Summary
Background.
Rotavirus (RV) infection is the first cause of acute viral gastroenteritis in children under five years of age all over the world; it mainly affects children between six and 24 months of age and can cause serious acute diarrhoea and dehydration. The aim of this study is to perform the budget impact analysis of universal rotavirus vaccination in the Local Health Unit (LHU) 11 Empoli, Tuscany, Italy.
Methods.
An ad hoc mathematical simulation model was developed to evaluate the budget impact analysis of 5-years universal rotavirus vaccination. Particularly, incidence of rotavirus gastroenteritis (RVGE), hospitalizations, nosocomial diarrhoea, medical consultations, prescriptions and accesses to emergency department were taken into account in the analysis. The direct medical costs due to RV diarrhoea and the costs of vaccination campaign were considered as the main outcome measures in the study.
Results.
The adoption of universal rotavirus vaccination campaign for five years in the LHU 11 Empoli would result in relevant savings due to the health cares avoided. These savings would overlapped the costs of vaccination yet from the second year after the introduction of vaccination. The saving for the Health Service would be 1.5 million Euro after five years of campaign.
Conclusions.
Universal vaccination against rotavirus results clinically and economically favourable for both the Health Service and the Society perspectives.
Key words: Rotavirus, Vaccination, Budget impact
Introduction
Rotavirus (RV) infection is the first cause of acute viral gastroenteritis in children under five years of age all over the world; it mainly affects children between six and 24 months of age and can cause serious acute diarrhoea, resulting in dehydration (which can lead to death if rehydration therapy is not adequately administered) [1-9]. Most of the children infected by RV are under three years of age and many children get sick more than once.
Every year, in the developing countries, rotavirus acute viral gastroenteritis (RVGE) causes the death of about 444,000 children; while in USA these diseases are responsible for a number of hospitalization between 58,000 and 70,000 [10]. In Europe, each year, among the population under five years of age (about 23.6 millions) there are approximately 3.6 millions cases of RVGE, 231 deaths, at least 87,000 hospitalizations and 700,000 medical examinations related to this disease [2].
In industrialized countries, deaths caused by RV are rare because of easy and rapid access to primary cares, but the burden of this disease is very relevant, due to the high frequency of the infection [2].
Several studies, performed in different countries, show the impact of RVGE on primary cares and on hospitalizations, also in the economic perspective; these studies often demonstrate discordant results, probably due to the different study designs applied (methods, populations and aim) [11-21].
In Italy, RV disease has a remarkable clinical impact with repercussions on National Health System (medical examinations, accesses to emergency department, hospitalizations) and on families (absences from work, costs of drugs, dietetic products, diapers, etc.) [22].
As a matter of fact, RV is the main responsible for the hospitalizations due to diarrhoea (about 10,000 hospitalizations per year) in children under five years of age (about 1% in a birth cohort), with an average duration of hospitalization amounting to five days during the last ten years [23-25]. Infection can also be contracted in hospital, causing an increase of the average duration of hospitalization of five days [23, 24].
In Italy, RVGE must be notified in the Second Group of infection diseases (according to Ministerial Decree 15/12/1990) as "infectious diarrhoea, not caused by Salmonella spp.", but official surveillance data on incidence of this disease are not available. However, there is a national surveillance system addressed to the characterization of circulating RV strains; this system is part of the European surveillance system [26].
RVGE is an infection preventable by vaccination. Nowadays, two different vaccines against RV are available in Europe: Rotarix (GSK) and Rotateq (Sanofi Pasteur); they must be orally administered, respectively in two and three doses, in children sixth weeks old.
To evaluate the allocative efficiency of this vaccination, considering the limited resources of Health System, and to support the decision makers in management decisions, an economic evaluation on the introduction of universal vaccination against RV for all the newborns in the LHU 11 Empoli, in Tuscany (Italy), for a period of five years, was carried out through a budget impact analysis.
