Patients who received gonadotropin-releasing hormone agonist (GnRHa) with chemotherapy were compared with patients who were treated with chemotherapy alone to determine whether GnRHa can increase the pregnancy rate in survivors. The results indicated that adding GnRHa to chemotherapy significantly increases the odds ratio for spontaneous conception, in addition to cyclic ovarian function. It is suggested that GnRHa cotreatment should be added before and during gonadotoxic chemotherapy.
Keywords: Fertility preservation, Pregnancies after gonadotoxic chemotherapy, Gonadotropin-releasing hormone agonist, Premature ovarian failure
Abstract
Background.
The use of gonadotropin-releasing hormone analogs (GnRHas) for fertility preservation is not unequivocally accepted. It is controversial whether GnRHa can increase the pregnancy rate in survivors.
Patients and Methods.
This is a retrospective cohort study. Every patient referred for fertility preservation was offered cryopreservation of embryos, ova, and ovarian tissue and GnRHa. The patients were consecutively included. The primary outcome was spontaneous pregnancies. The secondary outcome was cyclic ovarian function (COF) versus premature ovarian failure (POF). These outcomes were assessed 2 years or more after chemotherapy.
Results.
We compared 286 patients who received gonadotropin-releasing hormone agonist (GnRHa) with chemotherapy with 188 patients who were treated with chemotherapy alone. Ovarian function could be determined in 217 patients. Overall, 87% (127 of 146) of the patients in the GnRHa group retained COF and 13% (19 of 146) suffered POF, whereas in the control group, 49% (35 of 71) experienced COF and 51% (36 of 71) suffered POF (p = .0001). The odds ratio (OR) for preserving COF was 6.87 for the patients who received GnRHa (95% confidence interval [CI] 3.4–13.4). Overall 60% (112 of 188) of the survivors conceived: 69.3% (84 of 122) of the patients in the GnRHa group compared with 42.4% (28 of 66) in the control group (p = .006). In the GnRHa group, 123 healthy newborns were delivered, versus 40 in the controls. Spontaneous pregnancies occurred in 65.6% (80 of 122) of the survivors in the GnRHa group versus 37.9% (25 of 66) in the control group (p = .0004, OR 3.12, 95% CI 1.7–5.8).
Conclusion.
Adding GnRHa to chemotherapy significantly increases the OR for spontaneous conception, in addition to COF. It is suggested that GnRHa cotreatment should be added before and during gonadotoxic chemotherapy.
Implications for Practice:
The use of gonadotropin-releasing hormone analogs (GnRHa) for fertility preservation is not unequivocally accepted and is even controversial. This study compared 286 patients who received GnRHa with chemotherapy with 188 patients who were treated with chemotherapy alone. Ovarian function could be determined in 217 patients. The odds ratio for preserving cyclic ovarian function was 6.87 for the patients who received GnRHa. Furthermore, the total and spontaneous pregnancy rate was significantly higher for those who received the agonist (p = .006). Adding GnRHa to chemotherapy significantly increased the odds ratio for spontaneous conception, in addition to preserving regular ovarian function. It is suggested that GnRHa cotreatment should be administered to young women in conjunction with gonadotoxic chemotherapy.
Introduction
The increase in cancer incidence at young age and the significant increase in long-term survival after treatment have brought about a ubiquitous interest in fertility preservation in young patients exposed to gonadotoxic chemotherapy, creating a new specialty in reproductive medicine: oncofertility. Therefore, the late effects of cancer treatment have recently gained a worldwide interest [1–4], and the protection against iatrogenic infertility caused by chemotherapy assumes a high priority. Resumption of menses may not be an accurate marker of fertility, because infertility and diminished ovarian reserve are observed in women who resume normal menstrual cycles after treatment with chemotherapy [1–4]. In addition, spontaneous pregnancies can occur in women with chemotherapy-induced amenorrhea despite menopausal levels of gonadotropins [1–4]. Therefore, caution should be used when extrapolating surrogate markers, such as resumption of menses and markers of ovarian reserve, to clinical outcomes, such as fertility [1–4].
Several options have been put forward for preserving female fertility: ovarian transposition, cryopreservation of embryos, unfertilized oocytes, and/or ovarian tissue, and administration of GnRHa in an attempt to decrease the gonadotoxic effects of chemotherapy by simulating a prepubertal hormonal milieu [1–6]. Unfortunately, none of the suggested methods is ideal, and none guarantees future fertility in survivors. The in vitro maturation of primordial follicles to fertilizable metaphase-II oocytes is a future endeavor with enormous potential, but it requires overcoming many technological obstacles and is not clinically available yet. We have recently summarized the case for and against gonadotropin-releasing hormone agonist (GnRHa) for fertility preservation [1, 3, 4]. Indeed, GnRHa has been used by many groups of clinicians for minimizing the gonadotoxic effects of chemotherapy, with the rationale and philosophy that preventing premature ovarian failure (POF) in survivors is preferable to treating it, following the dictum: “an ounce of prevention is worth a pound of cure” [1].
To date, 25 studies (15 retrospective and 10 prospective, randomized controlled trials [RCTs]) have reported on 2,145 patients treated with GnRHa in parallel to chemotherapy, showing a significant decrease in POF rate in survivors versus nine studies reporting on 593 patients, not supporting GnRHa use [1]. However, the American Society of Clinical Oncology (ASCO), the American Society for Reproductive Medicine (ASRM), and the European Society for Medical Oncology (ESMO) concluded that GnRHa is not considered a proven effective method of fertility preservation [5–7]. Another claim against GnRHa use for fertility preservation was that decreasing the rate of POF and preserving cyclic ovarian function (COF) is not identical to fertility, and only pregnancy in survivors can unequivocally settle the debate. Therefore, we have evaluated the pregnancies in survivors of chemotherapy with or without GnRHa cotreatment.
