Unfit condition in frail elderly patients limits antineoplastic prescription and consequently patient survival. RAS and BRAF wild-type status could help select an elderly and unfit population that could benefit from anti-epidermal growth factor receptor single agent therapy. The results of this study show that single-agent off-label panitumumab was effective and well-tolerated as first-line treatment in frail elderly patients deemed unfit for chemotherapy for metastatic RAS and BRAF wild-type colorectal cancer.
Keywords: Elderly, Frail, Metastatic colorectal cancer, Panitumumab
Abstract
Background.
No prospective trials have specifically addressed the efficacy and safety of panitumumab in elderly patients with metastatic colorectal cancer (CRC). We aimed at assessing the efficacy and safety of single agent panitumumab in “frail” elderly patients diagnosed with metastatic RAS and BRAF wild-type CRC.
Materials and Methods.
Forty elderly patients (aged ≥75 years) with metastatic RAS-BRAF wild-type CRC received off-label prescriptions of single-agent panitumumab at seven Italian institutions. Treatment was administered as first line in patients with absolute contraindication to any chemotherapy or as second-line treatment after failure of a fluoropyrimidine-based treatment, in the presence of contraindication to irinotecan. The outcome measures included objective response rate (ORR), as well as progression-free survival (PFS), disease control rate (DCR), overall survival (OS), and safety.
Results.
The median PFS and OS were 6.4 months (95% confidence interval [CI]: 4.9–8 months) and 14.3 months (95% CI: 10.9–17.7 months), respectively. ORR was 32.5%, and DCR was 72.5%. Dose reductions related to adverse events (AEs) were reported in 9 (23%) patients, but no permanent treatment discontinuation caused by was reported. The most frequent grade 3 AE was skin rash, with an incidence of 20%.
Conclusion.
Panitumumab is effective and well-tolerated in frail elderly patients with RAS-BRAF wild-type metastatic CRC and deemed unfit for chemotherapy. A randomized study is needed to confirm these data.
Implications for Practice:
Treatment of elderly patients with metastatic colorectal cancer represents a difficult challenge in clinical practice. A significant proportion of frail elderly patients do not receive treatment, reflecting ongoing uncertainty of clinical benefit and toxicity of chemotherapy. Unfit condition in this cohort of patients further limits antineoplastic prescription and consequently patient survival. RAS and BRAF wild-type status could help select an elderly and unfit population that could benefit from anti-epidermal growth factor receptor single agent therapy. In the present study, single-agent off-label panitumumab was effective and well-tolerated as first-line treatment in frail elderly patients deemed unfit for chemotherapy for metastatic RAS and BRAF wild-type colorectal cancer.
Introduction
Anti-epidermal growth factor receptor (EGFR) monoclonal antibodies—cetuximab and panitumumab—improved the outcome of patients with advanced KRAS wild-type colorectal cancer (CRC), as single agents or in combination with chemotherapy [1–3]. However, panitumumab monotherapy is authorized only after failure of all three chemotherapy drugs, that is, as third- or further-line treatment following fluoropyrimidine-, oxaliplatin-, and irinotecan-containing regimens [2].
In the era of personalized medicine, anti-EGFRs achieved a response rate >40% in patients selected for KRAS, BRAF, NRAS, and exon 20 PI3KCA “quadruple wild-type” status [4, 5]. Recently, pan-RAS mutations were validated as negative predictive factors for anti-EGFR therapy in several retrospective, nonprespecified analyses of randomized clinical trials [6–8]. Thus, the prescription pattern of both cetuximab and panitumumab was restricted by the European regulatory authority (European Medicines Agency) to RAS wild-type patients. Moreover, we recently confirmed that the addition of anti-EGFRs does not seem to confer a benefit over standard treatment in RAS-wt/BRAF-mut patients [9].
Despite the high prevalence of CRC in the elderly population [10], these patients have been historically excluded or underrepresented in most clinical trials. As a result, there is not sufficient evidence on the appropriate management of elderly patients with metastatic CRC, and clinical decisions in routine practice are based on data extrapolated from nonelderly population. Regarding anti-EGFRs, weekly cetuximab was investigated in the elderly in a few retrospective or small prospective studies [11–14]. At present, the safety and efficacy of panitumumab in frail patients is not well-established. Moreover, limited available data mainly regard “fit” elderly patients retrospectively selected or candidates to clinical trials. In this study, we aimed at assessing the safety and efficacy of single agent panitumumab in frail elderly patients diagnosed with advanced RAS-BRAF wild-type CRC and deemed unfit for chemotherapy.
