Summary
Introduction.
The study objective is to evaluate critical points in the process of pre-analytical histology in an Anatomic Pathology laboratory. Errors are an integral part of human systems, including the complex system of Anatomic Pathology. Previous studies focused on errors committed in diagnosis and did not consider the issues related to the histology preparation of routine processes.
Methods.
Root Cause Analysis was applied to the process of histology preparation in order to identify the root cause of each previously identified problem. The analysis started by defining an 'a priori' list of errors that could occur in the histology preparation processes. During a three-month period, a trained technician tracked the errors encountered during the process and reported them on a form. 'Fishbone' diagram and 'Five whys' methods were then applied.
Results.
8,346 histological cases were reviewed, for which 19,774 samples were made and from which 29,956 histologies were prepared. 132 errors were identified. Errors were detected in each phase: accessioning (6.5%), gross dissecting (28%), processing (1.5%), embedding (4.5%), tissue cutting and slide mounting (23%), coloring, (1.5%), labeling and releasing (35%).
Discussion.
Root cause analysis is effective and easy to use in clinical risk management. It is an important step for the identification and prevention of errors, that are frequently due to multiple causes. Developing operators' awareness of their central role in the risk management process is possible by targeted training. Furthermore, by highlighting the most relevant points of interest, it is possible to improve both the methodology and the procedural safety.
Key words: Root cause analysis, Anatomic Pathology, Laboratory errors
Introduction
The term 'clinical risk management' was introduced in the United States around 1970 to describe a tool that was used to check claims, lawsuits and requests of indemnification of patients who suffered adverse events caused by inefficient health care.
However, the consequences that may derive from the errors committed during clinical practice became of universal interest only in 1999, when a report published by the Institute for Medicine in the United States stated that a high number of deaths - between 44,000 and 98,000 every year - were caused by errors in clinical practice [1]. Since the publication of this report, the safety of patients has also become of interest to healthcare organisations and regulating bodies (such as the Joint Commission on Healthcare Organizations) that consider this topic as crucial for hospitals, in order to achieve accreditation and to meet the quality requirements of consumers' associations [2].
According to Khon [1], error is an integral part of human actions and the more complex a system is, the higher is the degree of risk. A healthcare system should be considered as a complex system with a number of variables (complexity of operations, specificity of individual patients, involvement of different professionals). Therefore, the risk of an error or incident is always present.
Anatomic Pathology can be taken as an example of a complex system, where errors could occur during different phases of the diagnostic process. The absence of a 'gold standard' for this discipline [3] makes the detection of errors complicated. However, they are easily and promptly identified when validated standard operating guidelines exist. There exists a small number of reports in medical literature concerning errors and their identification process in Anatomic Pathology. However, they mainly focus on inter-laboratory quality control and do not address the quality and the effective management of the specimens.
In the area of medical diagnostics, detecting such errors is heavily influenced by the subjectivity, education and attitude of the operator. The number of errors committed during the preparation of histology slides is also significant. A correct process is crucial for a correct and valid diagnosis, but this process is not automated and, therefore, it is dependent on the ability of the single operator [4, 5].
As data provided for laboratory medicine, and specifically by Anatomic Pathology, represent a central element of the whole diagnostic and therapeutic process, increasing attention is given to errors and potential 'pitfalls' of the diagnostic pathway in these areas that were of marginal interest in the past or were only considered for clinical chemistry (numerical and quantitative) data [6]. However, to date, there are few papers concerning systemic analysis of errors within AP than papers concerning more specific clinical sectors.
This study attempted to widen the culture of procedural error prevention within Anatomic Pathology.
Assuming that education and training of personnel on the subject of error and the analysis of errors avoiding a punitive approach results in an increased level of attention in the workplace, and starting from the taxonomy defined by previous works [4], the study helped to increase the related knowledge and to detect deep causes of procedural errors that are committed within Anatomic Pathology.
This research did not focus on wrong diagnoses [7, 8], but on the problems related to the histology preparation of routine processes. Errors related to histology diagnosis, therefore, were not taken into consideration. The objective of the study was to evaluate the critical points in the process of pre-analytical histology in an Anatomic Pathology laboratory.