Patients and Methods
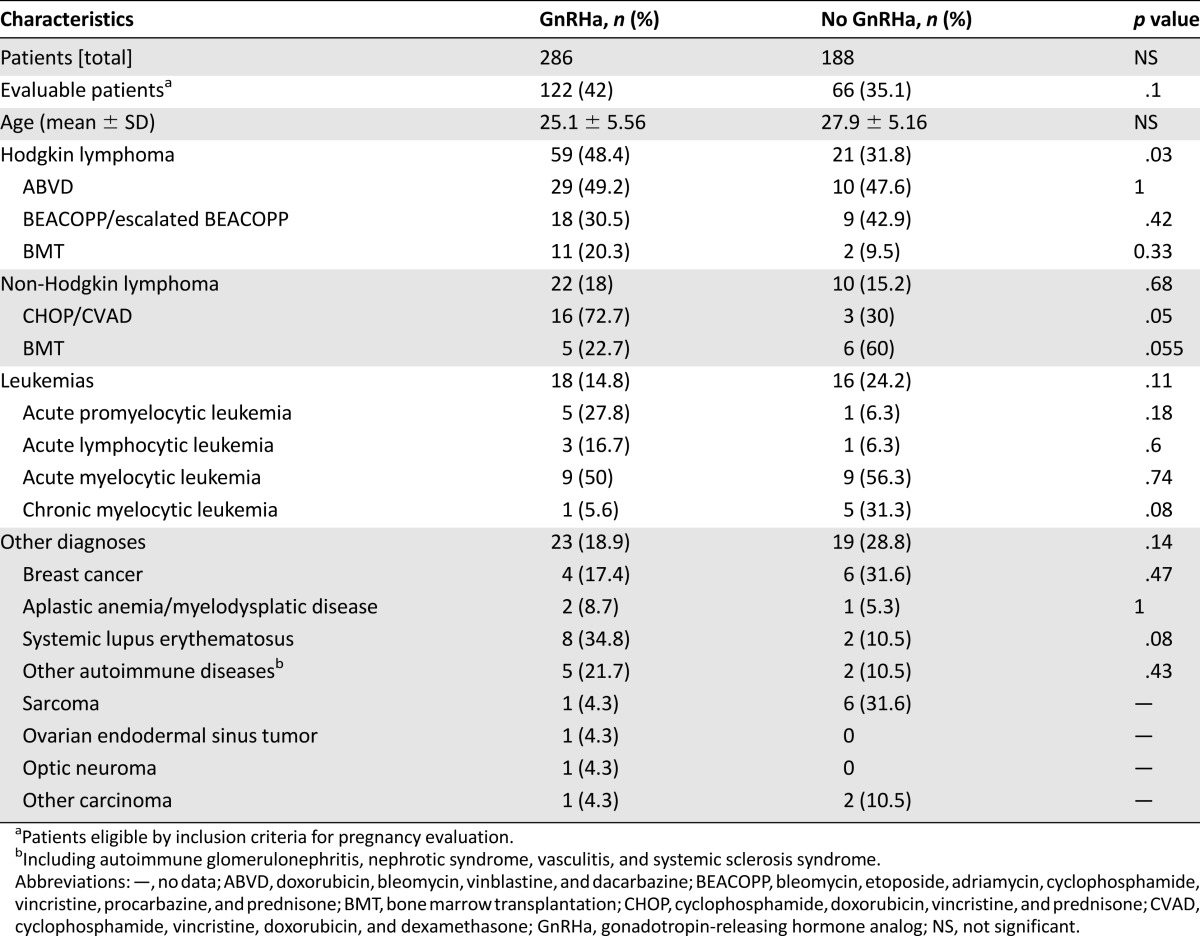
This is a retrospective cohort study. Starting in 1989 [1], female patients 14–40 years old, who had been referred to our reproductive endocrinology clinic before chemotherapy were offered in vitro fertilization (IVF) and cryopreservation of embryos (and in the last decade also ova), cryopreservation of ovarian tissue, and a monthly depot injection of 3.75 mg of GnRHa (D-TRP6-GnRHa; Decapeptyl CR; Ferring, Saint-Prex, Switzerland, https://www.ferring.com/en/home) in conjunction with chemotherapy. The patients were consecutively included. Until now, we have administered GnRHa before and during chemotherapy to 286 female patients (treatment group), whereas 188 did not receive the GnRHa cotreatment (control group) (Table 1) [1]. There was no significant difference in age, disease type and stage (diagnoses), cumulative chemotherapy, or radiation exposure between the two groups [1, 4, 5, 8]. The control group consisted of patients who elected not to receive the GnRHa cotreatment or were referred after starting chemotherapy. Every survivor, if more than 2 years after chemotherapy, was interviewed regarding menstrual cycles regularity and pregnancies—spontaneous or induced by treatment, singleton versus multiple, and outcome—miscarriage or delivery, fetal outcome (sex, weight, Apgar score, and mode of delivery). The study was approved by the institutional ethical committee for human experimentation of the Rambam Health Care Campus and the Ministry of Health, and patients signed informed consent.
Table 1.
The number of patients treated with or without GnRHa for various indications
The GnRHa administration was timed as early as possible, usually within 7–14 days before starting chemotherapy. In 55 patients, for whom the hematologists or oncologists indicated urgency to the initiation of chemotherapy, the interval was shorter (<7 days).