Materials and Methods
Patient Population
From September 2010 to February 2015, 40 elderly patients with metastatic CRC received off-label single-agent panitumumab at 7 Italian institutions. Key inclusion criteria were age ≥75 years; frailty status according to the definition of Hurria et al. [15], that is, higher risk for cancer treatment toxicity because of age-associated conditions such as functional losses, cognitive impairment, or physiologic changes; RAS and BRAF wild-type status per local assessment; life expectancy ≥12 weeks; and Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤2. We included patients who received panitumumab as first-line treatment for absolute contraindication to any chemotherapy (stratum A) or as second-line treatment after failure of a fluoropyrimidine-based treatment (with or without oxaliplatin or bevacizumab), in the presence of contraindication to irinotecan (stratum B).
Patients received single-agent panitumumab at the dosage of 6 mg/kg every 2 weeks until progressive disease (PD), unacceptable toxicity, or consent withdrawal. The study was approved by the institutional review board of the participating institutions, and all patients signed written informed consents for study analyses.
Study Endpoints and Assessments
The primary endpoint of our study was objective response rate (ORR) according to RECIST 1.1 [16]. Disease reassessments were performed by means of contrast-enhanced computed tomography scans every 8 weeks. Secondary endpoints included disease control rate (DCR), defined as the sum of RECIST responses and stable disease (SD) lasting at least 4 months; progression-free survival (PFS), which was measured from the beginning of the study treatment until the evidence of disease progression or death, whichever occurred first; overall survival (OS), measured from the beginning of the study treatment until death or last follow-up; and incidence of adverse events graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [17]. The dose adjustments or supportive medications were left to the treating physician, like in his clinical practice.
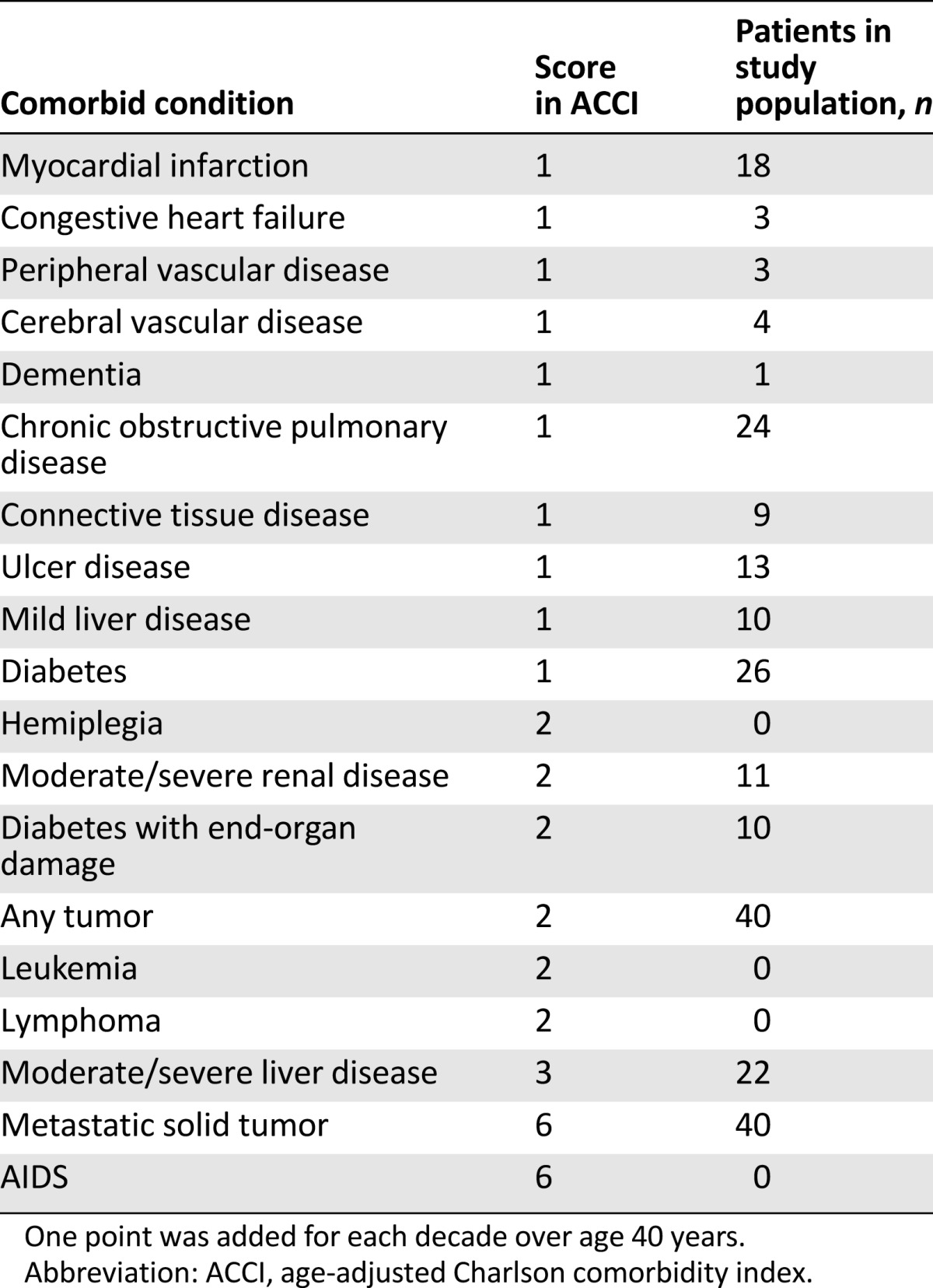
