Summary
Background
Every year, more than 32 million pregnancies in sub-Saharan Africa are at risk of malaria infection and its adverse consequences. The effectiveness of the intermittent preventive treatment with sulfadoxine–pyrimethamine strategy recommended by WHO is threatened by high levels of parasite resistance. We aimed to assess the efficacy and safety of two alternative strategies: intermittent screening with malaria rapid diagnostic tests and treatment of women who test positive with dihydroartemisinin–piperaquine, and intermittent preventive treatment with dihydroartemisinin–piperaquine.
Methods
We did this open-label, three-group, randomised controlled superiority trial at four sites in western Kenya with high malaria transmission and sulfadoxine–pyrimethamine resistance. HIV-negative pregnant women between 16 and 32 weeks’ gestation were randomly assigned (1:1:1), via computer-generated permuted-block randomisation (block sizes of three, six, and nine), to receive intermittent screening and treatment with dihydroartemisinin–piperaquine, intermittent preventive treatment with dihydroartemisinin–piperaquine, or intermittent preventive treatment with sulfadoxine–pyrimethamine. Study participants, study clinic nurses, and the study coordinator were aware of treatment allocation, but allocation was concealed from study investigators, delivery unit nurses, and laboratory staff. The primary outcome was malaria infection at delivery, defined as a composite of peripheral or placental parasitaemia detected by placental histology, microscopy, or rapid diagnostic test. The primary analysis was by modified intention to treat. This study is registered with ClinicalTrials.gov, number NCT01669941.
Findings
Between Aug 21, 2012, and June 19, 2014, we randomly assigned 1546 women to receive intermittent screening and treatment with dihydroartemisinin–piperaquine (n=515), intermittent preventive treatment with dihydroartemisinin–piperaquine (n=516), or intermittent preventive treatment with sulfadoxine–pyrimethamine (n=515); 1368 (88%) women comprised the intention-to-treat population for the primary endpoint. Prevalence of malaria infection at delivery was lower in the intermittent preventive treatment with dihydroartemisinin–piperaquine group than in the intermittent preventive treatment with sulfadoxine–pyrimethamine group (15 [3%] of 457 women vs 47 [10%] of 459 women; relative risk 0.32, 95% CI 0.18–0.56; p<0.0001), but not in the intermittent screening and treatment with dihydroartemisinin–piperaquine group (57 [13%] of 452 women; 1.23, 0.86–1.77; p=0.26). Compared with intermittent preventive treatment with sulfadoxine–pyrimethamine, intermittent preventive treatment with dihydroartemisinin–piperaquine was associated with a lower incidence of malaria infection during pregnancy (192.0 vs 54.4 events per 100 person-years; incidence rate ratio [IRR] 0.28, 95% CI 0.22–0.36; p<0.0001) and clinical malaria during pregnancy (37.9 vs 6.1 events; 0.16, 0.08–0.33; p<0.0001), whereas intermittent screening and treatment with dihydroartemisinin–piperaquine was associated with a higher incidence of malaria infection (232.0 events; 1.21, 1.03–1.41; p=0.0177) and clinical malaria (53.4 events; 1.41, 1.00–1.98; p=0.0475). We recorded 303 maternal and infant serious adverse events, which were least frequent in the intermittent preventive treatment with dihydroartemisinin–piperaquine group.
Interpretation
At current levels of rapid diagnostic test sensitivity, intermittent screening and treatment is not a suitable alternative to intermittent preventive treatment with sulfadoxine–pyrimethamine in the context of high sulfadoxine–pyrimethamine resistance and malaria transmission. However, dihydroartemisinin–piperaquine is a promising alternative drug to replace sulfadoxine–pyrimethamine for intermittent preventive treatment. Future studies should investigate the efficacy, safety, operational feasibility, and cost-effectiveness of intermittent preventive treatment with dihydroartemisinin–piperaquine.
Funding
The Malaria in Pregnancy Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine.
Introduction
In sub-Saharan Africa, more than 32 million pregnancies occur annually in malaria-endemic regions and in the absence of pregnancy-specific interventions. 12 million women delivering live babies (45% of all liveborn deliveries) would be exposed to malaria infection, which would cause an estimated 900 000 low birthweight deliveries due to preterm labour and intrauterine growth retardation.1–3 WHO recommends use of longlasting insecticide-treated nets throughout pregnancy and inter mittent preventive treatment with sulfadoxine–pyrimethamine in the second and third trimesters in malaria-endemic regions in sub-Saharan Africa.4 Intermittent preventive treatment with sulfadoxine–pyrimethamine is very effective for reducing the adverse outcomes of malaria during pregnancy,4,5 but is threatened by the emergence of widespread parasite resistance.6–9 Sulfadoxine–pyrimethamine is well tolerated, safe in the second and third trimesters, affordable, widely available, and can be given as a single dose, allowing for directly observed therapy in the antenatal clinics.4,10 The search for safe, effective, and well-tolerated alternative drugs to replace sulfadoxine–pyrimethamine for intermittent preventive treatment during pregnancy has proven elusive.11–16
Dihydroartemisinin–piperaquine is a fixed-dose artemisinin–based combination treatment with several properties that make it a potentially suitable replacement for sulfadoxine–pyrimethamine, but the drug has not yet been assessed for intermittent preventive treatment during pregnancy. Dihydroartemisinin–piperaquine is well tolerated, highly effective at clearing infections, and, importantly, the piperaquine component provides at least 4 weeks of post-treatment prophylaxis, the longest of all four fixed-dose artemisinin–based combination treatments currently available, including during pregnancy.17,18
An alternative strategy—intermittent screening and treatment in pregnancy—involves screening of asymptomatic women for malaria parasites with rapid diagnostic tests at every scheduled antenatal clinic visit, and treatment of only those who test positive. In west Africa, where sulfadoxine–pyrimethamine resistance is low, studies showed that intermittent screening and treatment with sulfadoxine–pyrimethamine, amodiaquine-arte-sunate,19 and artemether-lumefantrine20 was non-inferior to intermittent preventive treatment with sulfadoxine–pyrimethamine.
We did this study to establish whether intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin–piperaquine are superior to the existing strategy of intermittent preventive treatment with sulfadoxine–pyrimethamine for the control of malaria during pregnancy in a region of western Kenya with intense year-round malaria transmission and high sulfadoxine–pyrimethamine resistance.
Methods
Study design and participants
We did this open-label, three-group, randomised controlled superiority trial at four rural health facilities in Siaya County, western Kenya. In this region, 96% of parasites harbour high-level sulfadoxine–pyrimethamine resistance due to a series of five (quintuple) mutations in the parasite genes that encode the targets of pyrimethamine (dhfr) and sulfadoxine (dhps), and 5.8% carry the additional Pfdhps-A581G mutation (sextuple mutant; Desai M, unpublished). Sulfadoxine–pyrimethamine either failed to clear parasites or new infections occurred by day 42 in 45% of asymptomatic parasitaemic pregnant women who had received the drug for intermittent preventive treatment in this study region (Desai M, unpublished).
Eligible participants were HIV-negative pregnant women between 16 and 32 weeks’ gestation who had a viable pregnancy, no history of receiving intermittent preventive treatment with sulfadoxine–pyrimethamine during the pregnancy, were resident in the study region, and were willing to deliver in the study hospital. We excluded women with high-risk pregnancies, severe anaemia, or those unable to give consent.
The study protocol received ethics approval from the Kenya Medical Research Institute and the US Centers for Disease Control and Prevention.
Randomisation and masking
We randomly assigned participants (1:1:1), via computer-generated permuted-block randomisation (block sizes of three, six, and nine), to receive intermittent screening and treatment with dihydroartemisinin–piperaquine, intermittent preventive treatment with dihydroartemisinin–piperaquine, or intermittent preventive treatment with sulfadoxine–pyrimethamine. The randomisation assignments were put in sequentially numbered and sealed opaque envelopes, which were distributed to the study facilities and opened sequentially upon enrolment of a study participant by the study nurse. Study participants, study clinic nurses, and the study coordinator were aware of the treatment allocation, but allocation was concealed from study investigators, delivery unit nurses, and laboratory staff.
