Oxytocin (OT) matters for social functioning, and optimal evaluations of therapeutic efficacy matter for individuals with psychiatric and neurodevelopmental disorders. Recently, Guastella and Hickie (1), experts in the field of autism therapeutics, provided a comprehensive review of the potential of OT for improving social cognition in patients with autism spectrum disorder (ASD) and emphasized the need for better approaches to evaluate OT therapies in clinical settings. The ancient 500 million-year-old OT system has undergone tremendous physiologic transformations in neuronal morphology, axonal projections, and receptor distribution in the brain to shape species-specific social features, such as maternal motivation, pair bonding, and social learning (2). By using pharmacologic approaches and transgenic mice with mutations in the genes encoding OT or its receptor, researchers refined the role of OT in social information processing and social recognition (3). In recent decades, OT has become the sweetheart of social neuroscientists because of its effects on social behavior and its potential for enhancing social skills in individuals with psychiatric disorders. Long-term administration of OT to individuals with ASD was recently shown to be safe and to increase social reciprocity (4,5) and eye gaze (4). These behavioral improvements were associated with increased functional connectivity between anterior cingulate cortex and dorsomedial prefrontal cortex (4), which is known to be significantly altered in ASD. These promising findings are in agreement with the recent discovery that long-term intranasal administration of OT restored social behavior in a mouse model of autism (6). The key challenge in intranasal OT research is to translate optimistic research findings into potential therapies yielding sustainable improvements in social functioning in individuals with ASD.
In their review, Guastella and Hickie thoughtfully highlighted the limitations of current clinical trials and the complexity in crossing from the laboratory to meaningful clinical evaluation of therapeutics. Today, we are exposed to extreme opinions on the effects of intranasal OT that do not help move autism therapy forward. In their timely and very important article, Guastella and Hickie provided constructive criticisms and concerns that touch on the heart of the problem of intranasal OT and autism therapy from a clinical and neuroscience perspective. Such “gold standard” approaches in deciphering complex neurobehavioral deficits are crucial for providing advances in the domain of psychiatry. Several points were addressed in this review, including the importance of dose studies, different types of delivery of OT, and safety of intranasal OT. I will highlight and expand on some of the most important issues that could be the source of a lack of consistency in the efficacy of any therapeutics in autism.
Phenotypic heterogeneity in ASD is one of the most vital points that Guastella and Hickie addressed in their review. Heterogeneity in ASD is due to patient diversity in several areas, including genetics, epigenetics, and comorbidities (hyperactivity, anxiety, intellectual disability, seizures, gastrointestinal dysfunctions). This heterogeneity matters; for example, intrinsic differences in affiliation within prairie voles or between monogamous prairie voles and promiscuous meadow voles are associated with differences in responses to OT administration. This dissociation in affiliation is associated with both individual and species-dependent patterns of OT receptor (OXTR) expression (2), which is associated with specific polymorphisms in the Oxtr gene (7). Along these lines, we previously found that the acute effects of exogenous OT were more pronounced in individuals with ASD with “active but odd” clinical characteristics compared with individuals with ASD with “aloof” characteristics (8). This finding could be due to different expression of OT receptors in the brain. The rs53576 polymorphism of the OT receptor gene was found to account for the variability in the effects of OT on the blood oxygen level–dependent activity of reward brain regions in response to reciprocated cooperation in healthy subjects (9). Thus, we expect that an individual’s characteristics, including socioemotional aptitudes, OXTR distribution in the brain, OXTR genetic polymorphisms, and numerous other factors, can impact the trajectory of effects of intranasal OT on social outcomes.
Guastella and Hickie stated that “given this heterogeneity, it is not surprising there is not a single medical treatment for the behavioral phenotype of social impairments” (1). One possible way for approaching this complex issue of heterogeneity is to disentangle homogeneous subgroups of ASD based on the specific phenotype of social dysfunctions after controlling for other comorbid symptoms, such as hyperactivity and anxiety. Understanding the essential phenotype of social dysfunctions is crucial. Is theory of mind or social motivation the core deficit of ASD? It is more likely that there are different sociobehavioral endophenotypes contributing to diversity within ASD.
These questions lead to another related essential point that Guastella and Hickie made, which is the need for objective outcome measures and biomarkers that account for the nature of autism. How do we measure these different social phenotypes of subgroups of individuals with ASD? Objective outcome measures matter; for example, subjective self-report statements can be highly affected by external factors (e.g., social reputation). A battery of implicit and objective tools that can measure the different subdomains of social functioning will be crucial.