In order to quantify the clinical status of the patients in the study, we used the age-adjusted Charlson Index (ACCI). In ACCI, comorbid conditions are weighted and scored, with additional points added for age [18]. ACCI ranges from 0 to 43, with excellent predictive validity for a variety of clinical outcomes in oncology and geriatric and internal medicine. Despite the breadth of the range, a score >5 is generally an expression of severe clinical condition.
Statistical Analysis
Assuming ORRs of 5% and 45% as null and alternative hypotheses, respectively, with 2-tail α and β errors of 0.05 and 0.1, respectively, 10 first-line patients had to be treated with panitumumab monotherapy. The treatment would have been judged promising if at least three patients had achieved response. Assuming ORRs of 5% and 25% as null and alternative hypotheses, respectively, with 2-tail α and β errors of 0.05 and 0.1, respectively, 25 second-line patients had to be treated with panitumumab monotherapy. The treatment would have been judged promising if at least four patients had achieved response.
Survival curves for PFS and OS, medians and their 95% confidence intervals were estimated applying the Kaplan-Meier method. Cox multiple regression analysis for PFS and OS was used to assess the prognostic role of variables significantly associated with OS at univariate analyses. The following variables were tested: gender (male vs. female), ECOG PS (0–1 vs. 2), number of metastatic sites (1–2 vs. >2), skin rash (<G2 vs. ≥G2). The significance level was set at p < .05 for each test. Statistical analysis was carried out using SPSS package version 22 (SPSS software, IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/).
Results
Patient Population
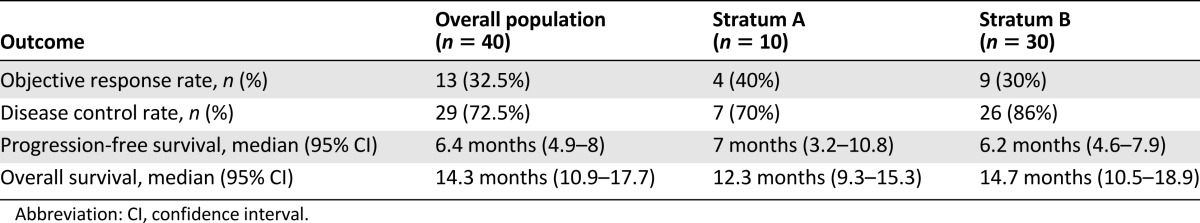
Forty patients were included. Patient demographics and disease characteristics are shown in Table 1. In particular, median age was 81 years (range, 76–90), and ECOG PS was mainly 1 (80%) or 2 (18%). The median value of ACCI was 11 (range, 9–15), and individual patient’s comorbid conditions are reported with the relative ACCI score in Table 2. Panitumumab was administered as first line (stratum A) in 10 (25%) patients and as second line (stratum B) in the remaining 30 (75%). In stratum B, previous treatments were oxaliplatin-based doublets at personalized dosage (43%), capecitabine monotherapy (37%), or capecitabine and bevacizumab (20%).