Procedures
Standardised questionnaires were administered to all participants and comprised demographic and socioeconomic information, use of insecticide-treated nets and indoor residual spraying, medical and obstetric history, medication use, and clinical assessment. Gestational age was ascertained by history of last menstrual period and physical examination. A 5 mL venous blood sample was collected for routine and study-specific testing, including haemoglobin concentrations, syphilis, and malaria (microscopy and filter paper for PCR), and urine was collected to test for leucocytes and proteinuria. All participants received a longlasting insecticide-treated net on enrolment.
Women received their allocated intervention at enrolment and at each scheduled focused antenatal care visit during the second and third trimesters of pregnancy. Women in the intermittent preventive treatment with sulfadoxine–pyrimethamine group received three tablets of quality-assured sulfadoxine–pyrimethamine (Durbin, Middlesex, UK; tablets contained 500 mg of sulfadoxine and 25 mg of pyrimethamine). Women in the intermittent preventive treatment with dihydroartemisinin–piperaquine group received a standard 3 day course with dihydroartemisinin–piperaquine (Eurartesim, Sigma Tau, Pomezia, Italy; tablets contained 40 mg of dihydroartemisinin and 320 mg piperaquine) and women in the intermittent screening and treatment with dihydroartemisinin–piperaquine group were screened for malaria with a histidine-rich protein-2—plasmodium lactate dehydro genase combination rapid diagnostic test (First Response Malaria pLDH/HRP2 Combo Test, Premier Medical Corporation, India). Women who tested positive received a standard 3 day course of dihydroartemisinin–piperaquine. Dosing of dihydroartemisinin–piperaquine was based on body weight at enrolment: two, three, or four tablets a day for bodyweights of 24–35.9 kg, 36–74.9 kg, and 75 kg or more, respectively. Sulfadoxine–pyrimethamine and the first dose of dihydroartemisinin–piperaquine were given as directly observed therapy. If vomiting occurred within the first 30 min, the full dose was repeated. Participants who vomited a repeat dose were given parenteral quinine or artemether-lumefantrine, but were not withdrawn from study follow-up. Participants receiving dihydroartemisinin–piperaquine were given the remaining two doses to take at home. All women were visited at home 2 days after enrolment to assess for drug safety, adherence, and use of longlasting insecticide-treated nets; additionally, a systematic sample of every fifth participant was visited at home in subsequent visits.
Women with symptoms of malaria were not excluded. If they were enrolled in the intermittent preventive treatment with sulfadoxine–pyrimethamine group, they were screened and, if found to have positive rapid diagnostic test results, given artemether-lumefantrine as per national guidelines. The first course of sulfadoxine–pyrimethamine was then given at the next scheduled visit.
All participants were required to make three or four additional scheduled visits, depending on the gestational age at enrolment, at intervals of 4–6 weeks, roughly mirroring the focused antenatal care schedule in Kenya. At each of these visits, participants received their allocated study intervention. Information about bednet use, symptomatology, and concomitant drug use was obtained and a physical examination done. Additionally, blood samples were taken for assessment of anaemia, malaria PCR, and microscopy. All women were retested for HIV in the third trimester as per Kenyan guidelines.
Participants who presented to the study clinic for unscheduled visits were examined by study staff and blood was taken for haemoglobin and malaria testing with rapid diagnostic tests for point of care (if needed) and malaria microscopy. Participants were assessed for adverse and serious adverse events during scheduled and unscheduled visits. Clinical malaria was treated with artemether-lumefantrine in the sulfadoxine–pyrimethamine group and with dihydroartemisinin–piperaquine in the other groups, or with artemether-lumefantrine if the malaria occurred within 4 weeks of the last dose of dihydroartemisinin–piperaquine.
At delivery, maternal peripheral blood was collected for malaria testing (by rapid diagnostic tests, microscopy, and PCR) and haemoglobin concentrations. Blood was collected from the maternal side of the placenta for malaria rapid diagnostic tests, microscopy, and PCR. An umbilical cord blood sample was collected for malaria rapid diagnostic tests and assessment of haemoglobin con centrations. A placental biopsy and placental impression smears were also collected. Newborn babies were weighed on a digital scale that was calibrated on a daily basis and measured weight to the nearest 10 g. Study nurses examined newborn babies for congenital malformation within the first 24 h of birth. The Ballard score was used to determine the gestational age at delivery.21
Participants and their babies were seen between 6 and 8 weeks post-delivery and babies were reassessed for congenital anomalies. Clinical and treatment findings for mother and babies were recorded. A blood sample was taken from the infant for malaria testing with rapid diagnostic tests and microscopy.
Outcomes
The primary outcome was malaria infection at delivery, defined as a composite of peripheral or placental parasitaemia detected by placental histology, microscopy, or rapid diagnostic tests (appendix). The main secondary outcomes were incidence of malaria infection and clinical malaria during pregnancy, defined as fever or recent history of fever in the presence of malaria parasites; prevalence of adverse newborn morbidity at birth, defined as a composite of either preterm delivery (<37 weeks’ gestation, low birthweight (<2500 g), or small for gestational age (<10th percentile relative to an external growth reference;22 anaemia (haemoglobin <110 g/L) during pregnancy or at delivery.
Statistical analysis
This trial was designed to detect a 50% decrease in malaria infection at delivery, from 12% to 6%, with 80% power at an α of 0.025 to allow for multiple comparison groups, which required a sample size of 432 women per group, or a total of 1296 women. To allow for a 20% loss, including both loss to follow-up and missing placental information, 1554 women were needed for recruitment. Observational studies of women receiving routine care in the study region estimated the prevalence of malaria at delivery to be roughly 18% (with a combination of histology and blood smear results). To allow for reductions in malaria transmission, we used a more conservative prevalence of infection of 12% at delivery. One interim analysis was planned (appendix).
We did the primary analysis in the modified intention-to-treat population, defined as all eligible women who were randomised, received at least one study intervention, and contributed to the outcome. The per-protocol population included women who received, on at least three separate occasions at least 4 weeks apart, either the scheduled study intervention or a protocol-approved alternative treatment for symptomatic malaria that replaced the need for the scheduled intervention, or those who delivered before completion of the three-visit schedule but received the intervention at least once. Women in the per-protocol population were also required to have taken all the daily study doses on each occasion, and to have contributed to the endpoint.
We compared treatment groups for binary responses with unadjusted log-binomial models and for continuous responses with unadjusted linear regression models. Multivariable log-binomial models were fit for the composite primary outcome and newborn morbidity at birth; however, the model did not converge for morbidity and a Poisson regression model was used instead. Count variables were expressed as incidence rate and compared using Poisson regression with follow-up time calculated from enrolment to delivery or time to loss to follow-up. We did analysis with SAS (version 9.2). This study is registered with ClinicalTrials.gov, number NCT01669941.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. VW, JW, JG, and MD had full access to the all the data in the study and MD and FOtK had final responsibility for the decision to submit for publication.
Results
Between Aug 21, 2012, and June 19, 2014, we randomly assigned 1546 women to receive intermittent screening and treatment with dihydroartemisinin–piperaquine (n=515), intermittent preventive treatment with dihydroartemisinin–piperaquine (n=516), or intermittent preventive treatment with sulfadoxine–pyrimethamine (n=515); 1368 (88%) women comprised the intention-to-treat population for the primary outcome (figure 1). Baseline characteristics were similar between groups (table 1). At enrolment, 58–63% of women were anaemic, the mean gestational age was 22.9 weeks (SD 4.8), and about a third of women had malaria parasites (table 1). The median number of intervention visits by study group was three (IQR 3 to 4) and was similar between groups (p=0.29). The median time between intervention visits was 33 days (IQR 29–42). In the intermittent screening and treatment group, 348 (68%) women received no courses of dihydroartemisinin–piperaquine, 140 (27%) received one course, 27 (5%) received two courses, and no women received three courses (appendix). The numbers of women who did not contribute to the primary outcome at delivery (n=178) did not differ by treatment group (figure 1). The prevalence of malaria (by microscopy) at baseline did not differ between women lost to follow-up before delivery (31 [17%] of 178 women) compared with those followed up successfully (214 [15%] of 1381 women).
