Summary
Biphosphonate (BPN) are widely used in clinics to treat metastatic cancer and osteoporosis thus representing a problem not only for patients but also for workers involved in their preparation and administration. A similar exposure occurred years ago in match-making workers undergoing bone alterations similar to those consequent to BPN exposure. Osteonecrosis of the jaw (ONJ) is a main adverse effect related to BPN administration, which is performed in millions of patients worldwide for osteoporosis and cancer therapy, thus representing an emerging problem in public health. In susceptible patients, BPN induce severe, progressive, and irreversible degeneration of facial bones, resulting in avascular ONJ often triggered by dental surgery. BPN induced ONJ occurs in subjects depending on lifestyle factors of both environmental and endogenous origins. Exogenous risk factors include cigarette smoke, alcohol consumption, bacterial infections, and cyclosporine therapy. Endogenous risk factors include systemic diseases such as diabetes or hypertension and adverse polymorphisms of genes involved in metabolism (CYPs, MTHFR), thrombosis (Factor V, Prothrombin), and detoxification (MDR).
Available molecular findings provide evidence that ONJ is related to risk-factors associated with environmental mutagenesis and gene-environment interactions. This issues may be useful to identify susceptible subjects by molecular analyses in order to prevent ONJ occurrence.
Key words: Jaw osteonecrosis, Biphosphonate, Single nucleotide polymorphisms
Introduction
Biphosphonate (BPN) are widely used in clinics to treat metastatic cancer and osteoporosis thus representing a problem not only for patients but also for workers involved in their preparation and administration. A similar exposure occurred years ago in match-making workers undergoing bone alterations similar to those consequent to BPN exposure. Osteonecrosis of the jaw (ONJ) is a main adverse effect related to BPN administration, which is performed in millions of patients worldwide for osteoporosis and cancer therapy [1].
In susceptible patients, BPN induce severe, progressive, and irreversible degeneration of facial bones, resulting in avascular ONJ triggered by dental surgery. BPN induced ONJ occurs in subjects undergoing exposure to DNA-damaging agents as resulting from chemo and radiotherapy of neoplastic diseases or lifestyle factors. Thus, multiple risk factors of both environmental and endogenous origin contribute to BPN-related ONJ.
BPNs association with ONJ was first described in patients with cancer such as multiple myeloma, breast cancer, and prostate cancer treated with intravenous BPN [2]. Nowadays BPN treatment is considered the main risk factor for the development of ONJ. This disease mainly appears in patients receiving intravenous BPNs for the treatment of cancer or oral BPNs for the treatment of osteoporosis [1]. Numerous risk factors of ONJ have been described mainly dealing with the interaction between genetic and environmental factors. Gene–environment interactions underline how genetic and environmental factors jointly influence the risk of developing a disease. Recent studies shed light on a substantial genetic component in the etiology of dentistry disease, paralleled by the established effect of environmental factors such as smoking, alcohol, radiation, and drugs [3].
The purpose of this review is to analyze how environmental factors interact with genetic assets to contribute to the development of ONJ. This knowledge may be useful to identify individuals at risk for developing this pathology.
Exogenous risk factors and oral osteonecrosis
Biphosphonates and Osteonecrosis of the jaw
The structure of bisphosphonates (BPNs) is similar to that of endogenous pyrophosphate, a natural potent inhibitor of osteoclast-mediated bone resorption [4]. Consequently, BPNs have the same properties of inorganic pyrophosphates, but are resistant to hydrolysis, as well as pyrophosphatase enzymes. Etidronate, precursor of pamidronate, is the base molecule of the more potent second-generation drugs. The third generation structure of BPN was generated by the addition of amethyl group to the N-alcylic chain [5] (Tab. I).
Tab. I.
Biphosphonates approved for clinical use by United States of America Food and Drugs Administration (FDA). N-BPs = Nitrogen-containing Biphosphonates.
| Drug compound | N-BPNs | Medical uses | Administration route |
|---|---|---|---|
| Alendronate | Yes | Osteoporosis, Paget's disease | Oral |
| Clodronate | No | Hypercalcemia of malignancy | IV/Oral |
| Etidronate | No | Paget's disease, hypercalcemia of malignancy, osteoporosis | Oral |
| Ibandronate | Yes | Osteoporosis, Paget's disease | Oral/IV |
| Pamidronate | Yes | Hypercalcemia of malignancy, Paget's disease, osteoporosis | IV |
| Risedronate | Yes | Osteoporosis, Paget's disease | Oral |
| Tiludronate | No | Paget's disease | Oral |
| Zoledronate | Yes | Hypercalcemia of malignancy | IV |
The principal mechanism of BPN action in vivo is the interaction with the mineralized component of bone tissue, preventing bone reabsorption via inhibition of osteoclast precursors [2].
BPNs can be classified into 2 groups based on the structure of R2 side chains. Nitrogen containing BPNs (eg, alendronic acid, risedronic acid, zoledronic acid) are much more potent with regard to their bone antiresorptive activity than the non nitrogen-containing BPNs (eg, etidronic acid). Among the nitrogen containing BPNs, those with a tertiary nitrogen in a ring structure (hetrocyclic N-BPs; eg, risedronic acid and zoledronic acid) have the highest potency, [6], inhibit the mevalonate pathway (i.e, the cholesterol synthesis pathway) and induce caspase activity [7] and are the strongest inhibitors of farnesyl pyrophosphate synthase, which is a key branch-point enzyme in the mevalonate pathway [8], partially explaining their anticancer effect. Zoledronic acid is the BP most extensively evaluated in vitro for its antitumor activity, including its effect on colon cancer cells. Osteonecrosis of the jaw has been reported among long term users of intravenous BPNs.
