Catumaxomab (CATU) is approved for i.p. treatment of malignant ascites (MA) resulting from certain carcinomas. Outpatient treatment seems appropriate in selected individuals. The feasibility of CATU in outpatients with MA was investigated. Outpatient i.p. CATU therapy is safe and effective in producing good ascites control in most individuals, allowing for subsequent systemic therapy in a substantial proportion of patients.
Keywords: Catumaxomab, Epithelial cell-adhesion molecule, Intraperitoneal therapy, Malignant ascites, Outpatient treatment, Trifunctional antibody
Abstract
Background.
Catumaxomab (CATU) is a trifunctional antibody approved for intraperitoneal (i.p.) treatment of malignant ascites (MA) related to carcinomas expressing the epithelial cell-adhesion molecule (EpCAM). CATU is mostly given to hospitalized patients, although outpatient treatment seems appropriate in selected individuals. This observational trial sought to obtain more detailed information regarding the feasibility of CATU in outpatients with MA related to various gynecologic tumors, including epithelial ovarian (EOC) and metastatic breast cancer (MBC).
Materials and Methods.
A total of 30 patients were included, 17 with EOC, 7 with MBC, and 6 with other malignancies. The patients had failed a median of 5 (range 1–12) previous systemic treatments. CATU was administered via an indwelling i.p. catheter at four increasing doses (i.e., 10, 20, 50, and 150 µg) given at 4-day intervals over 2 weeks. Toxicities were scored according to the Common Terminology Criteria for Adverse Events, version 4.03. Puncture-free survival (PuFS) was calculated from the start of CATU until the next puncture for MA, death, or loss to follow-up. Overall survival (OS) was calculated from the start of CATU to death from any reason or loss to follow-up. We also investigated various clinical parameters to predict PuFS and OS. These included age, tumor type, performance status, intensity of pretreatment, presence of extraperitoneal metastases, relative lymphocyte count at baseline, patient adherence to therapy, and the patients’ ability to undergo systemic treatment after CATU.
Results.
CATU was exclusively given on an outpatient basis, and 19 patients (63.3%) received all four planned i.p. instillations. Toxicity was the reason for discontinuation in only 2 patients. Toxicity was generally manageable, with abdominal pain, nausea/vomiting, fatigue, and fever the predominant adverse effects. Secondary hospitalization was necessary for 7 patients (23.3%), with a general deteriorated condition in 5 and fever/infection or abdominal pain in 1 patient each. Subsequent systemic treatment was possible in 11 patients (36.7%). Only 5 patients (16.7%) required a second puncture after i.p. CATU. The median PuFS was 56 days, and the median OS was 79.5 days. Positive predictors of both PuFS and OS were performance status, absence of extraperitoneal tumor, the capability to receive all four CATU infusions, and the ability to undergo subsequent systemic treatment.
Conclusion.
Outpatient i.p. CATU therapy for MA related to various gynecologic carcinomas is safe and effective in producing good ascites control in most individuals, allowing for subsequent systemic therapy in a substantial proportion of patients.
Implications for Practice:
Intraperitoneal treatment with the trifunctional antibody catumaxomab (CATU) was possible in a selected population of 30 outpatients with malignant ascites due to epithelial female genital tract or breast carcinoma. Toxicity was largely manageable. Patients in good condition at baseline, without extraperitoneal tumor and/or liver metastases, and with the ability to complete all four planned CATU instillations and the capability of undergoing subsequent systemic therapy benefited the most in terms of both puncture-free and overall survival. Outpatient i.p. CATU is safe and effective in a selected group of patients with malignant ascites due to various gynecologic malignancies and could be cost-saving compared with an inpatient approach.
Introduction
Malignant ascites (MA) is one of the most frequent sequelae of peritoneal spread of both intra- and extra-abdominal tumors. The major underlying causes of MA include gynecologic malignancies (e.g., epithelial ovarian, primary peritoneal, fallopian tube, uterine, cervical, and breast cancer), gastrointestinal carcinomas (e.g., stomach, pancreatic, colorectal, and esophageal cancer), lung cancer, malignant mesothelioma, and malignant lymphoma [1]. In many cancer patients, the occurrence of MA associated with peritoneal carcinomatosis is related to a poor prognosis [1, 2]. Patients who develop MA often experience troublesome symptoms such as abdominal swelling and pressure, dyspnea, pelvic pain, bloating, bowel dysfunction (constipation and/or diarrhea), or urination, which alone or combined might significantly deteriorate their performance status and, subsequently, their quality of life (QoL). Until now, the therapeutic options in this demanding oncologic situation have been limited and mainly included the administration of diuretics, repeated punctures, peritoneovenous shunting, and intravenous (i.v.) or intraperitoneal (i.p.) chemotherapy [3–7]. Although exhibiting short-lived effects, repeated paracentesis has been considered the standard of care to palliate patients with MA for a long time, because it is easy to perform, applicable to outpatients, inexpensive, and not risky for the vast majority of individuals [3, 4, 7].
Catumaxomab (CATU) is a nonhumanized, trifunctional, bispecific, monoclonal antibody combining two half-antibodies of both mouse (IgG2a) and rat (IgG2b) origin. One Fab fragment (the mouse IgG2a) binds to the epithelial cell-adhesion molecule (EpCAM), which is present on the surface of most epithelial tumors of nonsquamous differentiation [8]. The rat IgG2b antigen-binding site recognizes CD3, a part of the T-cell receptor complex [9]. The Fc foot of the antibody binds to accessorial cells, which express the Fcγ receptors I and III (macrophages, dendritic cells, natural killer cells) [10]. By bridging the gap between tumor cells and different components of the immune system, CATU is thought to facilitate the establishment of an autologous T-cell-mediated antitumor immunoresponse. Indeed, i.p. application of CATU results in the release of cytokines derived by immune cells such as interleukin (IL)-1β, IL-2, IL-6, IL-12, and dendritic cell cytokine 1 [11]. In two early clinical trials, i.p. CATU was found to be feasible and active against MA related to epithelial malignancies [12, 13]. This resulted in the initiation of a pivotal, randomized phase II/III study in patients with symptomatic MA caused by different epithelial malignancies. In this trial, the superiority of CATU compared with repeated paracentesis was demonstrated in terms of a significant improvement in puncture-free survival (PuFS) and a trend toward improved overall survival (OS), which was even significant in the subgroup of patients with gastric cancer [14]. In April 2009, CATU was approved in the European Union for the treatment of MA in patients with EpCAM-positive epithelial tumors for which a standard therapy is not available or is no longer feasible [15]. Most notably, CATU is the only specific treatment approved for this indication so far. The side effects of CATU, which are mostly related to cytokine release, such as fever, chills, nausea/vomiting, fatigue, and abdominal pain, were frequent but generally short-lived and were rarely severe [15, 16]. CATU was able to significantly delay the deterioration of QoL compared with paracentesis alone in the pivotal trial as was shown by Wimberger et al. [17]. The subsequent CASIMAS (Catumaxomab Safety Phase IIIb Study With Intraperitoneal Infusion in Patients With Malignant Ascites Due to Epithelial Cancer) trial, which aimed at reducing the cytokine-related side effects of CATU by premedication with low-dose prednisolone at 25 mg, failed to show any improvement in either toxicity or efficacy compared with CATU alone. However, that study was also able to demonstrate that reducing the infusion time from 6 to 3 hours adversely influenced neither toxicity nor efficacy [18].