Figure 1. Trial profile.
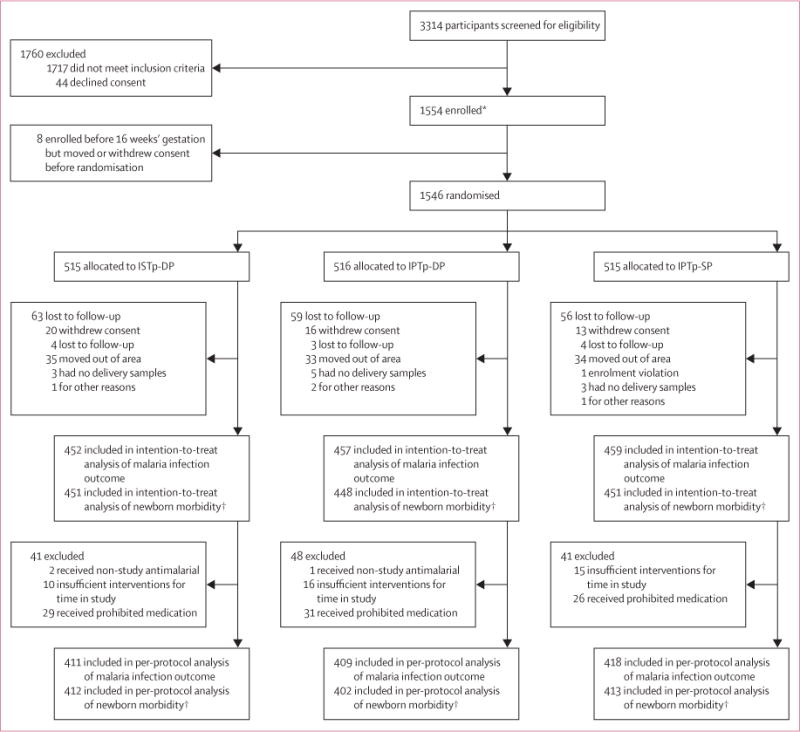
ISTp-DP=intermittent screening and treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-DP=intermittent preventive treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-SP=intermittent preventive treatment during pregnancy with sulfadoxine–pyrimethamine. *Women reporting to antenatal care clinics before 16 weeks’ gestation were booked into the trial, but were not randomised or given intervention until their next visit at 16 weeks’ gestation or more. †Composite of preterm delivery, low birthweight, or small for gestational age.
Table 1.
Baseline characteristics
| ISTp-DP group (n=515) | IPTp-DP group (n=514)* | IPTp-SP group (n=514)* | |
|---|---|---|---|
| Study site | |||
| Bondo | 153 (30%) | 153 (30%) | 154 (30%) |
| Lwak | 126 (24%) | 125 (24%) | 125 (24%) |
| Madiany | 160 (31%) | 159 (31%) | 159 (31%) |
| Siaya | 76 (15%) | 77 (15%) | 76 (15%) |
|
| |||
| Age (years) | 23.4 (5.9) | 23.4 (5.5) | 23.5 (6.0) |
|
| |||
| Gravidity | |||
| Primigravid or secundigravid (first or second pregnancy) | 267 (52%) | 263 (51%) | 292 (57%) |
| Multigravid (third pregnancy or more) | 248 (48%) | 251 (49%) | 222 (43%) |
|
| |||
| Gestational age (weeks)† | 22.9 (47) | 23.0 (4.0) | 22.8 (4.4) |
|
| |||
| Weight (kg) | 61.1 (8.3) | 61.8 (9.3) | 61.5 (9.1) |
|
| |||
| Height (cm) | 164.1 (6.8) | 164.3 (67) | 164.3 (6.9) |
|
| |||
| Educational status rank | |||
| Low | 121 (23%) | 119 (23%) | 118 (23%) |
| Medium | 231 (45%) | 222 (44%) | 224 (44%) |
| High | 162 (32%) | 168 (33%) | 169 (33%) |
|
| |||
| SES index | |||
| Low | 181 (35%) | 162 (32%) | 173 (34%) |
| Medium | 174 (34%) | 170 (33%) | 167 (33%) |
| High | 159 (31%) | 179 (35%) | 171 (33%) |
|
| |||
| Slept under a longlasting insecticide-treated net during the previous night | 292 (57%) | 292 (57%) | 294 (57%) |
|
| |||
| Haemoglobin (g/L) | 105 (160) | 106 (15) | 105 (15) |
|
| |||
| Anaemia (haemoglobin <110 g/L) | 325 (63%) | 302 (59%) | 307 (60%) |
|
| |||
| Malaria infection (by PCR) | 178/513 (35%) | 157/509 (31%) | 168/510 (33%) |
|
| |||
| Symptomatic malaria infection (by microscopy) | 14 (3%) | 8 (2%) | 4 (<1%) |
|
| |||
| Syphilis infection | 7/515 (1%) | 3/514 (<1%) | 7/514 (1%) |
Data are n (%), mean (SD), or n/N (%), unless otherwise specified. ISTp-DP=intermittent screening and treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-DP=intermittent preventive treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-SP=intermittent preventive treatment during pregnancy with sulfadoxine–pyrimethamine. SES=socioeconomic status.
Two women were excluded in the IPTp-DP group and one woman was excluded in the IPTp-SP group because of withdrawal of consent before baseline intervention was given (n=1), loss of study data file (n=1), and enrolment violation (n=1).
Determined by history of last menstrual period and physical examination.
Compared with women who received intermittent preventive treatment with sulfadoxine–pyrimethamine, prevalence of malaria infection at the time of delivery was lower in the intermittent preventive treatment with dihydroartemisinin–piperaquine group, but not in the intermittent screening and treatment with dihydroartemisinin–piperaquine group (figure 2A). Their effect was not modified by gravidity (pinteraction=0.74), and similar results were obtained after adjustment for baseline covariates (figure 2A), both in per-protocol analyses (appendix) and when PCR was added to the composite outcome (table 2).
Figure 2. Malaria at time of delivery (A) and newborn morbidity outcome (B) by treatment group in the intention-to-treat population.
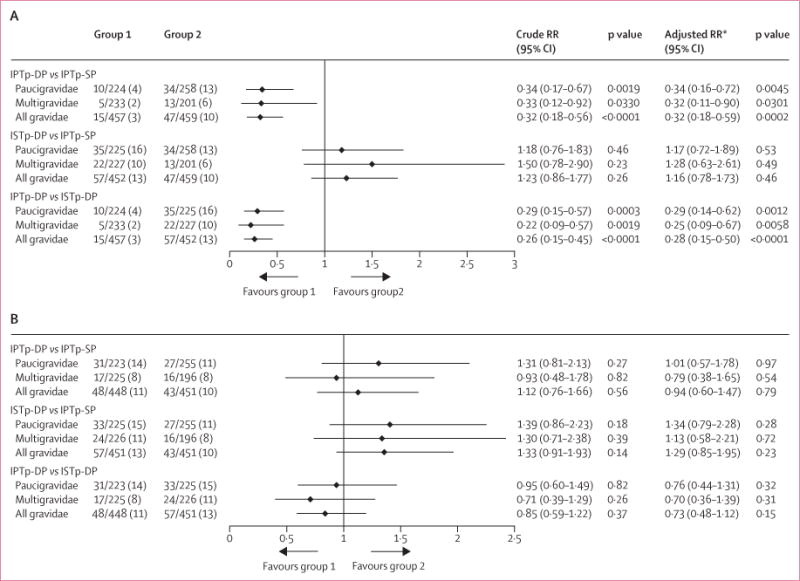
Data are n/N (%), unless otherwise specified. Malaria infection at delivery detected by placental histology, microscopy, or rapid diagnostic tests. RR=relative risk. ISTp-DP=intermittent screening and treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-DP=intermittent preventive treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-SP=intermittent preventive treatment during pregnancy with sulfadoxine–pyrimethamine. *Adjusted for site, gravidity (in all-gravidities model), malaria at enrolment by PCR, rain or seasonality 6 months before delivery, maternal height (malaria infection model only), use of longlasting insecticide-treated nets during pregnancy, haemoglobin concentration at enrolment, gestational age at enrolment, and educational status of mother.