BPNs are hydrophilic molecules thereby not passing directly through biological membranes. Evidence suggests that BPNs are held in bone for several years even after the drug therapy is discontinued [9]. BPNs bind tightly to hydroxyapatite crystals situated in bone surfaces and there remain for long time, since they are not susceptible to enzymatic degradation by osseous pyrophosphatase. BPNs are constantly and gradually released from bone tissue into haematic circulation, entering in osteoclasts by endocytosis [10].
However, the etiopathogenic mechanisms of this pathological condition are poorly understood. Although, several pathways have been proposed for Bisphosphonate- Related Osteonecrosis of the Jaw (BRONJ) occurrence, no single model can explain all morphological changes observed at the macro- and microscopic level. A recent research suggests that BPNs may promote an anti-angiogenic effect which contributes directly to the clinical features associated with BRONJ. Remarkably, the antiangiogenic effect promoting BRONJ might be in keeping with the anti-neoplastic action of BPNs [11].
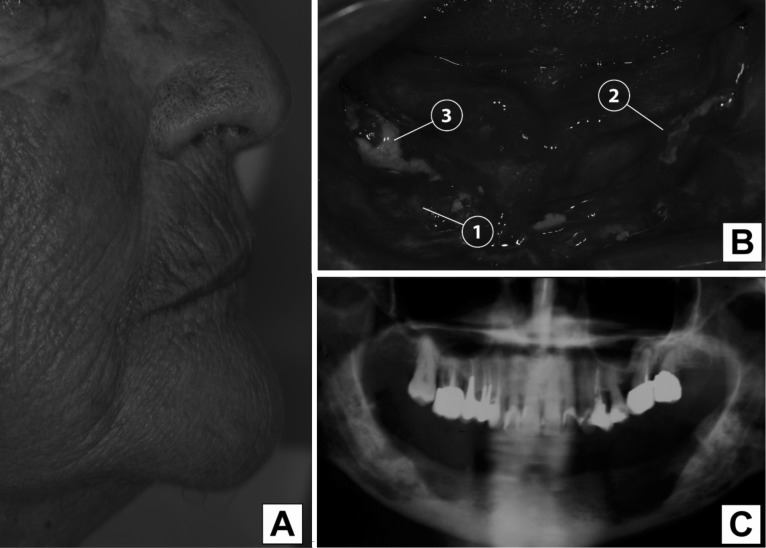
Recent studies revealed that BPNs induce osteoclast apoptosis by the farnesyl-diphosphate (FPP) synthase inhibition in the mevalonate pathway and subsequent accumulation of isopentenyl pyrophosphate [12]. BPNs inhibit osteoclast-mediated bone resorption and, consequently, the suppressive effect of BPNs on bone tissue renewal debilitate the bone by inhibiting the removal of damaged cells from normal bone and increasing bone mineralization [13]. This phenomenon, which recognizes the jawbone as target tissue, leads to ONJ with BPNs accumulation in skeletal sites for high bone turnover (Fig. 1). About 80% of ONJ cases reported heavy lesions, and 69% of cases occured after alveolar surgery, such as tooth extraction. Painful symptoms are reduced during chronic phases of the pathology and granulation tissue appears to delimit inflammation areas. The possible formation of fistulous tissue allows spontaneous draining of the purulent exudate, reducing the edema by defining the osteonecrotic area (Fig. 1). ONJ is often asymptomatic for some time before clinical manifestation. As long as the overlying mucosa is intact and infection is not introduced into the bone, which has limited healing potential, there may be no clinical signs or symptoms of the underlying bone pathology [14].
Fig. 1.
Osteonecrosis of the jaw as induced by BPNs in an edentulous patient. Is reported an example of 80-year-old woman diagnosed with multiple myeloma was on long-term treatment with biphosphonates before developing ONJ in relation with dental extractions. (A) Sagittal view of the patient's profile. (B) Macroscopic appearance of oral osteonecrosis of the jaw. It can be seen that: 1) granulation tissue delimiting inflammation areas; 2) purulent exudate; 3) exposed osteonecrotic areas. (C) Bone loss in jaw is evident in particular in the lower right part of the Figure (panoramic x-Ray).
The epidemiological relevance of ONJ
Osteoporosis is an age-related disease that causes disability, deterioration in the quality of life, and high costs in terms of public health [15]. BPNs are used in the treatment of osteoporosis, and are taken by 73% of osteoporotic patients. Almost 190 million prescriptions of oral BPNs are dispensed worldwide each year [16]. The type of BPN used may play a role in the development of ONJ. This complication has been described after exposure to the nitrogen-containing BPNs pamidronate and zoledronic acid. Zoledronic acid presents a higher risk than pamidronate [17]. Probably because of the higher inhibitory effect of zoledronic acid on bone turnover compared with pamidronate. Furthermore, zoledronic acid causes greater reduction in collagen type-I degradation products (N-telopeptide) than pamidronate [18], confirming its greater antiresorptive activity. Wessel et al. indicate that zoledronate independently increases the risk of adverse effects; in fact, individuals undergoing zoledronate therapy exhibit a 30-fold increased risk of developing ONJ [19]. Despite, a recent study [20] shows that intravenous BPN therapy for osteoporosis does not measurably increase the risk of ONJ among postmenopausal women. Among cancer patients receiving high-dose intravenous BPNs, it was estimated an ONJ incidence of 1-12% [21]. Cartson et al. found that patients with conditions requiring intravenous BPNs had a much higher risk of developing adverse conditions in the jaw compared with patients taking oral BPNs [22].