In addition, various subgroup analyses of these phase II/III trials were initiated to better define the prognostic indicators for improved clinical outcomes after i.p. CATU therapy. In particular, a good performance status, less-intensive pretreatment, the diagnosis of either ovarian or gastric cancer, the absence of extraperitoneal lesions, including liver metastases, a relative lymphocyte count (RLC) of >13% before therapy, a humoral response to CATU indicated by the appearance of human anti-mouse antibodies, and the ability to undergo subsequent systemic treatments were found to have a positive impact on both ascites control and survival [14, 19–22].
Until now, most CATU treatments have been performed after hospitalization of the patients for approximately 2 weeks. Most physicians are still concerned about the potentially occurring toxicities of CATU, although the side effects are generally manageable and mostly of short duration. However, the main reason for inpatient CATU treatment is that the drug was primarily administered as a 6-hour i.p. infusion, which is highly inconvenient for an outpatient setting. As a consequence of the CASIMAS trial, the European Medicines Agency has now approved the 3-hour i.p. instillation of CATU, greatly facilitating its outpatient use. In the CARMA study, a recent multicenter, prospective, noninterventional trial of i.p. CATU in a real-world population of patients with MA from various epithelial tumors, approximately 27% of individuals were treated on an outpatient basis, demonstrating that outpatient CATU therapy is both feasible and safe with careful patient selection [23, 24]. The present, observational, single-institution study was thus undertaken to evaluate the efficacy and feasibility of CATU given under routine clinical conditions to outpatients with MA related to various gynecologic malignancies. We also sought to evaluate the prognostic value of various parameters, such as performance status, tumor type, pretreatment intensity, baseline RLC, the absence or presence of extraperitoneal tumor spread, including liver metastases, patient adherence to CATU therapy, and patients’ ability to undergo subsequent systemic treatment for both ascites control and survival in a real-world population of patients.
Patients and Methods
Patients
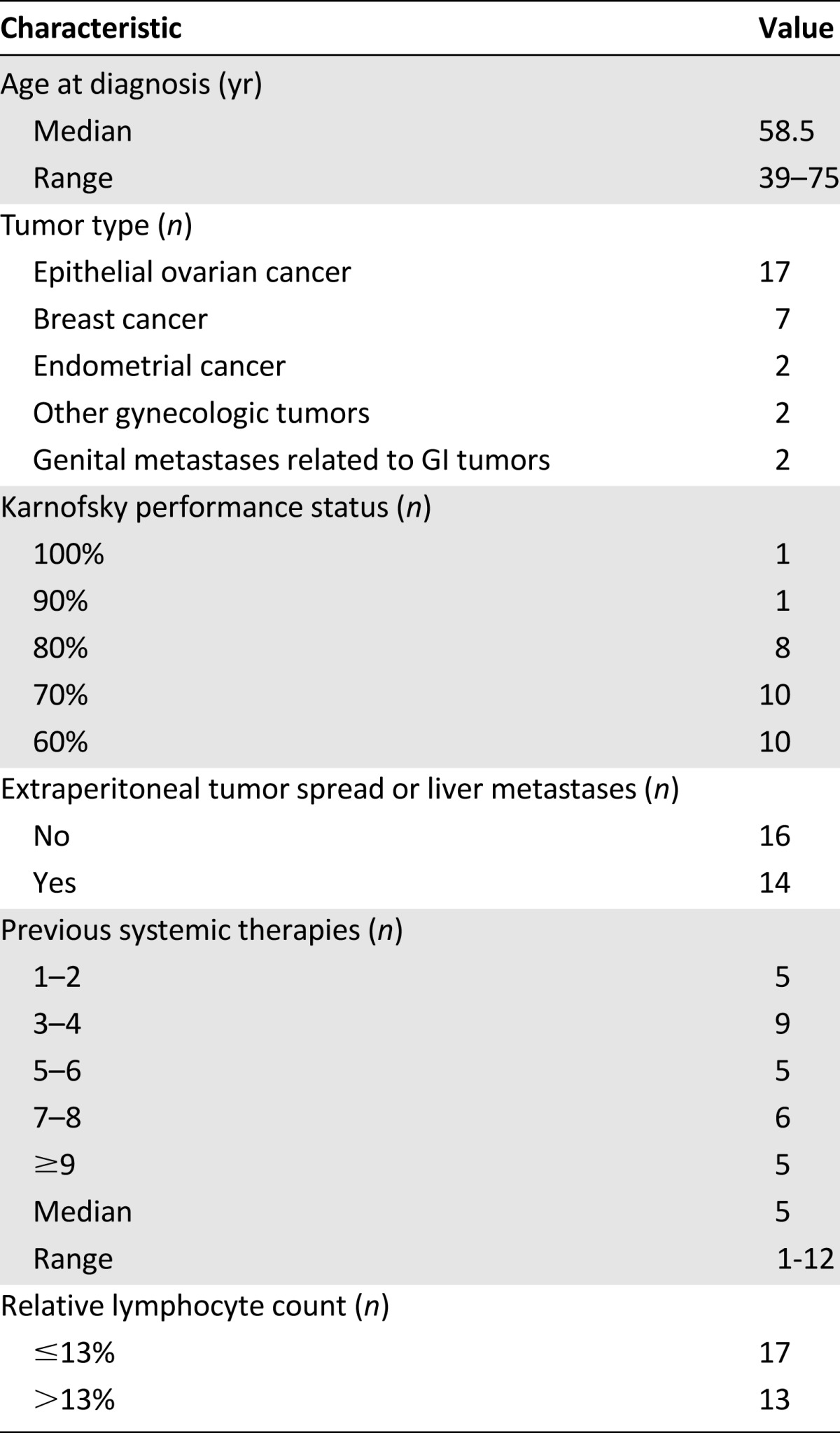
From 2010 to 2014, 30 adult outpatients with symptomatic MA related to various gynecologic malignancies or metastatic breast cancer received i.p. CATU, in accordance with its approval status. Two patients with nongynecologic tumors presenting with overt metastases involving the female genital tract were also included. The patients’ baseline characteristics are summarized in Table 1. Patients were required to have a Karnofsky performance status (KPS) score of ≥60% and an estimated life expectancy of at least 8 weeks. The patients were required to not have been exposed to any antineoplastic medication (i.e., chemotherapy, endocrine agents, or any other targeted drugs), high daily doses of systemic corticosteroids (i.e., dexamethasone or betamethasone >8 mg as a single injection or equivalent doses of prednisone, prednisolone, or methylprednisolone), or radiotherapy simultaneously administered with CATU. Additionally, treatment with mistletoe lectins or other immunomodulating agents was not allowed during the CATU treatment period. Patients also did not qualify for outpatient CATU treatment if any of the following were present: previous treatment with mouse and/or rat antibodies, severe malnutrition or cachexia with a body mass index after ascites drainage of less than 19 kg/m2 and/or hypoproteinemia with a serum albumin level less than 20 g/L, ascites related to conditions other than peritoneal carcinomatosis (i.e., renal or cardiac insufficiency, severe hepatic dysfunction and/or portal vein obstruction, peritoneal inflammation), active infections or nonhealing wounds, a history of organ transplantation, HIV-positivity or any other permanent immunodeficiency, and any other uncontrolled severe medical disorder.
