Summary
Introduction.
Nigeria is a country saddled with a high tuberculosis (TB) and human immunodeficiency virus (HIV) burden but the possible combination of these communicable diseases with diabetes mellitus (DM) has been overlooked. We undertook to determine the burden of HIV and DM in persons with TB by documenting the prevalence rates of these disorders.
Methods.
This is a cross-sectional Study that was conducted within 54TB/DOT centers in Lagos State. A total of 3,376 persons with TB who were on antiTB drugs were screened for HIV and DM using standardized tests. Statistical analysis was performed using Students t test and chi square.
Results.
The frequency of occurrence of DM in TB and that of HIV in TB were comparable (4.8% Vs 3.5%). The Study subjects with DM were older, had higher waist circumference measurements and had higher proportions of hypertension compared to the subjects without DM. The combination of HIV and DM in TB was found in (0.3%). We also noted that DM in TB and HIV in TB occurred more frequently in the third and fourth decades of life.
Conclusion.
This study demonstrated the potential co existence of HIV, DM and Tuberculosis. It is therefore important that these two diseases are sought for in patients with TB considering the changing epidemiology of these diseases particularly in developing countries like Nigeria.
Key words: Diabetes mellitus, Tuberculosis, Screening, HIV
Introduction
Diabetes mellitus (DM) is one of the four priority Non communicable diseases identified by the World Health Organisation (WHO) and is assuming epidemic proportions with devastating human, social and economic consequences [1]. DM affects 5-6% of the global population and in Nigeria, the estimated prevalence of DM was 2.2% as at 1997 with present estimates ranging from 6-8% [2]. Human immunodeficiency virus is a blood borne retrovirus typically transmitted via sexual intercourse, shared intravenous drug paraphernalia and via mother to child transmission. In Nigeria, the human immunodeficiency virus (HIV) prevalence among the general population is 3.6% with an estimated 3.1 million people affected [3]. Tuberculosis is a communicable disease caused by any of the several species of Mycobacteria usually Mycobacteria tuberculosis or tubercle bacillus. Nigeria is ranked fourth among the 22 worst affected countries with tuberculosis (TB) in the world and the first in Africa. In Nigeria, Lagos State carries 8.4% of the nation's TB burden and this consistently has been responsible for about 11% of the cases of TB registered in Nigeria [4].
In sub-Saharan Africa, the HIV epidemic is accelerating the already massive TB epidemic with a documented increase in the incidence of TB from 146 per 100,000 to 345 per 100,000 in 2003 [5]. The prevalence of HIV among TB patients increased from 2.2% in 1991 to 19.1% in 2001 and to 25% in 2010 thus underscoring the fact that the TB scenario in the country is HIV driven [6]. The multiple effects of HIV infection on the natural history of TB include an increase in the risk for reactivation of latent infection and for exogenous infection [7]. The existing link between DM and TB is well recognised with evidence that DM is not only an important risk factor for the acquisition of TB but also that TB might induce glucose intolerance and worsen glycaemic control in people with diabetes [8]. There is a global growing awareness and concern on the possible relationship between TB and DM thus informing the recent collaboration between WHO and the International Union Against Tuberculosis and Lung Disease (Union) to the effect of putting together a document for the care and control of both diseases [9]. In countries with high TB burden like China and India the reported prevalence rates of DM and TB are 16% [10] and 25-44% [11, 12] respectively. In SubSaharan Africa, specifically from Tanzania, the documented prevalence of DM in TB is 16.7% [13].
There is a growing body of evidence to substantiate the claim that some infectious diseases may be potential risk factors for some non communicable diseases[14]. TB and HIV are communicable diseases of public health significance that may be closely associated with the development of DM [14].
The objective of this Report is the determination of the burden of DM and HIV in persons with established diagnoses of pulmonary TB.
Methods
This was a cross sectional Study carried out in Lagos, Nigeria. Lagos State is a cosmopolitan state, located in the south Western region of Nigeria and has a population of about 20 million people.
Participant Recruitment and Data Collection: Patients with confirmed diagnoses of TB and on treatment for TB were recruited consecutively from 54 DOT centres during the Study period, which was from September 2010 to March 2012. Consenting patients with pulmonary TB of age ≥ 12 years and who had commenced anti-TB drugs were enrolled into the study. Patients who were pregnant, those with features of extrapulmonary TB and those who did not give Consent were excluded from the recruitment exercise. A diagnosis of TB was made if the patient presented with clinical symptoms suggestive of TB and either a positive sputum smear on Ziehl Nielsen or radiological indices of TB. Diagnosed patients were registered and treated with antiTB drugs in accordance with the WHO Guidelines [15]. The anti-TB drugs used in the intensive phase being Rifampicin, Isoniazid, Ethambutol and Pyrazinamide and in the maintenance phase being Rifampicin and Isoniazid.