Table 2.
Malaria-related perinatal outcomes by treatment group in the intention-to-treat population (all gravidities)
| ISTp-DP group | IPTp-DP group | IPTp-SP group | Crude prevalence ratio or difference (95%CI); p value | Crude prevalence ratio or difference (95%CI); p value | Crude prevalence ratio or difference (95%CI); p value | |
|---|---|---|---|---|---|---|
| ISTp-DP vs IPTp-SP | IPTp-DP vs IPTp-SP | IPTp-DP vs ISTp-DP | ||||
| Maternal haemoglobin (g/L; third trimester) | 110 (14); n=384 | 110 (14); n=370 | 111 (15); n=375 | −0.07 (−0.30 to 0.14); p=0.53 | −0.05 (−0.26 to 0.15); p=0.61 | 0.01 (−0.20 to 0.22); p=0.91 |
| Maternal anaemia (haemoglobin <110 g/L; third trimester) | 176/384 (46%) | 186/370 (50%) | 176/375 (47%) | 0.98 (0.84 to 1.14); p=0.76 | 1.07 (0.92 to 1.24); p=0.36 | 1.10 (0.95 to 1.27); p=0.22 |
| Maternal moderate anaemia (haemoglobin <90 g/L; third trimester) | 30/384 (8%) | 21/370 (6%) | 31/375 (8%) | 0.95 (0.58 to 1.53); p=0.82 | 0.69 (0.40 to 1.17); p=0.17 | 0.73 (0.42 to 1.25); p=0.25 |
| Maternal haemoglobin at delivery (g/L) | 116 (16); n=421 | 117 (15); n=426 | 116 (17); n=427 | 0.01 (−0.20 to 0.23); p=0.90 | 0.14 (−0.08 to 0.35); p=0.21 | 0.12 (−0.09 to 0.34); p=0.26 |
| Maternal anaemia at delivery (haemoglobin <110 g/L) | 142/421 (34%) | 115/426 (27%) | 147/427 (34%) | 0.98 (0.81 to 1.18); p=0.81 | 0.78 (0.64 to 0.96); p=0.0165 | 0.80 (0.65 to 0.98); p=0.0311 |
| Maternal moderate anaemia at delivery (haemoglobin <90 g/L) | 20/421 (5%) | 19/426 (4%) | 20/427 (5%) | 0.93 (0.52 to 1.68); p=0.82 | 0.87 (0.48 to 1.58); p=0.64 | 0.93 (0.50 to 1.71); p=0.81 |
| Malaria infection (third trimester) | 75/473 (16%) | 34/478 (7%) | 90/470 (19%) | 0.83 (0.63 to 1.09); p=0.18 | 0.37 (0.26 to 0.54); p<0.0001 | 0.45 (0.31 to 0.66); p<0.0001 |
| Peripheral or placental malaria at delivery (any measure including PCR, excluding past infections) | 79/452 (17%) | 26/457 (6%) | 73/459 (16%) | 1.10 (0.82 to 1.47); p=0.52 | 0.36 (0.23 to 0.55); p<0.0001 | 0.33 (0.21 to 0.50); p<0.0001 |
| Maternal peripheral malaria infection at delivery (any measure) | 65/452 (14%) | 16/457 (4%) | 54/459 (12%) | 1.22 (0.87 to 1.71); p=0.21 | 0.30 (0.17 to 0.51); p<0.0001 | 0.24 (0.14 to 0.41); p<0.0001 |
| Placental malaria (any measure including PCR and past infections on histology) | 190/414 (46%) | 139/421 (33%) | 159/426 (37%) | 1.23 (1.05 to 1.45); p=0.0121 | 0.88 (0.74 to 1.06); p=0.19 | 0.72 (0.61 to 0.85); p=0.0002 |
| Peripheral or placental malaria at delivery (any measure including PCR and past infections on histology) | 199/452 (44%) | 140/457 (31%) | 166/459 (36%) | 1.22 (1.04 to 1.43); p=0.0159 | 0.85 (0.70 to 1.02); p=0.08 | 0.70 (0.59 to 0.83); p<0.0001 |
| Maternal parasite density at delivery | 107 (45–250); n=32 | 177 (49–634); n=9 | 76 (37–154); n=29 | 0.34 (−0.73 to 1.42); p=0.53 | 0.84 (−0.76 to 2.45); p=0.30 | 0.50 (−1.08 to 2.09); p=0.54 |
| Fetal cord haemoglobin (g/L) | 141 (24); n=400 | 143 (23); n=401 | 144 (26); n=403 | −0.30 (−0.64 to 0.04); p=0.08 | −0.14 (−0.47 to 0.20); p=0.43 | 0.16 (−0.18 to 0.50); p=0.35 |
| Fetal anaemia (haemoglobin <125 g/L cord blood) | 80/400 (20%) | 81/401 (20%) | 76/403 (19%) | 1.06 (0.80 to 1.41); p=0.68 | 1.07 (0.81 to 1.42); p=0.63 | 1.01 (0.77 to 1.33); p=0.94 |
| Corrected birthweight | 3234.0 (483.8) | 3188.2 (433.0) | 3275.5 (463.2) | −41.5 (−104.4 to 21.4); p=0.19 | −87.3 (−150.1 to −24.5); p=0.0065 | −45.8 (−108.5 to 16.9); p=0.15 |
| Gestational age at birth (weeks) | 39.1 (1.8); n=451 | 39.0 (2.2); n=448 | 39.1 (1.8); n=451 | −0.08 (−0.33 to 0.17); p=0.53 | −0.11 (−0.36 to 0.14); p=0.39 | −0.03 (−0.28 to 0.22); p=0.82 |
| Birthweight for gestational age (Z score) | 0.43 (1.09); n=416 | 0.29 (0.97); n=417 | 0.51 (1.1); n=422 | −0.08 (−0.22 to 0.06); p=0.28 | −0.22 (−0.36 to −0.07); p=0.0027 | −0.14 (−0.28 to 0.003); p=0.06 |
| Small for gestational age | 36/416 (9%) | 27/417 (6%) | 25/422 (6%) | 1.46 (0.89 to 2.39); p=0.13 | 1.09 (0.65 to 1.85); p=0.74 | 0.75 (0.46 to 1.21); p=0.24 |
| Low birthweight | 19/413 (5%) | 22/414 (5%) | 18/409 (4%) | 1.05 (0.56 to 1.96); p=0.89 | 1.21 (0.66 to 2.22); p=0.54 | 1.16 (0.63 to 2.10); p= 0.64 |
| Preterm birth | 29/451 (6%) | 23/448 (5%) | 21/451 (5%) | 1.38 (0.80 to 2.38); p=0.25 | 1.10 (0.62 to 1.96); p=0.74 | 0.80 (0.47 to 1.36); p=0.41 |
| Stillbirth | 10/454 (2%) | 4/452 (1%) | 16/453 (4%) | 0.62 (0.29 to 1.36); p=0.24 | 0.25 (0.08 to 0.74); p=0.0126 | 0.40 (0.13 to 1.27); p=0.12 |
| Fetal loss* | 11/454 (2%) | 7/452 (2%) | 17/453 (4%) | 0.65 (0.31 to 1.36); p=0.25 | 0.41 (0.17 to 0.99); p=0.0463 | 0.64 (0.25 to 1.63); p=0.35 |
| Any adverse birth outcome† | 64/454 (14%) | 48/452 (11%) | 54/454 (12%) | 1.19 (0.85 to 1.66); p=0.32 | 0.89 (0.62 to 1.29); p=0.54 | 0.75 (0.53 to 1.07); p=0.11 |
| Congenital malaria infection | 5/514 (1%) | 2/504 (<1%) | 0/508 | NA | NA | NA |
| Infant clinical malaria by 6–8 weeks (cumulative) | 9/368 (2%) | 11/366 (3%) | 5/360 (1%) | 1.76 (0.59 to 5.24); p=0.31 | 2.11 (0.73 to 6.08); p=0.17 | 1.20 (0.50 to 2.90); p=0.68 |
| Neonatal death | 6/454 (1%) | 4/452 (1%) | 12/453 (3%) | 0.50 (0.19 to 1.32); p=0.16 | 0.33 (0.11 to 1.03); p=0.06 | 0.67 (0.19 to 2.36); p=0.53 |
| Perinatal death | 16/454 (4%) | 8/452 (2%) | 27/453 (6%) | 0.59 (0.32 to 1.08); p=0.09 | 0.30 (0.14 to 0.65); p=0.0022 | 0.50 (0.22 to 1.16); p=0.11 |
| Infant deaths by 6–8 weeks (end of follow-up) | 6/454 (1%) | 4/452 (1%) | 13/453 (3%) | 0.46 (0.18 to 1.20); p=0.11 | 0.31 (0.10 to 0.94); p=0.0383 | 0.67 (0.19 to 2.36); p=0.53 |
Data are mean (SD); n, n/N (%), or geometric mean (95% CI), unless otherwise specified. ISTp-DP=intermittent screening and treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-DP=intermittent preventive treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-SP=intermittent preventive treatment during pregnancy with sulfadoxine–pyrimethamine.