Table 1.
Patient demographics, disease characteristics, and therapy
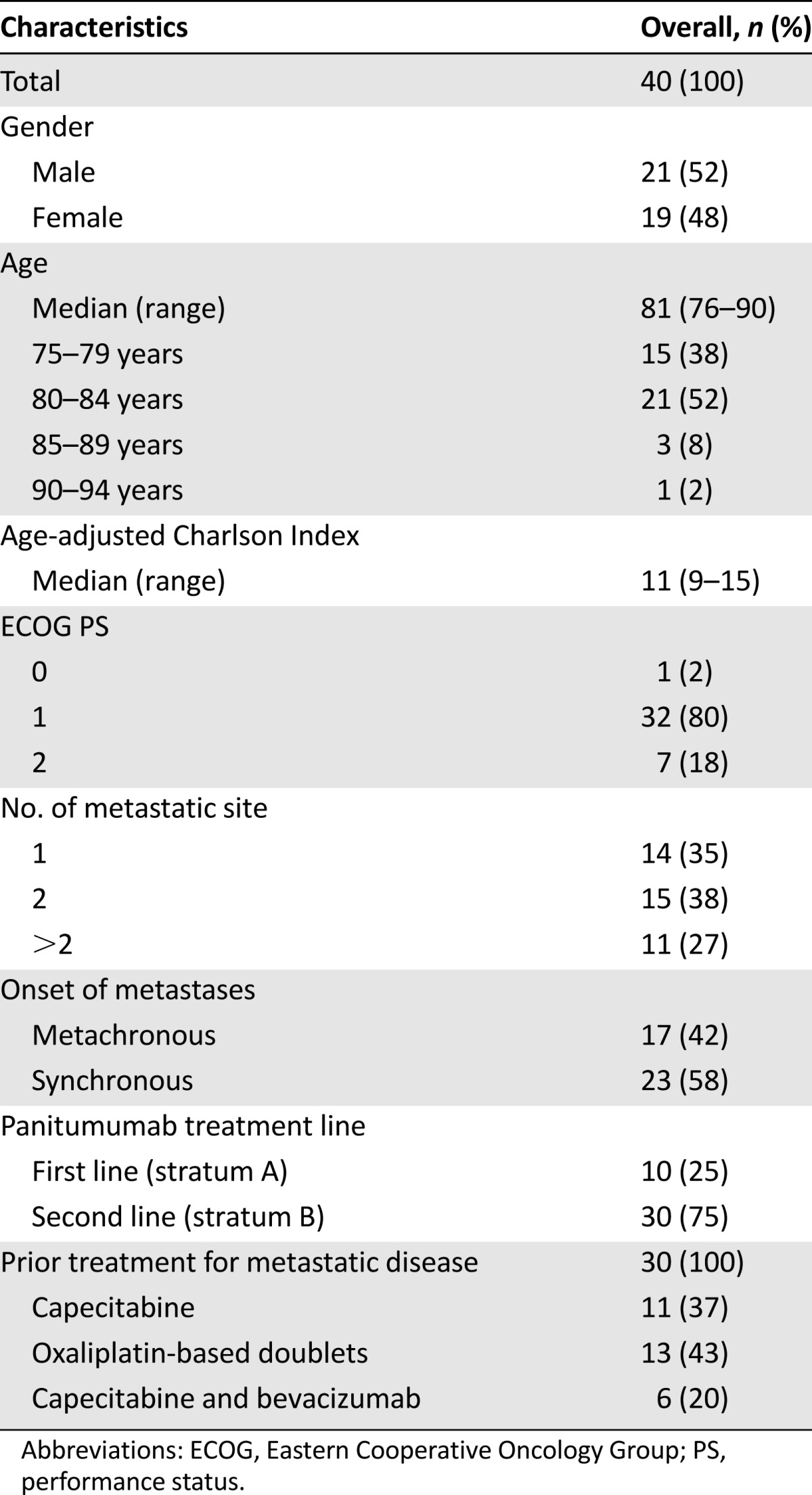
Table 2.