Measurement of fasting plasma glucose concentration was performed in participants using capillary blood with glucose meters that provide plasma equivalent readings. (The Finetest Auto-coding™, Infopia Co., Ltd. Korea). The diagnosis of DM was made based on the 1999 World Health Organization (WHO) guidelines which state that a fasting plasma glucose of ≥ 7 mmol/ is in the diabetic range [16].
In Lagos State all TB patients receiving care at the DOT facilities are routinely screened for HIV. Blood samples were tested for the presence of HIV using Elisa kit (Genescreen HIV -1/2) and all reactive samples were confirmed with a repeat test (Gene 11 Sanofi, Pasteur, Paris).
Ethical approval for this study was obtained from the Lagos State Ministry of Health which directly oversees the DOT centers within the State and informed consent was obtained from all study participants.
Results
A total of 3,376 persons with tuberculosis were screened for HIV and DM. The males were 1932 and females 1444 thus making up 57% and 43% respectively of the Study population.
The Mean age of the Study population was 34.9 (12.97) years. Males were older than females and this difference was statistically significant (35.7(12.7) Vs 33.8(13.1), p = 0.001). The majority of the Study subjects had some form of education as only 120 (3.5%) were non-literates. Of the Study subjects who were literate, the proportion of persons with primary, secondary and tertiary education were 586(18%), 2101(65%) and 571(17%) respectively. Sputum smear positivity was noted in 2809 (83%) of the subjects. HIV infection was found to be present in 118(3.5%) of the Study subjects.
Diabetes mellitus in tuberculosis
Of the 3,376 TB patients screened, 162 (4.8%) were found to have DM. Of these 77 (47.5%) already had a previous diagnosis of DM thus about half of the patients with DM were newly diagnosed cases. A family history of DM was documented in 290 (8.5%) of the Study populace and hypertension in 59 persons making up 1.7% of the Study populace. Well over half-82%- of persons with TB and DM had sputum smear positivity for TB. The median age of persons with DM in TB was 40 years. A comparison between persons with TB and DM and those without DM showed that persons with DM were older and tended to have hypertension as a co-morbidity. These results are shown in Table I.
Tab. I.
Comparison of Sociodemographic parameters between TB patients with DM and TB patients without DM.
| Variable | DM | Non DM | p |
|---|---|---|---|
| Age (years) | 40.7 (12.8) | 34.6 (12.9) | 0.0001 |
| Waist circumference | 74.5 (9.8) | 72.6 (9.1) | 0.017 |
| Gender (M:F) % | 57:43 | 59:41 | 0.7 |
| Hypertension | 12 (7.4%) | 47 (1.5%) | 0.001 |
HIV/DM/TB
The number of TB patients who had HIV coinfection was 118 thus making up 3.5% of the Study group. Patients with TB and HIV were older than those without HIV and this difference was statistically significant. 38.2(10.1) Vs 34.8(13), p = 0.004. Well over half (70%) of persons with TB and DM were sputum smear positive for TB.
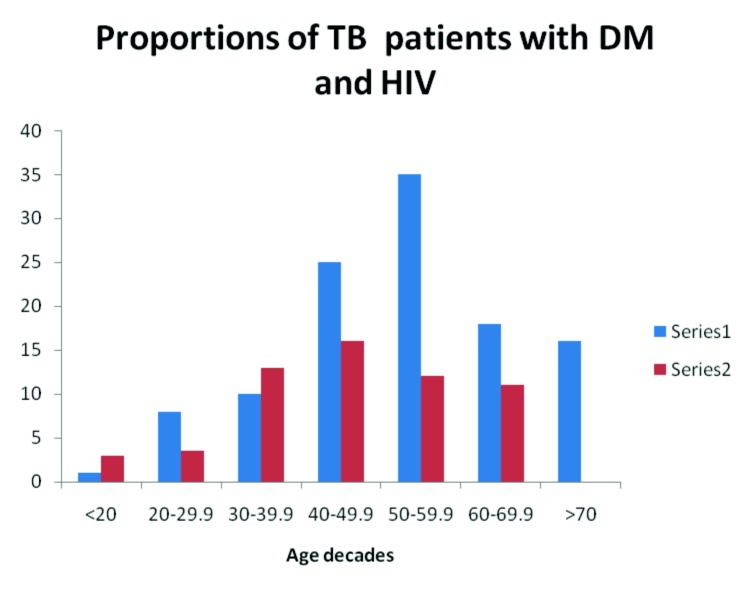
DM and HIV were present more commonly in patients between 40-60 years of age. The distribution of HIV and DM according to age decades is shown in Figure 1.