The need for behavioral measures and subgroups of social functioning leads to a more basic question, to which there are several subjective answers: what do we mean by social functioning? Several types of adaptive social behaviors are essential for our daily social interactions (e.g., theory of mind, discriminating friends from foes, recognizing implicit emotions, cooperation, prosocial actions). However, instead of using these higher order social skills as parameters for defining and measuring ASD deficits or determining the effects of intranasal OT, it would be optimal to target fundamental mechanisms that underlie these adaptive skills. Hence, I propose that there is a need for a theoretical framework of social functioning that divides social aptitudes into subdomains of fundamental processes from a neurodevelopmental perspective.
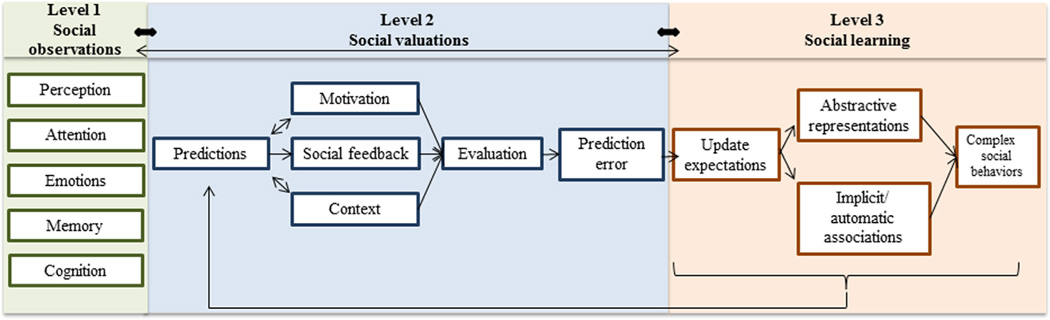
Guastella and Hickie mentioned the importance of translating laboratory findings to clinically relevant outcomes. A precise approach for understanding social functions matters. In line with the Research Domain Criteria policy of the National Institute of Mental Health, I propose a multilevel model of social learning (MSL) (Figure 1). This model is an illustration of a theoretical framework that defines common mechanisms that underlie any complex social behaviors. The first level of social observations entails the detection of social cues and observation of behavior of others, which are essential for calculating social expectations. The second level of social valuations consists of anticipating actions or thoughts based on social feedback, motivation value, and self-interest in a specific context. Social feedback can be complex, ranging from explicit and readable outcomes to more implicit or concealed intentions. The motivation value consists of our affiliative tendencies to approach others, to perceive social cues as rewarding, and to care for social approval and positive reputation. An evaluation of all these factors is necessary to generate a prediction error signal that will update our current behavior, actions, or thoughts. The third level, social learning, stands on the capacity to develop abstract representations and explicit and implicit associations of social signals within a generalized context. These three levels are intrinsically interactive and are dependent on innate predispositions, such as cognitive capacities, personalities, physiologic states, neural circuitry, and genetics. A theory-based model for social functioning such as the MSL can enable us to disentangle the different domains of social dysfunctions in ASD (e.g., social salience, social motivation, social valuation, and mental flexibility) and to better understand their neural correlates (Figure 2). For this MSL to be useful, we need to construct specific tests to evaluate the influence of therapies on each of these components in each level. Such evaluation would help us to more fully understand the processes most influenced by intranasal OT.
Figure 1. Multilevel model of social learning.
The first level (green) consists of simple observations of social cues and basic representations of the social world. The second level (blue) includes a more complex level of social learning and the calculation of prediction errors based on expectations and social outcomes. The third level (orange) is the most complex level of social learning and includes the capacity to update expectations and behaviors and to construct abstractive social knowledge that does not apply to one particular context. All three levels interact with each other (arrows); in particular, learning new implicit associations and expectations (level 3) can help generate predictions for future interactions in novel contexts (level 2) with different weights for self-interests and interests of others.