Materials and methods
An had hoc mathematical simulation model, in Excel (Microsoft, Redmont, USA), was developed in order to perform the budget impact analysis of the introduction of RV vaccination in the LHU 11 Empoli.
The clinical and economic impact related to the implementation of universal RV vaccination in a five-years period was compared with a no-vaccination scenario.
In the mathematical simulation, a vaccine coverage of 90%, constant for five years, was supposed.
Considering the short horizon time of analysis, no discount rates were applied to costs and benefits. The economic evaluation considered the birth cohorts of the LHU 11 Empoli, that includes about 2,300 newborns per year.
After the literature review, the following parameters were included in the study: incidence of RVGE, hospitalizations, accesses to emergency department, and medical consultations. Particularly, an analysis of the National Health System's direct medical costs associated to the burden of RV disease was carried out. To achieve the budget analysis, not-medical direct costs (transport to the hospital, diapers consumptions, rehydration solutions, drugs, special foods, etc.), and indirect ones (working days lost by the parents due to their sick children) were not considered, because they were both Societal expenses.
The parameters used in the mathematical simulation model are shown in Table I. Particularly, percentages and costs related to RVGE cases, hospitalizations, nosocomial diarrhoea cases, medical consultations, prescriptions and accesses to emergency department, were obtained from two Italian studies concerning economic evaluations [23, 27].
Tab. I.
Parameters used in the mathematical simulation model to perform the budget impact analysis.
| Incidence % |
Average costs per case (Euro) | |
|---|---|---|
| RVGE cases | 45.45% | - |
| Hospitalizations due to RV diarrhoea | 1.82 % | 1,463 |
| Nosocomial diarrhoea cases | 0.91% | 2,000 |
| Medical consultations | 22.73% | 23.80 |
| Accesses to emergency department | 7.70% | 352.72 |
| Prescriptions | 9.98 |
VACCINE DATA
Data on the efficacy of the RV vaccine used in the mathematical simulation model were obtained from a clinical study performed in several European countries, including Italy [28].
The cost of the RV vaccine in the LHU 11 Empoli, currently of 36.50 Euro for each dose, plus 10% for taxes (price in 2013), was used to develop the economic analysis. No other costs (for example the cost for the further organization of the vaccine centre) was added because the vaccine, already available in co-payment, would have been administered at the same time of the first two vaccination sessions, according to the Tuscan vaccination schedule [29].
SENSITIVITY ANALYSIS
To evaluate the impact of the uncertainty related to the input data on the results, a sensitivity analysis was carried out, applying a variation of ± 20% on the cost of the RV vaccine and on the percentages of hospitalizations, nosocomial diarrhoea cases, medical consultations, and accesses to emergency department. In addition, a variation on the vaccine coverage (from 90% to 80% and 70%) was also applied [23].
Results
Data obtained from the simulation carried out using the mathematical model show that the universal vaccination against RV, in five years in the LHU11 Empoli, would cause a relevant reduction (47%) of the RVGE cases: 26,134 cases of RVGE in no-vaccine scenario and 13,762 cases in vaccine scenario. In Table II, the cases of RV diarrhoea prevented with the introduction of the RV vaccination are shown.
Tab. II.
Clinical impact of RV vaccination in LHU 11 Empoli: RVGE cases prevented with the introduction of vaccination, divided by age.
