Summary
Introduction.
Enterovirus (EV) and parechovirus (PeV) can either infect humans asymptomatically or can cause gastroenteritis, respiratory symptoms and, sometimes, severe disease. As the number of newly identified EV and PeV genotypes keeps increasing, diagnostic methods need to be updated. To this end, we described a novel multiplex one-step real-time RT-PCR to detect EV and human PeV (HPeV) simultaneously in fecal samples collected from children with rotavirus group A (RV-A)-related gastroenteritis.
Methods.
The specificity and sensitivity of the EV/HPeV realtime RT-PCR were evaluated with two 2011 Quality Control for Molecular Diagnostics (QCMD) panels for EV and HPeV detection. RNA was extracted from 111 RV-A-positive fecal samples collected from children up to 5 years of age who had been hospitalized for gastroenteritis from September 2010 to August 2011.
Results.
The EV/HPeV real-time RT-PCR showed a 100% sensitivity and specificity for EV and 91% and 91.7% for HPeV, respectively. Of the 111 RV-A-positive stool specimens, 28 (25.2%) were EV-positive and 7 (6.3%) were HPeV-positive. No clinical differences between children with single or double infections were observed.
Discussion.
In our study, the frequency of EV and HPeV infections was surprisingly high, thus underlining the importance of including EV and HPeV detection in diagnostic panels. The multiplex real-time RT-PCR presented in this paper can therefore be a useful method in a diagnostic setting.
Key words: Enterovirus, Parechovirus, Multiplex one-step real-time RT-PCR
Introduction
The Picornaviridae family currently consists of 46 species grouped into 26 genera [1]. Three of these genera include human pathogens: Enterovirus (EV), Hepatovirus (hepatitis A virus), and Parechovirus (PeV). EVs and human PeVs (HPeV) may be associated with gastroenteritis as well as upper and lower respiratory tract infections [2-4], and can be detected in fecal and respiratory samples [5-7]. Both viruses have tropism for neuronal cells, therefore implying their involvement in aseptic meningitis, encephalitis, encephalomyelitis [3, 8] and white matter abnormalities [9, 10].
In the last few years, advances in metagenomics and molecular biology have enabled the in-depth analysis of picornaviruses, the discovery of new EV and PeV genotypes, as well as a continuous update of their taxonomy [11]. The enterovirus genus now consists of 12 species and contains more than 100 genotypes: human EVs (A-D), non-human EVs (E-H and J), and rhinoviruses A-C - the most recently categorized within the EV genus [12]. The parechovirus genus consists of two species: zoonotic Ljungan virus and HPeV, the latter containing 16 genotypes [1]. As the number of genotypes increases, their molecular detection methods need to be updated in order to ensure high sensitivity and specificity, particularly in a diagnostic setting.
This paper presents the novel multiplex one-step realtime RT-PCR assay for the simultaneous detection of EV and HPeV for clinical fecal samples collected from hospitalized children with acute gastroenteritis in Lombardy (northern Italy) from September 2010 to August 2011.
Methods
MULTIPLEX ONE-STEP REAL-TIME RT-PCR ASSAY
The assay was performed by using the following primer/ probe sets: 5'-GGTGCAAGAGTCTATTGAGC-3' (EV-08 forward), 5'-CACCCAAGTAGTCGGTTCC- 3' (EV-08 reverse), and 5'-CCGGCCCCTGAATG- 3' (EV-probe) specific for the 5'nontranslated region (5'-NTR) (nt. 415-555) of EVs, as published by Nielsen et al. [13]; 5'-GTAACASWWGCCTCTGGGSCCAAAAG- 3' (AN345-forward), 5'-GGCCCCWGRTCAGATCCAYAGT-3' (AN344-reverse), and 5'-CCTRYGGGTACCTYCWGGGCATCCTTC- 3' (AN257-probe) specific for the 5'-NTR (nt. 421-615) of HPeVs as published by Nix et al. [14]. The following two fluorescent dyes were used: 6-FAM for the EV-probe and VIC for the HPeV-probe. BHQ1 quencher was used for both probes.
The multiplex one-step real-time RT-PCR for EV and HPeV detection was prepared with 5 μl of RNA in a total volume of 25 μl with the AgPath-ID One-Step RT-PCR kit (Ambion®, Life Technologies, USA). The reaction mixture contained 1 μM of each EV primer, 0.4 μM of each HPeV primer and 0.2 μM of each probe and was carried out in a 7300 Real-time PCR System (Applied Biosystem®, Life Technologies, USA) with the following thermal profile: 50 °C × 30 min, 95 °C × 15 min, and 50 cycles at 95 °C × 15 sec, 58 °C × 30 sec, and 72 °C × 10 sec.