An increase in the perceived responsibility of each person involved in a process, results in the creation of a culture of prevention and risk analysis. Initially, therefore, errors found in the processes of accessioning, gross dissecting, processing, embedding, tissue cutting and slide mounting, coloring, and labeling and releasing, were collected and classified. These were then assessed, in order to identify contributing factors and causes of the most common and serious errors. Finally, the identification of solutions was undertaken, in order to reduce the errors and the consequent clinical risk.
Root cause analysis, which is one of the techniques used for detection of an error and identification of the cause, is of particular relevance. It is a structured investigation aimed at identifying the deep cause of a problem, and the necessary actions to eliminate the problem [9]. It is also a technique that, by analyzing the errors in a system, identifies the causes through an inductive method, i.e. through questions which explore the reason behind every action and the source of every possible deviation.
The main objective of this methodology is to understand in detail what happened, why it happened and what can be done to prevent it to happen again. It is, therefore, focused on the factors, the activities and the decisions that lead to an error [8].
To be effective, root cause analysis should be applied to all areas where an error may occur during the clinical process of patient care and involve both the therapeutic and the diagnostic activity in the laboratory.
Methods
Error detection
The study, that was performed during a trimester in a laboratory of a leading hospital in Lombardy, defined, on the basis of literature and of the experience gained by the staff involved, an 'a priori' list of errors that can occur in the processes (Tab. I) and then constructed a survey form to record: the date of the detected error; the type of error according to the classification by Reason [10] (based on rules, based on knowledge, slips, lapses), whether of omission or commission; the index of severity (Table II presents the severity rating scale adopted); the process in which the error was detected (accessioning, gross dissecting, processing, embedding, tissue cutting and slide mounting, coloring slides, labeling and releasing slides); and the operator who committed the error (piece selector, inclusor, cutter, secretary, freezer).
Tab. I.
Errors that may occur in the processes
| Accessioning | Gross dissecting | Processing | Embedding | Tissue cutting and slide mounting | Coloring slides | Labeling and releasing slides |
|---|---|---|---|---|---|---|
| The specimen was not in the container | Incorrect numbering of the slides containers | Unfinished program | Loss of specimen | Loss / exhaustion of specimen | Insufficient time for dewaxing | Error of number reported on the slide labels |
| Inconsistency between the specimen and request | Incorrect numbering of the containers of biological material | Instrumental error / error in temperature | Contamination | Number was reported incorrectly | Exhaustion of reactive | Exchange of slides |
| Request or supply not received | Error in checking of the request | Exchange of reagents | Exchange of specimen | Collection of incorrect section | Wrong coloring | Broken slide |
| Specimen wrongly accessioned | Incorrect choice of the containers | Mistaken choice of the program | Incorrect selection of paraffin | Contamination | Detachment of section from the slide | Slides were not delivered |
| Incomplete request | Loss of specimen | Excessive number of containers | The specimen was badly positioned | Thickness selection error | Breakage of slide | Specimen to be decalcified not reported |
| Allocation number error | Mistaken specimen | Loss of specimen | Damaged sample | Misuse of coloring solutions | Mistaken requests | |
| Worksheet wrongly attached | Contaminated specimen | Error in identification of block to be cut | Error in assembling | |||
| Registration error | The specimen was not decalcified | Error in coloring or lack of coloring | Error due to automatic coloring | |||
| Incorrect type of fixative | The specimen was not loaded or Incorrectly loaded | Collection of slides in incorrect dyes | Error in the choice of the program | |||
| Incorrectly mounted slide |
Tab. II.
Severity rate scale adopted in the study.
| Index | Consequence |
|---|---|
| 1 | No effect |
| 2 | Repetition of a process |
| 3 | Repetition of multiple processes |
| 4 | Lengthening of response time |
| 5 | Re-sampling for sampling error |
| 6 | Partial loss of material - re-sampling |
| 7 | Loss of material: repeat sampling (non invasive) |
| 8 | Loss of material: repeat sampling (invasive) |
| 9 | Loss of material: it is not possible to repeat sampling |
| 10 | Incorrect diagnosis |
The survey form also included an area where the technician could suggest the possible causes of the error. The form was filled by trained technical staff, who had been previously identified as being responsible for the immediate recording of errors.