Table 1.
Baseline patient characteristics (n = 30)
Treatment
A few days before the initiation of CATU therapy, all patients underwent a diagnostic puncture of the abdominal cavity to confirm the malignant nature of the ascites by cytological examination of the sample. If indicated (i.e., in invasive lobular breast cancer and cervical carcinoma), EpCAM positivity was determined by subsequent immunohistochemistry. In all other cases, immunohistochemical EpCAM staining was performed whenever possible.
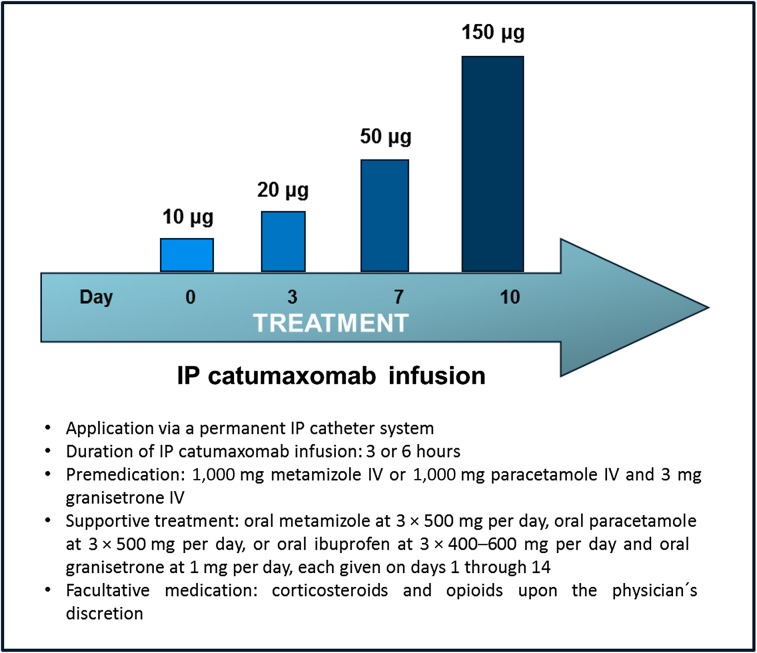
On the day of the first CATU instillation, a complete laboratory evaluation was performed, including a white blood cell count with leukocyte differentiation. Thereafter, a permanent i.p. catheter system was inserted into the abdominal cavity under aseptic conditions with abdominal ultrasound guidance. Consecutively, CATU was infused for either 3 or 6 hours (depending on the actual approval status) via the indwelling catheter on days 1, 4, 7, and 10 of a 2-week interval at four increasing dosages (i.e., 10, 20, 50, and 150 µg) using an automated infusion pump. Before each CATU application, all available ascites was drained. Standard premedication included both i.v. antiemetics (granisetrone at 3 mg), and i.v. antipyretics/pain killers (metamizole or paracetamole, either at 1,000 mg) diluted in 250 mL of normal saline. Both oral antiemetics (granisetrone 1 mg/day) and pain killers/antipyretics (metamizole 3 × 500 mg/day, paracetamole 3–4 × 500 mg/day, ibuprofen 3 × 400–600 mg/day) were routinely given on days 2, 3, 5, 6, 8, 9, and 11–14 of the entire treatment interval. Moreover, patients with a low pretreatment serum albumin level (i.e., <30 g/L) received a supplement of 100–200 mL of a 20% human albumin solution after each CATU application. According to the results of the CASIMAS trial, corticosteroids were not routinely administered. However, corticosteroids, as well as opioids, could be freely given at the physician’s discretion, providing the acute dose did not exceed 8 mg for dexamethasone or equivalent doses for low-potent compounds. In patients with no detectable amount of MA before the last CATU infusion, the i.p. catheter was removed immediately after. In all other cases, the i.p. catheter was removed on day 14, providing that no clinically relevant amount of ascites was detectable. The treatment scheme is illustrated in Figure 1.
Figure 1.
Treatment protocol of intraperitoneal catumaxomab.
Abbreviations: IP, intraperitoneal; IV, intravenous.
Treatment Evaluation and Statistical Analysis
Side effects were recorded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. The efficacy of CATU therapy was determined in accordance with previous phase III and IV trials [14, 18, 23, 24]. The puncture-free interval (PuFI) was calculated from the start of CATU until the next paracentesis required by the occurrence of symptomatic MA. OS was calculated from the start of CATU to death from any reason or loss to follow-up. Puncture-free survival (PuFS) was calculated from the start of CATU to the next puncture required by the reappearance of MA or death from any reason or loss to follow-up, whichever occurred first. The median OS and PuFS were both determined using Kaplan-Meier estimates, which also gave the 3-, 6-, and 12-month proportions for both OS and PuFS. Only 11 patients presented with measurable tumor lesions using the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). Therefore, the tumor response according to the RECIST 1.1 was not considered a valid measure to determine the effectiveness of i.p. CATU in the present study.
Our study also aimed to determine the value of different prognostic parameters in the clinical routine use of CATU. These included age (<60 vs. ≥60 years), tumor type (ovarian vs. nonovarian histology), the absence or presence of extraperitoneal lesions and/or liver metastases, pretreatment KPS score (80%–100% vs. 60%–70%), pretreatment RLC (<13% vs. ≥13%), intensity of pretreatment (four or fewer vs. more than four previous systemic treatments), patient adherence to CATU treatment (one to three vs. all four planned instillations), and the ability to undergo systemic therapy after CATU. Univariate statistical comparisons between the various prognostic subgroups were performed using log-rank tests. Adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated using the Cox proportional hazards model. For all statistical comparisons, p < .05 indicated significance.