| RVGE cases prevented with the vaccination | Children age | |||||
|---|---|---|---|---|---|---|
| 0 years | 1 year |
2 years |
3 years |
4 years |
Total | |
| 1st year of vaccination | 825 | 0 | 0 | 0 | 0 | 825 |
| 2nd year of vaccination | 825 | 825 | 0 | 0 | 0 | 1,650 |
| 3rd year of vaccination | 825 | 825 | 825 | 0 | 0 | 2,474 |
| 4th year of vaccination | 825 | 825 | 825 | 825 | 0 | 3,299 |
| 5th year of vaccination | 825 | 825 | 825 | 825 | 825 | 4,124 |
| Total | 4,124 | 3,299 | 2,474 | 1,650 | 825 | 12,372 |
Costs for hospitalizations, nosocomial diarrhoea cases, medical consultations, prescriptions and accesses to emergency department prevented with the RV vaccination are shown in Table III. The LHU 11 Empoli could save about 882,000 Euro for hospitalizations, 495,000 Euro for nosocomial diarrhoea cases, 156,000 Euro for medical consultations, 66,000 Euro for prescriptions and 785,000 Euro for the accesses to emergency department in five-years vaccination period.
Tab. III.
Costs for hospitalizations, nosocomial diarrhea cases, medical consultations, prescriptions, accesses to emergency department caused by RVGE and prevented with the vaccination (Euro).
| Hospitalizations for RV diarrhoea | Nosocomial diarrhoea cases | Medical consultations | Prescriptions | Accesses to emergency department | Total | |
|---|---|---|---|---|---|---|
| 1st year of vaccination | 58,791.53 | 33,027.54 | 10,426.73 | 4,372.22 | 52,347.10 | 158,965.13 |
| 2nd year of vaccination | 117,583.07 | 66,055.08 | 20,853.47 | 8,744.44 | 104,694.21 | 317,930.26 |
| 3rd year of vaccination | 176,374.60 | 99,082.62 | 31,280.20 | 13,116.65 | 157,041.31 | 476,895.39 |
| 4th year of vaccination | 235,166.13 | 132,110.16 | 41,706.93 | 17,488.87 | 209,388.42 | 635,860.51 |
| 5th year of vaccination | 293,957.66 | 165,137.70 | 52,133.66 | 21,861.09 | 261,735.52 | 794,825.64 |
| Totale | 881,872.99 | 495,413.10 | 156,400.99 | 65,583.27 | 785,206.57 | 2,384,476.93 |
VACCINATION COSTS
Vaccination against RV for a birth cohort (2,300 newborns per year) in the LHU 11 Empoli would amount 166,000 Euro per year, with a total cost of 831,000 Euro after five years from the introduction of the vaccine in the schedule.
BUDGET IMPACT ANALYSIS
According to the mathematical method, the annual cost related to the RV disease for the LHU 11 Empoli without the adoption of an immunization program would be about 916,000 Euro (Tab. IV).
Tab. IV.
RV disease costs with and without RV vaccination and budget difference (Euro).
| Budget difference (Euro) | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| RV disease costs without vaccination | 916,139 | 916,139 | 916,139 | 916,139 | 916,139 |
| Costs for remaining cases of RV disease with vaccination | 757,174 | 598,208 | 439,243 | 280,278 | 121,313 |
| Vaccination cost | 166,221 | 166,221 | 166,221 | 166,221 | 166,221 |
| Budget difference | -7,256 | 151,709 | 310,674 | 469,640 | 628,605 |
| Total budget difference in 5 years-period | 1,553,372 | ||||
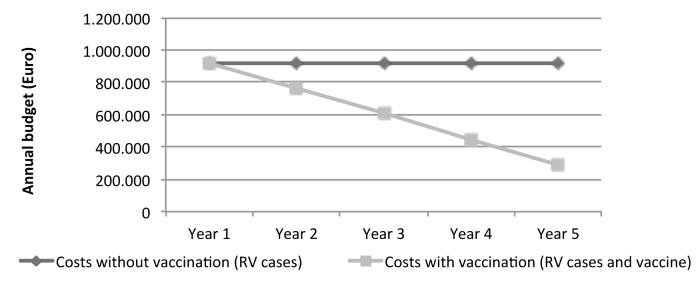
During the first year of RV vaccination, the costs for the Health Unit to take care of the remaining cases of RV disease, added to the costs related to vaccination, would exceed the costs related to the disease burden; from the second year, savings are registered and increase in the following years as shown in Figure 1. The total savings for the LHU 11 Empoli would be of 1,553,372 Euro at the end of the five years.