The activity of the technical staff was planned weekly where; two technicians were assigned to the procedures of accessioning and gross dissecting, two technicians to the procedures of processing and embedding, and four technicians to the procedures of tissue cutting and slide mounting, coloring, and labeling and releasing. The ability to detect and, at the same time, report the error instead of reporting it at the end of the process or even at the end of the working day prevented the loss of information. In addition, a person was assigned to fill the survey form prevented the collection of errors, thus reducing the risk of missing reports.
Analysis of errors and identification and analysis of the causes
In the first phase there was a preliminary assessment of all functional errors in the selection of cases to be submitted to root cause analysis, in particular, errors with gravity greater than 5 or high frequency of detection. The selected causes were then analyzed using two of the methods provided for in root cause analysis.
On the basis of assessments, that highlighted the absence of intermediate or higher levels of severity for the errors reported, it was decided to select, for the root cause analysis, errors with a higher frequency of detection; in particular, the causes of errors in the transcription of the number of identification from the generation of the code to the delivery phase of the prepared histology.
After the analysis of these causes, there was a further assessment to evaluate the potential risk; the incorrect transcription of the identification number may lead to an incorrect diagnosis and, also, to additional or unnecessary examinations or possible wrong therapy.
A multidisciplinary team of professionals trained to use root cause analysis was assembled. Team consisted of two technicians of the histology laboratory, the internal quality manager of the hospital and a medical doctor, trained in risk management, who works in a different hospital. The heterogeneity of the team allowed to highlight different points of view and to increase the robustness of results.
A 'fishbone' diagram [11] was built by the team, through a brainstorming activity that identified possible causes, contributing factors and, in addition, issues relating to the working environment, the system, staff and the external environment.
Starting from the reported errors, the 'fishbone' diagram was applied in order to identify potential causes and areas where the errors developed (Fig. 1).
Fig. 1.
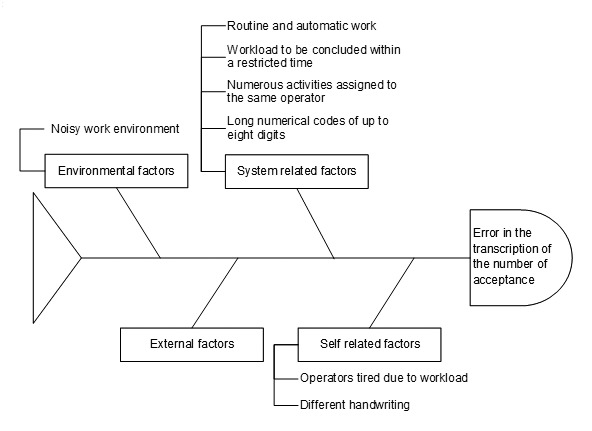
Incorrect transcription of the number of acceptance 'fishbone diagram'.
In particular, this was undertaken to identify causes related to environmental factors (workplace noise), causes related to staff (operators tired due to workloads, different handwriting) as well as causes related to system factors (many overlapping activities related to the same operator, workloads to be conducted in a restricted time, routine and automatic work, long numerical codes of up to eight digits). In this analysis there were no causes linked to factors outside of histology.
In the second phase, for each of the causes identified, the technique of 'Five whys?' [12] was applied in order to reach the root cause of each problem. However, it was not possible to answer the five "whys" in all the analyses carried out as the technique becomes exhausted when the root cause is identified. Finally, possible corrective measures were assessed in order to eliminate the identified causes.
Results
Errors highlighted in the individual processes
Over the whole period of the study 8,346 histological cases were reviewed, 19,774 samples were made and 29,956 histologies were prepared. The analytical process is reported as a flow-chart in Figure 2.
Fig. 2.
