PD-L1 and PD-L2 expression in tumor cells and the quantities of PD-1+ tumor-infiltrating lymphocytes were immunohistochemically evaluated in metastatic clear cell renal cell carcinoma patients treated with vascular endothelial growth factor (VEGF)-tyrosine kinase inhibitors (TKIs), and their associations with VEGF-TKI responsiveness and clinical outcome were analyzed. This study demonstrates that PD-L1 expression is a predictor for unfavorable response to VEGF-TKI and a prognostic indicator for poor overall survival and progression-free survival.
Keywords: Metastatic clear cell renal cell carcinoma, Programmed death ligand-2, Programmed death ligand-1, Vascular endothelial growth factor pathway-tyrosine kinase inhibitor, Responsiveness, Prognosis
Abstract
Background.
Vascular endothelial growth factor pathway (VEGF)-tyrosine kinase inhibitors (TKIs) are used as the first-line treatment for patients with metastatic clear cell renal cell carcinoma (mCCRCC). Recently, programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) blockade emerged as promising therapy for renal cell carcinoma. However, the expression pattern and prognostic implication of programmed death-ligands (PD-Ls) in mCCRCC patients receiving VEGF-TKI remain unclear.
Patients and Methods.
PD-L1 and PD-L2 expression in tumor cells and the quantities of PD-1+ tumor-infiltrating lymphocytes were immunohistochemically evaluated in 91 mCCRCC patients treated with VEGF-TKI, and their associations with VEGF-TKI responsiveness and clinical outcome were analyzed.
Results.
PD-L1 immunopositivity was observed in 17.6% and significantly associated with a high International Society of Urological Pathology grade (p = .031) and sarcomatoid features (p = .014). PD-L2 immunopositivity was observed in 39.6% and was not associated with any of the assessed clinicopathological variables. PD-L1-positive cases showed poor VEGF-TKI responsiveness (p = .012) compared with PD-L1-negative cases. In univariate survival analysis, PD-L1 immunopositivity was significantly associated with shorter overall survival (OS) (p = .037) and progression-free survival (PFS) (p = .043). Multivariate survival analysis revealed that PD-L1 expression was independently associated with poor OS (p = .038) and PFS (p = .013) in addition to tumor necrosis (p = .006; p = .029, respectively) and Memorial Sloan Kettering Cancer Center score (p = .018; p = .032, respectively). PD-L2 expression was neither associated with VEGF-TKI responsiveness nor patients’ outcome.
Conclusion.
PD-L1 expression was significantly related to lack of VEGF-TKI responsiveness and independently associated with shorter survival in mCCRCC patients after VEGF-TKI treatment. PD-L1 may have a predictive and prognostic value for determining the value of VEGF-TKI treatment in patients with mCCRCC.
Implications for Practice:
Vascular endothelial growth factor pathway (VEGF)-tyrosine kinase inhibitors (TKIs) are essential for the treatment of metastatic renal cell carcinoma patients, but the treatment suffers from a lack of predictive markers. This study demonstrates that PD-L1 expression is a predictor for unfavorable response to VEGF-TKI and a prognostic indicator for poor overall survival and progression-free survival in patients with metastatic clear cell renal cell carcinoma receiving VEGF-TKI.
Introduction
Systemic treatment for patients with metastatic clear cell renal cell carcinoma (mCCRCC) has shifted away from cytokine-based treatment and toward therapies targeting angiogenesis-related factors [1]. Tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor (VEGF) signaling, such as sunitinib, sorafenib, and pazopanib, have been approved by the Food and Drug Administration; currently, VEGF-TKI therapy or combining interferon-α immunotherapy with the anti-VEGF monoclonal antibody bevacizumab are recommended as a first-line systemic treatment for patients with mCCRCC [2, 3]. These therapeutic agents have prolonged the survival of patients with mCCRCC, but 20%–30% of patients derive no benefit from first-line VEGF-TKI treatment [4–6]. In addition, patients develop acquired resistance to the VEGF-TKI and occasionally experience adverse effects relating to treatment [4, 5, 7]. Although several clinicopathological factors were suggested as potential predictive markers for VEGF-TKI therapy, validated biological predictors of treatment response and clinical outcome, which would be valuable for deciding whether treatment for mCCRCC patients should include VEGF-targeted therapy, are lacking [8].