To minimize selection bias, every female patient before chemotherapy between 14 and 40 years who was referred to us was included either in the study group, if referred before chemotherapy and electing to receive GnRHa, or in the control group. Every consecutive patient was offered the GnRHa. Those who agreed to receive it were included in the treatment group, whereas those who elected not to receive the analog were included in the control group. The offer was put forward to every new patient without selection.
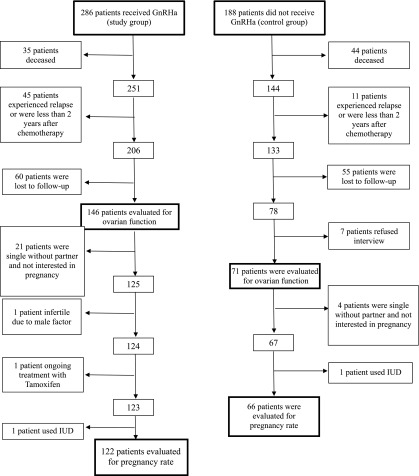
The inclusion criteria were (a) premenopausal, 14–40 years old; (b) first-line therapy; or (c) patients who had not received any chemotherapy for at least 2 years after completing the first chemotherapy and GnRHa (to enable a reliable follow-up and evaluation). Patients who had progressed or relapsed within 2 years were excluded. There were seven patients in the study group and six in the control group who were not eligible for inclusion in the evaluation because of progressive disease or relapse within less than 2 years after completion of the initial treatment (not significant [NS]) (Fig. 1).
Figure 1.
Flow sheet explaining patient dropout.
Abbreviations: GnRHa, gonadotropin-releasing hormone analog; IUD, intrauterine device.
POF was defined as persistent hypergonadotropic amenorrhea (follicle-stimulating hormone >40 U/L on at least two occasions) and low, menopausal, E2 levels, whereas COF was defined as regular spontaneous menstrual cycles, normal gonadotropins and E2 levels, ovulatory progesterone, visualization of ovarian follicles or corpora lutea, and/or spontaneous conception. Pregnancy rate was calculated in those survivors who had a partner, were interested in fertility, and were more than 2 years after chemotherapy, by telephone interview for those who were not under regular follow-up.