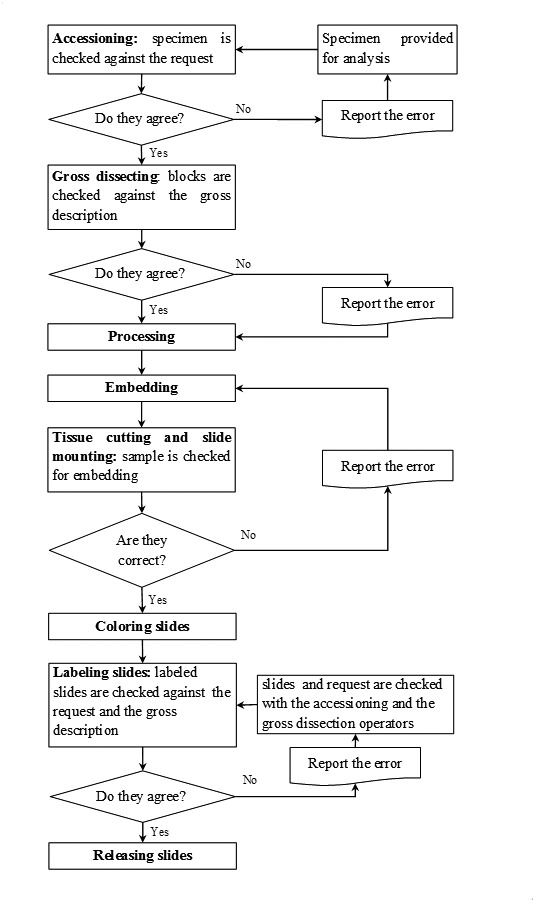
Flow chart of the histologies processing process.
From February 1st 2010 to April 31st 2010, 132 errors were identified as reported in Table III.
Tab. III.
Type and number of errors identified.
| Type of error | Errors, No. (%) |
|---|---|
| accessioning | 9 (6.5) |
| inconsistency between the specimen and request | 3 |
| requests or supply not received | 3 |
| allocation number error | 1 |
| worksheets wrongly attached to the request | 2 |
| gross dissecting | 37 (28) |
| incorrect numbering of the slides container | 26 |
| errors in checking of the request | 3 |
| incorrect choice of the containers | 3 |
| specimen not decalcified | 3 |
| specimen incorrectly loaded | 2 |
| processing | 2 (1.5) |
| exchange of reagents | 1 |
| instrumental error | 1 |
| embedding | 6 (4.5) |
| specimen was badly positioned | 5 |
| contamination | 1 |
| tissue cutting and slide mounting | 30 (23) |
| error in identification of block to be cut | 7 |
| the number was reported incorrectly | 15 |
| lack of coloring information | 6 |
| collection of incorrect section | 1 |
| thickness selection error | 1 |
| coloring slides | 2 (1.5) |
| misuse of coloring solutions | 1 |
| detachment of the section from the slide | 1 |
| labeling and releasing slides | 46 (35) |
| errors of number reported on the slide label | 44 |
| slides were not delivered | 2 |
| Total | 132 (100) |
During the different phases of processing there was no detection of the following errors: during the accessioning phase there were neither errors related to specimens which were not in the container, nor to specimens intended for another laboratory, incomplete requests, registration nor type of fixative used. During gross dissecting, there were no errors in the numbering of the containers of biological material, loss of specimen, specimen mismatching and sample contamination. During processing, there were no reported errors in the process temperature, the choice of the program, unfinished program, the quantity of loaded containers and in the loss of specimen. During embedding, there were no errors for incorrect selection of paraffin, mismatching or loss of specimen. During tissue cutting and slide mounting, there were no errors of loss, exhaustion or contamination of specimen, damage of samples on the slides and of the incorrect dyes, i.e. different from requested ones. During coloring, there were no errors related to insufficient time for dewaxing, to exhaustion of reagents, to wrong coloring, to breakage of slides, to wrong side mounting of the slide and, finally, to the use of automatic coloring. During labeling and releasing there were no errors in the mismatching of slides or of requests, either of specimen to be decalcified not reported on the request and, finally, of breakage of slides.