We previously reported that several clinicopathological factors, including tumor necrosis and sarcomatoid features, are associated with shorter survival after VEGF-TKI therapy in patients with CCRCC [9]. A recent study analyzed the molecular subtype of primary CCRCC in patients who developed metastatic tumors and were treated with sunitinib. The study showed that ccrcc1/ccrcc4 tumors had a lower response rate, shorter overall survival (OS), and shorter progression-free survival (PFS) than ccrcc2/ccrcc3 tumors [10]. Among the molecular subtypes analyzed, ccrcc4 tumors exhibited sarcomatoid differentiation with a strong inflammatory, Th1-oriented, but suppressive immune microenvironment, along with high expression of programmed death-1 (PD-1) and its ligand, PD-L1 [10]. Meanwhile, PD-L1 expression in tumor cells and inflammatory cells was associated with aggressive pathologic features and poor prognosis in patients with CCRCC [11]. Recently, PD-1/PD-L1 pathway blockade showed therapeutic benefit in patients with renal cell carcinoma (RCC) [12, 13], thus being raised as a novel therapeutic strategy. In advanced non-small cell cancer, anti-PD-1 inhibitor (pembrolizumab) had an acceptable side effect profile and showed antitumor activity and PD-L1 expression in tumor cells correlated with improved efficacy of pembrolizumab [14]. Therefore, PD-L1-High tumor may be an appropriate subset for PD-1 monotherapy as a first-line therapy in RCC, because they may have a worse prognosis and potentially greater likelihood of responding to therapy. However, the relationship between PD-1 or PD-L1 expression and efficiency of VEGF-TKI treatment remains unclear. Therefore, we investigated the clinicopathological and prognostic significance of the expression of PD-1 and PD-1 ligands PD-L1 and PD-L2 on tumors in mCCRCC patients receiving VEGF-TKI treatment.
Materials and Methods
Patients
A total of 193 patients with metastatic renal cell carcinoma (mRCC) who received VEGF-TKI treatment at the Asan Medical Centre (AMC) between 2006 and 2011 were retrospectively collected. In total, 16 patients with non-clear cell-type tumors and 77 patients without nephrectomy specimens of their primary tumor were excluded. Among the remaining 100 cases, 9 were excluded because of a lack of sufficient pathological material for analysis. Finally, a total of 91 cases were included in this study. Formalin-fixed, paraffin-embedded tissue samples taken from resected primary tumor at the time of initial diagnosis were exclusively collected, and 1.0-mm-core tissue microarray (TMA) blocks were constructed with 2 representative cores for each case. The histological subtypes were classified according to the 2004 World Health Organization Tumor Classification, and each tumor was graded according to the 2013 International Society of Urological Pathology (ISUP) grading system [15, 16]. Patient clinical information was obtained from the medical records. Tumor response to VEGF-TKI therapy was assessed according to the Revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1) [17]. Patients with complete or partial response (CR or PR) were considered responders, whereas those with stable or progressive disease (SD or PD) were considered nonresponders. The Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk scores were calculated according to the presence of six risk factors: a Karnofsky performance status (KPS) of <80%, anemia, time from diagnosis to treatment of <1 year, hypercalcemia, thrombocytosis, and neutrophilia [3]. This study was approved by the Institutional Review Board of AMC (S2014-1122-0002).
Immunohistochemistry for PD-L1, PD-L2, and PD-1
Sections from the TMA blocks were immunostained using the Ventana Benchmark XT automated staining system (Ventana Medical Systems, Tucson, AZ, http://www.ventana.com) according to the manufacturer’s protocol. The following primary antibodies were used for immunohistochemistry: anti-PD-L1 (1:100; E1L3N; rabbit monoclonal; Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com), anti-PD-L2 (1:3000; #176611; mouse monoclonal; R&D Systems, Minneapolis, MN, http://www.rndsystems.com), and anti-PD-1 (1:100; MRQ-22; mouse monoclonal; Cell Marque, Rocklin, CA, http://www.cellmarque.com). The expression levels of PD-L1 and PD-L2 were evaluated according to the intensity and extent of membranous staining on the tumor cells. The staining was scored as follows: 0, no expression or expression in <5% of tumor cells; 1, weak expression in ≥5% of tumor cells; 2, moderate expression in ≥5% of tumor cells; and 3, strong expression in ≥5% of tumor cells. The samples were subsequently subdivided into negative (those with scores of 0–1) or positive (those with scores of 2–3) subgroups (supplemental online Fig. 1).