Comorbid conditions evaluated by ACCI
Activity and Efficacy
The results of the study in terms of activity and efficacy are resumed in Table 3. In the overall population, no complete response (CR) was observed. Of 40 patients, 13 (32.5%) achieved a partial response (PR), whereas 16 (40%) and 11 (27.5%) had SD (all lasting ≥4 months) and PD as the best response, respectively. Therefore, the ORR was 32.5%, and the DCR (CR + PR + SD ≥ 4 months) was 72.5%. According to the statistical plan, the study met its primary endpoint in both stratum A (ORR 40%) and stratum B (ORR 30%).
Table 3.
Activity and efficacy of treatment in the overall population, and separately in stratum A (panitumumab first line) or stratum B (panitumumab second line)
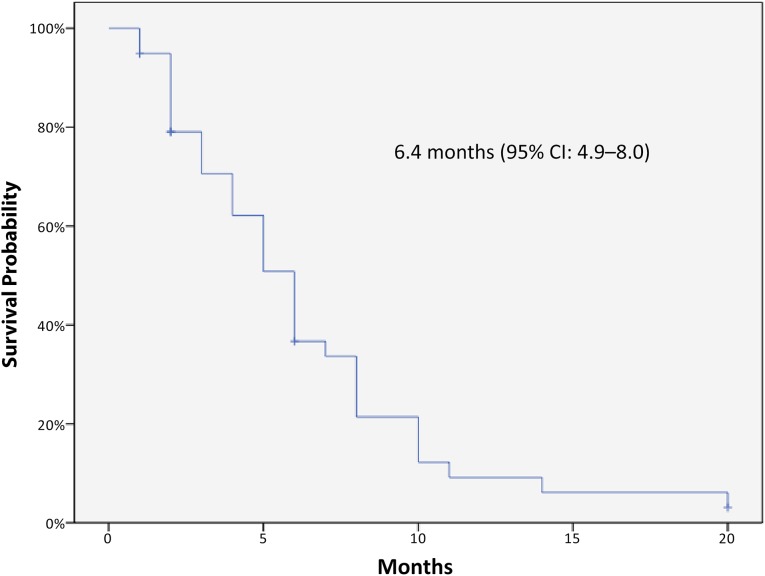
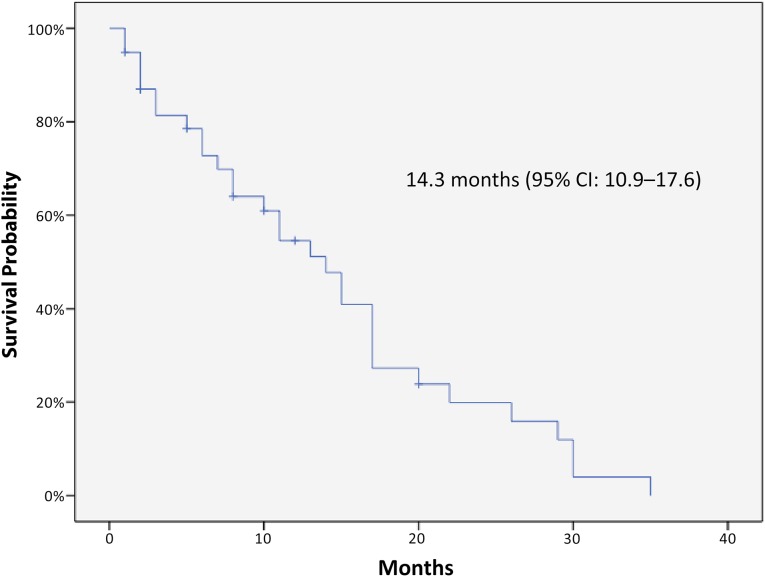
Kaplan-Meier curves for PFS and OS of the 40 patients are displayed in Figures 1 and 2, respectively. The median PFS was 6.4 months (95% CI: 4.9–8 months), and the median OS was 14.3 months (95% CI: 10.9–17.7 months). No relevant differences in terms of outcomes were observed according to panitumumab treatment line. In stratum A (panitumumab first line) median PFS and OS were 7 months (95% CI: 3.2–10.8) and 12.3 months (95% CI: 9.3–15.3), respectively. In stratum B (panitumumab second-line) median PFS and OS were 6.2 months (95% CI: 4.6–7.9) and 14.7 months (95% CI: 10.5–18.9), respectively. None of the patients received further postprogression treatments. The median PFS was not significantly modified in the subgroup analysis, whereas median OS was significantly better in patients with PS ECOG 0–1. A Cox regression test confirmed a significant positive impact on OS for patients with lower PS ECOG (p = .003).