Results
The median age at the start of CATU was 58.5 years (range, 39–75 years); more than half the patients (53.3%) were 60 years old or older. Of all the patients included, 17 (56.7%) had EOC and 13 (43.3%) non-EOC tumors. Ten patients (33.3%) had a KPS score of ≥80% and 20 patients (66.7%) a KPS score of 60%–70%. In accordance with the physical impairment of most patients, only 13 (43.3%) presented with a pretreatment RLC of 13% or higher. Of the 30 patients, 16 (53.3%) had intraperitoneal lesions only and 14 (46.7%) had been diagnosed with extraperitoneal metastases and/or liver involvement before CATU therapy. The patients had been exposed to a median of 5 (range, 1–12) previous systemic treatments. Most had been heavily pretreated, with more than four preceding therapy regimens having failed in 16 patients (53.3%).
In all 30 patients, CATU was completely administered on an outpatient basis; 19 patients (63.3%) were able to complete i.p. therapy as planned and 11 (36.7%) received fewer than four CATU applications. The reasons for therapy discontinuation were deteriorated performance status and patient request in 4 each, nontolerated side effects in 2, and premature loss of the indwelling catheter in 1 patient. Eleven patients (36.7%) were able to undergo subsequent systemic therapy after i.p. CATU. The toxicities related to i.p. CATU therapy are summarized in Table 2. Adverse effects were frequent but rarely exceeded CTCAE grade 2. The major toxicities of CATU were nausea/vomiting, fever, local pain, and fatigue. These could be effectively controlled in most patients using the aforementioned supportive regimen. Secondary hospitalization was necessary in only 7 patients (23.3%) owing to a generally deteriorated condition related to the underlying disease in 5 and fever/infection or abdominal pain/subileus in 1 patient each.
Table 2.
Toxicities related to intraperitoneal catumaxomab therapy
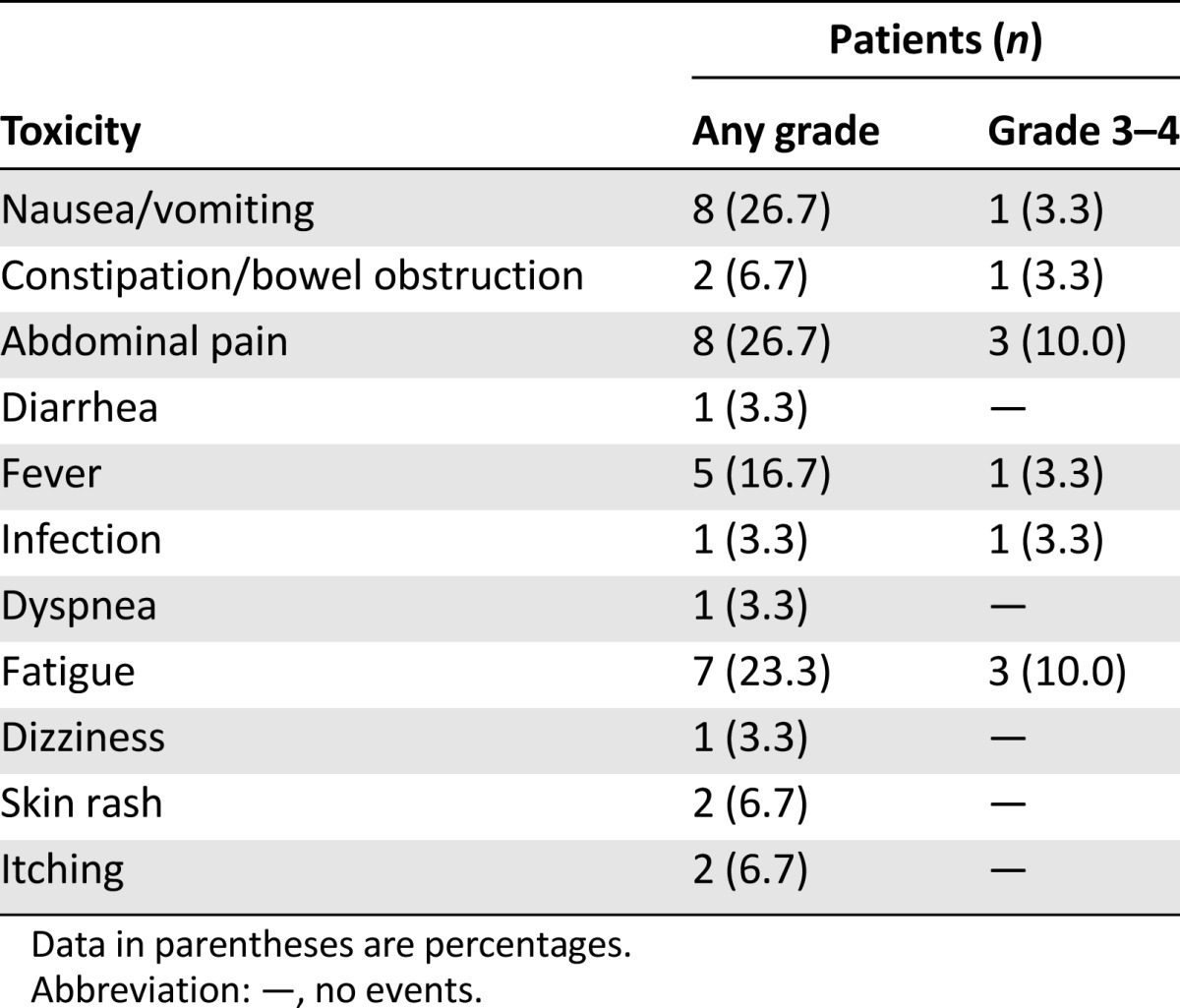
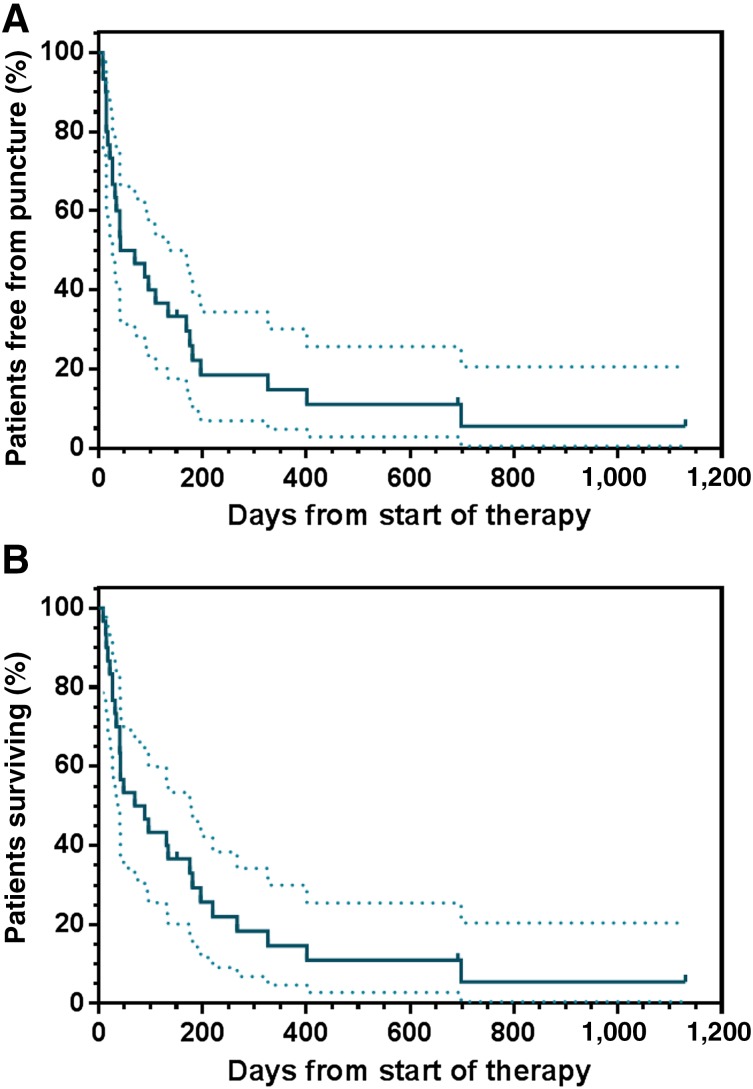
Only 5 patients (16.7%) required a secondary paracentesis because of symptomatic MA subsequent to CATU treatment. In these 5 individuals, the median PuFI was 15 days (range, 8–169 days). In the remainder, malignant ascites was completely controlled until death or the end of the observation period. Albeit not considered a measure of therapeutic effectiveness, none of the 11 individuals presenting with measurable tumor before study entry experienced progressive disease during i.p. CATU treatment. A partial response according to RECIST 1.1 was observed in 2 of these patients (18.1%). At present, 3 patients are still alive and free of MA. The median PuFS in the entire study population was 56.0 days, and the median OS was 79.5 days (Fig. 2). The 3-, 6-, and 12-month OS rates were 46.7%, 33.0%, and 14.7%, respectively. The corresponding rates for PuFS were 43.3%, 25.9%, and 14.7%.
