Abstract
Lessons Learned
Our results highlight some of the challenges in the management of soft tissue sarcomas, which requires close cooperation between surgeons and medical oncologists and a careful selection of patients. The incidence of hepatotoxicity was a concerning finding and had been previously reported in patients treated with pazopanib.
Although pharmacokinetic analysis was not part of this study, concomitant treatment with pazopanib has been recently reported to increase docetaxel exposure, which may explain the increased toxicity of combination regimens. It remains possible that lower doses of combined gemcitabine, docetaxel, and pazopanib may be tolerable. However, caution should be exercised in future trials investigating similar combinations.
Background.
For extremity soft tissue sarcomas (STS), surgical resection remains the standard of care, and the addition of chemotherapy is controversial. This was a phase Ib/II trial of neoadjuvant therapy for patients with STS.
Methods.
Patients with high grade, extremity STS of >8 cm and amenable to definitive resection were treated with up to four 21-day cycles of 900 mg/m2 gemcitabine on days 1 and 8, 75 mg/m2 docetaxel on day 8, and 400 mg of pazopanib daily (GDP), followed by surgery and, if indicated, radiation therapy. Primary and secondary endpoints (phase Ib portion) were the safety and rate of pathologic response.
Results.
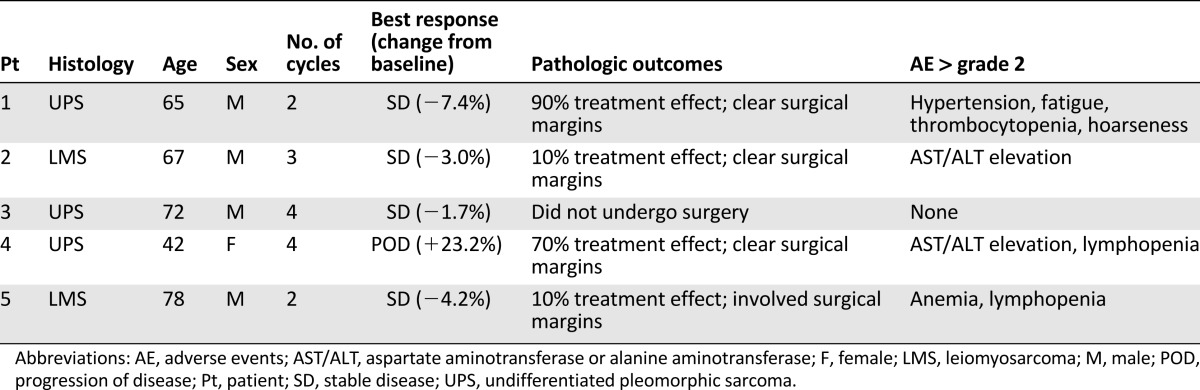
The trial was discontinued because of slow accrual after inclusion of five patients (leiomyosarcoma: two; undifferentiated pleomorphic sarcoma: three). Two patients completed four treatment cycles: one underwent surgery and one had insufficient response and received additional therapies. Three patients discontinued treatment because of toxicity. Grade 3 adverse events included hypertension, fatigue, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation, hoarseness, and myelotoxicity. There were no complete or partial responses. One patient had ≥90% pathologic response. Among four patients who underwent resection, three remain free of disease, and one patient eventually relapsed.
Conclusion.
GDP combination used in the neoadjuvant setting resulted in significant toxicity; despite pathologic responses, no objective responses occurred.