In fact at least 50% of intravenous BPNs is bio-available for incorporation into the bone matrix compared with an average of 1% of oral BPNs absorbed by the gastrointestinal tract [23]. Approximately 94% of all osteonecrosis cases are due to intravenous BPNs used and 6% are linked to oral BPNs [24, 25]. Among 368 cases of ONJ, 15 (4.1%) occurred in patients receiving BPNs to treat osteoporosis, considerably less than the 337 cases (91.6%) observed in patients receiving BPN to treat multiple myeloma, breast cancer, or prostate cancer. This difference is attributable to the different route of administration and dose used in these clinical situations, i.e., high dose parenteral administration in the context of cancer and low dose oral administration in the case of osteoporosis [25].
Lower ONJ incidence levels have been reported in those countries demonstrating a high level of health and oral hygiene (UK, Sweden) [26], suggesting an important role for bacterial infection as a contributing risk factor. Moreover, bacterial flora could produce septic conditions, involving bacteria such as Staphilococcus aureus and Pseudomonas aeruginosa. It remains unclear whether infection plays a primary causative role or is secondary to the appearance of the lesion, and whether it initially arises in the bone or soft tissue [27].
Currently available published incidence data for BRONJ are based on retrospective studies and estimates of cumulative incidence range from 0.8 to 12% and 60-70% of cases are preceded by a dental surgical procedure [28]. A summary of ONJ risk factors related on BPNs is reported in Table II.
Tab. II.
Risk factors identifying BPN-treated patients at high risk for developing ONJ following dental surgery.
| Risk factor | Rank | Conferred risk | References |
|---|---|---|---|
| BPN administration route | intravenous > oral | High | [22, 25] |
| BPN type BPN dose BPN therapy duration |
Zoledronate > Pamidronate high > low long > short |
High High Medium |
[18, 19] |
| Cause of BPN therapy | Cancer > osteoporosis | Medium | [20, 21] |
| BPN treatment cycles | 18 cycles or more > 12 cycles or less | High | [62] |
| Oral hygiene | Poor > high | Medium | [26] |
| Cyclosporine therapy | Yes > no | Low | [41] |
| Antiangiogenic therapies (e.g.interferon, etc.) | Yes < no | Low | [45] |
| Smoking | Yes < no | Medium | [46] |
| Multiple myeloma | Yes < no | High | [27] |
| Systemic blood diseases and lymphomas | Yes < no | Medium | [27] |
| Autoimmune diseases | Yes > no | Low | [46]; |
| Local radiotherapy | Yes > no | High | [3] |
| Local dental and periodontal diseases | Yes > no | Medium | [27, 49] |
| Obesity | Yes > no | Low | [19] |
| CYP2C8 gene polymorphism | TT homozygosity > heterozigosity and wild type | High | [51] |
| Prothrombin gene polymorphisms | G20210 > A | Low | [53] |
| Leiden V factor gene polymorphisms | Arg5406 > Gln | Low | [53] |
| Plasminogen-activating inhibitor-1 gene polymorphism | 4G/4G homozygosity > heterozigosity and wild type | Low | [55] |
| Multidrug resistance gene 1 polymorphism | Exon 26: C3435 > T; Exon 21: G2677 > T/A |
Low | [56] |
| TNF-alpha gene polymorphism | G307 > A | Low | [58] |
The role of dental procedures in the development of ONJ
Dental trauma, such as dental extraction, is the most common risk factor for ONJ, although cases have occurred spontaneously [29]. In fact, while dental extraction greatly increases the need for repair of alveolar bone, the anti-resorptive function of BPNs may reduce the ability to perform this repair, thus causing ONJ. Disruption of blood flow could be another contributing factor [30]. Badros et al. [31] reported a significant association between the occurrence of ONJ in dental extraction (p = 0.009) and age (OR = 1.09; p = 0.003) in patients with multiple myeloma receiving intravenous BPN treatment.
Implant therapy is not allowed, in patients who underwent intravenous BPNs. In contrast, surgery is usually not contraindicated with use of oral BPNs, but the patient should be informed of potential complication [32]. The jaw is highly susceptible to osteonecrosis because it is characterized by greater cellular turnover than the maxilla and other bones. Furthermore, the jaw has a terminal circulation, hampering the establishment of additional circulation during ischemic episodes. Ruggiero et al. highlighted ONJ prevalence in the jaw, its possible development from even simple surgical treatments, as well as it has s a vascular nature and predisposing septic parameters. In parallel, chewing causes constant trauma to the jaw, and exacerbates the instability of localized microfractures, [33].
Occupational risk
In vivo studies show that BPNs have toxic and genotoxic power [34], and therefore should be considered as "dangerous drugs" during the preparation and handling. According to Polovich [35] "hazardous drugs" represents a potential health risk to health care workers who may be exposed during preparation or administration. These drugs require special handling because of their inherent toxicities. Drugs that meet one or more of the following criteria should be handled as hazardous [36].
Carcinogenicity
Teratogenicity or developmental toxicity
Reproductive toxicity
Organ toxicity at low doses
Genotoxicity
Structure or toxicity similar to drugs classified as hazardous using the above criteria.
