/JCM.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wessel AK, Liew J, Kwon T, et al. Role of Pseudomonas aeruginosa peptidoglycan-associated outer membrane proteins in vesicle formation. J Bacteriol. 2013;195:213–219. doi: 10.1128/JB.01253-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vos D, Bouton C, Sarniguet A, et al. Sequence diversity of the oprI gene, coding for major outer membrane lipoprotein I, among rRNA group I pseudomonads. J Bacteriol. 1998;180:6551–6556. doi: 10.1128/jb.180.24.6551-6556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vos D, Lim A, Jr., Vos P, et al. Detection of the outer membrane lipoprotein I and its gene in fluorescent and non fluorescent pseudomonads: implications for taxonomy and diagnosis. J Gen Microbiol. 1992;139:2215–2223. doi: 10.1099/00221287-139-9-2215. [DOI] [PubMed] [Google Scholar]