Fig. 1.
Distribution of the proportions of TB patients with DM and HIV.
Series 1: TB patients with DM, Series 2: TB patients with HIV infection.
The HIV/DM/TB prevalence was 11-(0.3%). Patients with DM and HIV were older than those with HIV and no DM. A comparison of the clinical parameters of persons with TB/HIV/DM versus those with TB/HIV and no DM are shown in Table II.
Tab. II.
Comparison of the clinical parameters of persons with TB/HIV/DM versus those with TB/HIV and no DM.
| Variable | DM/HIV | Non DM/HIV | p |
|---|---|---|---|
| Age (years) | 42.6 (13.2) | 34.9 (12.9) | 0.04 |
| Waist circumference | 80 (12.8) | 72.7 (9.1) | 0.009 |
| Gender (M:F) % | 6:5 | 59:41 | 0.7 |
| Hypertension | 2 (18.2%) | 3 (2.5%) | 0.001 |
| Smear positivity | 9 (72.7%) | (82.6%) | 0.3 |
Discussion
Nigeria like most developing countries is experiencing an epidemiological transition with the burden of Non communicable diseases (NCD) like DM poised to overwhelm the healthcare system that is already overburdened by HIV/AIDS, TB and other communicable diseases such as malaria.
The relation between HIV and TB has been long established and in Africa, the percentage of persons with HIV co-infection has steadily risen in geometric proportions from 4% in 2004 to 68% in 2011 [17]. Sub- Saharan Africa has borne the brunt of the HIV and TB co-epidemic and accounts for 79% of the global burden of HIV infection-associated TB cases in 2007 [18]. We report the frequency of occurrence of HIV in persons with TB to be 3.5% and notably occurring in the fourth and fifth decades of life. This is against a background median prevalence of HIV of 17% which had earlier been documented in Nigerians with TB [19]. The implications of this entwined infections are oft times grave as they may result in multidrug and extreme drug resistance TB, development of other opportunistic infections and ultimately a reduction in both quality and quantity of life.
Diabetes mellitus is a chronic metabolic disease that is of public health significance in developing and developed countries. In sub- Saharan Africa objective documentation of the predisposition for persons with TB to develop DM was done by Mugusi et al [20] who noted that DM tended to occur four times more in persons with TB in comparison to persons without TB. The projected global geometric increase in DM is unfortunately bound to occur more in developing countries of which an estimated 70% are TB endemic countries [21]. In this Report we determined from our screening that 50% of the persons with DM were newly diagnosed. Our data is comparable to that from Indonesia where 61% of persons with DM with TB were noted to be newly diagnosed [22]. The clinical correlates of DM in TB included older age and higher mean waist circumference and this pattern was also documented in persons with DM and HIV compared to those with HIV but no DM. We did not classify our DM patients but given the median age of 40 years of persons with TB who had DM and the clinical correlates we are of the opinion that a large majority of these persons, have type 2DM. Reports from Africa and elsewhere indicate that patients with DM and TB are usually older and have higher body mass indices than TB patients without DM [21]. From the foregoing it is pertinent to note that the documented clinical correlates in our Report are essentially known risk factors for the development of type 2 DM.It is instructive to note that DM was also documented in persons who were less than 20 years of age. Sputum smear positivity in patients with TB and DM was high and same scenario obtained for persons with TB and HIV. The association of DM with particularly for smear positive cases of TB has been reported in Indian populations with TB [11]. The importance of this finding though unclear may be related to delayed sputum conversion which has been reported [22] as a feature of persons with TB and concomitant DM.
Screening for hypertension though was not a stated objective of this Report, we undertook to screen for hypertension. The reason for this is because hypertension is a co-morbidity that is oft reported in Nigerians with DM – a recent Nigerian Report found that as many as half of persons with DM also have hypertension [23]. The prevalence of hypertension in our Study populace is 1.7% but that in the subjects with DM was 7.4%.
Although the combination of DM and HIV in persons with TB was less than one percent, the implication of these comorbidities may translate into increased morbidity and mortality. In resource poor settings like Nigeria with high burden of TB, opportunistic screening for DM should be a priority. We have shown that the combination of DM and TB is comparable to that of HIV and DM but unfortunately DM often goes undiagnosed in persons with TB.