Some of these features are typical of BPNs and therefore during the preparation and administration of these drugs should be taken preventive safety measures. Preventive measures such as: the use of disposable gowns, gloves, masks, shoe covers, and the use of vertical laminar flow hoods to maintain the sterility of the product and the protection of the work surface of the operator. The work surface must be free of materials, with the exception of BPN drugs and must be cleaned with 70% alcohol and moist paper (guidelines ASHP) [36]. Therefore, the workers handling the BPNs must undergo health surveillance pursuant to Legislative Decree 81/2008 on the same basis in workers exposed to processing and handling of other dangerous drugs.
Environmental mutagens and oral osteonecrosis
Bisphosphonates and DNA damage
BPNs realize their antiosteoclast effects through modification of the Rho family GTPases, which include Rac GTPases [37]. Deletion of Rac1 gene results in a reduction of osteoclast formation because of its effects on preosteoclast chemotaxis, actin assembly, and RANKLmediated reactive oxygen-species generation. Rac1 deletion in knock-out mice increased trabecular-bone volume and trabecular number as compared with wildtype and Rac2-null mice [38].
Two aromatic bis-(2-chloroethyl) amino-bisphosphonic acids were investigated for their genotoxic potential as determined in S. typhimurium and mammalian cells in vitro. Both compounds induced a two-fold increase in his+ revertants in S. typhimurium TA1535 following metabolic activation with subcellular liver fractions, thus demonstrating the mutagenic attitude of BPNs. In vivo toxicity and genotoxicity parameters were determined in liver and bone marrow cells after treatment. The genotoxicity of both compounds was exerted in several tissues but was lower in bone marrow cells than in liver cells [34].
BPNs induce apoptosis in osteoclasts but prevent apoptosis in osteoblasts. Both effects are related to BPNs binding to connexin 43. The prevention of osteoblastic apoptosis is mediated by Cx43 hemi channel opening and related activation of the extracellular signal-regulated kinases). However, Cx43, is also involved in BPNs pro-apoptotic effects in osteoclast [39].
Exogenous risk factors for oral osteonecrosis other than BPNs: drugs, smoke, and ionizing radiation
Exogenous risk factors for ONJ include cigarette smoke, alcohol consumption, bacterial infections, and cyclosporine therapy. Endogenous risk factors include systemic diseases such as diabetes or hypertension and adverse polymorphisms of genes involved in metabolism such as CYPs, MTHFR.
Available molecular findings provide evidence that ONJ recognizes risk-factors associated with environmental mutagenesis and gene-environment interactions.
Glucocorticoid administration is a risk factor for osteonecrosis [40]. Calcineurin-inhibitors, particularly cyclosporine, may increase the risk of osteonecrosis because of vasoconstricting effects [41]. Cyclosporine is a cyclic hydrophobic endecapeptide derived from the metabolic products of two species of fungi, Trichoderma polysporum and Cylindrocarpon lucidum. The antimicrobial activity of cyclosporine is weak but this compound has a remarkable inhibitory effect on lymphocyte proliferation [42]. Cyclosporine inhibits many of the processes involved in T-cell-mediated immune responses; in particular, a concentration of 10/20 ng/ml inhibits the synthesis of interleukin-2, limiting the amplification of cytotoxic T lymphocytes. Cyclosporine has a selective activity against T lymphocytes: T suppressor lymphocytes remain unaffected, while cytotoxic T lymphocytes and T helper cells are susceptible to drug. Cyclosporine is a widely used immunosuppressive agent, predominantly for transplant patients. It is well recognized that transplant patients are prone to developing squamous carcinoma of the skin and mucosa. Cyclosporine is a specific ligand for calcineurin, a ubiquitously expressed cellular serine/threonine phosphatase that plays important roles in the immune system and cardiac muscle. Many genes were identified to be responsive to cyclosporine treatment, including regulatory molecules involved in apoptosis, DNA damage repair, and cell-cycle regulation [43]. The mechanisms involved in the pathogenesis of cyclosporin A-induced gingival hyperplasia are not well understood. In mice treated intraperitoneally with increasing doses of cyclosporine A, oral mucosa has increased vascularity, thickening of the epithelial tissue and connective tissue, edema, mononuclear infiltrate and gingival hyperplasia [44]. Anti angiogenetic agents induce a risk of ONJ when administered concurrently with BPNs [45]. This situation frequently occurs in patient undergoing long-term treatment with interferon for chronic viral hepatitis or for anti-cancer therapies.
Cigarette smoke is an extremely complex mixture of mutagens representing a risk factor for a variety of inflammatory, neoplastic, and vascular diseases. The direct effect of smoke on blood vessel significantly contribute to ONJ. In fact, smoke causes increased vasoconstriction and thrombosis in the bone, leading to ischemic states that may underlie the pathophysiology of osteonecrosis [46]. It has been demonstrated that smoke increase mtDNA deletetion (common deletion 4977) in artery and inhibits the activator of plasminogen inhibitor 1 increasing the thrombosis risk [47].
In addition, ionizing radiation constitute a risk factor for ONJ [3]. In fact, 93% of patients with ONJ was previously treated with a radiotherapy regimen with doses of over 6,500 cGy. This leads to a adverse effects exerted by ionizing radiation towards bone tissue including clastogenic DNA damage, oxidative stress, stem cell depletion [3] (Tab. II).