Figure 2.
Long-term results in all outpatients exposed to intraperitoneal catumaxomab treatment. (A): Puncture-free survival. (B): Overall survival. Dashed lines represent the 95% confidence intervals.
The predictive value of the different clinical parameters for both PuFS and OS are presented in Table 3 for PuFS and Table 4 for OS. Compared with those with a KPS score of 70 and lower, patients with a KPS score of 80%–100% showed a significantly longer PuFS (326 vs. 33 days; p < .0001; HR, 0.24; 95% CI, 0.07–0.35) and OS (326 vs. 41.5 days; p = .0018; HR, 0.26; 95% CI, 0.10–0.45). Another baseline characteristic that indicated a significantly improved likelihood to benefit from CATU was the absence of extraperitoneal tumor or liver metastases. Compared with the patients with extraperitoneal lesions, the PuFS of patients with limited disease involving the abdominal cavity only was 169 versus 29.5 days (p = .0091; HR, 0.40; 95% CI, 0.14–0.72), and the OS was 181 versus 37.5 days (p = .0047; HR, 0.28; 95% CI, 0.12–0.68). In contrast, all other observed trends regarding both PuFS and OS, which favored older patients and those with ovarian histology, less intensive pretreatment, and a RLC ≥13%, did not reach statistical significance.
Table 3.
Puncture-free survival after intraperitoneal catumaxomab related to various clinical subgroups
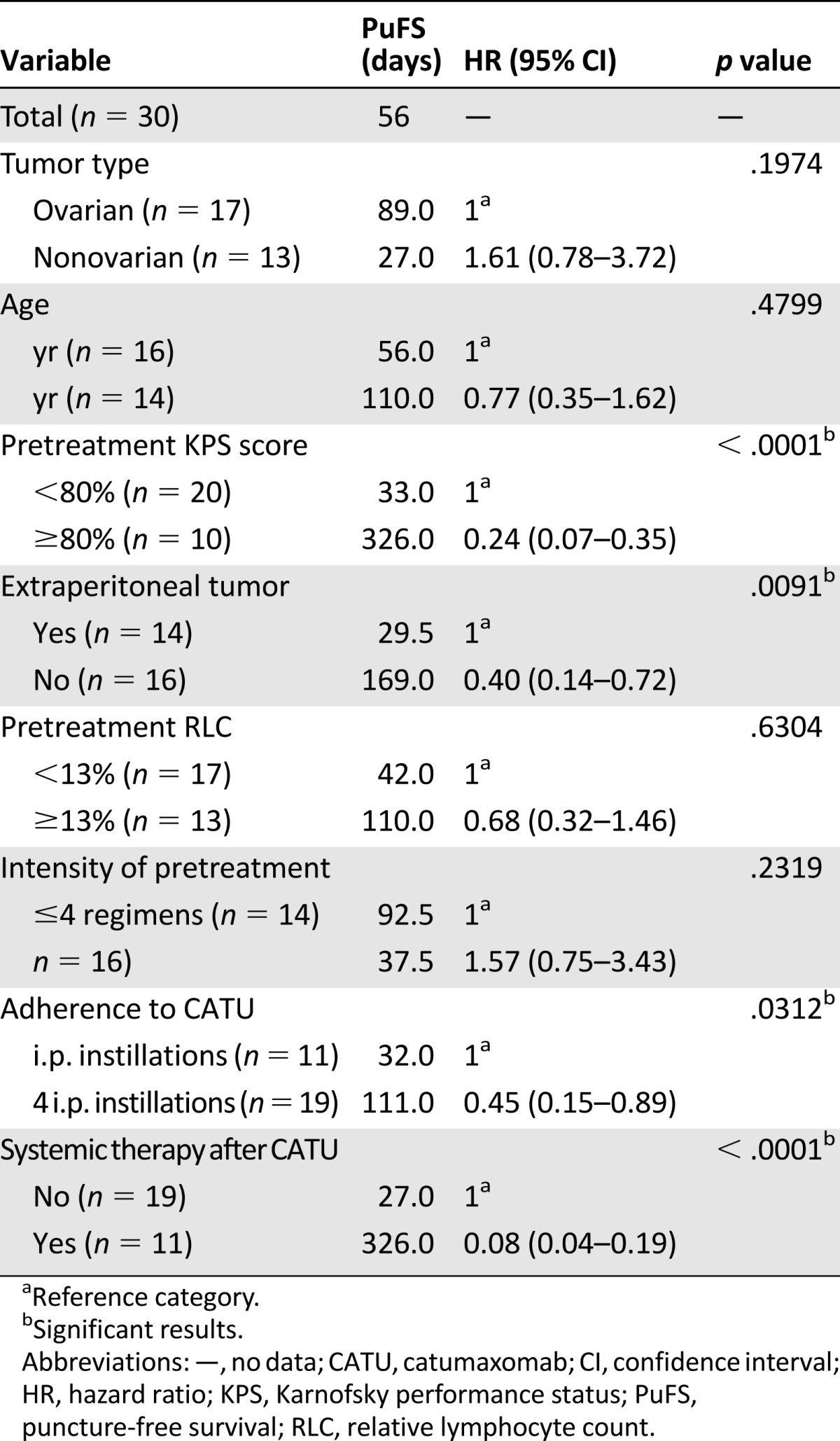
Table 4.
