Summary
Herpes Zoster (HZ) and its main complication, post-herpetic neuralgia (PHN), represent an important public health issue because of their relevant burden within older adult population and the actual suboptimal therapeutic management of the diseases.
Incidences of HZ and PHN are comparable all over the world and are closely related with the population age. Epidemiological data collected in Italy about HZ and its complications confirmed the trend registered in North America and Europe. Moreover HZ related burden is exacerbated by a significant economic impact related to both direct and indirect costs.
Since 2006 a live, attenuated varicella zoster virus vaccine, that contains VZV Oka strain [Zostavax, Merck & Co., Inc.], was licensed for the prevention of HZ and PHN in adults aged ≥ 60 years. Since 2011, the licensure has been extended to adults between 50 and 59 years. The vaccine has demonstrated a good immunogenicity, efficacy and safety profiles in two pivotal phase III clinical trials and the effectiveness was further confirmed after vaccine licensure. Pharmaco-economic studies concluded that HZ vaccine is cost-effective in most European countries and generally supported the economic value of this vaccination.
The vaccine is actually recommended in USA, Canada and several European countries. The opportunity to reduce the burden of these diseases by the recommendation of HZ vaccination have been evaluated and suggested also in our Country and some Regions have been recently introduced the vaccine in their immunization plan. If the good results, already obtained with HZ vaccine in other countries, will be confirmed by these Italian pilot experiences, vaccination programs should be made uniform in all Country in order to ensure an equitable offer of this important preventive tool.
Key words: Herpes Zoster, Vaccine, Italy
Introduction
Herpes zoster is an acute disease, presenting with dermatologic manifestations and neurological pain, caused by the reactivation of the varicella zoster virus (VZV). During primary infection, VZV infects skin nervous endings and remains latent in the sensory ganglia of the spinal dorsal root cord and cranial nerves. Age and immune system depression can favor virus reactivation and leads to the peculiar vesicular rash with unilateral and dermatomeric localization, known as HZ [1].
The most common complication of HZ is constituted by the PHN, a painful syndrome which interests the course of the nerve up to cutaneous dermatome corresponding to the viral site of infection and reactivation [2-4].
PHN commonly occurs with one or more accesses or with paroxysmal pain, burning, allodynia and hyperalgesia and current trends define PHN as a chronic neuropathic resilient pain HZ-related that persists or develops after at least 90 days after wound healing skin and can continue for months or years [5]. As demonstrated in several epidemiological studies, incidence and severity of PHN increase with age; furthermore acute pain and rash severity were recognized as important risk factors for PHN [6-9].
Therapeutic treatments currently available for HZ and PHN are not able to ensure a satisfactory management of the diseases [5]. This therapeutic gap, together with the relevant burden of the diseases, leave unmet medical needs that could be satisfied by vaccination programs.
Epidemiology
Incidence of HZ and PHN. Incidence of HZ is comparable all over the world and is closely related with the population age. A recent systematic review, summarizing incidence rates of HZ reported in 49 studies performed in North America, Europe and Asia, showed that, in these three continents, overall HZ incidence rate ranged between 3 and 5 cases per 1,000 person/years [10]. In these countries the occurrence of HZ is age-dependent and the incidence by age-group shows a similar pattern, with rates of 6-8 cases per 1,000 person/year in the sixth decade and 8-12 per 1,000 person/year in the eighth decade [10].
Similarly, the incidence rate of HZ in Europe is estimated with a frequency of 2-3/1,000 person/year in the adults aged between 20 and 50 years, 5/1,000 in the sixth decade, 6-7/1,000 from the seventh to the eighth one, and increases up to > 1/100 in 90-year-old people [11].
An Italian study, dealing with the immune-competent adult population older than 50 years, with an observation period of two years (from 2003 to 2005), reported an incidence rate of 6.3 per 1,000 person/year, estimating that, in a population of approximately 24.2 million people aged over 50, at least 153,000 new cases of HZ occur yearly [12]. This Italian research further confirmed that HZ incidence increases with age, therefore older patients have a greater risk of developing HZ [12].