The number of PD-1+ tumor-infiltrating lymphocytes (TILs) was manually counted for each core, and the mean number per unit area (mm2) was calculated for each case. PD-1+ TILs were observed to have increased in the cases with ≥5 TILs per mm2.
Statistical Analysis
All of the statistical analyses were performed using SPSS (version 18.0; SPSS, Chicago, IL, http://www-01.ibm.com/software/analytics/spss/). The relationships between the groups were compared using the chi-square test, Fisher’s exact test, or Student’s t test. OS was defined as the time interval between the date of TKI treatment initiation and the date of death caused by RCC. PFS was defined as the time interval between the date of TKI treatment initiation and the date of disease progression, relapse, or death from RCC. The Kaplan-Meier method with the log-rank test and the multivariate Cox proportional hazard regression model were used for survival analyses. To assess model accuracy (discrimination) for patient survival, Harrell’s bias-corrected concordance index (C-index) was calculated. Model generation was repeated 1,000 times with the bootstrap resampling technique. Two-sided p values <.05 were considered statistically significant.
Results
Patient Characteristics
The median follow-up period for the patients in this study was 34.6 months (range, 2.3–171.7 months). Of 91 total patients, 54 (59.3%) patients had metastatic disease at the time of initial diagnosis (i.e., nephrectomy), and 73 (80.2%) had died by the time of analysis. The median time between the date of diagnosis and the date of VEGF-TKI treatment initiation was 2.9 months (range, 0–126.2 months). Before VEGF-TKI therapy, 11 patients (12.1%) had undergone interferon-α or interleukin-2 immunotherapies, 6 (6.6%) had received cytotoxic chemotherapy, and 