The impact of DM in TB has been found to portend poor treatment outcome which could be in the form of drug resistance, relapse of TB and death. The presence of TB in persons with DM may lead to poor glycaemic control. Nigeria is facing a dual burden of communicable and NCDs. The magnitude of DM in TB and HIV and DM in TB is not yet known.
Conclusion: The dual burden of DM and HIV in persons with TB is of moderate magnitude. Given that the DM/ TB combination is comparable to that of HIV/TB, we recommend that opportunistic screening for DM be offered to persons with TB.
Acknowledgement
I wish to acknowledge the Staff of Structured Healthcare Initiatives and Staff of the DOT centres in which these TB patients were recruited for the Study. I also acknowledge the World Diabetes Foundation who sponsored this work.
References
- 1.Zimmet P, Shaw J, Alberti KG. Preventing Type 2 Diabetes and the Dysmorphic syndrome in the real world: a realistic view. Diabet Med. 2003;20:693–702. doi: 10.1046/j.1464-5491.2003.01052.x. [DOI] [PubMed] [Google Scholar]
- 2.Akinkugbe OO, Akinyanju OO. Final Report-national Survey on non-communicable diseases in Nigeria. Lagos: Federal Ministry of Health; 1997. pp. 65–68. [Google Scholar]
- 3. www.unaids.org. HIV and AIDs Estimates, Nigeria.
- 4.Global Tuberculosis Report, 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 5. WHO , author. Global tuberculosis control: surveillance, planning, financing. WHO report 2005. Geneva: World Health Organisation; 2005. [Google Scholar]
- 6. Nigeria Tuberculosis Fact Sheet. Photos.state.gov.libraries/Nigeria/487468/pdfs/JanuaryTuberculosisFactSheets.pdf.
- 7.DeReimer K. Quantitative impact of human immunodeficiency virus infection on tuberculosis dynamics. Am J Respir Crit Care Med. 2007;176:936–936. doi: 10.1164/rccm.200603-440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stop TB Initiative (World Health Organization); World Health Organization, Department of Chronic Diseases and Health Promotion; International Union against Tuberculosis and Lung Disease , author. Collaborative framework for care and control of tuberculosis and diabetes. Geneva: World Health Organization; 2011. [Google Scholar]
- 10.Li L, Lin Y, Mi F, et al. Screening of patients with tuberculosis for diabetes mellitus in China. Trop Med Int Health. 2012;17:1294–1301. doi: 10.1111/j.1365-3156.2012.03068.x. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan V, Kumpatla S, Aravindalochanan V, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS One. 2012;7:e41367–e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balakrishnan S, Vijayan S, Nair S, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS One. 2012;7:e46502–e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Tuberculosis Report, 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 14.Young F, Critchley JA, Johnstone LK, et al. A review of co-morbidity between infectious and chronic disease in Sub Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Global Health. 2009;5:9–9. doi: 10.1186/1744-8603-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization , author. Treatment of tuberculosis: guidelines for national program. Geneva: World Health Organization; 2006. [Google Scholar]
- 16. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus , author. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization , author. Tuberculosis and HIV data and statistics. www.who.int/hiv/topics/tb/data/en/index1.html.
- 18.Bekkee L-G, Wood R. The changing natural history of Tuberculosis and HIV coinfection in an urban area of Hyperendemicity. Clin Infect Dis. 2010;50:S208–S214. doi: 10.1086/651493. [DOI] [PubMed] [Google Scholar]
- 19.Odaibo GN, Gboun MF, Ekanem EE, et al. HIV infection among patients with pulmonary tuberculosis in Nigeria. Afr Med J Sci. 2006;35:S93–S98. [PubMed] [Google Scholar]
- 20.Mugusi F, Swai AB, Aberti KG, et al. Increased prevalence of diabetes mellitus in patients with pulmonary tuberculosis in Tanzania. Tubercle. 1990;71:271–276. doi: 10.1016/0041-3879(90)90040-f. [DOI] [PubMed] [Google Scholar]
- 21.Ruslami R, Aarnoutse RE, Alisjahbana B, et al. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289–1299. doi: 10.1111/j.1365-3156.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez-Corona ME, Cruz-Hervert LP, Garcia-Garcia L, et al. Association of diabetes and tuberculosis; impact on treatment and post-treatment outcomes. Thorax. doi: 10.1136/thoraxjnl-2012-201756. doi:10.1136/thoraxjnl- 2012-201756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unadike BC, Eregie A, Ohwovoriole AE. Prevalence of hypertension amongst patients with diabetes mellitus in Benin City, Nigeria. Niger J Clin Pract. 2011;14:300–302. doi: 10.4103/1119-3077.86772. [DOI] [PubMed] [Google Scholar]