Fig. 1.
Costs of RV disease without RV vaccination compared with disease costs after the introduction of RV vaccination (as sum of RV disease costs and vaccination campaign costs).
As shown in Figure 1, the savings due to the adoption of the RV vaccination would be registered after the second year of immunization program.
Also changing the input data in the mathematical method, the adoption of the universal RV vaccination program for the newborns in the LHU 11 Empoli is still economically favourable for the payer (LHU) (Tab. V).
Tab. V.
Total costs and saving in the five-years period: sensitivity analysis.
| RV disease costs without vaccination (Euro) | Costs due to remaining cases of RV disease with vaccination (Euro) | Budget difference (Euro) | |
|---|---|---|---|
| Model | 4,580,693 | 3,027,322 | 1,553,372 |
| Vaccination coverage 80% | 4,580,693 | 2,934,977 | 1,645,717 |
| Vaccination coverage 70% | 4,580,693 | 2,842,632 | 1,738,062 |
| % Hospitalizations: -20% | 4,311,501 | 2,913,184 | 1,398,317 |
| % Hospitalizations: +20% | 4,816,236 | 3,127,192 | 1,689,045 |
| % Nosocomial diarrhoea: -20% | 4,339,193 | 2,900,148 | 1,439,046 |
| % Nosocomial diarrhoea: +20% | 4,799,193 | 3,142,384 | 1,656,810 |
| % Medical consultations: -20% | 4,492,705 | 2,983,574 | 1,509,131 |
| % Medical consultations: +20% | 4,651,978 | 3,062,764 | 1,589,214 |
| % Accesses to emergency department: -20% | 4,276,472 | 2,876,063 | 1,400,410 |
| % Accesses to emergency department: +20% | 4,824,070 | 3,148,328 | 1,675,742 |
| Vaccine cost: -20% | 4,580,693 | 2,860,687 | 1,720,007 |
| Vaccine cost: +20% | 4,580,693 | 3,159,802 | 1,420,892 |
Discussion and conclusions
Nowadays, also in Italy, infections caused by Rotavirus in children from zero to five years of age results in an high clinical and economic impact, in spite of the availability of effective vaccines.
The results of the analysis carried out show that the adoption of an universal vaccination programme against RV, in five years in the LHU 11 Empoli, Tuscany (Italy), would determine considerable savings related to the treatments and therapies avoided with the implementation of immunization campaign. These savings would exceed costs for vaccination since the second year of the vaccine programme. Therefore, also in more limited time horizon of analysis, for example 3 years, typical of budget impact evaluations, our results continued to be favourable with saving amounting to 455,128 Euro.30 As a matter of fact, the RV vaccination cost represents the 0.5% of the annul pharmaceutic budget in the LHU 11 Empoli, without considering the obtained saving.
The results of the study would be more favourable, from the point of view of the Society, considering also notmedical direct costs and indirect costs avoided with the introduction of immunization.
Data obtained agree with other studies carried out in European countries, for example in France, but disagree with analysis performed in England and in the Netherlands, where vaccination against RV is not cost-effective in the current situation, and with the studies concerning United States, where RV vaccination would be cost-effective but it would not determine savings for the payer [10, 21, 30-32]. In spite of this, the RV universal vaccination for newborns was recently implemented in England.
In Italy, two studies demonstrated that the introduction of RV vaccination was favourable, particularly in terms of cost-effectiveness, at national and regional level [23, 27].
In addition, our results are consistent with data obtained in two other studies on RV vaccination conducted in the Province of Genoa (Northern Italy), and should be considered conservative if the RV hospitalisation code is attributed only to 65% of RV-positive cases and, consequently, hospitalisation related to RV is underestimated, as showed in that analyses [33, 34].