Overall survival after intraperitoneal catumaxomab related to different clinical subgroups
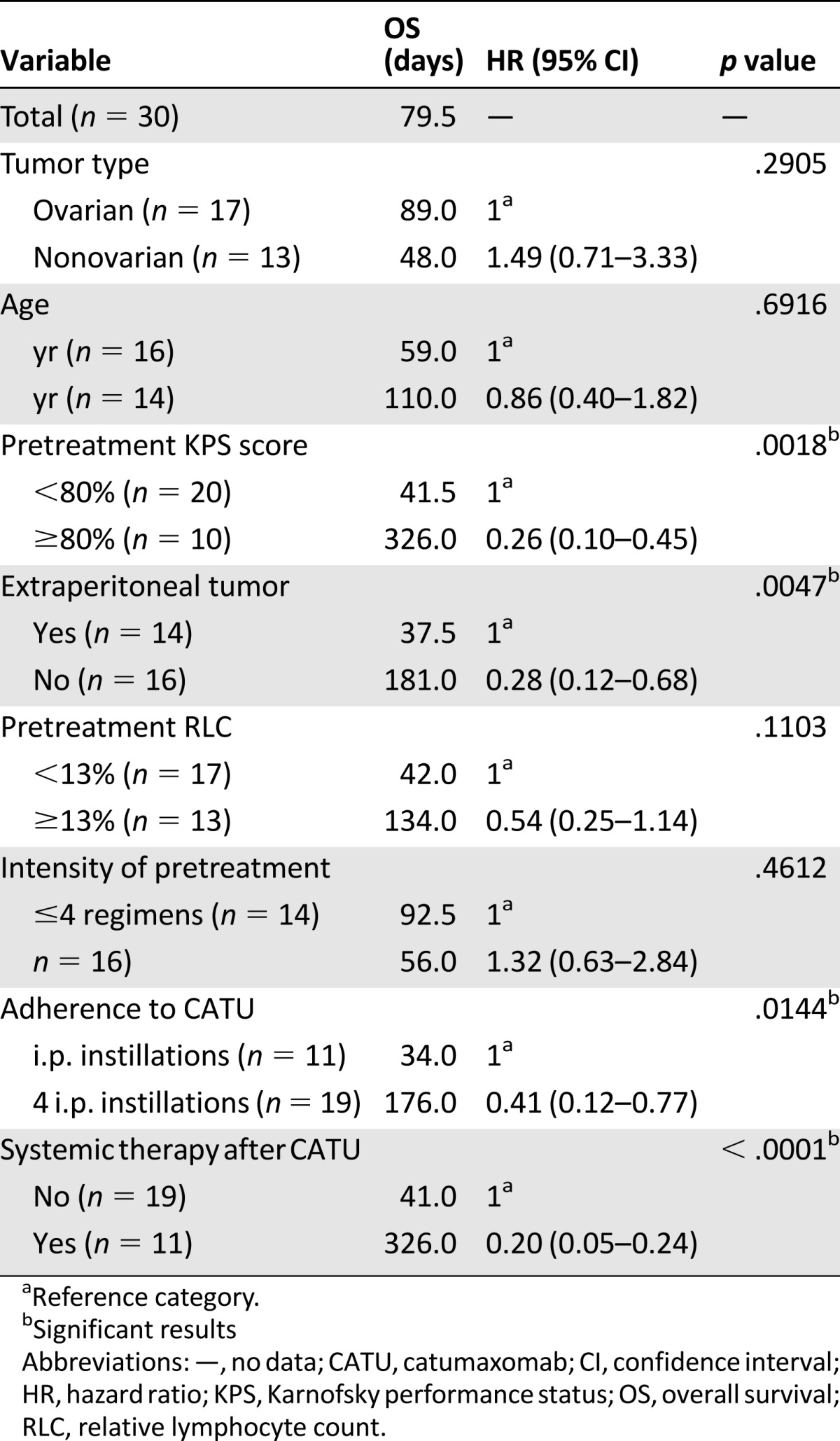
Both treatment-related parameters were significantly predictive for an improved outcome regarding both control of MA and survival. In contrast to those who received fewer than four CATU applications, patients who completed the treatment as planned exhibited a significant longer PuFS, with 110 versus 32 days (p = .0312; HR, 0.45; 95% CI, 0.15–0.89), and OS, with 176 versus 34 days (p = .0144; HR, 0.41; 95% CI, 0.12-0.77). Moreover, patients who received subsequent systemic treatment after i.p. CATU exhibited a highly significant benefit compared with those who were not able to undergo such treatment, with a PuFS of 326 versus 27 days (p < .0001; HR, 0.08; 95% CI, 0.04–0.19) and an OS of 326 versus 41 days (p < .0001; HR, 0.20; 95% CI, 0.05–0.24).
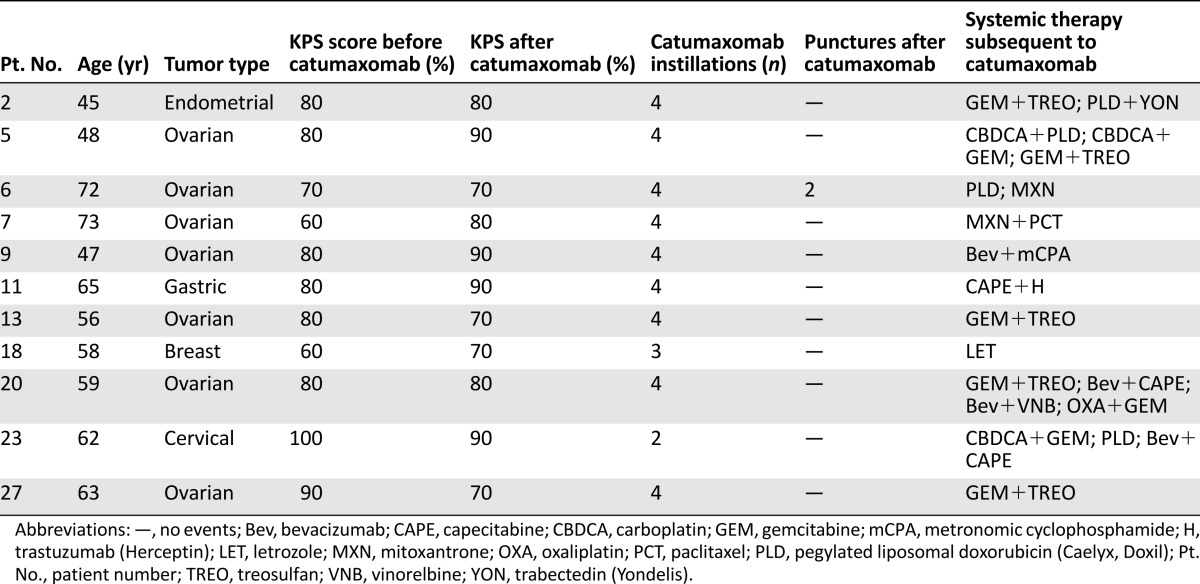
The individual characteristics of the 11 patients who had undergone systemic antineoplastic therapy after CATU are summarized in Table 5. These patients were able to receive one to four subsequent antineoplastic treatments, including endocrine agents, chemotherapy, and targeted agents. Eight of these patients presented with a baseline KPS score of ≥80%, which represented 80% of all individuals with an initial KPS score of 80% or more. In 5 of these patients, the KPS score remained unchanged or improved during CATU treatment. Three of the patients who underwent subsequent systemic treatment presented with a baseline KPS score of 70% or lower (i.e., 15% of the entire subgroup). In these 3 patients, the KPS score did not alter or even increased during i.p. CATU.