Comparison of both study groups regarding pregnancies was done using χ2 test (Pearson and Fisher’s exact tests). Evaluation of time to achievement of pregnancy was compared between the two groups using the unpaired t test. Cumulative pregnancy rate was compared between groups using the Kaplan-Meier method of estimation for right censored data distributions. p < .05 was considered statistically significant. All analyses were carried out using SPSS software (SPSS software, IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/) and R software, version 2.12.2 (R Foundation, Vienna, Austria, http://www.rproject.org).
Results
In the past 25 years, overall, 286 patients had received GnRHa along with chemotherapy and 188 had not (controls) (Table 1). However, ovarian function could be determined only in 217 patients: 146 in the GnRHa group and 71 in the control group (Fig. 1). The shortest follow-up was 2 years, and the longest was 25 years. There was no significant difference in the cohort of follow-up between the two groups. Overall, 87% (127 of 146) of the patients in the GnRHa group experienced COF, and 13% (19 of 146) suffered POF, whereas in the control group, 49% (35 of 71) experienced COF, and 51% (36 of 71) suffered POF, p = .0001 (Table 2). The odds ratio (OR) for preserving COF was 6.87 for the patients who received GnRHa in addition to chemotherapy (95% confidence interval [CI] 3.4–13.4). None of the patients who were diagnosed with POF for more than 24 months resumed COF.
Table 2.
Comparison of the pregnancy rate in the overall cohort of patients treated with or without GnRHa
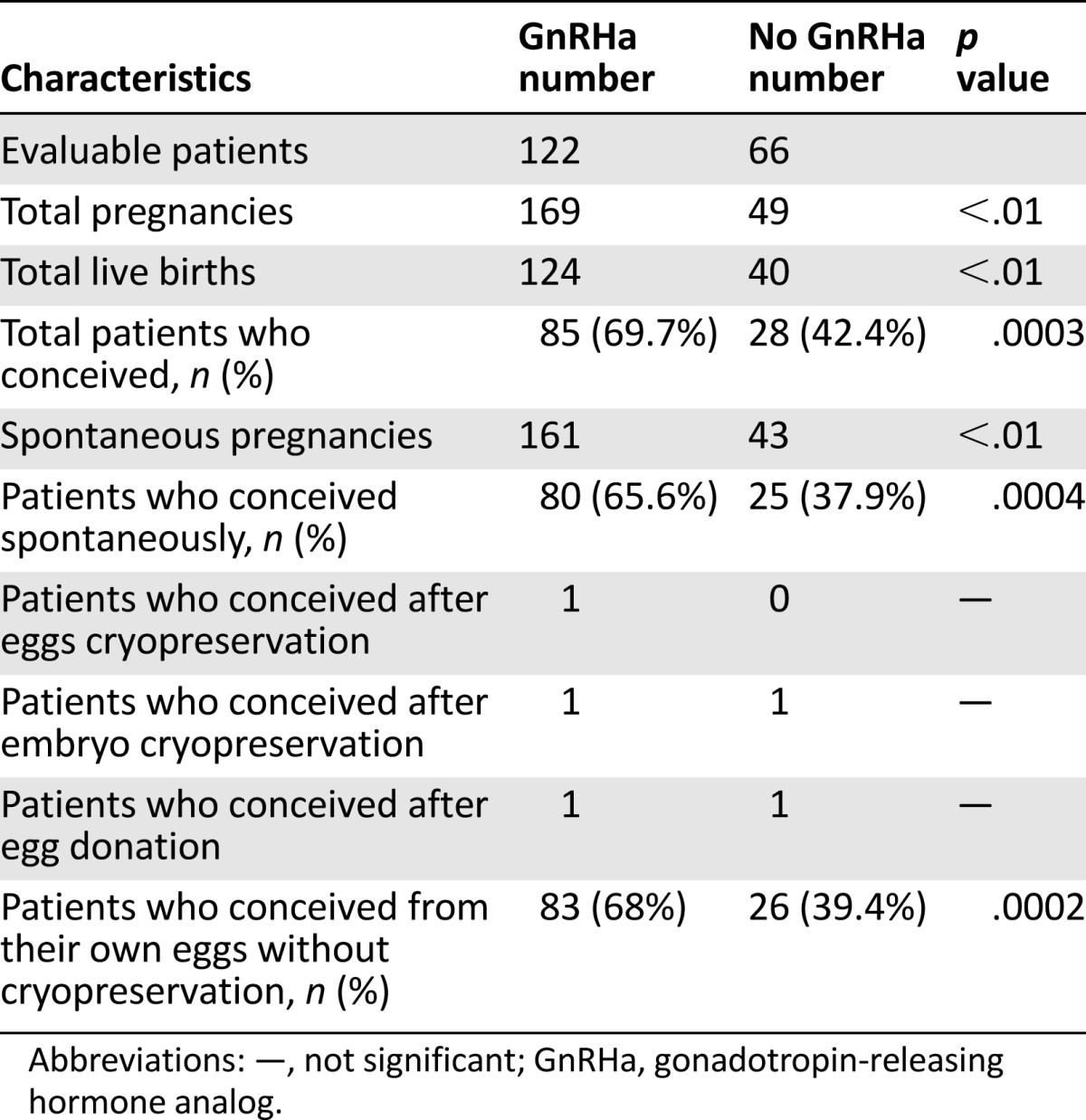
In the GnRHa group, 51% (62 of 122) had children before chemotherapy versus 66% (44 of 66) in the controls (NS). In the GnRHa group, 29% claimed, before chemotherapy, they were not planning pregnancies versus 26% in the controls (NS).
In total, 188 female patients were evaluable, regarding pregnancy (Fig. 1). Of these, 80 patients were treated for Hodgkin lymphoma, 32 for non-Hodgkin lymphoma, 34 for leukemia, and the remaining 42 for other diseases (breast cancer, sarcoma, autoimmune diseases such as systemic lupus erythematosus, necessitating gonadotoxic chemotherapy such as