SENSITIVITY AND SPECIFICITY OF THE MULTIPLEX ONE-STEP REAL-TIME RT-PCR
The sensitivity and specificity of the multiplex onestep real-time RT-PCR were evaluated with two Quality Control for Molecular Diagnostics (QCMD) panels [15]: the QCMD 2011 Enterovirus RNA EQA Programme (2011 EV-QCMD), and the QCMD 2011 Parechovirus RNA EQA Programme (2011 PeV-QCMD). The 2011 EV–QCMD panel consisted of 12 samples: 10 were EV-positive (Coxsackievirus A16, A21, and A24; EV68; EV71; Echovirus 11 and 30; stock dilutions: 10-3- 10-7), one contained human Rhinovirus 16, and one was viral transport medium (VTM). The 2011 PeV-QCMD panel consisted of 11 samples: 8 were HPeV-positive (HPeV 1-5; stock dilutions: 10-3-10-7), one contained human Rhinovirus 16, one contained Coxsackievirus A21, and one was VTM.
The inter- and intra-assay variation of the one-step realtime RT-PCR were calculated and expressed by coefficient of variation (%CV).
CLINICAL FECAL SPECIMENS
From September 2010 to August 2011, fecal samples were collected from children aged 0-5 years old (62.2% males, median age: 12 months; inter-quartile range [IQR]: 12 months) who had been hospitalized for acute rotavirus-A related (RV-A) gastroenteritis, in Lombardy (northern Italy). Rotavirus infection was diagnosed on admission to hospital by commercial routine immunochromatographic or latex-agglutination tests. Overall, in this study, 111 RV-A-positive fecal samples were analyzed anonymously by the multiplex one-step real-time RT-PCR method described previously.
At the time of hospitalization, all children presented with clinical signs of gastroenteritis, i.e. fever and/or abdominal pain and/or diarrhea and/or vomiting. Diarrhea was the most frequently reported symptom (88/111: 79.3%), followed by vomiting (78/111: 70.3%) and fever (67/111: 60.4%). Abdominal pain was detected in 38 cases (34.2%) and only in association with one or more symptoms. The majority of children (84/111: 75.7%) presented with more than one gastroenteritis sign and all symptoms were present in 20.6% (23/111) of children studied.
RNA was extracted for testing with the commercial kit Invisorb® Spin Virus RNA Mini kit, (Stratec Molecular, Germany). To monitor RNA extraction each clinical sample was tested for the presence of human
RNase P gene (RNP) by using a specific primer/probe set (TaqMan® RNase P Assay, ABY® dye/QSY® probe, Life Technologies, USA). Five μl of each sample's RNA were added to a reaction mixture that consisted of 12.5 μl AgPath-ID One-Step RT-PCR kit (Ambion®, Life Technologies, USA), 0.4 μM of each RNase P primer, 0.2 μM of probe and biological grade water up to 20 μl. The reaction was carried out at the same conditions of the multiplex one-step real-time RT-PCR, as described above. Each sample should exhibit RNP reaction curves that cross the threshold line at or before 40 cycle threshold (CT). A sample was considered positive to EV/HPeV when a curve crosses the threshold before or at 40 CT.
Results
SENSITIVITY AND SPECIFICITY OF THE MULTIPLEX ONE-STEP REAL-TIME RT-PCR ASSAY
The EV/HPeV real-time RT-PCR assay presented in this study detected all EV-positive samples included in the 2011 EV-QCMD and 2011 PeV-QCMD panels (sensitivity: 100%). The assay detected all EV-dilutions until the least concentrated (limit of detection: 10-7), which were identified by 55.4% of all laboratories participating in the 2011 EV-QCMD programme. Therefore, the EV/HPeV real-time RT-PCR detected all EV species included in 2011 EV- and PeV-QCMD panels and no cross-reactions were observed with the rhinoviruses included in the panels: the assay specificity was 100%.
Our multiplex assay identified all HPeV-positive specimens included in the 2011 PeV-QCMD panel except the PeV3 10-7 stock dilution, which was detected successfully only by 24.1% of all participants in the 2011 PeVQCMD programme. The limit of detection for HPeV was 10-6 and the assay sensitivity was 91%. The specificity of the multiplex was 91.7% for HPeV, because a cross-reaction between HPeV and Coxsackievirus A16 was identified. A singleplex assay for HPeV was set up with the same conditions as the multiplex assay and no differences in sensitivity and specificity were observed. No differences in terms of CT values (mean standard deviation: 0.97 for EV and 0.59 for HPeV) were observed when mixes of EV and HPeV concentrations were used. The intra-assay %CVs of the real-time RT-PCR assay were 0.99% and 2.09% for EV and HPeV, respectively. The inter-assay %CV was 4.1% for EV and 3.9% for HPeV.