Figure 1.
Kaplan-Meier curve of progression-free survival in the study population.
Abbreviation: CI, confidence interval.
Figure 2.
Kaplan-Meier curve of overall survival in the study population.
Abbreviation: CI, confidence interval.
Safety
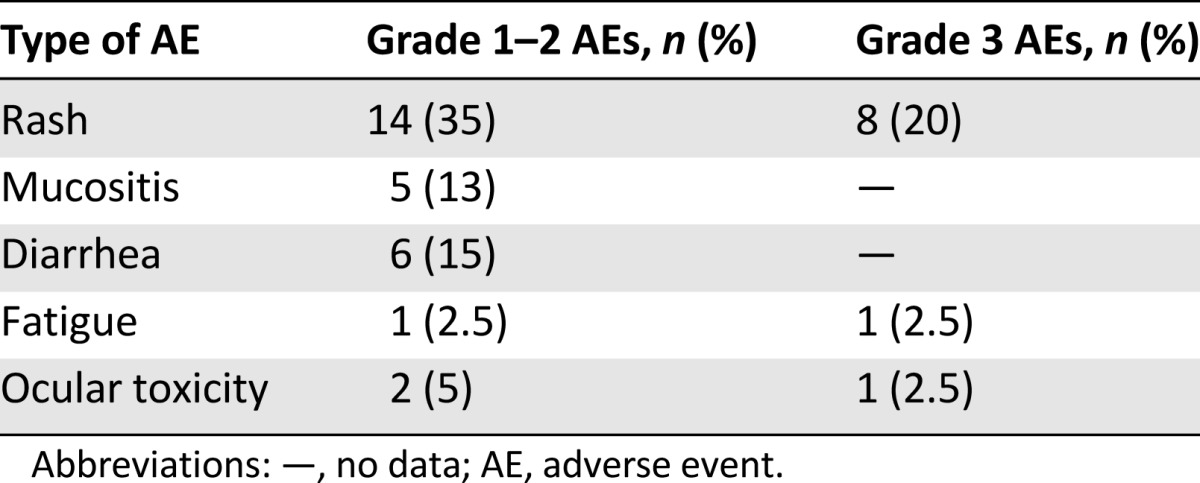
All patients were evaluable for safety and had at least one adverse event (AE) of all grades. In 10 (25%) patients, grade 3 AEs were reported, whereas no grade 4 or 5 AEs were observed. Dose reductions related to AEs were performed in 9 (23%) patients, but no AE-related treatment permanent discontinuation was observed. The most frequent grade 3 AEs were skin rash with an incidence of 20%, followed by fatigue and ocular toxicity (2.5% each). All reported AEs are listed in Table 4.
Table 4.
AEs in all 40 patients
Discussion
The present study aims at addressing an unmet clinical need: to investigate the safety and efficacy of panitumumab monotherapy in the usually neglected population of elderly and frail metastatic CRC patients not candidate to other therapeutic options. We specifically selected patients who were deemed by their treating physicians suboptimal candidates to first-line chemotherapy or second-line irinotecan-containing therapy because of high likelihood of treatment-associated toxicities. In fact, retrospective analyses of the pivotal irinotecan trials suggested that clinical benefit from this agent was largely confined to patients in good general condition (ECOG PS 0) [19, 20], and both advanced age and impaired PS have been reported to increase irinotecan-associated toxicities [21–25].
Currently, the most robust data concerning the efficacy and safety of a targeted therapy in elderly patients with advanced CRC are derived from the AVEX study [26]. This study was the only prospective, randomized, phase III trial addressing the role of bevacizumab in elderly mCRC patients aged ≥70 years deemed ineligible for combination chemotherapy by their treating physicians. The addition of bevacizumab to capecitabine monotherapy consistently improved clinical outcomes over capecitabine alone, with no unexpected AEs and no impact on quality of life. However, it must be pointed out that patients enrolled in this study were clearly fit enough to receive the study treatment. In the real-world setting, cardiovascular comorbidities and patient frailty may limit the applicability of this combination.