4 (4.4%) had received both types of therapy. The most common TKI used was sunitinib (n = 70; 76.9% of patients), followed by sorafenib (n = 18; 19.8%) and pazopanib (n = 3; 3.3%). Overall, 9 (9.9%) had a KPS value of <80, and 12 (13.2%) showed a poor MSKCC score. The median time between the date of diagnosis and the date of death was 28.3 months (range, 2.3–132.1 months). The median time between the VEGF-TKI treatment initiation and the date of death was 20.1 months (range, 0.9–75.4 months). The estimated 5-year OS and PFS rates of the patients were 24.7% and 19.5%, respectively.
The Association Between PD-L1 and PD-L2 Expression With Clinicopathological Features in the Tumor Cells of Patients With mCCRCC
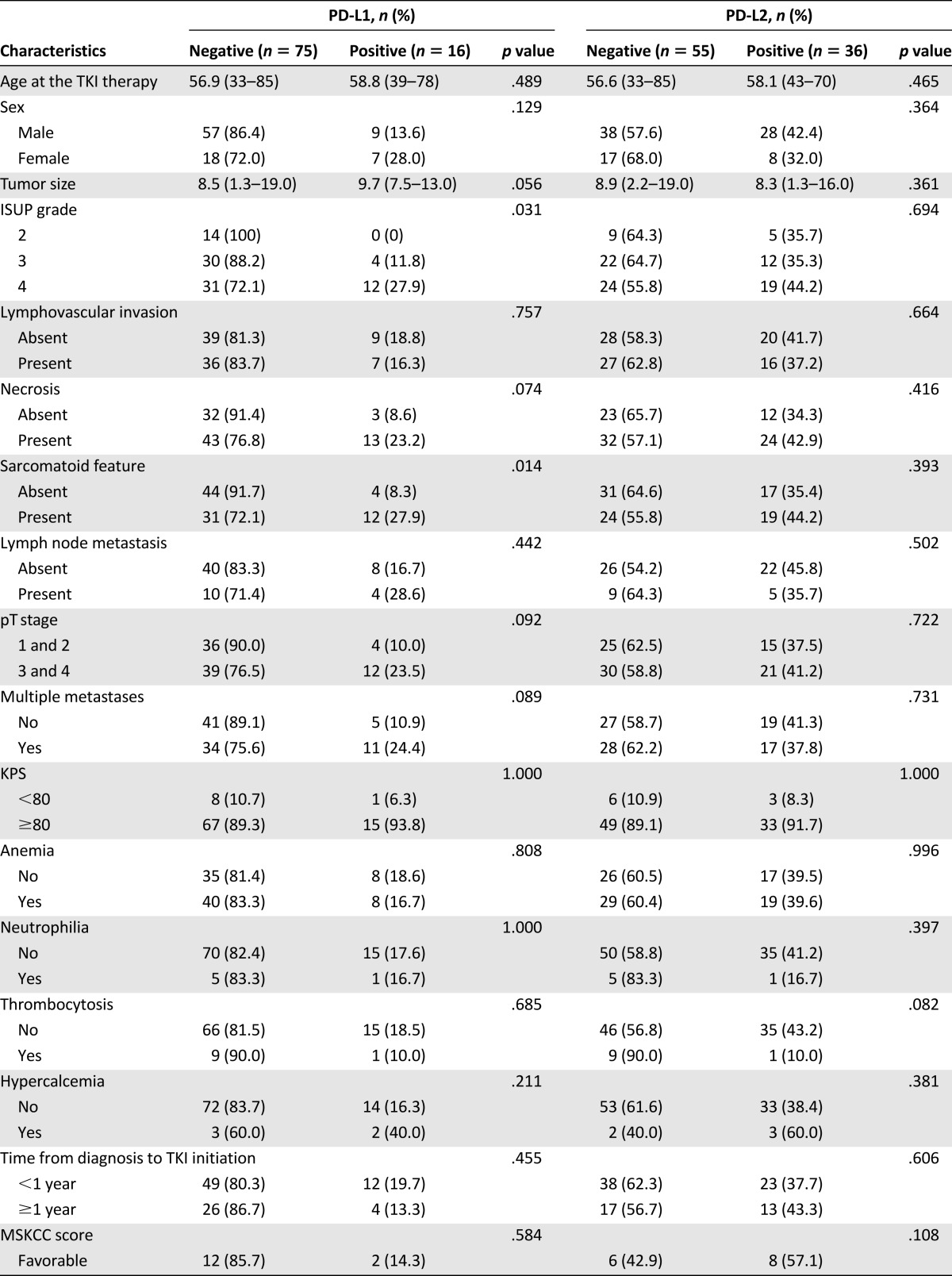
Overall, PD-L1 and PD-L2 expression was observed in 17.6% (16 of 91) and 39.6% (36 of 91) of patients with mCCRCC, respectively. The frequency of PD-L1 and PD-L2 immunopositivity did not vary for patients with different TKI regimens (p = .312 and p = .621, respectively). PD-L1 immunopositivity was significantly associated with a high ISUP grade (i.e., grade 3 or 4; p = .031), and the presence of sarcomatoid features (p = .014). Tumor necrosis (p = .074), advanced pT stage (i.e., stage 3 or 4; p = .092), and multiple metastasis (p = .089) were more frequently observed in the PD-L1-positive group than in the PD-L1-negative group, although the difference was not statistically significant. There was no significant correlation between PD-L2 expression and any of the clinicopathological variables (Table 1). Tumor samples from the metastatic sites were available from three patients, and the PD-L1 and PD-L2 expression patterns in the primary renal mass and metastatic lesion were identical in all these three cases.
Table 1.