EV AND HPEV DETECTION IN CLINICAL FECAL SPECIMENS
During the 12-month study period, 111 RV-A-positive stool samples were collected and analyzed with the multiplex assay. Twenty-eight (28/111: 25.2%) were positive for EV and 7 (7/111: 6.3%) for HPeV. No triple infections (EV/HPeV/RV-A) were detected.
The median CT values of EV-positive samples was 34.31 (IQR: 2.70; range: 18.22-39.75); the median CT values of HPeV-positive samples was 36.66 (IQR: 1.01; range: 28.25-39.02).
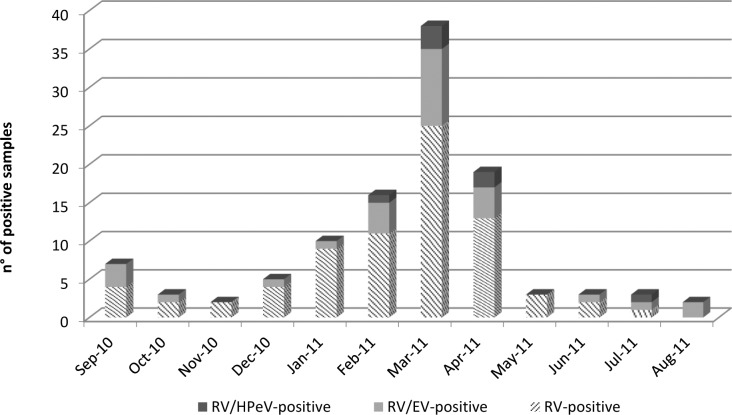
Figure 1 displays the monthly trend of RV-A-positive specimens with the detection of EV and HPeV. The occurrence of RV-A infections peaked in late winter/ beginning of spring (March 2011). RV-A/EV co-infections were found throughout the year, especially (10/28: 35.7%) during springtime (March-April 2011). RV-A/ HPeV co-infections were observed from February to April and in July 2011.
Fig. 1.
Number of positive samples for EV, HPeV and RV–A in children ≤5 years hospitalized with gastroenteritis from September 2010 to August 2011.
In our study, the mean age of children with co-infection was 19 months for RV-A/EV (range: 26 days – 60 months; median age: 12 months; IQR: 12 months) and 23.3 months for RV-A/HPeV (range: 5 – 48 months; median age: 24 months; IQR: 6 months). However, no significant differences (p-value>0.05) were found between the two groups. In addition, patients with a double infection did not show more severe clinical symptoms.
Discussion
As the number of novel picornaviruses identified over the last few years increases considerably, the continuous validation and update of existing EV and HPeV assays is essential.
We set up a multiplex one-step real-time RT-PCR assay combining the EV assay by Nielsen et al. [13] and the HPeV assay by Nix et al. [14]. The sensitivity and the specificity of the EV/HPeV real-time RT-PCR were tested on two 2011 EV/PeV QCMD panels and resulted with 100% for EV and 91% and 91.7% for HPeV, respectively. Contrary to another study [16], it is noteworthy that no cross-reactions between EVs and other viruses, such as rhinoviruses, were observed here.
Viral intestinal infections are the most common cause of acute infectious diarrhea in children [17]. Over the past decade, there have been major advances in the understanding of viral gastroenteritis etiology. Group A rotavirus is responsible for the majority of acute diarrhea in young children worldwide [18]. Furthermore, other viruses like norovirus, adenovirus, enterovirus, bocavirus, sapovirus, astrovirus, calicivirus, and, more recently, torovirus and parechovirus have also been identified thanks to the development of rapid molecular techniques [18, 19]. The frequencies of EV (about 25%) and HPeV (about 6%) infections observed in our study were much higher than those reported by Rovida et al. [20], who showed the frequencies of EV and HPeV infections in pediatric and adult populations with gastroenteritis to be 3% and 1%, respectively. Several studies have evaluated the clinical impact of mixed infections believed to be the cause of severe diarrhea in children under 5, and they have found that the frequencies of mixed infections fluctuate between 5% and 34% [19-23]. It is difficult to compare such findings as dual infections are often misdiagnosed or not investigated at all in routine laboratory work. For example, Rimoldi et al. [24] only included EV in their viral gastroenteritis panel and no samples were EV-positive, whereas HPeV was not considered at all.
Conclusions
The impact of EV and HPeV on childhood gastroenteritis is still unknown due to the lack of systematic testing of clinical samples. In our study, the high frequency of both EV and HPeV in clinical fecal samples underlines the importance of introducing EV and HPeV assays in routine laboratory practice.
A fast, sensitive and specific test, such as the EV/HPeV multiplex real-time RT-PCR described above, is a useful tool for the detection of these viruses in fecal samples as well as in other clinical samples.
Acknowledgments
The authors would like to thank miss Talya Raphael for her editorial assistance.