Two prospective phase 2 trials investigated the role of cetuximab in elderly patients with molecularly unselected, metastatic CRC. In a trial conducted by the Spanish group for digestive tumor therapy [13], first-line cetuximab monotherapy was administered to 41 fit elderly patients aged ≥70 years. The response rate was 14.6%, whereas PFS and OS were 2.9 and 11.4 months, respectively [13]. Different from our results, the modest efficacy observed in terms of ORR and PFS may reflect the absence of molecular selection based on RAS and BRAF status, whereas the relatively longer OS may be due to postprogression chemotherapy. In the second trial carried out by the same group, first-line cetuximab plus capecitabine combination was administered to 66 fit elderly patients. Twenty-nine evaluable patients had a KRAS wild-type status. In this subgroup, response rate was 48.3%, and the median PFS was 8.4 months [14]. However, based on the subgroup analysis of a large phase III randomized trial [27], the association of cetuximab with a capecitabine-based chemotherapy is not a preferred combination, and this may have negatively affected the efficacy of cetuximab in this study.
With regard to the safety profile, older age does not seem to negatively increase serious toxicities. Despite this higher-risk study population (median value of ACCI was 11), treatment with single-agent panitumumab seemed to be relatively well-tolerated. The well-described panitumumab-associated adverse event of grade 3 skin rash was seen in 20% of the patients, and no AEs-related permanent discontinuation of the treatment was reported. According to our results, panitumumab monotherapy is tolerable in frail elderly patients with advanced, RAS and BRAF wild-type CRC and aged ≥75 years. Results in terms of ORR confirm that panitumumab is active independently from the line of treatment and highlight the potential impact of this easy-to-manage treatment in inducing tumor shrinkage, thus delaying the occurrence of tumor-related symptoms and improving patient quality of life. The choice of panitumumab may offer some potential advantages over cetuximab given the possibility of a biweekly administration, as well as the extremely lower incidence of allergic reactions with no mandatory prophylaxis.
Conclusion
Although the small sample size must be taken into account, these encouraging safety efficacy results do provide new hope for a subset of patients for whom the current label precludes any chance to receive an active treatment. A prospective randomized trial is needed to further define the efficacy and tolerability of single-agent panitumumab in frail elderly patients with metastatic RAS and BRAF wild-type CRC judged unfit for chemotherapy.
Author Contributions
Conception/Design: Filippo Pietrantonio, Chiara Cremolini, Giuseppe Aprile, Sara Lonardi, Armando Orlandi, Alessia Mennitto, Rosa Berenato, Gianluca Tomasello, Maria Di Bartolomeo, Fotios Loupakis, Filippo de Braud
Provision of study material or patients: Filippo Pietrantonio, Chiara Cremolini, Giuseppe Aprile, Sara Lonardi, Armando Orlandi, Alessia Mennitto, Rosa Berenato, Carlotta Antoniotti, Mariaelena Casagrande, Valentina Marsico, Federica Marmorino, Giovanni Gerardo Cardellino, Francesca Bergamo, Gianluca Tomasello, Vincenzo Formica, Raffaella Longarini, Elisa Giommoni, Marta Caporale, Maria Di Bartolomeo, Fotios Loupakis, Filippo de Braud
Collection and/or assembly of data: Filippo Pietrantonio, Chiara Cremolini, Giuseppe Aprile, Sara Lonardi, Armando Orlandi, Alessia Mennitto, Rosa Berenato, Carlotta Antoniotti, Mariaelena Casagrande, Valentina Marsico, Federica Marmorino, Giovanni Gerardo Cardellino, Francesca Bergamo, Gianluca Tomasello, Vincenzo Formica, Raffaella Longarini, Elisa Giommoni, Marta Caporale, Maria Di Bartolomeo, Fotios Loupakis, Filippo de Braud
Data analysis and interpretation: Filippo Pietrantonio, Chiara Cremolini, Giuseppe Aprile, Sara Lonardi, Armando Orlandi, Maria Di Bartolomeo, Fotios Loupakis, Filippo de Braud
Manuscript writing: Filippo Pietrantonio, Chiara Cremolini, Giuseppe Aprile, Sara Lonardi, Armando Orlandi, Rosa Berenato, Maria Di Bartolomeo, Fotios Loupakis, Filippo de Braud