cyclophosphamide pulsatile treatment, and others) (Table 1). Overall 60% (112 of 188) of all eligible women conceived (Table 2); 69.7% (85 of 122) of the patients in the GnRHa group successfully conceived (between 1 and 6 times) compared with 42.4% (28 of 66) of the patients in the control group (between 1 and 4 times), p = .006 (Table 2). In the GnRHa group, 124 healthy newborns were delivered versus 40 in the controls (Tables 2, 3).
Table 3.
Comparison of the pregnancy rate in the patients treated with or without GnRHa according to their disease
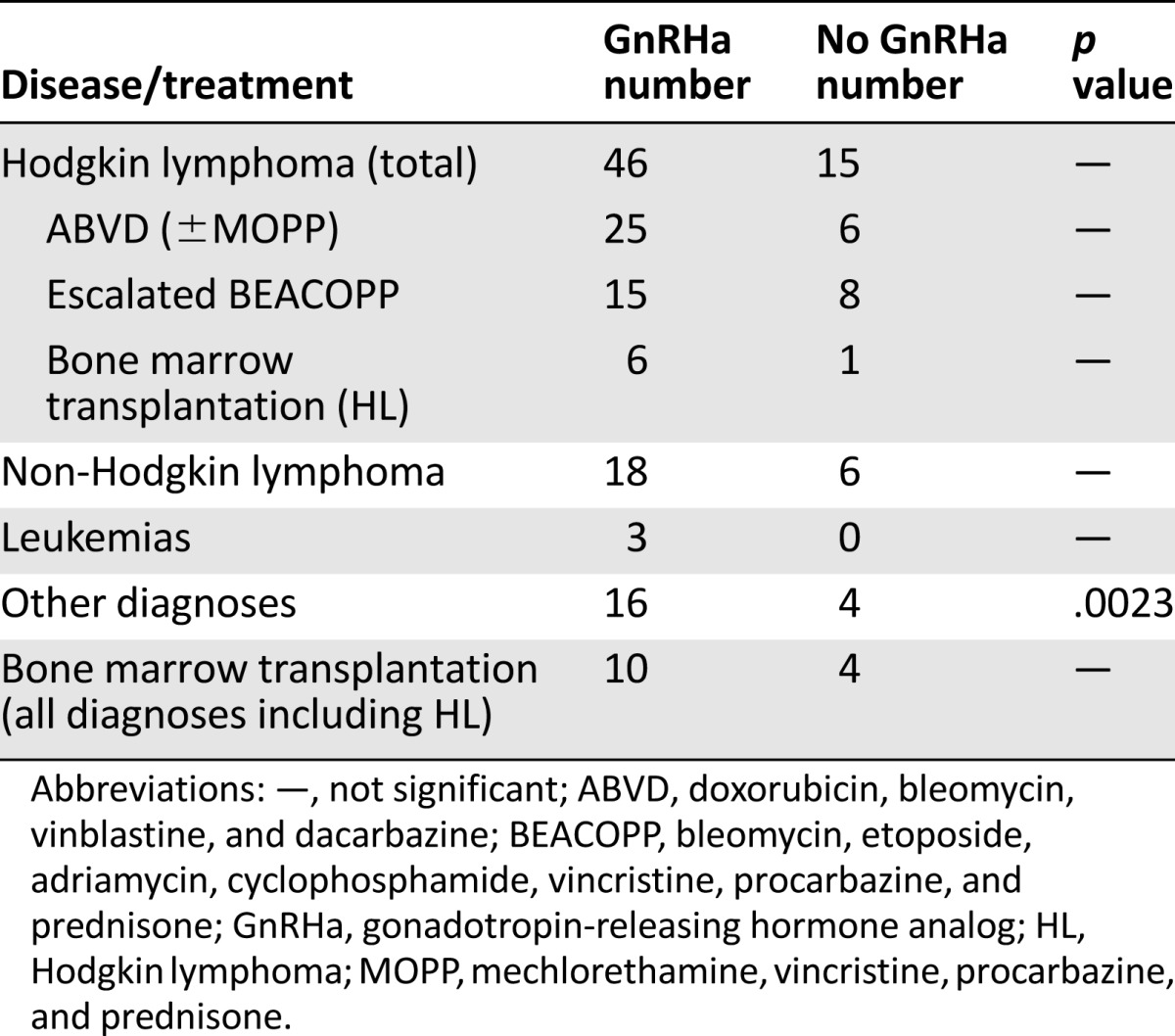
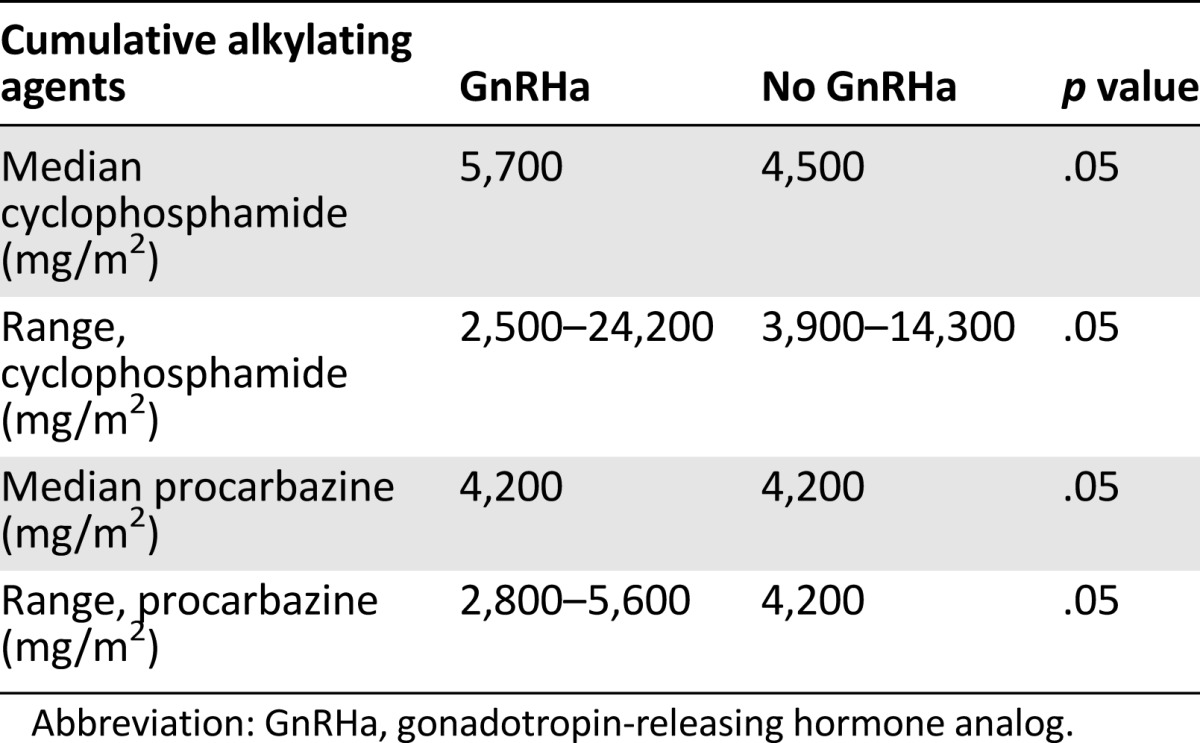
Spontaneous pregnancies occurred in 65.6% (80 of 122) of the survivors in the GnRHa group, and 67.8% (83 of 122) conceived from their own oocytes after chemotherapy exposure versus 37.9% (25 of 66) of the survivors in the control group who conceived spontaneously and 39.4% (26 of 66) of women conceived from their own oocytes after exposure to chemotherapy (p = .0004). The OR for spontaneously conceiving was 3.12 for the patients who received GnRHa in addition to chemotherapy versus those who received chemotherapy without the GnRHa (95% CI 1.7–5.8). In the GnRHa group, 19 patients (16%) spontaneously conceived despite receiving chemotherapy after age 30, with the oldest being 38 years old at chemotherapy, whereas in the control group only 5 patients (7%) were older than 30 at chemotherapy. In the GnRHa group, 11 patients spontaneously conceived after the age of 35 versus 5 in the control group. In the GnRHa group, the longest time of spontaneous conception after chemotherapy was 18 years versus 14 years in the controls. In the GnRHa group, the oldest patient who spontaneously conceived was aged 42 years (versus aged 37 years in the control group). The comparison of subgroups lacked the statistical power to evaluate significant differences because of small numbers. (Table 3). There was no difference in the cumulative alkylating agents between the groups (Table 4), as we have also previously published [9].