Correlation between PD-L1 and PD-L2 expression and clinicopathological features of patients with metastatic clear cell renal cell carcinoma receiving vascular endothelial growth factor-tyrosine kinase inhibitor
The Relationship Between VEGF-TKI Responsiveness and PD-L1, PD-L2, and PD-1 Expression
According to the RECIST criteria [17], CR was observed in on1 (1.1%) patient, and PR was observed in 36 (39.6%) patients, whereas 37 (40.7%) patients showed SD, and 17 (18.7%) had PD. An overall objective response (CR and PR) was observed in 37 patients (40.7%). As summarized in Table 2, PD-L1 immunopositivity in tumor cells was inversely correlated with the objective VEGF-TKI responsiveness; responder accounted for 46.7% of PD-L1-negative cases but 12.5% of PD-L1-positive cases (p = .012). Of note, higher expression of PD-L1 showed a worse response rate (supplemental online Fig. 2). The response rate to VEGF-TKI was 29.1% and 0% among PD-L1-positive patients in terms of PD-L1 score 1–3 as immunopositive and 3 as immunopositive, respectively. In contrast, PD-L2 immunopositivity in tumor cells was unrelated to VEGF-TKI responsiveness. Patients without PD-1+ TILs showed higher responsiveness (42.0%) than those with positivity for PD-1+ TILs (14.3%), but these results were not statistically significant.
Table 2.
Correlation between PD-L1, PD-L2, and PD-1 with vascular endothelial growth factor-tyrosine kinase inhibitor response

Prognostic Significance of PD-L1 Expression in Patients Treated With VEGF-TKI
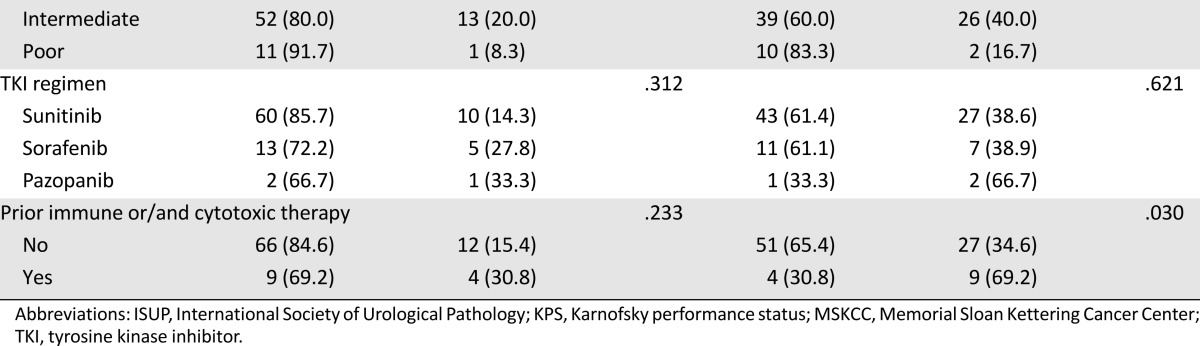
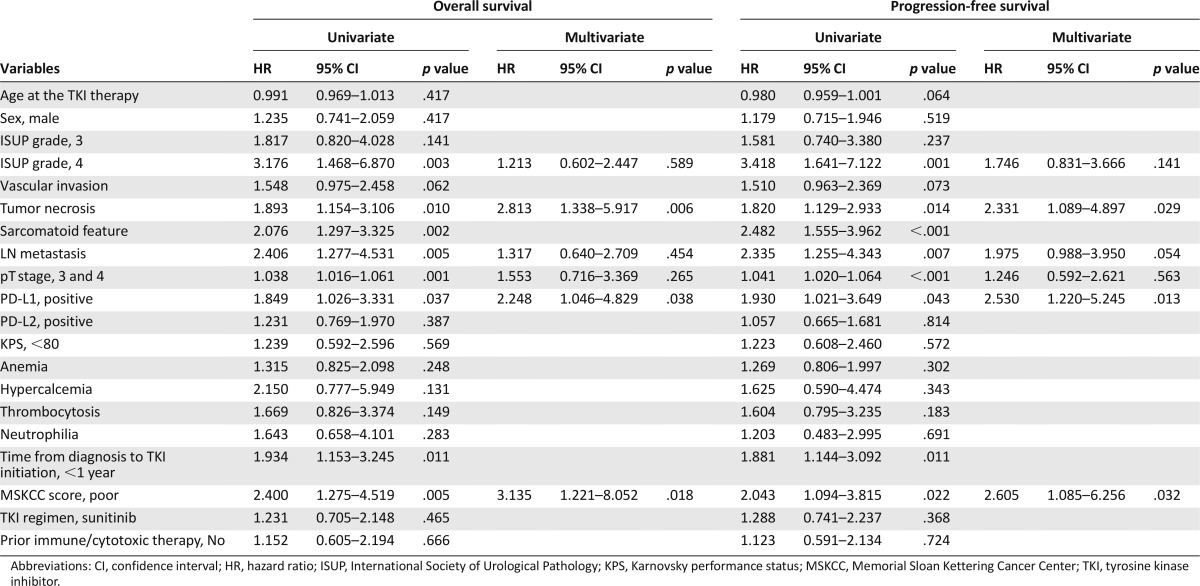
In the patients with mCCRCC, PD-L1 immunopositivity was associated with shorter OS and PFS (p = .037 and p = .043; Fig. 1A, 1B). The univariate survival analysis for cancer-specific death after TKI treatment showed that PD-L1 expression, ISUP grade, tumor necrosis, sarcomatoid feature, LN status, pT stage, and MSKCC score were significantly associated with OS (p < .05 for all) (Table 3). The multivariate Cox regression analysis, including PD-L1 expression, ISUP grade, tumor necrosis, LN status, pT stage, and MSKCC score, revealed that tumor necrosis (p = .006), MSKCC score (p = .018), and PD-L1 expression (p = .038) were independent prognostic factors for poor OS (Table 3). The C-index of this model was 0.723. The univariate survival analysis for tumor progression after TKI treatment also showed a significant association of PD-L1 expression, ISUP grade, tumor necrosis, LN status, pT stage, and MSKCC score with PFS (p < .05 for all) (Table 3). The multivariate Cox regression analysis revealed that tumor necrosis (p = .029), MSKCC score (p = .032), and PD-L1 expression (p = .013) were independent prognostic factors for poor PFS (Table 3), and the C-index of the PFS model was 0.729. The sarcomatoid features factor was excluded from the multivariate analysis because ISUP grade 4 tumors include tumors with rhabdoid and sarcomatoid differentiation and/or those showing tumor giant cells or showing extreme nuclear pleomorphism [16]. PD-L2 expression had no prognostic implication in patients treated with VEGF-TKI (Fig. 1C, 1D; Table 3).
Figure 1.
Kaplan-Meier analysis of PD-L1 and PD-L2 expression in metastatic clear cell renal cell carcinoma. (A, B): Overall survival (OS) (A) and progression-free survival (PFS) (B) rates were lower in patients showing PD-L1 immunopositivity compared with those showing PD-L1 immunonegativity. (C, D): OS (C) and PFS (D) were not associated with PD-L2 immunopositivity.
Table 3.
The univariate and multivariate Cox regression analyses for overall survival and progression-free survival in patients with metastatic clear cell renal cell carcinoma receiving vascular endothelial growth factor-tyrosine kinase inhibitor treatment
Discussion
This study demonstrates that PD-L1 expression is associated with unfavorable response to VEGF-TKI and is also a prognostic indicator for poor OS and PFS in patients with mCCRCC receiving VEGF-TKI. This result was consistent with that observed in recent VEG105192 and COMPARZ trials, which also showed that increased tumor PD-L1 expression was associated with shorter survival in mRCC patients receiving VEGF-TKI treatment [18, 19]. However, unlike previous studies, the present study analyzed the predictive and prognostic implication of PD-Ls for VEGF-TKI therapy in comparison with various clinicopathological features associated with aggressive tumor behavior including pT stage, grade, tumor necrosis, vascular invasion, and sarcomatoid features. In fact, PD-L1 immunopositivity in tumor cells was associated with sarcomatoid features, necrosis, advanced pT stage, and multiple metastases. By incorporating the diverse variables, we could demonstrate that PD-L1 expression is related with the responsiveness to VEGF-TKI and furthermore independently associated with clinical outcome of patients with mCCRCC after VEGF-TKI treatment. We also investigated the PD-L2 expression and showed that PD-L2 was not related to VEGF-TKI responsiveness and clinical outcome.