ONJ biomarkers
The decreased serum levels of telopeptide type I collagen is a biological marker in ONJ. To validate this hypothesis, Bagan et al. compared 15 patients with bisphosphonate-induced ONJ to a control group of 10 healthy age- and gender-matched individuals [48]. Their preliminary results showed decreased telopeptide of type I collagen serum levels in patients with ONJ, but further studies containing a greater number of patients are needed to prove the utility of this finding as a biological and prognostic marker in ONJ [48].
Endogenous risk factors and oral osteonecrosis
Systemic and local risk factors
ONJ may be associated with a number of different predisposing systemic conditions such as haemoglobinopathies, coagulopathies, lymphoproliferative disorders, Paget's disease, phosphorous exposure, and local conditions of sepsis (apical or periodontal), trauma (surgical or accidental) and radiotherapy. The exact role of these factors involved in the pathogenic process remains to be established [26]. Patients with multiple myeloma are at highest risk for ONJ, possibly due to inherent myeloma bone-metabolism abnormalities, such as increased activity of osteoclasts and inhibition of osteoblast function, leading to enhanced bone resorption.
Dental risk factors include periodontal disease, dental abscesses, surgical procedures involving the bone, and trauma from ill-fitting dentures, all of which may lead to mucosal lesions [26]. Oral conditions that predispose to tooth extraction, such as advanced caries, moderate to advanced periodontitis, and xerostomia, are indirect risk factors for ONJ [49]. The association of ONJ with poor dental health is established [50].
Obesity was also found to be associated with ONJ risk in cancer patients. Although it is currently unclear which pathway might cause an obese person to develop ONJ. In fact, obesity is potentially correlated with an increase in masticatory function that could lead to oral-bone microtraumas [19].
Gene polymorphisms
Like other degenerative diseases, ONJ is caused by a combination of environmental and genetic risk factors. The potential role of genetics in the development of ONJ has been explored in multiple myeloma patients undergoing BPN therapy. Four single nucleotide polymorphisms (SNPs) were considered (rs1934951, rs1934980, rs1341162, and rs17110453) mapped within the cytochrome P450-2C gene (CYP2C8). The analysis of SNP rs1934951 and 3 additional SNPs allocated on the same gene (i.e. rs1934980, rs1341162, and rs17110453) highlights an over-representation of the homozygous TT genotype in cases as compared with controls. Thus, individuals homozygous for the TT genotype have an increased risk of developing ONJ (OR = 12.75) [51]. CYP2C8 gene polymorphisms can affect several biologic pathways [52], which could be involved in the development of ONJ in patients treated with BPNs. Because ONJ is an avascular necrosis of the jawbone, the alteration of this pathway due to a variant of CYP2C8 could make development of osteonecrosis. These results suggest that the rs1934951 polymorphism on CYP2C8 gene is a risk factor for ONJ. This information could help us to exclude patients at high risk from BPN therapy, and to take specific preventive measures.
Another study examined the association between osteonecrosis and thrombotic polymorphisms in genes encoding factor V Leiden and the prothrombin (20210A) gene mutation. Factor V Leiden and the prothrombin 20210A gene mutations occurred significantly (p = 0.006) frequently in patients with osteonecrosis than in a population of 282 healthy volunteers (OR = 3.1, 95% CI = 1.4– 6.6). This study suggests that coagulation abnormalities in the form of factor V Leiden and prothrombin 20210A gene mutations might play a role in osteonecrosis [53].
Major thrombophilic mutations have been identified as risk factors for non-traumatic osteonecrosis in Caucasians. The factor V Leiden mutation was present in 18% of the patients compared with 4.6% of the control subjects, resulting in a statistically significant difference (OR = 4.5). Factor V Leiden polymorphism is also associated with osteonecrosis of the femoral head [54]. Genetic studies in subgroups of patients with steroidinduced osteonecrosis focused on coagulation, fibrinolytic factors and homocysteine metabolism. A positive association of plasminogen-activating inhibitor-1 (PAI), MTHFR and factor V Leiden polymorphisms with steroid- induced ON was reported [55].
High plasma PAI-1 concentrations were associated with coronary artery disease and other thrombotic disorders. Regarding the effect of PAI-1 polymorphism on osteonecrosis, it has been reported that 4G/4G PAI-1 homozygosis is a risk factor for osteonecrosis [56]. It has been demonstrated that cigarette smoke is a potent induced of PAI-1 gene expression [47] thus providing evidence for the interaction between smoking and an adverse genetic PAI-1 polymorphism as contributing to thrombosis and ONJ.
Polymorphisms in the multidrug resistance gene 1 (ABCB1), which encodes the drug-transport protein, Pglycoprotein leads to drug resistance to various agents, including steroids. It was found that the 3435TT exon 1 genotype had a protective effect against the development of ONJ. The same result was also observed with homozygosity in the mutant 2677T/A variants. As ONJ is a rare occurrence, systematic pharmacogenetic studies are needed to assess the possibility of genetic susceptibility [57]. The TNF cytokine is crucial to both immune and inflammatory responses. TNF up-regulates host defense mechanisms and also affects tissue physiology, including bone resorption, which is a crucial step in ONJ. Over-expression of TNF in the periodontium may be harmful to the host. Normally, TNF and other pro-inflammatory agents are regulated by IL-10, suggesting that some deficiency in this regulation mechanism may be linked to the disease. Although several polymorphisms have been reported in the TNF-A promoter, the majority of studies have been focused on the G/A polymorphism at position-307 because most of the other polymorphisms being functionally silent. TNF adverse polymorphism increase the risk of periodontal bone loss [58] and periodontitis [59]. Main genetic polymorphisms representing risk factors for ONJ are listed in Table II.