Table 4.
Cumulative doses of cyclophosphamide and procarbazine, the most toxic alkylating agents used to treat Hodgkin lymphoma
Discussion
To our best knowledge, this is the largest study on pregnancy rate (fertility) in survivors treated with or without GnRHa. The OR for preserving COF was 6.87 for the patients who received GnRHa in addition to chemotherapy (95% CI 3.4–13.4) and 3.12 (95% CI 1.7–5.8) (p < .001) for spontaneously conceiving versus those who were treated with chemotherapy without the GnRHa. Although, 25 studies (over 10 prospective RCTs) have shown a significant decrease in POF rate in survivors of chemotherapy and GnRHa, and there are nine publications not supporting GnRHa use [1]. The ASCO [5], the ASRM [6], and the ESMO [7] do not support GnRHa as a proven method for fertility preservation and consider it experimental. One of the main arguments against considering the GnRHa cotreatment as an established method for fertility preservation is that preserving COF and regular menses in survivors is only a surrogate marker not equivalent to fertility, that is, pregnancies [10]. The main weakness of studies evaluating the role of GnRHa in preserving ovarian function is the lack of data concerning the long-term maintenance of ovarian function and preservation of fertility [1, 11].
Therefore, we have evaluated the pregnancy rate in a large group of patients treated with or without GnRHa, in conjunction with gonadotoxic chemotherapy, for the last 25 years. Our data demonstrate a significant advantage for the survivors who received the GnRHa both in keeping COF and in their ability to conceive spontaneously.
Relevant to this highly debatable issue, Behringer et al. [12] also found that the use of GnRHa during chemotherapy has significantly increased the probability to become pregnant (OR = 12.87; p = .001). Similarly, Del Mastro et al. [11] and the authors of several other recent meta-analyses of RCTs [1] concluded that the significant reduction (p = .013) in POF rate, associated with GnRHa, provides convincing evidence in support of the efficacy of this preventive strategy. In addition, the Cochrane database [13] supports the use of GnRHa cotreatment for fertility preservation. Most recently, Moore et al. [14] published the results of an NIH-sponsored prospective RCT trial, in which 257 premenopausal breast cancer patients received chemotherapy with or without GnRHa [14]. The GnRHa-treated patients had better-preserved ovarian function across multiple endpoints and improved fertility (more successful pregnancies) than the controls [14], in keeping with our findings. Unexpectedly, the combination led to more favorable disease-free survival (DFS) and overall survival (OS) rates [14]. At 2 years, the POF rate was 22% for the standard chemotherapy arm compared with 8% for the GnRHa arm (OR = 0.30, 95% CI [0.09, 0.97]; p = .04) [14]. Successful pregnancy was achieved by 12 of 18 women who attempted pregnancy and who were treated with chemotherapy alone compared with 22 of 25 successful pregnancies in the GnRHa arm (adjusted OR 2.45; p = .03). In addition, women in the GnRHa group gave birth to 18 babies versus 12 in the standard chemotherapy group. Secondary ovarian outcomes also favored the GnRHa group, as well as better DFS and OS. The rate of DFS in the standard chemotherapy arm was 78% compared with 89% in the GnRHa arm (hazard ratio [HR] 0.49 adjusted for age, regimen, and stage, 95% CI [0.24, 0.97]; p = .04). In addition, the 4-year OS rate was 82% in the standard chemotherapy arm compared with 92% in the GnRHa arm (HR 0.43 adjusted for age, regimen, and stage, 95% CI [0.18, 1.0]; p = .05).
Similarly, Lambertini et al. [15] presented updated results from the PROMISE-GIM6 study after 7.3 years of follow-up. PROMISE-GIM6 was a multicenter, open-label trial of 281 premenopausal women with early-stage breast cancer who were candidates for neoadjuvant or adjuvant chemotherapy. Eighty percent of subjects had hormone receptor-positive disease. Patients were randomly assigned to chemotherapy alone (n = 133) or chemotherapy with GnRHa (n = 148). The 1-year results showed a highly significant 72% reduction in the risk of treatment-related early menopause in women receiving adjuvant GnRHa versus chemotherapy alone [15]. In the updated analysis, women in the PROMISE-GIM6 study were more than twice as likely to become pregnant over 7 years when treated with the adjuvant GnRHa compared with chemotherapy alone (HR = 2.56) [15]. The authors concluded that the similar disease-free survival in both groups confirmed the safety of the GnRHa cotreatment in both hormone receptor-positive and hormone-negative breast cancer patients [15]. Thus, two recent RCTs have found significantly higher partial response (PR) in the GnRHa versus controls, with either better survival in hormone receptor negative breast cancer patients [14] or similar survival in hormone receptor positive women [15].
When the gonadotoxicity of the chemotherapeutic protocols is either very low, such as when using ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) protocol for Hodgkin disease, or very high (> 90%), such as in the use of escalated BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone) protocol for advanced Hodgkin disease, the needed power to detect a difference between the GnRHa arm and control arm requires hundreds of patients; therefore, studies of 17–60 patients that did not find an advantage of the GnRHa cotreatment were not powered to find a possible advantage [1, 5–7, 10]. Another methodological error, possibly leading to inaccurate conclusions in this equivocal issue, is premature evaluation of ovarian function [1, 5–7, 10, 11, 16]. Indeed, Demeestere et al. [16], who initially did not find a difference in POF rate after 1 year, have presented the 2-year follow-up of their patients [16] claiming that “the number of patients who totally restored their ovarian function was significantly higher in the GnRHa group (p = .049) confirming results of [anti-Müllerian hormone (AMH)]” [16]. This supports the possibility that short follow-up may be responsible for the discrepancy between studies and lead to incorrect conclusions [1].