The tumor microenvironment and immune surveillance system are thought to play an important role for tumor growth and progression and also to be involved in the treatment responses to targeted therapy [20, 21]. Beuselinck et al. [10] demonstrated that the ccrcc4 molecular subtype was closely related to a poor response to sunitinib treatment. This tumor subtype also showed increased activity in the hypoxia pathway, a strong inflammatory immune environment, high levels of regulatory T-cell markers, such as Foxp3, increased numbers of myeloid-derived suppressor cells, and high PD-L1 expression. Hugonnet et al. [22] evaluated the level of initial tumor hypoxia and the changes in these levels after initiation of sunitinib treatment in mRCC using 18F-fluoromisonidazole PET/CT; the results demonstrated that patients with initially hypoxic lesions exhibited shorter PFS upon treatment than patients with nonhypoxic metastatic targets. PD-L1 has been shown to enhance and sustain Foxp3 expression and T regulatory cell suppressive functions, and under hypoxic conditions, PD-L1 expression in tumor cells leads to cytotoxic T-cell apoptosis [23, 24]. These previous studies suggested a possible mechanism between PD-L1, hypoxic microenvironment, and poor therapeutic efficiency of VEGF-TKI in mRCC patients. Moreover, recent early-phase clinical trials reported that combined therapy using PD-1/PD-L1 axis blockade and sunitinib or pazopanib showed encouraging antitumor activity and tolerable adverse events [25]. We think that the poor prognostic implication of PD-L1 expression in mCCRCC patients with VEGF-TKI treatment observed in this study may provide a rationale for combined PD-1/PD-L1 blockade and VEGF-TKI therapy. Therefore, PD-L1 may have predictive and prognostic potential and therapeutic relevance for the determination of VEGF-TKI treatment value in patients with mCCRCC.
However, PD-L2 was associated with neither TKI responsiveness nor prognosis in patients with mCCRCC receiving VEGF-TKI treatment. Our previous study (S.-J. Shin, Y.K. Jeon, P.-J. Kim, Y.M. Cho, J. Koh, D.H. Chung, H. Go, manuscript submitted for publication) revealed that PD-L2 expression in primary CCRCC was associated with LN metastasis and poor prognosis. Both PD-L1 and PD-L2 are expected to play a key role in cancer-induced immune suppression in the tumor microenvironment [26, 27]. However, Ghiotto et al. [28] showed that PD-L1 interacts with not only PD-1 but also CD80, and significant conformational changes occur upon PD-L1/PD-1 interaction, which is not the case for PD-L2/PD-1 interaction. Furthermore, PD-L1 competes with PD-L2 for PD-1 binding. The affinity of PD-L2 to PD-1 is much higher than that to PD-L1, but PD-L2 expression is more dependent on environmental cues than PD-L1 [27]. Thus, it is possible that the effects of PD-L1 and PD-L2 expression on TKI responsiveness and prognosis could be distinct.
Conclusion
We demonstrated that PD-L1 expression was independently associated with poor prognosis in mCCRCC patients undergoing VEGF-TKI treatment and that PD-L1 may be a predictive factor of the response of patients to TKI treatment. PD-1 pathway blockade as a monotherapy and combination with VEGF-TKI therapy in patients with high-PD-L1 tumors will need to be assessed prospectively to determine the optimal treatment for patients with mCCRCC.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Footnotes
For Further Reading: Christian Rothermundt, Alexandra Bailey, Linda Cerbone et al. Algorithms in the First-Line Treatment of Metastatic Clear Cell Renal Cell Carcinoma—Analysis Using Diagnostic Nodes. The Oncologist 2015;20:1028-1035.
Implications for Practice: The data provided in the present report should not be considered to serve as treatment recommendations for the management of treatment-naïve patients with multiple metastases from metastatic clear cell renal cell carcinoma outside a clinical trial; however, the data highlight the different treatment options and the criteria used to select them. The diversity in decision making and how results from phase III trials can be interpreted and implemented differently in daily practice are demonstrated.