Conclusions
Risk factors of both exogenous and endogenous origin play an important pathogenic role in the development of ONJ. It is important to identify patients at high risk for developing ONJ after dental surgery. This approach should be useful to (a) identify those patients in which dental surgery could cause a risk rather than a benefit; (b) apply preventive intervention before dental surgery to decrease ONJ risk; (c) define time and type (less or more invasive) of surgical intervention. The American Dental Association has produced guidelines regarding the dental management of patients prescribed BPNs [60]. A recognized therapeutic protocol that allows for clear identification of BNP-related ONJ does not currently exist. Common guidelines present in the literature consist of a first approach to acute phases with broad antibiotic therapy and antimicrobial rinses such as 0.12% chlorhexidine, and, for second incidences, substitution with a specific therapy after the antibiogram.
Since the half-life of BPNs in human bone can reach more than 10 years [61] it has been generally questioned whether the interruption of BPNs will be of any benefit [62, 63]. Migliorati et al. suggest that, until prospective studies of BPN-related ONJ provide information about effective treatment protocols, the best approach is prevention, with the dental practitioner and the physician working collaboratively [64]. Translation of basic scientific findings into clinical practice is the essential premise for the development of future preventive strategies. Risk for ONJ appearance is greatly influenced by genotoxic factors related to oxidative damage such as those related to bacterial infections, inflammation, smoking, and ionizing radiation. Administration of chemopreventive drugs is a possible strategy to overcome this mechanisms in high risk patients, such an approach being usually referred as targeted chemoprevention [65, 66]. Furthermore BPN being mutagenic and genotoxic represent an occupational risk for workers involved in their production and administration thus have to be considered "hazardous drugs".
ACKNOWLEDGMENTS
This study was supported by the University of Genoa, Italy.
References
- 1.Shane E, Goldring S, Christakos S, et al. Osteonecrosis of the jaw: more research needed. J. J. Bone Miner Res. 2006;21:1503–1505. doi: 10.1359/jbmr.060712. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 3.Baldi D, Izzotti A, Bonica P, et al. Degenerative periodontal diseases and oral osteonecrosis: the role of gene-environment interactions. Mutat Res. 2009;667:118–131. doi: 10.1016/j.mrfmmm.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Fehm T, Beck V, Banys M, et al. Biphosphonate-induced osteonecrosis of the jaw (ONJ): incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecologic Oncol. 2009;112:605–609. doi: 10.1016/j.ygyno.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Wilder L, Jaeggi KA, Glatt M, et al. Higly potent geminal bisphosphonates. From pamidronate disodium to zolendronic acid. J Med Chem. 2002;45:3721–3738. doi: 10.1021/jm020819i. [DOI] [PubMed] [Google Scholar]
- 6.Oizumi T, Yamaguchi K, Funayama H, et al. Necrotic actions of nitrogen-containing biphosphonates and their inhibition by chlodronate, a non-nitrogen-containing biphosphonate in mice: potential for utilization of clodronate as a combination drug with a nitrogen-containing biphosphonate. Basic Clinical Pharmacol Toxicol. 2009;104:384–392. doi: 10.1111/j.1742-7843.2008.00374.x. [DOI] [PubMed] [Google Scholar]
- 7.Shmeeda H, Amitay Y, Tzemach D, et al. Liposome encapsulation of zoledronic acid results in major changes in tissue distribution and increase in toxicity. J Control Release. 2013;167:265–275. doi: 10.1016/j.jconrel.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Gao J, Liu J, Qiu Y, et al. Multi-target-directed design, syntheses, and characterization of fluorescent bisphosphonate derivatives asmultifunctional enzyme inhibitors in mevalonate pathway. Biochim Biophys Acta. 2013;1830:3635–3642. doi: 10.1016/j.bbagen.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Black DM, Schwartz AV, Ensrud KE. Effects of continuing or stopping aledronate after 5 years of treatment: the fracture intervention trial long term extension – a randomized trial. J Am M Ass. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 10.Berenson JR, Rosen LS, Howell A, et al. Zolendronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. Cancer;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Sharma D, Ivanovski S, Slevin M, et al. Bisphosphonate-related osteonecrosis of jaw (BRONJ): diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect. Vascular Cell. 2013;5:1–1. doi: 10.1186/2045-824X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mönkkönen H, Ottewell PD, Kuokkanen J, et al. Zoledronic acid-induced IPP/ApppI production in vivo. Life Sci. 2007;81:1066–1070. doi: 10.1016/j.lfs.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Roelofs AJ, Thompson K, Gordon S, et al. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:62225–62305. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 14.Mcleod NMH, Davies BJB, Brennan PA. Biphosphonates osteonecrosis of the jaws; an increasing problem for the dental pratictioner. British Dental J. 2007;203:641–644. doi: 10.1038/bdj.2007.1065. [DOI] [PubMed] [Google Scholar]