Spontaneous abortion or stillbirth.
Small for gestational age, low birthweight, preterm birth, or fetal loss.
Prevalence of low birthweight, small for gestational age, and preterm delivery did not differ significantly between groups, overall and within each gravidity strata in both the intention-to-treat and the per-protocol populations (figure 2B, appendix). However, the mean birthweight was lower in the intermittent preventive treatment with dihydroartemisinin–piperaquine group than in the intermittent preventive treatment with sulfadoxine–pyrimethamine group (table 2, appendix).
Women in the intermittent preventive treatment with dihydroartemisinin–piperaquine group had fewer malaria infections, a lower incidence of clinical malaria, and fewer all-cause sick-clinic visits during pregnancy than those in the intermittent preventive treatment with sulfadoxine–pyrimethamine group (figure 3). Anaemia at time of delivery, stillbirths, and infant mortality within 6–8 weeks were likewise reduced (table 2). Women in the intermittent screening and treatment with dihydroartemisinin–piperaquine group had more malaria infections during pregnancy than did those in the inter mittent preventive treatment with sulfadoxine–pyrimethamine group (figure 3). The prevalence of any malaria at delivery (by rapid diagnostic tests, microscopy, histology [including past infections], or PCR in peripheral or placental blood) was higher in the intermittent screening and treatment with dihydroartemisinin–piperaquine group than in the intermittent preventive treatment with sulfadoxine–pyrimethamine group (table 2).
Figure 3. Incidence of malaria infection, clinical malaria, and all-cause sick-clinic visits during pregnancy by treatment group in the intention-to-treat population.
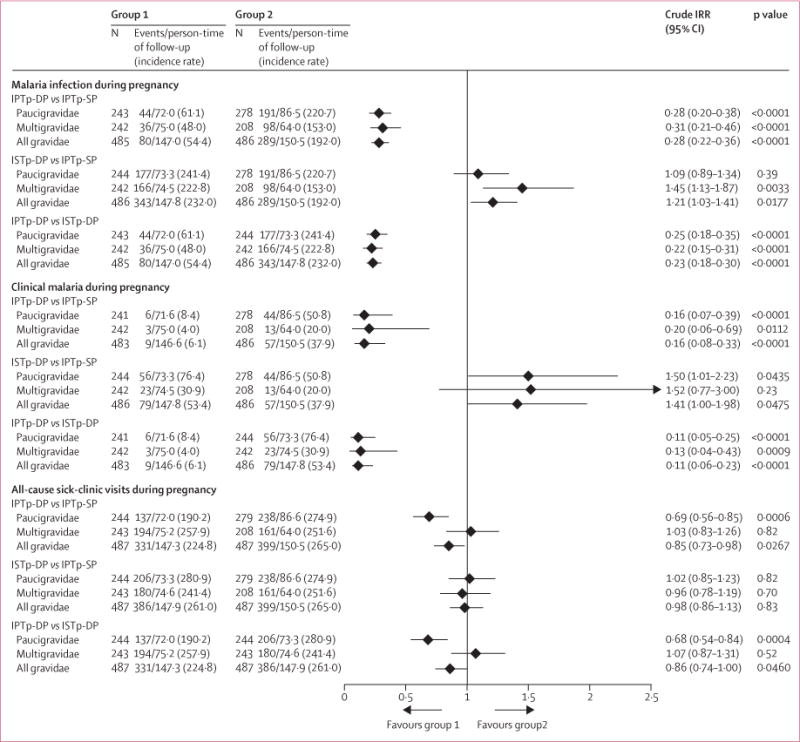
Data are the number of women with an event and the number of events/person-time of follow-up, with incidence rate per 100 person-years, unless otherwise specified. Malaria infection defined by microscopy or PCR. IRR=incidence rate ratio. ISTp-DP=intermittent screening and treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-DP=intermittent preventive treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-SP=intermittent preventive treatment during pregnancy with sulfadoxine–pyrimethamine.
The first dose of dihydroartemisinin–piperaquine was well tolerated by most women: nine women had a single episode of vomiting and three vomited the repeat dose (appendix). 65 adverse events were potentially associated with drug tolerability, with the highest incidence reported in women in the intermittent preventive treatment with sulfadoxine–pyrimethamine group (appendix). We recorded 303 maternal and infant serious adverse events, which were least frequent in the intermittent preventive treatment with dihydroartemisinin–piperaquine group (table 3). Three women died (n=1 in the intermittent screening and treatment with dihydroartemisinin–piperaquine group, n=2 in the inter mittent preventive treatment with sulfadoxine–pyrimethamine group); all deaths were unrelated to the intervention or malaria. The frequency of congenital malformations was 2% in the intermittent screening and treatment with dihydroartemisinin–piperaquine group, 2% in the intermittent preventive treatment with dihydroartemisinin–piperaquine group, and 3% in the intermittent preventive treatment with sulfadoxine–pyrimethamine group (p=0.41).
Table 3.