In this study, a vaccination coverage of 90% was supposed in order to evaluate the greatest impact of the RV vaccination. This assumption implies the underlying rationale that the co-administration of RV vaccine with hexavalent vaccine would determine the rapid achievement of high level of vaccination coverage.
However, the sensitivity analysis demonstrates that, also reducing the vaccination coverage to 70%, as in other studies reported in literature, the favourable economic issue of rotavirus vaccination still stand [31, 32].
In this study, the effect of herd immunity was not taken into account: according to some authors, it would increase the overall effectiveness of RV vaccination [32, 34, 37].
The study has some limitations: epidemiological data and costs were collected from a recent study and they were not directly calculated in our area, due to a gap of specific data [27].
However, data used in the mathematical simulation were consistent with local data obtained from health archives, considering that the underestimation about RV diarrhoea cases and hospitalizations is about 40% [24], due to the high number of cases of gastroenteritis with not-defined aetiology, related to the lack of sensitivity of the hospital discharge data system[20, 31, 32, 35, 38].
Indeed, the examination of the hospital discharge data of the period between 2004 and 2013 [36] 2013 [39] in the LHU 11 Empoli, shows that 337 children between zero and five years of age were hospitalized because of RV disease or they extended the period of hospitalization due to RV. They determine a total cost exceeding 500,000 Euro, assuming real costs of hospitalization equal to Diagnosis- Related Groups (DRG) [37, 40] charges (even if it was demonstrated that these costs often exceeded the applied charges, especially in paediatric wards) [35, 38]. These hospitalizations concerned only diarrhoea cases in which a clear RV aetiology could be proved and these data are consistent with those reported in the study [1-3]. In this analysis, the costs for National Health System and costs for the families, that might result from possible adverse drug reactions to vaccination, were not taken into account, because of the rarity of these events. In addition, costs for possible paediatric consultations at home were not considered in the mathematical simulation.
The budget impact analysis carried out demonstrates that the adoption of RV universal vaccination for the newborns cohorts in 5 years would be clinically and economically favourable for both the Health Service and, consequently, the Society.
References
- 1.Giaquinto C, Damme P, Huet F, et al. Reveal Study Group, author. Costs of community-acquired pediatric rotavirus gastroenteritis in 7 European countries: the REVEAL Study. J Infect Dis. 2007;195(Suppl 1):S36–S44. doi: 10.1086/516716. [DOI] [PubMed] [Google Scholar]
- 2.Damme P, Giaquinto C, Huet F, et al. Reveal Study Group, author. Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004-2005: the REVEAL study. J Infect Dis. 2007;195(Suppl 1):S4–S16. doi: 10.1086/516714. [DOI] [PubMed] [Google Scholar]
- 3.Giaquinto C, Damme P, Huet F, et al. Reveal Study Group, author. Clinical consequences of rotavirus acute gastroenteritis in Europe, 2004-2005: the REVEAL study. Infect Dis. 2007;195(Suppl 1):S26–S35. doi: 10.1086/516717. [DOI] [PubMed] [Google Scholar]
- 4.Leung Ak, Kellner Jd, Davies Hd. Rotavirus gastroenteritis. Adv Ther. 2005;22:476–487. doi: 10.1007/BF02849868. [DOI] [PubMed] [Google Scholar]
- 5.Bresee Js, Glass Ri, Ivanoff B, et al. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine. 1999;17:2207–2222. doi: 10.1016/s0264-410x(98)00376-4. [DOI] [PubMed] [Google Scholar]