Final approval of manuscript: Filippo Pietrantonio, Chiara Cremolini, Giuseppe Aprile, Sara Lonardi, Armando Orlandi, Alessia Mennitto, Rosa Berenato, Carlotta Antoniotti, Mariaelena Casagrande, Valentina Marsico, Federica Marmorino, Giovanni Gerardo Cardellino, Francesca Bergamo, Gianluca Tomasello, Vincenzo Formica, Raffaella Longarini, Elisa Giommoni, Marta Caporale, Maria Di Bartolomeo, Fotios Loupakis, Filippo de Braud
Disclosures
Chiara Cremolini: Bayer, Roche, Amgen (C/A), Bayer, Sanofi Aventis (ET); Filippo de Braud: Istituto Nazionale Tumori (E), Bristol-Myers Squibb, Merck Serono, Servier, Dephaphorum (H); Fotios Loupakis: Roche, Amgen, Bayer, Merck Serono, Sanofi, Lilly (C/A), Roche, Amgen (ET). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 2.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 4.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 5.Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, et al. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One. 2009;4:e7287. doi: 10.1371/journal.pone.0007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 9.Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur J Cancer. 2015;51:587–594. doi: 10.1016/j.ejca.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 11.Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol. 2008;67:255–262. doi: 10.1016/j.critrevonc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Fornaro L, Baldi GG, Masi G, et al. Cetuximab plus irinotecan after irinotecan failure in elderly metastatic colorectal cancer patients: Clinical outcome according to KRAS and BRAF mutational status. Crit Rev Oncol Hematol. 2011;78:243–251. doi: 10.1016/j.critrevonc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Sastre J, Aranda E, Grávalos C, et al. First-line single-agent cetuximab in elderly patients with metastatic colorectal cancer: A phase II clinical and molecular study of the Spanish group for digestive tumor therapy (TTD) Crit Rev Oncol Hematol. 2011;77:78–84. doi: 10.1016/j.critrevonc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Sastre J, Grávalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: Clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. The Oncologist. 2012;17:339–345. doi: 10.1634/theoncologist.2011-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32:2587–2594. doi: 10.1200/JCO.2013.55.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available at http://www.hrc.govt.nz/sites/default/files/CTCAE%20manual%20-%20DMCC.pdf. Accessed September 24, 2015.
- 18.Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg. 2004;8:1061–1067. doi: 10.1016/j.gassur.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Knight RD, Miller LL, Pirotta N, et al. First-line irinotecan (C), fluorouracil (F), leucovorin (L) especially improves survival (OS) in metastatic colorectal cancer (MCRC) patients (PT) with favorable prognostic indicators. Proc Am Soc Clin Oncol. 2000;19:255a. [Google Scholar]
- 20.Camptosar (irinotecan, CPT-11): First-line therapy of metastatic colorectal cancer. Paper presented at: Oncologic Drug Advisory Committee Meeting; March 16, 2000; Bethesda, MD.
- 21.Bleiberg H, Cvitkovic E. Characterisation and clinical management of CPT-11 (irinotecan)-induced adverse events: The European perspective. Eur J Cancer. 1996;32AS(suppl 3):S18–S23. doi: 10.1016/0959-8049(96)00293-6. [DOI] [PubMed] [Google Scholar]
- 22.CPT-11 for the treatment of patients with metastatic colorectal cancer that has progressed following initial 5-FU based chemotherapy. Paper presented at: Oncologic Drug Advisory Committee Meeting; June 13, 1996; Bethesda, MD.
- 23.Rougier P, Bugat R. CPT-11 in the treatment of colorectal cancer: Clinical efficacy and safety profile. Semin Oncol. 1996;23(suppl 3):34–41. [PubMed] [Google Scholar]
- 24.Rougier P, Bugat R, Douillard JY, et al. Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol. 1997;15:251–260. doi: 10.1200/JCO.1997.15.1.251. [DOI] [PubMed] [Google Scholar]
- 25.Rothenberg ML, Cox JV, DeVore RF, et al. A multicenter, phase II trial of weekly irinotecan (CPT-11) in patients with previously treated colorectal carcinoma. Cancer. 1999;85:786–795. [PubMed] [Google Scholar]
- 26.Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 27.Adams RA, Meade AM, Madi A, et al. Toxicity associated with combination oxaliplatin plus fluoropyrimidine with or without cetuximab in the MRC COIN trial experience. Br J Cancer. 2009;100:251–258. doi: 10.1038/sj.bjc.6604877. [DOI] [PMC free article] [PubMed] [Google Scholar]