Serious adverse events
| ISTp-DP (n=510 women, n=454 infants)*
|
IPTp-DP (n=510 women, n=459 infants)†
|
IPTp-SP (n=508 women, n=458 infants)‡
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Number with event |
Total events | Incidence per 100 person-years (95% CI) |
Number with event |
Total events | Incidence per 100 person-years (95% CI) |
Number with event |
Total events | Incidence per 100 person-years (95% CI) |
|
| Women | |||||||||
|
| |||||||||
| Any event | 55 (11%) | 82 | 40.72 (32.80–50.56) | 32 (6%) | 37 | 18.27 (13.23–5.21) | 61 (15%) | 85 | 41.35 (33.43–51.15) |
| Pregnancy, puerperium, and perinatal disorders | 31 | 37 | 18.37 (13.31–25.36) | 25 | 28 | 13.82 (9.54–20.02) | 44 | 52 | 25.30 (19.28–33.20) |
| Infections and infestations | 21 | 25 | 12.41 (8.39–18.37) | 6 | 6 | 2.96 (1.33–6.59) | 16 | 22 | 10.70 (7.05–16.26) |
| Gastrointestinal disorders | 6 | 6 | 2.98 (1.34–6.63) | 2 | 2 | 0.99 (0.25–3.95) | 3 | 3 | 1.46 (0.47–4.53) |
| Surgical and medical procedures | 3 | 3 | 1.49 (0.48–4.62) | 0 | 0 | 0 | 1 | 1 | 0.49 (0.07–3.45) |
| Blood and lymphatic system disorders | 1 | 1 | 0.50 (0.07–3.53) | 0 | 0 | 0 | 2 | 2 | 0.97 (0.24–3.89) |
| Musculoskeletal and connective tissue disorders | 2 | 2 | 0.99 (0.25–3.97) | 0 | 0 | 0 | 1 | 1 | 0.49 (0.07–3.45) |
| Nervous system disorders | 1 | 1 | 0.50 (0.07–3.53) | 1 | 1 | 0.49 | 1 | 1 | 0.49 (0.07–3.45) |
| Reproductive system and breast disorders | 2 | 2 | 0.99 (0.25–3.97) | 0 | 0 | 0 | 1 | 1 | 0.49 (0.07–3.45) |
| Injury, poisoning, and procedural complications | 1 | 1 | 0.50 (0.07–3.53) | 0 | 0 | 0 | 1 | 1 | 0.49 (0.07–3.45) |
| Respiratory, thoracic, and mediastinal disorders | 2 | 2 | 0.99 (0.25–3.97) | 0 | 0 | 0 | 0 | 0 | 0 |
| General disorders and administration site conditions | 1 | 1 | 0.50 (0.07–3.53) | 0 | 0 | 0 | 0 | 0 | 0 |
| Immune system disorders | 1 | 1 | 0.50 (0.07–3.53) | 0 | 0 | 0 | 0 | 0 | 0 |
| Vascular disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.49 (0.07–3.45) |
|
| |||||||||
| Infants§ | |||||||||
|
| |||||||||
| Any event | 26 (6%) | 31 | 6.15 (4.32–8.74) | 22 (5%) | 27 | 5.30 (3.64–773) | 36 (8%) | 41 | 7.86 (5.77–10.72) |
| Congenital, familial, and genetic disorders | 8 | 11 | 2.18 (1.21–3.94) | 10 | 11 | 2.16 (1.20–3.90) | 14 | 16 | 3.14 (1.93–5.13) |
| Pregnancy, puerperium, and perinatal disorders | 6 | 6 | 1.19 (0.53–2.65) | 6 | 6 | 1.18 (0.53–2.62) | 8 | 8 | 1.57 (0.79–3.14) |
| Infections and infestations | 8 | 9 | 1.78 (0.93–3.43) | 3 | 5 | 0.98 (0.41–2.36) | 4 | 4 | 0.79 (0.30–2.09) |
| General disorders and administration site conditions | 1 | 1 | 0.20 (0.03–1.41) | 4 | 4 | 0.79 (0.29–2.09) | 7 | 7 | 1.18 (0.53–2.63) |
| Respiratory, thoracic, and mediastinal disorders | 4 | 4 | 0.79 (0.30–2.11) | 1 | 1 | 0.20 (0.03–1.39) | 5 | 5 | 0.98 (0.41–2.36) |
| Gastrointestinal disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.20 (0.03–1.40) |
ISTp-DP=intermittent screening and treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-DP=intermittent preventive treatment during pregnancy with dihydroartemisinin–piperaquine. IPTp-SP=intermittent preventive treatment during pregnancy with sulfadoxine–pyrimethamine. Total follow-up time:
201.4 years in women, 48.0 years in infants;
202.6 years in women, 48.7 years in infants;
205.5 years in women, 48.4 years in infants.
Excluding twin births, including liveborn and stillborn infants.
Discussion
Our findings show that intermittent preventive treatment with dihydroartemisinin–piperaquine is associated with a 68% reduction in the risk of malaria infection at delivery, an 84% reduction in the incidence of clinical malaria during pregnancy, and a 22% reduced risk of anaemia at delivery compared with the current intermittent preventive treatment with sulfadoxine–pyrimethamine strategy. The risk of stillbirths and early infant mortality were also reduced in the intermittent preventive treatment with dihydroartemisinin–piperaquine group, both in paucigravid and multigravid women; however, the intervention was not associated with improvements in birthweight. Intermittent screening and treatment with dihydroartemisinin–piperaquine was not superior to the existing preventive strategy with sulfadoxine–pyrimethamine, and was associated with a higher incidence of malaria infection and clinical malaria during pregnancy.
We did our study in a region with high sulfadoxine–pyrimethamine resistance, with near saturation of the quintuple mutant parasites but where the sextuple mutant is prevalent at less than 6%, characteristic of much of east and southern Africa. The beneficial effect shown for intermittent preventive treatment with dihydroartemisinin–piperaquine might be even greater in regions where the prevalence of the sextuple dhfr/dhps mutation is more frequent.23 Furthermore, we provided intermittent preventive treatment as part of the focused antenatal care package as per current WHO policy,24 and the median number of courses was three. Greater effectiveness might be achieved by monthly dosing regimens, as reported in trials in adults from Thailand, showing that monthly dosing with dihydroartemisinin–piperaquine provided almost complete chemoprophylaxis compared with dosing every 2 months.25
Despite the large reductions in the risk of malaria conferred by intermittent preventive treatment with dihydroartemisinin–piperaquine, birthweight was higher in the sulfadoxine–pyrimethamine group. The mean Z score for birthweight for gestational age was likewise higher in the intermittent preventive treatment with sulfadoxine–pyrimethamine group than in the intermittent preventive treatment with dihydroartemisinin–pipera quine group, despite the similar gestational age at birth, suggesting that the higher birthweight was mainly due to differences in intrauterine growth. There are several potential explanations for this difference. First, this could be a chance finding. However, intermittent preventive treatment with dihydroartemisinin–piperaquine could have saved pregnancies with borderline viability that might otherwise have resulted in fetal loss had these women been randomised to one of the other two less efficacious groups. This survival could have resulted in more livebirths with lower birthweights in the inter mittent preventive treatment with dihydroartemisinin–piperaquine group. Repeated courses of dihydroartemisinin–piperaquine are unlikely to have negatively affected fetal growth, despite the reduced risk of malaria, because there was no dose–response association between the number of courses received and birthweight for gestational age Z scores (p=0.92). Furthermore, sulfadoxine–pyrimethamine might still have some beneficial effect on birthweight. Although the ability of sulfadoxine–pyrimethamine to clear existing malaria infections or prevent new ones is clearly compromised in the region under study (Desai M, unpublished), only 5.8% of parasites carry the sextuple dhfr/dhps mutation, conferred by the Pfdhps-A581G mutation, indicating the highest level of resistance. Sulfadoxine–pyrimethamine is thus likely to have provided partial protection against malaria in women not infected with these highly resistant parasites, especially now that intermittent preventive treatment with sulfadoxine–pyrimethamine is given at least three times in the second and third trimester instead of twice as with the original regimen,24,26 possibly by suppressing parasite densities in the placenta (rather than clearing them) and thereby reducing placental inflammation. Furthermore, sulfadoxine–pyrimethamine has broad antimicrobial activity, which, in addition to antimalarial activity, might have conferred some protection against undetected bacterial infections, particularly Gram-positive bacteria,27 and this deserves further study. Because of the even broader antibacterial activity of azithromycin,27 assessment of the combined effect of azithromycin and dihydroartemisinin–piperaquine to improve birth outcomes could also be investigated in this part of the world with a high dual burden of malaria and sexually transmitted infections and reproductive tract infections in pregnancy.28,29
The absence of superiority of intermittent screening and treatment with dihydroartemisinin–piperaquine is consistent with findings from a multicentre non-inferiority trial in paucigravid women in four countries in west Africa,20 which showed that intermittent screening and treatment with artemether-lumefantrine was non-inferior to intermittent preventive treatment with sulfadoxine–pyrimethamine in terms of reductions in low birthweight. Even though that trial was done in regions with very low sulfadoxine–pyrimethamine resistance, it likewise showed that paucigravid women in the intermittent screening and treatment group had a higher incidence of clinical malaria (incidence rate ratio 1.66; p<0.001), and lower mean birthweights (−28 g; p=0.04) than those in the intermittent preventive treatment with sulfadoxine–pyrimethamine group. The lower mean birthweight with intermittent screening and treatment was also reported in the first trial of that strategy in Ghana, which compared intermittent screening and treatment with amodiaquine-artesunate with intermittent preventive treatment with sulfadoxine–pyrimethamine.19
The absence of superiority of intermittent screening and treatment with dihydroartemisinin–piperaquine could be explained by the high number of infections missed by rapid diagnostic tests, combined with the absence of a prophylactic benefit in women testing negative, allowing low-density infections to persist and new infections to occur before the next scheduled visit. This finding also suggests that sulfadoxine–pyrimethamine still provides some degree of benefit towards clearance or suppression of malaria infection and reduction in the incidence of clinical malaria, despite the restricted efficacy in clearance of existing infections and the shortened duration of post-treatment prophylaxis recorded in our previous in-vivo follow-up studies in this high-resistance study region (Desai M, unpublished). The brand of rapid diagnostic test used in this trial performed well in the diagnosis of Plasmodium falciparum at low densities assessed in the WHO product testing of malaria rapid diagnostic tests.30 Combined with the results of the present trial, this observation suggests that the performance of presently available tests is not sufficient for the screening of asymptomatic women who typically have low-density infection.