- 15.Lane NE. Epidemiology ethiology and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3–S11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 16.Leon A. Assael. Oral Bisphosphonates as a Cause of a Bisphosphonates- Related Osteonecrosis of the Jaws: Clinical Findings, Assessment of Risks, and Preventive Strategies. J Oral Maxillofac Surg. 2009;67:35–43. doi: 10.1016/j.joms.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and biphosphonates. N Engl J Med. 2005;353:99–102. doi: 10.1056/NEJM200507073530120. [DOI] [PubMed] [Google Scholar]
- 18.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletalmetastases in patients with breast cancer or osteolytic lesions of multiplemyeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 19.Wessel JH, Dodson TB, Zavras AI. Zoledronate smoking and obesity are strong risk factors for osteonecrosis of the jaw: a case–control study. J Oral Maxillofac Surg. 2008;66:625–631. doi: 10.1016/j.joms.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodson TB. Intravenous biphosphonate therapy and biphosphonate- related osteonecrosis of the jaws. J Oral maxillofac Surg. 2009;67:44–52. doi: 10.1016/j.joms.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Dimitrakopoulos I, Magopoulos C, Karakasis D. Bisphosphonate- induced avascular osteonecrosis of the jaws: a clinical report of 11 cases. Int J Oral Maxillofac Surg. 2006;35:588–593. doi: 10.1016/j.ijom.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Cartson V, Zhu S, Zavras A. Biphosphonates use and the risk of adverse jaw outcomes: a medical claims study of 714,217 people. J Am Dent Ass. 2008;139:23–30. doi: 10.14219/jada.archive.2008.0016. [DOI] [PubMed] [Google Scholar]
- 23.Ezra A, Golomb G. Adminitsration routes and delivery systems of biphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev. 2000;42:175–195. doi: 10.1016/s0169-409x(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 24.Woo SB, Hellstein JW, Kalmar JR. Narrative (corrected) review: biphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 25.Sedghizadeh PP, Stanley K, Caligiuri M, et al. Oral biphosphonate use and the prevalence of osteonecrosis of the jaw: an institutional inquiry. J Am Dent Assoc. 2009;140:61–66. doi: 10.14219/jada.archive.2009.0019. [DOI] [PubMed] [Google Scholar]
- 26.Hugoson A, Koch G, Göthberg C, et al. Oral health of individuals aged 3-80 years in Jönköping, Sweden during 30 years (1973–2003). I. Review of findings on dental care habits and knowledge of oral health. Swed. Dent J. 2005;29:125–138. [PubMed] [Google Scholar]
- 27.Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of biphosphonates: a critical review. Am J Med. 2009;122:S33–S45. doi: 10.1016/j.amjmed.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Ficarra G, Beninati F. Bisphosphonate-related Osteonecrosis of the Jaws: An Update on Clinical, Pathological and Management Aspects. Head Neck Pathol. 2007;1:132–140. doi: 10.1007/s12105-007-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards B, Gounder M, McKoy JM, et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: biphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008;9:1666–1672. doi: 10.1016/S1470-2045(08)70305-X. [DOI] [PubMed] [Google Scholar]
- 30.Wood J, Bonjean K, Ruerz S, et al. Novel antiangiogenic effects of the bisphosphonatecompound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 31.Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol. 2006;24:945–952. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- 32.Hwang D, Wang HL. Medical contraindications to implant therapy: part I: absolute contraindications. Implant Dent. 2006;15:353–360. doi: 10.1097/01.id.0000247855.75691.03. [DOI] [PubMed] [Google Scholar]
- 33.Ruggiero SL, Mehrota B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of biphosphonates: a review of 63 cases. J Oral maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Pool BL, Berger M, Schlehofer JR, et al. In vivo and in vitro investigations on biological effects of aromaticbis-(2-chloroethyl) amino-bisphosphonic acids, new agents proposed for chemotherapy of bone tumors: cytostatic activity in rat osteosarcoma; toxicity and genotoxicity in liver and bone marrow; mutagenicity in S. typhimurium. Invest New Drugs. 1988;6:67–78. doi: 10.1007/BF00195363. [DOI] [PubMed] [Google Scholar]
- 35.Polovich M. Safe handling of hazardous drugs. Online J Issues Nurs. 2004;9(3):6–6. 30. [PubMed] [Google Scholar]
- 36. ASHP guidelines on handling hazardous drugs Developed through the ASHP Council on Professional Affairs and approved by the ASHP Board of Directors on January 12, 2006. [Google Scholar]
- 37.Fukuda A, Hikita A, Wakeyama H, et al. Regulation of osteoclast apoptosis and motility by small GTPase binding protein Rac1. J Bone Miner Res. 2005;20:2245–2253. doi: 10.1359/JBMR.050816. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Lebowitz D, Sun C, et al. Identifying the Relative Contributions of Rac1 and Rac2 to Osteoclastogenesis. J Bone Miner Res. 2008;23:260–270. doi: 10.1359/jbmr.071013. [DOI] [PubMed] [Google Scholar]
- 39.Plotkin LI, Lezcano V, Thostenson J, et al. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J. Bone Miner Res. 2008;23:1712–1721. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King AE, Umland EM. Osteonecrosis of the jaw in patients receiving intravenous or oral biphosphonates. Pharmacother. 2008;28:667–677. doi: 10.1592/phco.28.5.667. [DOI] [PubMed] [Google Scholar]
- 41.Elder GJ. From marrow oedema to osteonecrosis: common paths in the development of post-transplant bone pain. Nephrology. 2006;11:560–567. doi: 10.1111/j.1440-1797.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 42.Borel JF. Comparative study of in vitro and in vivo drug effects on cell mediated cytotoxicity. Immunology. 1976;31:631–641. [PMC free article] [PubMed] [Google Scholar]