Furthermore these studies clearly demonstrated that incidence of PHN rises with age. In the afore-mentioned systematic review, the risk of developing PHN is estimated between 5% to more than 30% in the adults and in patients with HZ who are 50 or older, the risk of PHN increased to 25-50% [10]. In Europe, adult patients with HZ developed PHN lasting at least 1 month in the 6.5- 38% of cases and PHN lasting at least 3 months in the 2.6 to 27% of cases; [13] in Italy it was observed that at least 8% of the adult population with HZ presented a PHN lasting at least 1 month and the 6.2% experienced a PHN lasting at least 3 months. [12] Moreover it was shown that the most important risk factors for the development of PHN are determined by increasing age, female gender and decline of the immune system. [12]
Hospitalization rates for HZ and PHN in adult patients. The rates of HZ-related hospitalization, in the 49 countries included in the global systematic review, ranged widely from 2 to 25 per 100,000 person/years in studies examining all age groups. Hospitalization with a primary diagnosis of HZ were about 29-42% of the total HZrelated hospitalizations and these rates increase steeply in adults aged 50 or older [10].
In particular, in the US, HZ-associated hospitalization rates (confirmed with medical records) ranged from 10 per 100,000 in adults aged 60-69 to 65 per 100,000 in adults aged ≥ 80. Similarly, the rate of hospitalization with primary diagnosis of HZ ranged from 13 per 100,000 in adults aged 60-64 to 96 per 100,000 in adults aged ≥ 80 in Australia [10]. In Germany, the rates ranged from 31 per 100,000 in adults aged 60-64 to 100 per 100,000 in adults aged ≥ 80 [10].
Gialloreti et al. analyzed the Italian hospital discharge records related to primary diagnosis of HZ disease and reported an annual hospitalization rate equal to 10.34 per 100,000 person/years within immunocompetent patients older than 50 years. This figure raised to 20.31 per 100,000 when both the primary and secondary diagnosis are considered [12].
Costs related with HZ e PHN. In Italy, the annual costs related to the HZ and PHN disease accounted to 41.2 million euros, of which 28.2 million related to direct costs (21.5 million for treatment of acute HZ) and 13 million associated to indirect costs (12.2 in lost productivity related to acute episode of HZ) [12]. These figures corresponded to a direct cost of 166 € for each patient with a HZ episode and 560 € in patients with a PHN episode, furthermore the indirect costs were estimated as € 556 in patients with a HZ episode and € 795 in patients with PHN [12].
In the hospitalized patients the costs, evaluated for a single episode, were approximately € 2,592 ± 1,313 for acute HZ and € 5,400 ± 2,641 for PHN [12]. Similarly, in another study performed in the Piedmont, one of the largest Italian region, the costs related to hospitalization for a single case of HZ were estimated to amount to € 4,082.59 [14].
Herpes Zoster vaccine
Efficacy. Since 2006 a live, attenuated varicella zoster virus vaccine, that contains VZV Oka strain [Zostavax, Merck & Co., Inc.], was licensed for the prevention of HZ and PHN in adults aged ≥ 60 years and in 2008 the Advisory Committee on Immunization Practices (ACIP) recommended its use for the prevention of HZ and its complications in individuals aged ≥ 60 [15]. Since 2011, the vaccine was authorized also for administration in the adults between 50 and 59 by Food and Drug Administration (FDA) [16].
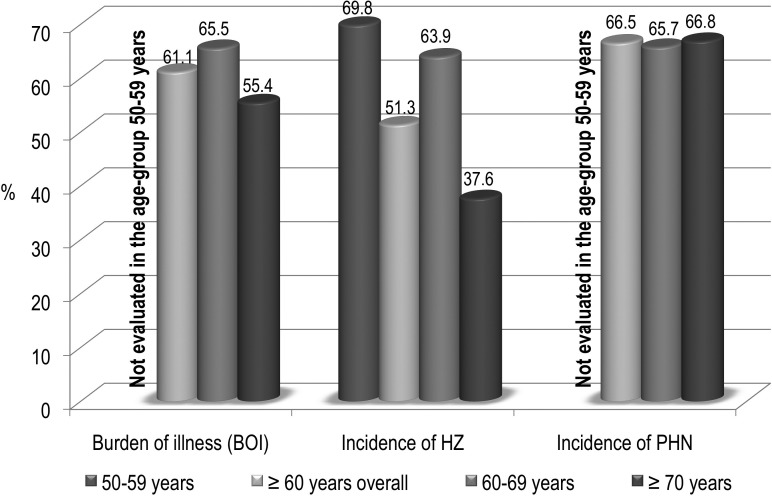
Short-term efficacy of zoster vaccine in adults aged ≥ 50 have been demonstrated in two pivotal clinical trials, including 38,546 subjects aged more than 60 years and 22,439 subjects aged 50-59 years, with respect to three major outcomes: burden of illness determined by HZ, incidence of HZ and incidence of PHN [17, 18].