Evaluation of Errors
The classification for each reported error took into account the following considerations:
98.5% of the errors were due to lack of attention and oversight and only 1.5% appeared to be due to a lack of knowledge of the process;
85% of the errors were of commission type, due to incorrect practical actions, while 15% of the errors were of an omission type or due to failure to perform an action;
10% of the errors had a severity index equal to 4; these errors resulted delayed report to physician;
2% had a severity index between 2 and 3, inducing the repetition of one or more processes;
88% had a severity index of 1 so they had no consequences.
It is important to consider that the majority of errors that were listed had no consequences for the patient (severity index not greater than 4) due to the ability of the working team in identifying and correcting them. However, as reported also in other works [13], these errors lead to a "near miss event" and required considerable time to be corrected.
73% of errors were detected only in the final process of labeling and releasing. In particular, all the errors related to copying and numbering committed during the gross dissecting process were recognized only at the time of delivery of the slides. Only 1.5% of errors was detected during the reading phase by the pathologist because of contamination and (or) material not consistent with that reported in the clinician's request.
85% of errors were detected during the processes of gross dissecting, tissue cutting and slide mounting, labeling and releasing. 80% of these errors appeared to be attributable to a number of incorrect transcriptions of containers identification, on slides and on labels applied to the slides at the time of delivery.
While considering it as a possible cause of error, the problem of different handwriting styles of each individual operator was not investigated.
Each proximate cause, identified in the 'fishbone' diagram, was investigated by the team using the question 'why?'.
Proximate cause 1: why is the work place noisy? Because safety cabinets are working and technician and pathologist need to talk. Why? Because safety cabinets are necessary to protect operators from dangerous substances. Pathologists describe specimens during gross dissection. Accessioning specimens are checked against request by two technicians: the first one reading the request the second one checking the specimen. Why? Because specimen description and identification number on blocks are handwritten by one technician during gross dissection. Why? Because automated blocks labeling system lacks in the laboratory.
Proximate cause 2: while considering it as possible cause of error it was not thought necessary to go deeper into the problem of different handwriting styles as an immutable feature of each individual operator. However the use of an automated specimen identification system would solve the problem.
Proximate cause 3: why are the operators tired? Because of personnel shortage.
Why? Handwriting of blocks, labels, slide and gross descriptions involves a number of technicians. Why? Automated specimen identification system is lacking in the laboratory.
Proximate cause 4: why is the work routine and automatic? Because the identification numbers on blocks slides and labels are handwritten. Why? Automated label maker for slides and blocks lacks in the laboratory. Why? Automated specimen identification system is lacking in the laboratory.
Proximate cause 5: why do activities overlap? Due to handwritten specimens and checking made during gross dissection. Why? Surgical pathology cases are accessioned in two large batches, one in the morning and one in the afternoon and the lack of automated label maker for blocks leads to overlapping. Why? Automated specimen identification system lacks in the laboratory. Surgical pathology cases are delivered from hospital twice a day. Why? Because histology and hospital are located in different buildings.
Proximate cause 6: numerical code of up to eight digits was not investigated because it is an unchangeable security system feature.
Proximate cause 7: why are workloads to be concluded in restricted times? Because large batches of accessioning specimens are checked in short time. Why? Because surgical pathology cases are delivered from hospital to histology twice a day, where inappropriate specimen or request have to be immediately returned to hospital. Why? Because histology and hospital are located in different buildings.
The answers identified were inserted into a chart with each answer below its parent, the result is a 'tree' where the roots are the deeper causes (Fig. 3).
Fig. 3.