Table 5.
Characteristics of patients with malignant ascites who received systemic antineoplastic therapy subsequent to intraperitoneal catumaxomab
Discussion
During the past 5 years, i.p. CATU has become an established palliative treatment for adult patients with MA related to peritoneal involvement of various EpCAM-positive epithelial tumors [14, 25, 26]. Until now, CATU has mostly been administered to inpatients, as indicated by the pivotal phase II/III trial and the subsequent CASIMAS study requiring a 14-day hospitalization period [14, 18]. However, inpatient CATU treatment is both cost-intensive and often inconvenient to patients and thus not always easy to perform. In the recent, large, multicenter, noninterventional CARMA study, we were able to show that i.p. CATU is a well-tolerated and valuable treatment for MA in routine clinical practice, although most patients had more advanced disease compared with those in the pivotal trial [14, 23, 24]. Just as seen in previous studies, patients in the CARMA study showed an improvement in QoL over time, indicated by a decrease in the sum Functional Assessment of Chronic Illness Therapy-Ascites Index score [17, 23, 27]. The same observation was made in a recently published phase II trial of i.p. CATU therapy in refractory ovarian cancer in which patients improved in most ascites-related symptoms, except for three (dyspnea, mobility, and fatigue) [28].
One of the most intriguing findings of the CARMA study was that outpatient treatment was possible in 27% of patients, provided careful preselection had occurred. This observation stimulated the initiation of the present study, in which we were able to demonstrate that outpatient CATU treatment of patients with MA related to various gynecologic tumors, mainly including recurrent ovarian and metastatic breast cancer, is both feasible and safe. All individuals in the present study were exclusively treated on an outpatient basis, and 19 (63.3%) were able to complete the CATU therapy as planned. This result is somewhat inferior to that achieved in phase II and phase III trials reporting treatment completion rates of 72% and 80%, respectively [14, 28]. However, our findings are in good agreement with those from the CARMA study, in which 65% of patients completed all four planned i.p. CATU instillations. In contrast to previous trials, both CARMA and the present study focused on a real-world population of individuals with MA. Moreover, our study represented a more “frail” group of patients, with only one third presenting with a pretreatment KPS score of 80% or better. In contrast, the corresponding proportions in the pivotal trial and in CARMA were 54% and 62% [14, 23, 24]. Additionally, the present population had been more intensively pretreated, with a median number of five previous systemic therapies. In both the pivotal trial and CARMA, patients with EOC had been exposed to three and patients with non-EOC to one to two previous systemic therapies. Just as in these clinical projects, however, discontinuation of treatment in our study was rarely the result of toxicity but more likely to be from rapid disease progression or patient refusal [14, 23, 28].
Despite the substantial proportion of individuals presenting with a reduced performance status, CATU-related toxicity was generally manageable, which might be partly attributable to our intensified supportive regimen, as described above. As could be expected, adverse effects were frequent, with nausea/vomiting, abdominal pain, fatigue, and fever dominating the toxicity profile of CATU. However, grade 3/4 toxicities were rarely observed and were not treatment limiting in the vast majority of patients. Accordingly, secondary hospitalizations were rare and mainly related to the progression of the underlying disease. Only 2 patients (6.7%) were hospitalized because of severe adverse effects of CATU. Because most previous nonrandomized and randomized trials abstained from including outpatients, our findings strongly argue in favor of the safety and feasibility of an outpatient CATU treatment and therefore largely confirm the observations previously made in a subset of patients in the CARMA study [23, 24].
In most patients included in the present study, CATU led to good clinical control of MA. After CATU treatment, only 5 patients required a subsequent abdominal puncture because of the reappearance of symptomatic MA. In these patients, the PuFI ranged from 8 to 169 days. All other patients included in the present study remained free from any subsequent paracentesis, which compares favorably with all other previous phase II-IV studies [14, 18, 23, 24, 28]. In our study, the median PuFS was 56 days, and the median OS was 79.5 days. These findings are generally in good agreement with those reported until now. In the pivotal phase II/III trial, the median PuFS was 46 days, and the median OS was 72 days [14]. In subsequent studies, the median PuFS ranged from 29.5 to 57 days and the median OS from 86 to 111 days [18, 23, 24, 28]. Although the median PuFS was among the highest ever reported to date, the median OS observed in the present study was somewhat inferior to those from all other aforementioned trials. This was undoubtedly attributable to the high proportion of relatively “frail” or heavily pretreated patients included in our study, which by far exceeded that of any other study of i.p. CATU therapy. Accordingly, it seems of particular interest that only 68 of 201 events (34%) indexed for the determination of PuFS in the pivotal study were deaths. The corresponding proportion in the present study was 22 of 27 events (81.5%) [14]. Moreover, almost all patients in the cited trials had been treated in a hospital, but all the patients included in the present study were exclusively treated in an outpatient setting.
Additionally, we sought to determine the parameters that might be able to indicate which patient with MA related to an EpCAM-positive epithelial malignancy would be mostly suitable for i.p. outpatient treatment with CATU. Considering previous reports, that determinants that were worthwhile to consider were ovarian versus nonovarian histology, patient age (<60 vs. ≥60 years) patient performance status (KPS score ≥80% vs. <80%), presence or absence of extraperitoneal metastases, intensity of previous systemic therapy (four or fewer vs. more than four protocols), pretreatment RLC, patient adherence to complete i.p. CATU therapy (four vs. one to three instillations), and the ability to undergo subsequent systemic treatment [14, 20–24]. Owing to the limited sizes of these patient subgroups, performing a multivariate analysis of variance did not seem appropriate. Nonetheless, we were able to demonstrate that a KPS score of ≥80%, the absence of extraperitoneal lesions, the ability to receive all four planned CATU applications, and the ability to undergo subsequent systemic therapies after outpatient i.p. CATU treatment were predictors of both improved PuFS and OS. The analysis of 11 individuals who had undergone systemic antineoplastic treatments after i.p. CATU seems of particular interest. Eight of these patients presented with a baseline KPS score of 80% or more, and the KPS score stabilized or improved in 5 of them. However, 3 of these patients had a baseline KPS score of 70% or lower, all of which remained unchanged or even increased. These findings argue in favor of the ability to undergo systemic antineoplastic therapy subsequently to i.p. CATU is the more reliable predictor of benefit compared with the baseline KPS.