Figure 1 summarized data about HZ vaccine efficacy among adults aged 50 through 59 and ≥ 60 years. Furthermore, both clinical trials and post-marketing studies demonstrated the optimal safety and tolerability profile of this vaccine [19].
Fig. 1.
Efficacy of Herpes Zoster vaccine registered in two phase III clinical trials [17, 18].
Duration of protection. Duration of protection in adults aged ≥ 60 has been studied in two consecutive researches. A persistent vaccine efficacy for HZ and PHN has been indicated in a short-term persistence substudy (STPS), performed within 14,000 subjects. In particular analysis of vaccine efficacy in each year after vaccination for the HZ burden of illness, the incidence of HZ and the incidence of PHN showed a decrease in vaccine efficacy after one year since administration of HZ vaccine, with a further decline thereafter. However, vaccine efficacy remained statistically significant for the incidence of HZ and the HZ burden of illness till five year after HZ vaccine administration [20].
Subsequently, a subgroup of 6,000 subjects were enrolled in a long-persistence study to evaluated the vaccine efficacy up to 11 years after vaccine administration. The results, estimated by a model, revealed that vaccine efficacy decreased over time in the study population compared to modelled control estimates: statistically significant vaccine efficacy for HZ Burden of Illness persisted up to 10 years after vaccination, whereas statistically significant vaccine efficacy for incidence of HZ persisted up to 8 years after vaccination [21].
A recent study, investigating the effect of chronological age on the level of protection provided by HZ vaccine over time with respect to HZ incidence, demonstrated that much of the reduction in vaccine efficacy over time since vaccination can be explained by increasing age, responsible for decline of immune response [22].
Effectiveness. After the licensure, the on-field effectiveness of HZ vaccine was confirmed by two large studies. A retrospective cohort study, performed among 75,761 vaccinated subjects cohort matched to 227,283 unvaccinated subjects, demonstrated that vaccination was associated with a reduced risk of HZ (hazard ratio = 0.45; 95% CI, 0.42-0.48); this reduction occurred in all age strata and among individuals with chronic diseases [23]. A larger cohort study, performed among more than 700,000 subjects aged > 65 in the period lasting from 2007 to 2009, confirmed these results, demonstrating a vaccine effectiveness, adjusted for age, gender, race, immunosuppression, low income, and comorbidity, of 0.48 (95% CI 0.39-0.56) [24]. This means that an overall vaccine effectiveness (VE) of 48% was demonstrated where VE was calculated as (1 – the adjusted hazard ratio).
Cost-effectiveness. A recent systematic review identified and analyzed 15 cost-effectiveness studies of vaccination against HZ and PHN performed in North America and Europe [25]. Most studies conducted in Europe and Canada concluded that HZ vaccine is likely to be costeffective and generally supported the economic value of this vaccination. Divergences in results among studies were largely attributable by authors to differing assumptions regarding duration of vaccine protection and a loss in quality of life associated with HZ and to a larger extent, PHN. Moreover, vaccine efficacy against PHN, age at vaccination, and vaccine cost strongly influenced the results [25].
A pharmaco-economic evaluation performed in Italy confirmed that vaccination program against HZ and PHN within subjects aged 60-79 years is cost-effective from both societal and third-payer standpoints in the Italian scenario [19].
Conclusions
HZ and its main complication, PHN, represent an important public health issue because of their relevant burden within older adult population and the actual suboptimal therapeutic management of the diseases.
The licensure since 2006 of a live attenuated HZ vaccine in adults aged more than 60 years, extended since 2011 in adults aged 50-59 years, had stimulated the interest by the public health to evaluate the introduction of HZ vaccination in these categories in order to reduce the healthcare and economic burden associated with HZ.