References
- 1. Picornaviridae home. Available from: http://www.picornaviridae.com/
- 2.Chen H, Yao Y, Liu X, et al. Molecular detection of human parechovirus in children with acute gastroenteritis in Guangzhou, China. Arch Virol. 2014;159:971–977. doi: 10.1007/s00705-013-1915-0. [DOI] [PubMed] [Google Scholar]
- 3.Harvala H, Simmonds P. Human parechovirus: Biology, epidemiology and clinical significance. J Clin Virol. 2009;45:1–9. doi: 10.1016/j.jcv.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 4. Enterovirus foundation. Available from: http://www.enterovirusfoundation.org/associations.shtml.
- 5.Benschop K, Minnaar R, Koen G, et al. Detection of human enterovirus and human parechovirus (HPeV) genotypes from clinical stool samples: polymerase chain reaction and direct molecular typing, culture characteristics, and serotyping. Diagn Microbiol Infect Dis. 2010;68:166–173. doi: 10.1016/j.diagmicrobio.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Harvala H, Robertson I, McWilliam Leitch EC, et al. Epidemiology and clinical associations of human parechovirus respiratory infections. J Clin Microbiol. 2008;46:3446–3453. doi: 10.1128/JCM.01207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia J, Espejo V, Nelson M, et al. Human rhinoviruses and enteroviruses in influenza-like illness in Latin America. Virol J. 2013;10:305–317. doi: 10.1186/1743-422X-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrerizo M, Trallero G, Echevarrı JE. Molecular characterization of enteroviruses associated with neurological infections in Spain, 2008. J Med Virol. 2013;85:1975–1977. doi: 10.1002/jmv.23693. [DOI] [PubMed] [Google Scholar]
- 9.Wu T, Fan XP, Wang WY, et al. Enterovirus infections are associated with white matter damage in neonates. J Paediatr Child Health. 2014;50:817–822. doi: 10.1111/jpc.12656. [DOI] [PubMed] [Google Scholar]
- 10.Pariani E, Pellegrinelli L, Pugni L, et al. Two cases of neonatal human parechovirus 3 encephalitis. Pediatr Infect Dis J. 2014;33:1191–1193. doi: 10.1097/INF.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 11.Simmonds P. Recombination and selection in the evolution of picornaviruses and other mammalian positive-stranded RNA viruses. J Virol. 2006;80:11124–11140. doi: 10.1128/JVI.01076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntyre CL, Knowles NJ, Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J Gen Virol. 2013;94:1791–1806. doi: 10.1099/vir.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen AC, Böttiger B, Midgley SE, et al. A novel enterovirus and parechovirus multiplex one-step real-time PCR-validation and clinical experience. J Virol Methods. 2013;193:359–363. doi: 10.1016/j.jviromet.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nix WA, Maher K, Johansson ES, et al. Detection of All Known Parechoviruses by Real-Time PCR. J Clin Microbiol. 2008;46:2519–2524. doi: 10.1128/JCM.00277-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. QCMD.org. Available from: http://www.qcmd.org/
- 16.Cabrerizo M, Calvo C, Rabella N, et al. Design and validation of a real-time RT-PCR for the simultaneous detection of enteroviruses and parechoviruses in clinical samples. J Virol Methods. 2014;208:125–128. doi: 10.1016/j.jviromet.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Pham NT, Trinh QD, Chan IT, et al. A novel RT-multiplex PCR for detection of Aichi virus, human parechovirus, enteroviruses, and human bocavirus among infants and children with acute gastroenteritis. J Virol Methods. 2010;169:193–197. doi: 10.1016/j.jviromet.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Parashar UD, Hummelman EG, Bresee JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosek M, Bern C, Guerrant RL, et al. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 20.Rovida F, Campanini G, Piralla A, et al. Molecular detection of gastrointestinal viral infections in hospitalized patients. Diagn Microbiol Infect Dis. 2013;77:231–235. doi: 10.1016/j.diagmicrobio.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentini D, Vitucci AC, Grandin A, et al. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis. 2013;32:909–915. doi: 10.1007/s10096-013-1825-9. [DOI] [PubMed] [Google Scholar]
- 22.Román E, Wilhelmi I, Colomina J, et al. Acute viral gastroenteritis: proportion and clinical relevance of multiple infections in Spanish children. J Med Microbiol. 2003;52:435–440. doi: 10.1099/jmm.0.05079-0. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Duan Z, Qian Y, et al. Changes in human Parechovirus profiles in hospitalized children with acute gastroenteritis after a three-year interval in Lanzhou, China. PLoS One. 2013;8:e68321–e68321. doi: 10.1371/journal.pone.0068321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimoldi SG, Stefani F, Pagani C, et al. Epidemiological and clinical characteristics of pediatric gastroenteritis associated with new viral agents. Arch Virol. 2011;156:1583–1589. doi: 10.1007/s00705-011-1037-5. [DOI] [PubMed] [Google Scholar]