Our study has some limitations. The study used an open-label design and participants and field staff were aware of treatment allocation. Loss to follow-up was 12%. Although there was no difference in numbers of women lost to follow-up between the treatment groups, and the baseline prevalence of malaria in those lost to follow-up did not differ from those who completed follow-up, we cannot completely rule out some level of bias. Only the first dose of dihydroartemisinin–piperaquine was provided via directly observed therapy and the remaining two doses were taken at home. Nevertheless, very high (>95%) compliance to the second and third doses was reported among the subsample of women visited by field staff at home on the last day of the course. We did not establish the effect of repeat dosing of dihydroartemisinin–piperaquine on cardiac repolari sation, but previous trials in young children did not show evidence that piperaquine-associated QT prolongation increases with monthly dosing.31 Furthermore, pregnancy does not affect the extent of piperaquine-associated QTc prolongation.32
Our data suggest that in a region with high malaria transmission, high sulfadoxine–pyrimethamine resistance, and in the context of high use of longlasting insecticide-treated nets, intermittent screening and treatment with dihydroartemisinin–piperaquine with presently available rapid diagnostic tests is not a suitable alternative to intermittent preventive treatment with sulfadoxine–pyrimethamine. By contrast, intermittent preventive treatment with dihydroartemisinin–piperaquine resulted in substantial reductions in clinical malaria and malaria infection, anaemia, stillbirths, and early infant mortality. The long half-life, good tolerability, safety, and once-daily dosing regimen are all desirable properties of dihydroartemisinin–piperaquine that make intermittent preventive treatment with this drug a potential suitable replacement to intermittent preventive treatment with sulfadoxine–pyrimethamine in regions where the efficacy of the latter strategy is threatened. Additional multicentre studies are now warranted to further establish the safety of intermittent preventive treatment with dihydroartemisinin–piperaquine and its effect on birth outcome, and the feasibility and cost-effectiveness of using a 3 day regimen.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed and the Malaria in Pregnancy Library between June 29, and Aug 30, 2015, for studies investigating intermittent screening and treatment and intermittent preventive treatment during pregnancy with alternative drugs in HIV-negative women in Africa. We first used the search terms (Malaria AND pregnan* AND intermittent) AND (screen* OR treat* OR clearance) AND (delivery OR malaria OR birth OR LBW or preterm OR premature) to identify studies that assessed the efficacy of intermittent screening and treatment measured at delivery. We then used the search terms (Malaria AND pregnan* AND intermittent) AND (prevent* OR prophyla* OR presumpt* OR chemoprevent* OR chemoprophyla* OR IPT*) AND (delivery OR malaria OR birth OR LBW or preterm OR premature) to identify studies that assessed the efficacy of intermittent preventive treatment with alternative drugs. Both searches were unrestricted by language or publication date.
Through these searches, and by scanning reference lists of articles and trial registers, we identified a total of eight publications in peer reviewed journals. The only two previous studies of intermittent screening and treatment were done in west Africa in regions of low sulfadoxine–pyrimethamine resistance and both showed non-inferiority of the screening and treatment strategy compared with the existing two-course strategy of intermittent preventive treatment with sulfadoxine–pyrimethamine. For previous trials designed to identify alternative drugs for intermittent preventive treatment, two trials assessed mefloquine and both showed high efficacy but low tolerability. One trial in Ghana assessed amodiaquine with and without sulfadoxine-pyrimethamine and showed no additional benefit compared with intermittent preventive treatment with sulfadoxine–pyrimethamine and low tolerability of the amodiaquine-based regimens. Two studies in west Africa assessed chloroquine for intermittent preventive treatment (one in combination with pyrimethamine) and all reported superiority of the intermittent preventive treatment with sulfadoxine–pyrimethamine strategy compared with intermittent preventive treatment with chloroquine. One further unpublished multicentre trial comparing the intermittent preventive treatment with sulfadoxine–pyrimethamine with intermittent preventive treatment with a fixed-dose combination of chloroquine and azithromycin was stopped early because chloroquine–azithromycin did not show superiority and was associated with more serious and treatment-emergent adverse events that resulted in treatment discontinuation or were regarded as treatment related. Thus, none of the previous trials of intermittent preventive treatment during pregnancy have identified an alternative to sulfadoxine–pyrimethamine that was effective, safe, and well tolerated by asymptomatic pregnant women.
Added value of this study
We present the first trial assessing the effect of intermittent preventive treatment with an artemisinin-based combination treatment—namely dihydroartemisinin–piperaquine—during pregnancy, and intermittent screening and treatment in an area of high sulfadoxine–pyrimethamine resistance and high malaria transmission. This trial is the third to assess intermittent screening and treatment as an alternative to intermittent preventive treatment with sulfadoxine–pyrimethamine.
Implications of all the available evidence
Our findings show that intermittent preventive treatment with dihydroartemisinin–piperaquine holds promise as an alternative to intermittent preventive treatment with sulfadoxine–pyrimethamine in regions with high malaria transmission and high resistance of the parasite to sulfadoxine–pyrimethamine. We also show that, although intermittent screening and treatment has been reported to be non-inferior in areas of low sulfadoxine–pyrimethamine resistance, it is not a suitable alternative in areas of high malaria transmission and high sulfadoxine–pyrimethamine resistance in view of the limitations of sensitivity of current rapid diagnostic tests.