The vaccine is actually recommended in USA and Canada in patients ≥ 60 years since 2006 and 2010, respectively. In Europe, vaccination is recommended in several countries (i.e. Germany, United Kingdom, Sweden, Austria, France) according to age-based strategies [19].
In Italy, available epidemiological and economic data about HZ and its complications are superimposable with similar data collected in North America and Europe. For these reasons, the opportunity to reduce the burden of these diseases by the recommendation of HZ vaccination have been evaluated and suggested also in our Country [19].
During 2014, some Italian regions, such as Liguria and Puglia, established to introduce HZ vaccination in the regional immunization plan by the active and free offer of the vaccine to specific age-group.
The administration of HZ vaccine within public health strategy in these Regions offers the opportunity to assess on-field its safety and tolerability profile and, importantly, to evaluate the impact of vaccination on healthcare services.
If the good results, already obtained with HZ vaccine in other countries, will be confirmed by these Italian pilot experiences, vaccination programs should be made uniform in all Country in order to ensure an equitable offer of this important preventive tool.
References
- 1.Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013;369:255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274–280. doi: 10.4065/84.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines. 2010;9:21–26. doi: 10.1586/erv.10.30. [DOI] [PubMed] [Google Scholar]
- 4.Watson P. Postherpetic neuralgia. Am Fam Physician. 2011;84:690–692. [PubMed] [Google Scholar]
- 5.Schmader K, Gnann JW, Jr, Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. J Infect Dis. 2008;197:S207–S215. doi: 10.1086/522152. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RW, Bouhassira D, Kassianos G, et al. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37–37. doi: 10.1186/1741-7015-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershon A. Combating Varicella Zoster Virus-related Diseases. Recommendations from the IHMF® Management Strategies Workshop held on 30 April-1 May 2005 and ratified at the 12th Annual Meeting of the IHMF®, Lisbon, Portugal, 28-30 October 2005.
- 8.Whitley RJ, Shukla S, Crooks RJ. The identification of risk factors associated with persistent pain following Herpes Zoster. J Infect Dis. 1998;178:S71–S75. doi: 10.1086/514274. [DOI] [PubMed] [Google Scholar]
- 9.Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545–1551. doi: 10.1212/01.wnl.0000123261.00004.29. [DOI] [PubMed] [Google Scholar]
- 10.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833–e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinchinat S, Cebrián-Cuenca AM, Bricout H, et al. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis. 2013;13:170–170. doi: 10.1186/1471-2334-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gialloreti LE, Merito M, Pezzotti P, et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230–230. doi: 10.1186/1471-2334-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opstelten W, Mauritz JW, Wit NJ, et al. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract. 2002;19:471–475. doi: 10.1093/fampra/19.5.471. [DOI] [PubMed] [Google Scholar]
- 14.Legami V, Gianino MM, Ciofi degli Atti M, et al. Epidemiology and costs of herpes zoster: background data to estimate the impact of vaccination. Vaccine. 2007;25:7598–7604. doi: 10.1016/j.vaccine.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention (CDC) , author. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729–731. [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention (CDC) , author. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528–1528. [PubMed] [Google Scholar]
- 17.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 18.Schmader KE, Levin MJ, Gnann JW, Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012;54:922–928. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabutti G, Franco E, Bonanni P, et al. Reducing the burden of Herpes Zoster in Italy. Hum Vaccin Immunother. 2015;11:101–107. doi: 10.4161/hv.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55:1320–1328. doi: 10.1093/cid/cis638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison VA, Johnson GR, Schmader KE, et al. Long- Term Persistence of Zoster Vaccine Efficacy. Clin Infect Dis. 2015;60:900–909. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Zhang JH, Betts RF, et al. Modeling the durability of ZOSTAVAX( ®) vaccine efficacy in people ≥ 60 years of age. Vaccine. 2015;33:1499–1505. doi: 10.1016/j.vaccine.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 23.Tseng HF, Smith N, Harpaz R, et al. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–166. doi: 10.1001/jama.2010.1983. [DOI] [PubMed] [Google Scholar]
- 24.Langan SM, Smeeth L, Margolis DJ, et al. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420–e1001420. doi: 10.1371/journal.pmed.1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai K, Preaud E, Baron-Papillon F, et al. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine. 2014;32:1645–1653. doi: 10.1016/j.vaccine.2014.01.058. [DOI] [PubMed] [Google Scholar]