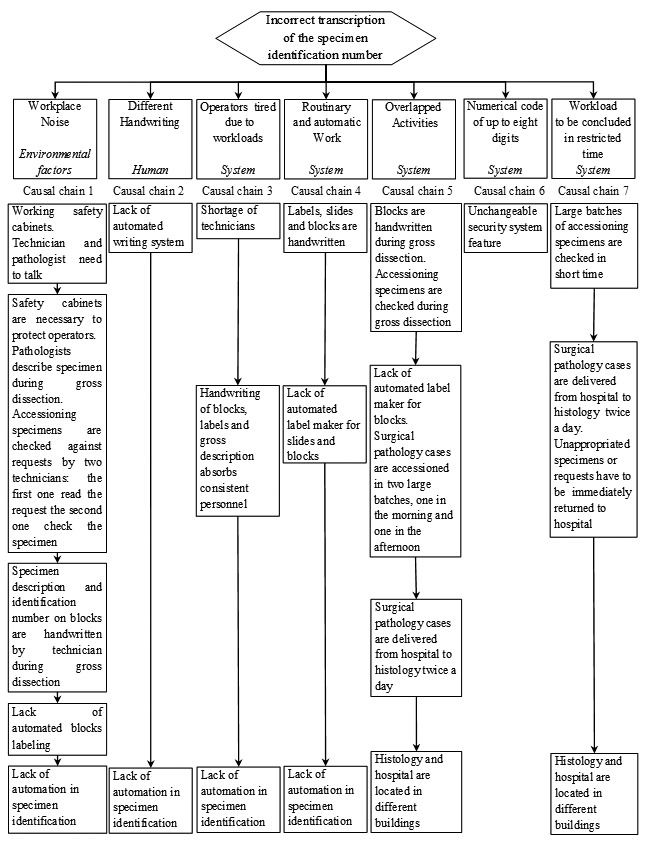
Incorrect transcription of the specimen identification number 'Five whys' technique results.
In the path to find the root causes there was no disagreement; all the causes identified by the members of the team were considered and analyzed.
Discussion and conclusions
The root cause analysis carried out in the study revealed two causes which can be considered as the common root causes to most problems.
The first cause was the different locations of the histology laboratory and the hospital from where the test material came from. The material was delivered only twice a day, with the consequent need for accessioning in a short time, requiring more human resources, sometimes involved in other processes, in this unique operation. This root cause is to be referred to pre-analytic phase.
The second cause was the lack of automation in the numerical identification of containers, in the dictation and typing of the macroscopic description of the test material and in the numbering of the labels and the slides. This was not only the reason why the errors occurred, but also a waste of time for the personnel. The lack of automation is a root cause which influence both pre-analytic and analytic phases. As indicated by other authors14 the introduction of technology and the adoption of automatic tracking system perfectly integrated with all the available technologies dramatically reduce errors in specimen processing.
In the study, the first cause, i.e. the problem of the location of histology, was not considered as it is beyond the possibilities of intervention. By contrast, the second cause allowed the possibility of some feasible solutions, such as the implementation of automatic systems for the management of the samples, e.g. a computerized system which includes the use of a unique bar code generated in the identification phase. Thus, by reading the bar code with an optical reader directly from the gross dissecting location, the pathologist can access the folder of the case, record in a voice recorder the description of the sample, and assign and print the number of the slides container. The focus of the study was the analysis of pre-analytic and analytic phases, therefore, root causes related to post analytic phase were not investigated.
The use of such systems would appear to be useful not only to obtain a decrease in the number of errors, but also to control the sample position during the processes. It was found that root cause analysis is a technique that is useful and easy to use in clinical risk management. In particular, as shown in the study, it allows the possibility to highlight points of reliable importance for improving the methodology and assuring procedural safety.
The initiation of an error analysis program could become a stimulus for the detection of errors. Operators who already use a quality management system would be encouraged to report events. They would also be aware that this will be brought to the attention of the risk manager for enhancing process quality improvements. In this regard, it is useful that all staff involved is adequately informed about the objectives which must be achieved and about the possibility of changes resulting from reporting an incident and applying RCA.
In addition, the tools used for the analysis of cases, in order to investigate the factors involved, have proved effective and easy to use. The 'fishbone' diagram is particularly beneficial, also from a graphical point of view, helping to identify problems related to different types of factors involved (external factors, factors related to the system, factors related to staff and environmental factors) and to undertake an initial identification of causes. Furthermore, the 'Five whys?' method allows an in depth analysis of the causes, distinguishing all the steps, from the identification of the problem to the root cause.