- 43.Tiu J, Li H, Rassekh C, et al. Molecular basis of posttransplant squamous cell carcinoma: the potential role of cyclosporine A in carcinogenesis. Laryngoscope. 2006;116:762–769. doi: 10.1097/01.mlg.0000205170.24517.28. [DOI] [PubMed] [Google Scholar]
- 44.Meller AT, Rumjanek VM, Sansone C, et al. Oral mucosa alterations induced by cyclosporin in mice: morphological features. J Periodontal Res. 2002;37:412–415. doi: 10.1034/j.1600-0765.2002.01002.x. [DOI] [PubMed] [Google Scholar]
- 45.Christodoulou C, Pervena A, Klouvas G, et al. Combination of biphosphonates and antiangiogenetic factors induces osteonecrosis of the jaw more frequently than biphosphonates alone. Oncology. 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 46.Assouline-Dayan Y, Chang C, Greenspan A, et al. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 47.Izzotti A, Cartiglia C, Longobardi M, et al. Alterations of gene expression in skin and lung of mice exposed to light and cigarette smoke. FASEB J. 2004;18:1559–1561. doi: 10.1096/fj.04-1877fje. [DOI] [PubMed] [Google Scholar]
- 48.Bagan JV, Jiménez Y, Gómez D, et al. Collagen telopeptide (serum CTX) and its relationship with the size and number of lesions in osteonecrosis of the jaws in cancer patients on intravenous bisphosphonates. Oral Oncol. 2008;4:1088–1089. doi: 10.1016/j.oraloncology.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Koka S, Clarke BL, Amin S, et al. Oral biphosphonate therapy and osteonecrosis of the jaw: what to tell the concerned patient. Int J Prosthodont. 2007;20:115–122. [PubMed] [Google Scholar]
- 50.Rayman S, Almas K, Dincer E. Biphosphonate-related jaw necrosis: a team approach management and prevention. Int J Dent Hygiene. 2009;7:90–95. doi: 10.1111/j.1601-5037.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 51.Sarasquete M, García-Sanz R, Marín L, et al. Bisphosphonaterelated osteonecrosis of the jaw is associated with polymorphisms of the cytochrome P450 CYP2C8 in multiple myeloma: a genome-wide single nucleotide polymorphism analysis. Blood. 2008:709–712. doi: 10.1182/blood-2008-04-147884. [DOI] [PubMed] [Google Scholar]
- 52.Ingelman-Sundberg M, Sim SC, Gomez A, et al. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther. 2007;116:496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Björkman A, Burtscher IM, Svensson PJ, et al. Factor V Leiden and the prothrombin 20210A gene mutation and osteonecrosis of the knee. Arch Orthop Trauma Surg. 2005;125:51–55. doi: 10.1007/s00402-004-0760-8. [DOI] [PubMed] [Google Scholar]
- 54.Chang JD, Hur M, Lee SS, et al. Genetic background of nontraumatic osteonecrosis of the femoral head in the Korean population. Clin Orthop Relat Res. 2008;466:1041–1046. doi: 10.1007/s11999-008-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohler HP, Grant P J. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 56.Asano T, Takahashi KA, Fujioka M, et al. ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics. 2003;13:675–682. doi: 10.1097/00008571-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Goldwaser BR, Chuang SK, Kaban LB, et al. Risk factor assessment for the development of osteoradionecrosis. J Oral Maxillofac Surg. 2007;65:2311–2316. doi: 10.1016/j.joms.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Cury PR, Joly JC, Freitas N, et al. Effect of tumor necrosis factor- alpha gene polymorphism on peri-implant bone loss following prosthetic reconstruction. Implant Dent Mar. 2007;16:80–88. doi: 10.1097/ID.0b013e31803277c1. [DOI] [PubMed] [Google Scholar]
- 59.Shapira L, Stabholz A, Rieckmann P, et al. Genetic polymorphism of the tumor necrosis factor (TNF)-alpha promoter region in families with localized early-onset periodontitis. J Periodontal Res. 2001;36:183–186. doi: 10.1034/j.1600-0765.2001.360307.x. [DOI] [PubMed] [Google Scholar]
- 60. American Dental Association Council on Scientific Affairs , author. Dental management of patients receiving oral biphosphonates therapy. J Amer Dent Ass. 2006;137:1144–1150. doi: 10.14219/jada.archive.2006.0355. [DOI] [PubMed] [Google Scholar]
- 61.Lin JT, Lane JM. Biphosphonates. J AM Acad Orthop Surg. 2003;11:1–4. doi: 10.5435/00124635-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Zarychanski R, Elphee E, Walton P, et al. Osteonecrosis of the jaw associated with pamidronate therapy. Am J Emathol. 2006;81:73–75. doi: 10.1002/ajh.20481. [DOI] [PubMed] [Google Scholar]
- 63.Wooltorton E. Patients receiving intravenous biphosphonates should avoid invasive dental procedures. CMAJ. 2005;172:1684–1684. doi: 10.1503/cmaj.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Migliorati CA, Castiglia J, Epstein J, et al. Managing the care of patients with biphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent As. 2005;136:1658–1668. doi: 10.14219/jada.archive.2005.0108. [DOI] [PubMed] [Google Scholar]
- 65.Flora S, Scarfì S, Izzotti A, et al. Induction by 7,12-dimethylbenz(a)anthracene of molecular and biochemical alterations in stem-derived human mammary epithelial cells, and protection by N-acetylcysteine. Int J Oncology. 2006;29:521–529. [PubMed] [Google Scholar]
- 66.Flora S, Izzotti A, D'Agostini F, et al. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer,with special reference tosmoking related end-points. Carcinogenesis. 2001;22:999–1013. doi: 10.1093/carcin/22.7.999. [DOI] [PubMed] [Google Scholar]