Abstract
经验
• 本研究强调了软组织肉瘤管理中的一些难点, 需要外科医生和肿瘤内科医生密切协作, 并且对患者进行细致筛选。研究中的肝毒性发生率的结果略令人担忧, 既往在帕唑帕尼用于治疗患者的研究中也曾报告过这一问题。
• 尽管本研究并不包括药代动力学分析, 但近期有报告显示同期给予帕唑帕尼治疗可增加多西他赛的暴露水平, 这也许解释了为何联合方案毒性增加。较低剂量的吉西他滨、多西他赛与帕唑帕尼联合方案仍然有可能为患者耐受, 但在将来研究类似联合方案的临床试验中应谨慎实施。
摘要
背景. 手术切除依然是四肢软组织肉瘤 (STS) 的标准治疗, 而是否加用化疗则存有争议。本项研究为在 STS 患者中开展新辅助治疗的 Ib/II 期临床试验。
方法. 纳入可接受根治术的高级别且肿瘤> 8 cm 的四肢 STS 患者, 给予吉西他滨 900 mg/m2(D1、D8)、多西他赛 75 mg/m2 (D8) 和帕唑帕尼 400 mg 每日一次 (GDP联合方案), 21天为一周期, 至多治疗 4 周期。后继以手术, 如有指征则进行放疗。主要和次要终点 (Ib期阶段) 为安全性和病理学缓解率。
结果. 本临床试验因在纳入 5 例患者 (平滑肌肉瘤 2 例、多形性未分化肉瘤 3 例) 后招募缓慢而中止。2 例患者完成了 4 周期治疗: 其中一例接受了手术, 另一例治疗反应不佳并接受了其他治疗。3 例患者因毒性事件停止治疗。3级不良事件包括高血压、疲乏、天门冬氨酸氨基转移酶 (AST) 或丙氨酸氨基转移酶 (ALT) 水平升高、声音嘶哑以及骨髓毒性。未观察到完全或部分缓解。1 例患者达到≥ 90%病理学缓解。4 例接受切除术的患者中, 3 例仍然处于无病状态, 1 例最终复发。
结论. GDP联合方案用于新辅助治疗导致了明显的毒性, 除病理学缓解外, 未观察到客观缓解。The Oncologist 2015;20:1245–1246
Author Summary
Discussion
The role of systemic therapy in the management of patients with localized STS is a topic of ongoing debate. We sought to investigate whether the addition of pazopanib to gemcitabine/docetaxel (GDP) in the neoadjuvant setting would be safe and result in an antitumor effect in patients with localized, high grade (limited to undifferentiated pleomorphic sarcoma, high grade leiomyosarcoma, and malignant peripheral nerve sheath tumor), extremity STS of >8 cm amenable to definitive resection. Activity of these agents has been previously documented in STS [2–5], and the use of pazopanib is supported by vascular endothelial growth factor (VEGF)-dependent signaling in sarcomas and evidence of antitumor effect in patients with metastatic disease.
This was a standard 3 + 3 phase Ib dose escalation. Of the 3 patients treated at dose level 1, 1 experienced dose-limiting toxicity (DLT) (grade 3 fatigue). The cohort was expanded, and 2 additional patients were treated at the same dose level before the trial was discontinued because of slow accrual. Although there were no further DLTs, the combination treatment was poorly tolerated; among 5 patients, 3 discontinued treatment because of toxicity. Although no grade 4 adverse events (AEs) occurred, 4 patients developed grade 3 AEs, which included hypertension, thrombocytopenia, hoarseness, and fatigue (1 patient each), as well as AST/ALT elevations and lymphopenia (2 patients each). There were no objective responses per RECIST; 4 patients had stable disease as best response, and 1 patient progressed. Overall, 4 patients underwent resection of the primary tumor, with significant treatment effect (≥90% pathologic response) observed in only 1 patient; 1 additional patient had 70% of pathologic response, and 2 patients had minor responses (≤10%). After a median follow-up of 27 months, 3 patients remain disease-free, and 1 patient developed lung metastases. Treatment/outcome details are provided in Table 1.
Table 1.
Efficacy and treatment summary
Studies investigating neo- or adjuvant systemic treatments in STS have yielded conflicting results. A meta-analysis suggested a marginal improvement in local recurrence, distant recurrence, and overall survival with adjuvant chemotherapy in patient with resectable STS; however, subsequent prospective trials have not confirmed this.
In conclusion, the question of whether neoadjuvant chemotherapy can improve the outcomes of patients with localized, high-grade extremity STS remains unanswered. The addition of pazopanib to gemcitabine and docetaxel resulted in significant toxicity. Despite pathologic responses, no objective responses occurred. As per current standards, there is no evidence to support the routine use of neoadjuvant chemotherapy in this setting.
Supplementary Material
Acknowledgment
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by GlaxoSmithKline.
Footnotes
Access the full results at: Dickson-15-245.theoncologist.com
ClinicalTrials.gov Identifier: NCT01418001
Sponsor: Memorial Sloan Kettering Cancer Center
Principal Investigator: William D. Tap
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.