Finally, root cause analysis can be applied within different contexts due to its focus on processes, and can therefore be successfully applied within hospitals accredited by National Health Service.
Thus, the proper identification of procedures to be submitted to this method, by carefully assessing the gravity and frequency of individual errors encountered in a routinely performed evaluation, is crucial.
The study identified errors, that are typical and specific of the laboratory where the analysis was performed, and it did not overlap with other studies, already published, relating to error collection databases. Most of the works concerning errors in surgical pathology are focused on misidentification or mislabeling cases or specimens [14, 15], it is therefore difficult to compare them with the results presented in this study.
In addition, the study framework, will help organisations and regulating bodies within the health care service to focus on two fundamental concepts:
the application of root cause analysis, to the processes that characterize a healthcare area, represents a useful tool to correct working habits that may generate errors and reduce safety;
the training of operators, to develop awareness of the fact that errors represent a central aspect of the risk management process.
References
- 1.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 2.Sirota RL. Error and error reduction in pathology. Arch Pathol Lab Med. 2005;129:1228–1233. doi: 10.5858/2005-129-1228-EAERIP. [DOI] [PubMed] [Google Scholar]
- 3.Foucar E. Error in anatomic pathology. Am J Clin Pathol. 2001;116(Suppl.1):S34–S46. doi: 10.1309/DDKV-E4YP-CJ5Q-3M4V. [DOI] [PubMed] [Google Scholar]
- 4.Zarbo RJ, Meier FA, Raab SS. Error detection in anatomic pathology. Arch Pathol Lab Med. 2005;129:1237–1245. doi: 10.5858/2005-129-1237-EDIAP. [DOI] [PubMed] [Google Scholar]
- 5.Sirota RL. Defining error in anatomic pathology. Arch Pathol Lab Med. 2006;130:604–606. doi: 10.5858/2006-130-604-DEIAP. [DOI] [PubMed] [Google Scholar]
- 6.Bonini P, Plebani M, Ceriotti F, et al. Errors in laboratory medicine. Clin Chem. 2002;48:691–698. [PubMed] [Google Scholar]
- 7.Nodit L, Balassanian R, Sudilovsky D, et al. Improving the quality of cytology diagnosis: root cause analysis for errors in bronchial washing and brushing specimens. Am J Clin Pathol. 2005;124:883–892. [PubMed] [Google Scholar]
- 8.Raab SS, Grzybicki DM, Zarbo RJ, et al. Anatomic pathology databases and patient safety. Arch Pathol Lab Med. 2005;129:1246–1251. doi: 10.5858/2005-129-1246-APDAPS. [DOI] [PubMed] [Google Scholar]
- 9.Anderson B, Fagenhaug T. RCA: Simplified tool and techniques. Milwaukee, WI: ASQ Quality Press; 2000. [Google Scholar]
- 10.Reason J. Human error. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 11.Fernandes CM, Walker R, Price A, et al. Root cause analysis of laboratory delays to an emergency department. J Emerg Med. 1997;15:735–739. doi: 10.1016/s0736-4679(97)00158-3. [DOI] [PubMed] [Google Scholar]
- 12.Ammerman M. The Root Cause Analysis Handbook: A Simplified Approach to Identifying, correcting and Reporting Workplace Errors. New York, NY: Quality Resources; 1998. [Google Scholar]
- 13.Smith ML, Raab SS. Near-miss event rates in a traditional surgical pathology accessioning and gross examination laboratory. Mod Pathol. 2009;22(suppl 1):366A–366A. Abstract 1663. [Google Scholar]
- 14.Nakhleh RE, Idowu MO, Souers RJ, et al. Mislabeling of cases, specimens, blocks, and slides: a college of american pathologists study of 136 institutions. Arch Pathol Lab Med. 2011;135:969–974. doi: 10.5858/2010-0726-CPR. [DOI] [PubMed] [Google Scholar]
- 15.Layfield LJ, Anderson GM. Specimen labelling errors in surgical pathology. Am J Clin Pathol. 2010;134:466–470. doi: 10.1309/AJCPHLQHJ0S3DFJK. [DOI] [PubMed] [Google Scholar]