Author Contributions
Conception/Design: Su-Jin Shin, Yoon Kyung Jeon, Yong Mee Cho, Heounjeong Go
Provision of study material or patients: Yoon Kyung Jeon, Yong Mee Cho, Jae-Lyun Lee, Doo Hyun Chung, Ji Young Park, Heounjeong Go
Collection and/or assembly of data: Su-Jin Shin
Data analysis and interpretation: Su-Jin Shin, Heounjeong Go
Manuscript writing: Su-Jin Shin, Heounjeong Go
Final approval of manuscript: Su-Jin Shin, Yoon Kyung Jeon, Yong Mee Cho, Jae-Lyun Lee, Doo Hyun Chung, Ji Young Park, Heounjeong Go
Disclosures
The authors indicated no financial relationships.
References
- 1.Srinivasan R, Ricketts CJ, Sourbier C, et al. New strategies in renal cell carcinoma: Targeting the genetic and metabolic basis of disease. Clin Cancer Res . 2015;21:10–17. doi: 10.1158/1078-0432.CCR-13-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rini BI. Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma. Cancer. 2009;115(suppl):2306–2312. doi: 10.1002/cncr.24227. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii65–vii71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Di Lorenzo G, Porta C, Bellmunt J, et al. Toxicities of targeted therapy and their management in kidney cancer. Eur Urol. 2011;59:526–540. doi: 10.1016/j.eururo.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Maroto P, Rini B. Molecular biomarkers in advanced renal cell carcinoma. Clin Cancer Res. 2014;20:2060–2071. doi: 10.1158/1078-0432.CCR-13-1351. [DOI] [PubMed] [Google Scholar]
- 9.Park JY, Lee JL, Baek S, et al. Sarcomatoid features, necrosis, and grade are prognostic factors in metastatic clear cell renal cell carcinoma with vascular endothelial growth factor-targeted therapy. Hum Pathol. 2014;45:1437–1444. doi: 10.1016/j.humpath.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Beuselinck B, Job S, Becht E, et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin Cancer Res. 2015;21:1329–1339. doi: 10.1158/1078-0432.CCR-14-1128. [DOI] [PubMed] [Google Scholar]
- 11.Thompson RH, Dong H, Kwon ED. Implications of b7-h1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–715s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 12.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 15.Eble JNEJ, Sesterhenn IA. World Health Organization Classification of Tumors. Lyon, France: IARC Press; 2004. [Google Scholar]
- 16.Delahunt B, Cheville JC, Martignoni G, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37:1490–1504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Figueroa DJ, Liu Y, Gagnon RC, et al. Correlation of PDL1 tumor expression and outcomes in renal cell carcinoma (RCC) patients (pts) treated with pazopanib (paz) J Clin Oncol. 2013;31:3021a. [Google Scholar]
- 19.Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of pd-l1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: Results from comparz, a randomized controlled trial. Clin Cancer Res. 2015;21:1071–1077. doi: 10.1158/1078-0432.CCR-14-1993. [DOI] [PubMed] [Google Scholar]
- 20.Olson OC, Joyce JA. Microenvironment-mediated resistance to anticancer therapies. Cell Res. 2013;23:179–181. doi: 10.1038/cr.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heine A, Held SA, Bringmann A, et al. Immunomodulatory effects of anti-angiogenic drugs. Leukemia. 2011;25:899–905. doi: 10.1038/leu.2011.24. [DOI] [PubMed] [Google Scholar]
- 22.Hugonnet F, Fournier L, Medioni J, et al. Metastatic renal cell carcinoma: Relationship between initial metastasis hypoxia, change after 1 month’s sunitinib, and therapeutic response: An 18F-fluoromisonidazole PET/CT study. J Nucl Med. 2011;52:1048–1055. doi: 10.2967/jnumed.110.084517. [DOI] [PubMed] [Google Scholar]
- 23.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barsoum IB, Smallwood CA, Siemens DR, et al. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 25.Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (MRCC) J Clin Oncol. 2014;32:5010a. [Google Scholar]
- 26.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozali EN, Hato SV, Robinson BW, et al. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340. doi: 10.1155/2012/656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghiotto M, Gauthier L, Serriari N, et al. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int Immunol. 2010;22:651–660. doi: 10.1093/intimm/dxq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.