Although our results suggest the GnRHa significantly increases the PR in survivors, it has to be taken with caution, because our study was not a RCT. Although there were no significant differences in the age and chemotherapy protocols between the compared groups, a possible bias caused by the patients’ decision whether to receive GnRHa cotreatment or not cannot be excluded. Our results and conclusions are in keeping with those of the recent prospective RCTs of Moore et al. [14], and Lambertini et al. [15].
Although no randomized trials assessed the role of ovarian tissue cryopreservation, many investigators refer to it as an established method of fertility preservation. The quality of evidence for recommending such strategies may be considered low, according to Fleisher et al. [17], because it is based on nonrandomized, case-control, or observational studies. On the contrary, the role of GnRHa therapy in preserving ovarian function has been assessed both in randomized trials and in nonrandomized, case-control studies [1, 3, 5, 8, 9, 11–15].
An argument against GnRHa use [18] claims that prepubertal children, whose hypogonadotropic milieu is simulated by the GnRHa, receiving high-dose chemotherapy given before hematopoietic stem cell transplantation still suffer from ovarian failure. Remérand et al. [19] have described four spontaneous pregnancies and successful deliveries in a patient after prepubertal high-dose busulfan and cyclophosphamide conditioning and bone marrow transplantation (BMT), demonstrating that successful pregnancies may occur in patients undergoing prepubertal BMT. Similarly, the only published case of repeated spontaneous pregnancies and two successful deliveries after repeated autologous BMTs and GnRHa treatment has been described in a postpubertal lymphoma patient, suggesting that the prepubertal milieu induced by the GnRHa cotreatment might have contributed to the preserved fertility despite repeated BMT [20]. Only 0.6%–3% of patients conceive after one BMT [21, 22]. Thus, the pregnancy odds after two BMTs are negligible (approximately 0.36%) [21].
Another argument against GnRHa use is that 8% of prepubertal children exposed to gonadotoxic chemotherapy may suffer POF by age 40 years. Indeed, three recent publications [23–25] have found that survivors of childhood cancer have an 8% risk of suffering POF before the age of 40 years, compared with <1% in the general population. This is in keeping with the published results [1, 4, 8, 9, 11] indicating that women of reproductive age receiving GnRHa in addition to chemotherapy suffer POF in approximately 8%–13% of cases (simulating prepubertal exposure), whereas those treated without the agonist have 30%–60% risk for POF. Because our study was not a RCT, we looked at the number of patients, in both arms, who had previous children and claimed they were not planning additional pregnancies, as a possible diverting bias. We did not find a significant difference between the two groups for this parameter; therefore, it is not likely that a difference in interest in future fertility had biased our results. Furthermore, our results are in keeping with the recent publication of the RCT multicenter South West Oncology Group study (SWOG, S0230) [14]. This recent publication reassures regarding the raised hypothetical interference of GnRHa with the efficiency of chemotherapy and possible survival in hormone receptor-negative patients [14]. The recent PROMISE study demonstrated similar survival in hormone receptor-positive breast cancer patients [15]. Thus, we may carefully suggest that the GnRHa cotreatment does not decrease survival and may possibly even increase it [14, 15].
Furthermore, GnRHa can effectively prevent the thrombocytopenia-associated menorrhagia in these treated patients [1]. Recently, it has also been shown that the GnRHa cotreatment is beneficial not only against regular chemotherapy but also for lymphoma patients undergoing stem cell transplantation [8].
Wong et al. [26], in the largest series of cancer patients treated with a GnRHa, reported a high pregnancy rate (71%) which is in keeping with our results. Furthermore, this [26] and other recent studies [14, 15, 27] suggest the safety of GnRHa cotreatment.
The recently published 14th St. Gallen International Breast Cancer Conference and expert consensus on the primary therapy of early breast cancer [28] support the use of GnRHa in breast cancer patients with hormone receptor-negative disease. This consensus stated that GnRHa therapy during chemotherapy proved effective to protect against POF and preserve fertility in young women with estrogen receptor-negative breast cancer undergoing chemotherapy [28]. This consensus states that the GnRHa cotreatment also increased the rate of subsequent successful pregnancies and did not compromise disease outcomes [28].
There are many unknown and equivocal matters regarding the important issue of fertility preservation, such as efficiency and safety of autotransplantation of cryopreserved ovarian tissue [1], efficiency of GnRHa, safety of ovarian stimulation with gonadotropins in breast cancer, and many others. None of the suggested avenues for fertility preservation guarantees unequivocal success in future fertility preservation. Even IVF and cryopreservation of a few embryos or ova cannot guarantee future pregnancy [1].
Conclusion
The addition of GnRHa to gonadotoxic chemotherapy may significantly increase pregnancy and COF in survivors. It is therefore recommended that GnRHa cotreatment be offered for fertility preservation, in addition to other methods. There is no contraindication to ovarian biopsy for cryopreservation combined with GnRHa administration and follicular aspiration. In cases in which conventional chemotherapy regimens such as those commonly used for young lymphoma patients are applied, GnRHa cotreatment may preserve ovarian function and prevent POF without necessitating the use of cryopreserved ova, embryos, or ovarian tissue.
Footnotes
Editor's Note: See the related commentary, “Temporary Ovarian Suppression With Gonadotropin-Releasing Hormone Agonist During Chemotherapy for Fertility Preservation: Toward the End of the Debate?” on page 1233 of this issue.