- 6.Clark Hf, Offit Pa. Vaccines for rotavirus gastroenteritis universally needed for infants. Pediatr Ann. 2004;33:536–543. doi: 10.3928/0090-4481-20040801-11. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization , author. State of the art of new vaccines research & development: initiative for vaccine research. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 8.Clark B, Mckendrick M. A review of viral gastroenteritis. Curr Opin Infect Dis. 2004;17:461–469. doi: 10.1097/00001432-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Parashar U, Gibson C, Bresse J, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widdowson M, Meltzer M, Zhang X, et al. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. 2007;119:684–697. doi: 10.1542/peds.2006-2876. [DOI] [PubMed] [Google Scholar]
- 11.Noel Js, Parker Sp, Choules K, et al. Impact of rotavirus infection on a paediatric hospital in the east end of London. J Clin Pathol. 1994;47:67–70. doi: 10.1136/jcp.47.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parashar U, Chung M, Holman R, et al. Use of state hospital discharge data to assess the morbidity from rotavirus diarrhea and to monitor the impact of a rotavirus immunization program: a pilot study in Connecticut. Pediatrics. 1999;104(3 Pt 1):489–494. doi: 10.1542/peds.104.3.489. [DOI] [PubMed] [Google Scholar]
- 13.Piednoir E, Bessaci K, Bureau-Chalot F, et al. Economic impact of healthcare-associated rotavirus infection in a paediatric hospital. J Hosp Infect. 2003;55:190–195. doi: 10.1016/j.jhin.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Gleizes O, Desselberger U, Tatochenko V, et al. Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J. 2006;25(1 Suppl):S12–S21. doi: 10.1097/01.inf.0000197563.03895.91. [DOI] [PubMed] [Google Scholar]
- 15.Muhsen K, Shulman L, Rubinstein U, et al. Rota Study Group, author. Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of israeli children < 5 years of age, 2007-2008. J Infect Dis. 2009;200(Suppl 1):S254–S263. doi: 10.1086/605425. [DOI] [PubMed] [Google Scholar]
- 16.Mast T, Walter E, Bulotsky M, et al. sving of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J. 2010;29:e19–e25. doi: 10.1097/inf.0b013e3181ca7e2e. [DOI] [PubMed] [Google Scholar]
- 17.Mast T, Chen P, Lu K, et al. Epidemiology and economic burden of rotavirus gastroenteritis in hospitals and paediatric clinics in Taiwan, 2005-2006. Vaccine. 2010;28:3008–3013. doi: 10.1016/j.vaccine.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Ehlken B, Laubereau B, Karmaus W, et al. Romod Study Group, author. Prospective population-based study on rotavirus disease in Germany. Acta Paediatr. 2002;91:769–775. doi: 10.1080/08035250213227. [DOI] [PubMed] [Google Scholar]
- 19.Mangen Mj, Duynhoven Yt, Vennema H, et al. Is it cost-effective to introduce rotavirus vaccination in the Dutch national immunization program? Vaccine. 2010;28:2624–2635. doi: 10.1016/j.vaccine.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Milne Rj, Grimwood K. Budget impact and cost-effectiveness of including a pentavalent rotavirus vaccine in the New Zealand childhood immunization schedule. Value Health. 2009;12:888–898. doi: 10.1111/j.1524-4733.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 21.Standaert B, Parez N, Tehard B, et al. Cost-effectiveness analysis of vaccination against rotavirus with RIX4414 in France. Appl Health Econ Health Policy. 2008;6:199–216. doi: 10.1007/BF03256134. [DOI] [PubMed] [Google Scholar]