Acknowledgments
The Malaria in Pregnancy Consortium which is funded through a grant from the Bill and Melinda Gates Foundation to the Liverpool School of Tropical Medicine (LSTM). The study drug dihydroartemisinin–piperaquine (Eurartesim) was provided free of charge by the manufacturer (Sigma Tau, Rome, Italy). We thank all the pregnant women who participated in this study. We also thank the study staffmembers (research nurses: Mary Owidhi, Rodgers Kongina, Epines Chanvangi, Caroline Marlene Achieng, Patience Dali; data management: Benjamin Odera, Linda Odeck, and Alloys Koloo; laboratory technicians: Mary Omwalo, Esther Odongo, Winnie Chebore, Malaki Ogalo; quality assurance: Sophie Omondi; and administration: Edna Otieno); the Siaya County Health Management team for their assistance and support in the conduct of this work within their health facilities; the KEMRI-CDC clinical monitoring department (Emily Kemunto, Emmanuel Rono, and Elizabeth Ayuo); Carl Henry from the clinical monitoring department at LSTM; Martina Oneko for being the local safety monitor for the trial; members of the data safety monitoring committee: Kevin Ault (Emory University School of Medicine, Atlanta, GA, USA), Jennita Reefhuis (CDC National Center on Birth Defects and Developmental Disabilities, Atlanta, GA, USA), Davidson Hamer (Boston University School of Public Health and Medicine, Boston, MA, USA), Kathleen Wannemuehler (CDC Center for Global Health, Atlanta, GA, USA) for their expertise and voluntary independent review of the safety data, protocol, and statistical analysis plan; and the following independent members of our Trial Steering Committee for their expertise and guidance: Laurence Slutsker (CDC Center for Global Health, Atlanta, GA, USA), David Schellenberg (London School of Hygiene & Tropical Medicine, London, UK), Tom Williams (Imperial College, London, UK). This manuscript has been approved by the Director of KEMRI. The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Contributors
MD, JG, and FOtK conceived the study and wrote the protocol. MD and FOtK acquired funding. MD and AK were the co-principal investigators, supported by JG and AL. AL did the fieldwork under supervision of PO, AK, KL, and MD. EJ acted as government liason with the Ministry of Health in Kenya. JG and AL maintained the safety database and supported safety reporting to the respective oversight committees, ethical boards, and the centralised safety registry of the Malaria in Pregnancy Consortium. KO and SK were responsible for the PCR and placental histology done at Kenya Medical Research Institute–Centers for Disease Control and Prevention (KEMRI-CDC) laboratories in Kisumu, Kenya. VW, JG, AL, and MD supported the data management. JW was the study statistician, did the primary data analysis, and, together with MD, JG, and FOtK, interpreted the data. JG, JW, MD, and FOtK co-wrote the statistical analysis plan. MD and FOtK wrote the first draft of the manuscript. All authors reviewed, revised, and approved the final version of the manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 2010;7:e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 3.Walker PG, ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health. 2014;2:e460–67. doi: 10.1016/S2214-109X(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 4.WHO. A strategic framework for malaria prevention and control during pregnancy in the African region. Brazzaville: WHO Regional Office for Africa; 2004. [Google Scholar]
- 5.Eisele TP, Larsen DA, Anglewicz PA, et al. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis. 2012;12:942–49. doi: 10.1016/S1473-3099(12)70222-0. [DOI] [PubMed] [Google Scholar]
- 6.Chico RM, Cano J, Ariti C, et al. Influence of malaria transmission intensity and the 581G mutation on the efficacy of intermittent preventive treatment in pregnancy: systematic review and meta-analysis. Trop Med Int Health. 2015 doi: 10.1111/tmi.12595. published online Sept 1. [DOI] [PubMed] [Google Scholar]
- 7.Alifrangis M, Nag S, Schousboe ML, et al. Independent origin of Plasmodium falciparum antifolate super-resistance, Uganda, Tanzania and Ethiopia. Emerg Infect Dis. 2014;20:1280–86. doi: 10.3201/eid2008.131897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington WE, Mutabingwa TK, Muehlenbachs A, et al. Competitive facilitation of drug-resistant Plasmodium falciparum malaria parasites in pregnant women who receive preventive treatment. Proc Natl Acad Sci USA. 2009;106:9027–32. doi: 10.1073/pnas.0901415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutman J, Kalilani L, Taylor S, et al. The A581G mutation in the gene encoding Plasmodium falciparum dihydropteroate synthetase reduces the effectiveness of sulfadoxine–pyrimethamine preventive therapy in Malawian pregnant women. J Infect Dis. 2015;211:1997–2005. doi: 10.1093/infdis/jiu836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman RD, Parise ME, Slutsker L, Nahlen B, Steketee RW. Safety, efficacy and determinants of effectiveness of antimalarial drugs during pregnancy: implications for prevention programmes in Plasmodium falciparum-endemic sub-Saharan Africa. Trop Med Int Health. 2003;8:488–506. doi: 10.1046/j.1365-3156.2003.01066.x. [DOI] [PubMed] [Google Scholar]
- 11.Clerk CA, Bruce J, Affipunguh PK, et al. A randomized, controlled trial of intermittent preventive treatment with sulfadoxine–pyrimethamine, amodiaquine, or the combination in pregnant women in Ghana. J Infect Dis. 2008;198:1202–11. doi: 10.1086/591944. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez R, Desai M, Macete E, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo-controlled trial. PLoS Med. 2014;11:e1001735. doi: 10.1371/journal.pmed.1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez R, Mombo-Ngoma G, Ouedraogo S, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-negative women: a multicentre randomized controlled trial. PLoS Med. 2014;11:e1001733. doi: 10.1371/journal.pmed.1001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briand V, Bottero J, Noel H, et al. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open-label equivalence trial comparing sulfadoxine–pyrimethamine with mefloquine. J Infect Dis. 2009;200:991–1001. doi: 10.1086/605474. [DOI] [PubMed] [Google Scholar]
- 15.Chandra RS, Orazem J, Ubben D, Duparc S, Robbins J, Vandenbroucke P. Creative solutions to extraordinary challenges in clinical trials: methodology of a phase III trial of azithromycin and chloroquine fixed-dose combination in pregnant women in Africa. Malar J. 2013;12:122. doi: 10.1186/1475-2875-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimani J, Phiri K, Kamiza S, et al. Azithromycin/chloroquine (AZCQ) versus sulfadoxine/pyrimethamine (SP) in intermittent preventive treatment of falciparum malaria infection in pregnant women (IPTp) in sub-Saharan Africa: an open-label randomized trial. European Congress of Clinical Microbiology and Infectious Diseases; Copenhagen, Denmark. April 25–28, 2015; p. EP168. [Google Scholar]
- 17.Nambozi M, Mulenga M, Halidou T, et al. Safe and efficacious artemisinin–based combination treatments for African pregnant women with malaria: a multicentre randomized control trial. Reprod Health. 2015;12:5. doi: 10.1186/1742-4755-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 19.Tagbor H, Bruce J, Agbo M, Greenwood B, Chandramohan D. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: a randomised controlled non-inferiority trial. PLoS One. 2010;5:e14425. doi: 10.1371/journal.pone.0014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagbor H, Cairns M, Bojang K, et al. A non-inferiority, individually randomized trial of intermittent screening and treatment versus intermittent preventive treatment in the control of malaria in pregnancy. PLoS One. 2015;10:e0132247. doi: 10.1371/journal.pone.0132247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 22.Schmiegelow C, Scheike T, Oesterholt M, et al. Development of a fetal weight chart using serial trans-abdominal ultrasound in an East African population: a longitudinal observational study. PLoS One. 2012;7:e44773. doi: 10.1371/journal.pone.0044773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naidoo I, Roper C. Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol. 2013;29:505–15. doi: 10.1016/j.pt.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 24.WHO. WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine–pyrimethamine (IPTp-SP) Geneva: World Health Organization; 2013. [Google Scholar]
- 25.Lwin KM, Phyo AP, Tarning J, et al. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin–piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother. 2012;56:1571–77. doi: 10.1128/AAC.05877-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayentao K, Garner P, van Eijk AM, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine–pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. 2013;309:594–604. doi: 10.1001/jama.2012.216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capan M, Mombo-Ngoma G, Makristathis A, Ramharter M. Anti-bacterial activity of intermittent preventive treatment of malaria in pregnancy: comparative in vitro study of sulphadoxine–pyrimethamine, mefloquine, and azithromycin. Malar J. 2010;9:303. doi: 10.1186/1475-2875-9-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chico RM, Hack BB, Newport MJ, Ngulube E, Chandramohan D. On the pathway to better birth outcomes? A systematic review of azithromycin and curable sexually transmitted infections. Expert Rev Anti Infect Ther. 2013;11:1303–32. doi: 10.1586/14787210.2013.851601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chico RM, Mayaud P, Ariti C, Mabey D, Ronsmans C, Chandramohan D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA. 2012;307:2079–86. doi: 10.1001/jama.2012.3428. [DOI] [PubMed] [Google Scholar]
- 30.FIND, TDR, WHO, CDC. Results of WHO product testing of malaria RDTs: round 4 (2012) Italy: World Health Organization; 2012. Malaria rapid diagnostic test performance. [Google Scholar]
- 31.Bigira V, Kapisi J, Clark TD, et al. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young ugandan children: a randomized controlled trial. PLoS Med. 2014;11:e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamin JM, Moore BR, Salman S, et al. Population pharmacokinetics, tolerability, and safety of dihydroartemisinin–piperaquine and sulfadoxine–pyrimethamine-piperaquine in pregnant and nonpregnant Papua New Guinean women. Antimicrob Agents Chemother. 2015;59:4260–71. doi: 10.1128/AAC.00326-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