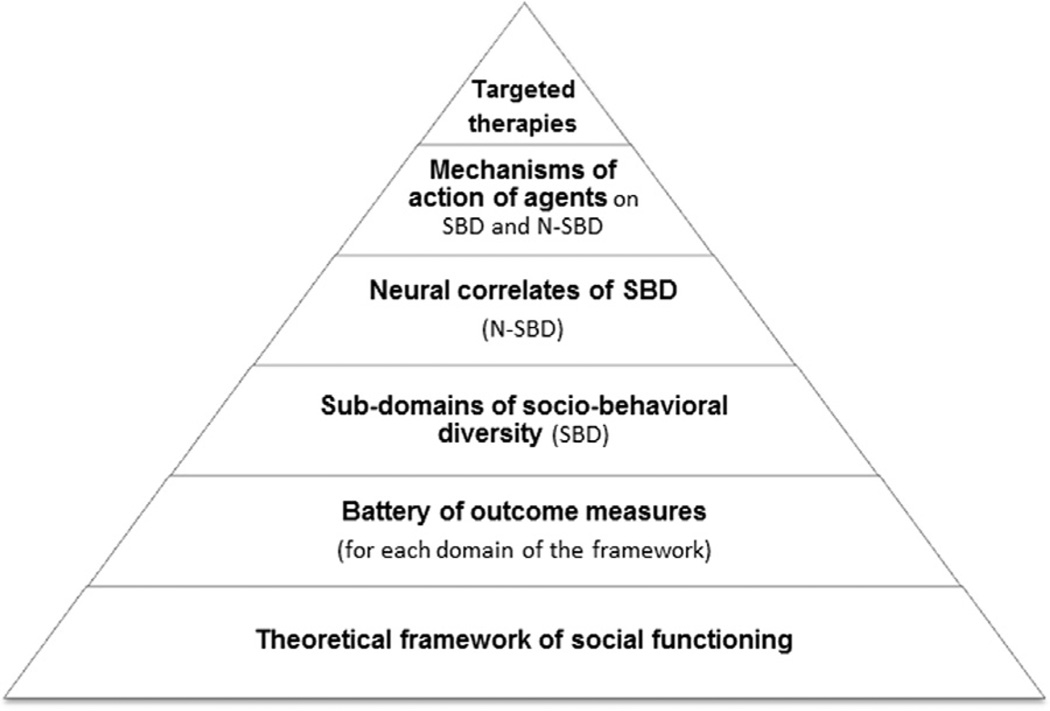
Figure 2. Hierarchical approach for optimal targeted therapies in psychiatric disorders.
The theoretical framework of social functioning is the foundation of targeted therapies. It delineates the different domains of the fundamental process of social learning that underlies all adaptive high-order behaviors. By using a battery of objective outcome measures, subdomains of sociobehavioral diversity (SBD) can be defined based on the different domains of the social framework. This can lead to a better understanding of neural and molecular underpinnings of these subdomains (N-SBD) and of neurobehavioral mechanisms of treatments (e.g., pharmacologic and behavioral therapies), including intranasal oxytocin. The goal of this approach is to create domain-specific therapies that are targeted toward patients’ specific social and neural deficits.
Guastella and Hickie mentioned some potential mechanisms of action of OT, such as its role in social salience, reward sensitivity, and social learning. We need a better understanding of the neurobehavioral mechanisms of OT, including its action on fundamental processes (e.g., within the MSL) and within subdomains of ASD, to optimize targeted therapies. It is possible that administering OT can affect more basic latent aptitudes related to emotion regulation, motivation, and attention and that in combination with targeted behavioral therapies we can maximize its effect on social cognition.
Guastella and Hickie concluded their review by stressing the complexity of evaluating the efficacy of OT in autism and that OT may have the “potential to provide a first medical treatment to improve social impairments for some” (1). Intranasal OT or other OT-based treatments, such as small molecule agonists, positive allosteric modulators, or drugs that can evoke endogenous release (10), might have great efficacy for treatment of social disorders. At this point, there is compelling evidence suggesting that the OT system is very likely to be a successful target for improving social functioning. We face many challenges, but by adopting translational approaches, developing theoretical frameworks, and encouraging collaborations between fundamental animal researchers and clinical scientists, we may significantly improve our ability to harness this powerful brain system to offer hope to families affected by ASD.
Acknowledgments
Early Career Investigator Commentaries are solicited in partnership with the Education Committee of the Society of Biological Psychiatry. As part of the educational mission of the Society, all authors of such commentaries are mentored by a senior investigator. This work was mentored by Larry J. Young.
The author would like to acknowledge funding support from NIH grants 1P50MH100023 and P51OD11132 to YNPRC.
I thank Larry J. Young for his mentorship, support, and editing.
Footnotes
Disclosures
The author reports no biomedical financial interests or potential conflict of interest.
References
- 1.Guastella AJ, Hickie IB. Oxytocin treatment, circuitry, and autism: A critical review of the literature placing oxytocin into the autism context. Biol Psychiatry. 2016;79:234–242. doi: 10.1016/j.biopsych.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–292. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–3412. doi: 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- 5.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.162. [published online ahead of print Oct 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra278. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King LB, Walum H, Inoue K, Eyrich NW, Young LJ. Variation in the oxytocin receptor gene predicts brain region specific expression and social attachment. Biol Psychiatry. doi: 10.1016/j.biopsych.2015.12.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng C, Lori A, Waldman ID, Binder EB, Haroon E, Rilling JK. A common oxytocin receptor gene (OXTR) polymorphism modulates intranasal oxytocin effects on the neural response to social cooperation in humans. Genes Brain Behav. 2015;14:516–525. doi: 10.1111/gbb.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modi ME, Inoue K, Barrett CE, Kittelberger KA, Smith DG, Landgraf R, et al. Melanocortin receptor agonists facilitate oxytoc-independent partner preference formation in the prairie vole. Neuropsychopharmacology. 2015;40:1856–1865. doi: 10.1038/npp.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]