- 22.Fontana M, Zuin G, Pancheri P, et al. Costs associated with outpationts diarrhoea in infants and toddlers: a nationwide study of the Italian Society of Taediatric Gastroenterology anh Hepatology (SIGEP) Dig Liver Dis. 2004;36:523–527. doi: 10.1016/j.dld.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Standaert B, Marocco A, Assael B, et al. Analisi di costo-efficacia della vaccinazione universale in Italia con il vaccino Rix4414 contro i rotavirus. PharmacoEconomics – Italian Research Articles. 2008;10(1):23–35. [Google Scholar]
- 24.Marocco A, Baarouk A, Gabutti G, et al. Ricoveri per enterite da Rotavirus in Italia valutati mediante analisi delle Schede di Dimissione Ospedaliera negli anni 2001-2003. Ig Sanità Pubbl. 2006;62:215–224. [PubMed] [Google Scholar]
- 25.Marsella M, Raimondi L, Bergamini M, et al. Epidemiology of rotavirus-associated hospital admissions in the province of Ferrara, Italy. Eur J Pediatr. 2009;168:1423–1427. doi: 10.1007/s00431-009-0942-z. [DOI] [PubMed] [Google Scholar]
- 26.Ruggeri Fm, Delogu R, Petouchoff T, et al. The Rotanet-Italy Study Group, author. Molecular characterization of rotavirus strains from children with diarrhea in Italy, 2007-2009. J Med Virol. 2011;83:1657–1668. doi: 10.1002/jmv.22163. [DOI] [PubMed] [Google Scholar]
- 27.Vitale F, Barbieri M, Dirodi B, et al. Una valutazione economica completa della vaccinazione estensiva contro i rotavirus con il vaccino RIX4414 a livello nazionale e regionale in Italia. Ann Ig. 2013;25:43–56. doi: 10.7416/ai.2013.1905. [DOI] [PubMed] [Google Scholar]
- 28.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, doubleblind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 29. http://web.rete.toscana.it/attinew/ [Google Scholar]
- 30.Fantelli V, Vooren K, Garattini L. Valutazione Economica. Budget Impact Analysis: stato dell'arte in letteratura e proposta per una definizione in Italia. Quaderni di Farmacoeconomia. 2011;15:7–14. [Google Scholar]
- 31.Jit M, Edmunds W. Evaluating rotavirus vaccination in England and Wales. Part II. The potential cost-effectiveness of vaccination. Vaccine. 2007;25:3971–3979. doi: 10.1016/j.vaccine.2007.02.070. [DOI] [PubMed] [Google Scholar]
- 32.Tucker Aw, Haddix Ac, Bresee Js, et al. Cost-effectiveness analysis of a rotavirus immunization program for the United States. JAMA. 1998;279:1371–1376. doi: 10.1001/jama.279.17.1371. [DOI] [PubMed] [Google Scholar]
- 33.Panatto D, Amicizia D, Giacchino R, et al. Burden of rotavirus infections in Liguria, Northern Italy: hospitalisations and potential savings by vaccination. Eur J Clin Microbiol Infect Dis. 2011;30:957–964. doi: 10.1007/s10096-011-1180-7. [DOI] [PubMed] [Google Scholar]
- 34.Panatto D, Amicizia D, Ansaldi F, et al. Burden of rotavirus disease and cost-effectiveness of universal vaccination in the Province of Genoa (Northern Italy) Vaccine. 2009;27:3450–3453. doi: 10.1016/j.vaccine.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 35.Parashar UD, Glass RI. Rotavirus vaccines – Early seccess, remaining questions. N Engl J Med. 2009;360:1063–1065. doi: 10.1056/NEJMp0810154. [DOI] [PubMed] [Google Scholar]
- 36.Glass RI. Unexpected benefits of rotavirus vaccination in the United States. J Infect Dis. 2011;204:975–977. doi: 10.1093/infdis/jir477. [DOI] [PubMed] [Google Scholar]
- 37.Gagneur A, Nowak E, Lemaitre T, et al. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine. 2001;29:3753–3759. doi: 10.1016/j.vaccine.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Azzari C, Massai C, Poggiolesi C, et al. Cost of varicella-related hospitalisations in an Italian paediatric hospital: comparison with possible vaccination expenses. Curr Med Res Opin. 2007;23:2945–2954. doi: 10.1185/030079907X242610. [DOI] [PubMed] [Google Scholar]
- 39. www.ministerosalute.it/programmazione/sdo/ [Google Scholar]
- 40. www.drg.it. [Google Scholar]