Author Contributions
Conception/Design: Zeev Blumenfeld
Provision of study material or patients: Zeev Blumenfeld, Hilli Zur, Eldad J. Dann
Collection and/or assembly of data: Zeev Blumenfeld, Hilli Zur, Eldad J. Dann
Data analysis and interpretation: Zeev Blumenfeld, Hilli Zur, Eldad J. Dann
Manuscript writing: Zeev Blumenfeld, Hilli Zur, Eldad J. Dann
Final approval of manuscript: Zeev Blumenfeld, Hilli Zur, Eldad J. Dann
Disclosures
The authors indicated no financial relationships.
References
- 1.Blumenfeld Z, Katz G, Evron A. ‘An ounce of prevention is worth a pound of cure’: The case for and against GnRH-agonist for fertility preservation. Ann Oncol. 2014;25:1719–1728. doi: 10.1093/annonc/mdu036. [DOI] [PubMed] [Google Scholar]
- 2.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy?: The role of GnRH agonist in addition to cryopreservation of embryos, oocytes, or ovaries. The Oncologist. 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update. 2008;14:543–552. doi: 10.1093/humupd/dmn022. [DOI] [PubMed] [Google Scholar]
- 5.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Practice Committee of American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril. 2013;100:1214–1223. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Peccatori FA, Azim HA, Jr, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi160–vi170. doi: 10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld Z, Patel B, Leiba R, et al. Gonadotropin-releasing hormone agonist may minimize premature ovarian failure in young women undergoing autologous stem cell transplantation. Fertil Steril. 2012;98:1266–1270.e1. doi: 10.1016/j.fertnstert.2012.07.1144. [DOI] [PubMed] [Google Scholar]
- 9.Blumenfeld Z, Avivi I, Eckman A, et al. Gonadotropin-releasing hormone agonist decreases chemotherapy-induced gonadotoxicity and premature ovarian failure in young female patients with Hodgkin lymphoma. Fertil Steril. 2008;89:166–173. doi: 10.1016/j.fertnstert.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Turner NH, Partridge A, Sanna G, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation in young breast cancer patients: The benefit remains uncertain. Ann Oncol. 2013;24:2224–2235. doi: 10.1093/annonc/mdt196. [DOI] [PubMed] [Google Scholar]
- 11.Del Mastro L, Ceppi M, Poggio F, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: Systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40:675–683. doi: 10.1016/j.ctrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Behringer K, Thielen I, Mueller H, et al. Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Ann Oncol. 2012;23:1818–1825. doi: 10.1093/annonc/mdr575. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Li J, Cui T, et al. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2011:CD008018. doi: 10.1002/14651858.CD008018.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923–932. doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambertini M, Boni L, Michelotti A et al. Long-term outcome results of the phase III PROMISE-GIM6 study evaluating the role of LHRH analog during chemotherapy as a strategy to reduce ovarian failure in early breast cancer patients. Paper presented at: ASCO Breast Cancer Symposium, San Francisco, California; September 4, 2014. Abstract 105. [Google Scholar]
- 16.Demeestere I, Brice P, Peccatori FA, et al. Gonadotropin-releasing hormone agonist for the prevention of chemotherapy-induced ovarian failure in patients with lymphoma: 1-year follow-up of a prospective randomized trial. J Clin Oncol. 2013;31:903–909. doi: 10.1200/JCO.2012.42.8185. [DOI] [PubMed] [Google Scholar]
- 17.Fleisher LA, Bass EB, McKeown P. Methodological approach: American College of Chest physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:17S–23S. doi: 10.1378/chest.128.2_suppl.17s. [DOI] [PubMed] [Google Scholar]
- 18.Nitzschke M, Raddatz J, Bohlmann MK, et al. GnRH analogs do not protect ovaries from chemotherapy-induced ultrastructural injury in Hodgkin’s lymphoma patients. Arch Gynecol Obstet. 2010;282:83–88. doi: 10.1007/s00404-009-1308-5. [DOI] [PubMed] [Google Scholar]
- 19.Remérand G, Merlin E, Froissart R, et al. Four successful pregnancies in a patient with mucopolysaccharidosis type I treated by allogeneic bone marrow transplantation. J Inherit Metab Dis. 2009;32(suppl 1):S111–S113. doi: 10.1007/s10545-009-1095-y. [DOI] [PubMed] [Google Scholar]
- 20.Blumenfeld Z, Zuckerman T. Repeated spontaneous pregnancies and successful deliveries after repeated autologous stem cell transplantation and GnRH-agonist treatment. The Oncologist. 2010;15:59–60. doi: 10.1634/theoncologist.2009-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salooja N, Szydlo RM, Socie G, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: A retrospective survey. Lancet. 2001;358:271–276. doi: 10.1016/s0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 22.Carter A, Robison LL, Francisco L, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: Report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. 2006;37:1023–1029. doi: 10.1038/sj.bmt.1705364. [DOI] [PubMed] [Google Scholar]
- 23.Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–896. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 24.Green DM, Sklar CA, Boice JD, Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: Results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–2381. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar AB, Wallace WH. Pregnancy in women who had cancer in childhood. Eur J Cancer. 2007;43:1890–1894. doi: 10.1016/j.ejca.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Wong M, O’Neill S, Walsh G, et al. Goserelin with chemotherapy to preserve ovarian function in pre-menopausal women with early breast cancer: Menstruation and pregnancy outcomes. Ann Oncol. 2013;24:133–138. doi: 10.1093/annonc/mds250. [DOI] [PubMed] [Google Scholar]
- 27.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies-improving the management of early breast cancer: St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]