Ovarian histology, patient age, pretreatment RLC, and the intensity of systemic pretreatment failed to be indicators of better PuFS or OS in our study. Regarding the tumor type, this appears to be somewhat amazing, because patients with ovarian cancer mostly did better compared with others in previous trials [14, 23, 24]. However, these studies recruited a large number of patients with nongynecologic cancers, which are known to carry a considerably worse long-term prognosis than most gynecologic malignancies. In our study, the group of nonovarian cancer patients mainly included those with breast or endometrial cancer. Compared with most gastrointestinal and other nongynecologic carcinomas, these tumors are generally associated with better clinical outcomes, which might explain, in part, why an ovarian histology failed to be a positive prognostic factor in the present study. Additionally, we were not able to unequivocally confirm the results of a previous report describing a baseline RLC exceeding 13% to be associated with a significantly better clinical outcome after i.p. CATU [22]. The reason for this finding is unclear at present but might be partly attributable to the relative small number of patients included, inasmuch as a clear trend favored patients with a higher median RLC before the start of i.p. CATU. The intensity of pretreatment also failed to be a prognostic indicator in our study. However, this result is in good agreement with those from previous publications, in particular when comparing the pivotal phase II/III trial with the CARMA study [14, 23, 24].
The findings from case reports argue in favor that i.p. CATU might well produce systemic antineoplastic effects beyond ascites control [29, 30]. In our study, a few patients presented with measurable lesions. The response according to RECIST 1.1 was therefore not used as an estimate of CATU effectiveness. Nonetheless, none of the 11 patients with measurable disease experienced tumor progression and 2 showed tumor regression. In agreement with previous reports, these findings show that i.p. CATU might exhibit a systemic antineoplastic effect, at least in a subset of patients.
A recently published survey evaluating the palliative treatment of MA in the clinical routine in Central Europe showed that repeated paracentesis is still the mainstay of therapy despite the presence of more effective options [31]. Catumaxomab was given to only 7% of patients. One of the major reasons might be the concern that specific therapy for MA is both costly and difficult to administer to outpatients. In our study, however, we were able to show that i.p. CATU is a feasible treatment of MA in outpatients with peritoneal carcinomatosis. Although not always translating into improved OS, ascites control was satisfactory in the vast majority of patients, including those with older age, more intensive pretreatment, or an impaired physical condition. Regarding the low rate of secondary hospitalizations in our study and assuming that the costs for CATU and all supportive medication would be similar for both hospitalized and nonhospitalized individuals, it can be hypothesized that outpatient CATU therapy for suitable individuals would be cost-effective or even cost-saving compared with inpatient treatment. However, because the pricing of both drugs and medical care differs in both inpatient and outpatient facilities and from country to country, a prospective cost evaluation is clearly required before outpatient i.p. CATU can be generally adopted as routine care for patients with malignant ascites.
Our study had some limitations, including the relative small sample size and the noninterventional setting. However, as in the CARMA study, it focused on a real-world population of patients, which might approach the daily clinical reality more precisely than possible with the highly selected groups of patients included in randomized trials. Moreover, to the best of our knowledge, it represents by far the largest cohort of outpatients treated with i.p. CATU for MA related to various gynecologic carcinomas and metastatic breast cancer published to date. In regard to our findings, candidates for outpatient CATU therapy would be those presenting with a good general condition with no evidence of extraperitoneal tumor spread who are able to receive both all four planned CATU instillations and subsequent systemic therapies.
In conclusion, our results indicate that i.p. CATU is safe, effective, and cost-saving in carefully selected outpatients with MA related to various gynecologic tumors or metastatic breast cancer. Therefore, this approach should be seriously considered for inclusion into the routine therapeutic armamentarium of this demanding clinical situation.
Acknowledgments
We are grateful to all patients and their families participating in this study and to the medical staff at the study center. We give special thanks to Katja Monreal, Gabriele Wessling, and Tim A. Kalisch for their editorial assistance.
Parts of this study have been previously presented as a proffered paper at the 45th Annual Meeting of Women’s Cancer, Tampa, FL, USA, March 2014; and as a poster at the ESMO Congress Madrid, Spain, September 2014, the 15th Biennial Meeting of the International Gynecologic Cancer Society, Melbourne, Australia, November 2014, and the 26th International Congress on Anti-Cancer Treatment, Paris, France, February 2015.
Author Contributions
Conception/design: Christian Martin Kurbacher, Jutta Anna Kurbacher
Provision of study material or patients: Christian Martin Kurbacher, Olympia Horn, Jutta Anna Kurbacher, Susanne Herz, Ann Tabea Kurbacher, Ralf Hildenbrand, Reinhardt Bollmann
Collection and/or assembly of data: Christian Martin Kurbacher, Olympia Horn, Jutta Anna Kurbacher, Susanne Herz, Ann Tabea Kurbacher, Ralf Hildenbrand, Reinhardt Bollmann
Data analysis and interpretation: Christian Martin Kurbacher, Jutta Anna Kurbacher, Ann Tabea Kurbacher
Manuscript writing: Christian Martin Kurbacher, Jutta Anna Kurbacher
Final approval of manuscript: Christian Martin Kurbacher, Olympia Horn, Jutta Anna Kurbacher, Susanne Herz, Ann Tabea Kurbacher, Ralf Hildenbrand, Reinhardt Bollmann
Disclosures
The authors indicated no financial relationships.
References
- 1.Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: A retrospective study. Ann Oncol. 2007;18:945–949. doi: 10.1093/annonc/mdl499. [DOI] [PubMed] [Google Scholar]
- 2.Garrison RN, Kaelin LD, Galloway RH, et al. Malignant ascites. Clinical and experimental observations. Ann Surg. 1986;203:644–651. doi: 10.1097/00000658-198606000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker G, Galandi D, Blum HE. Malignant ascites: Systematic review and guideline for treatment. Eur J Cancer. 2006;42:589–597. doi: 10.1016/j.ejca.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson J, Gilbert J. The development of clinical guidelines on paracentesis for ascites related to malignancy. Palliat Med. 2002;16:213–218. doi: 10.1191/0269216302pm509oa. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher DL, Saclarides TJ, Staren ED. Peritoneovenous shunts for palliation of the patient with malignant ascites. Ann Surg Oncol. 1994;1:378–381. doi: 10.1007/BF02303809. [DOI] [PubMed] [Google Scholar]
- 6.Zanon C, Grosso M, Aprà F, et al. Palliative treatment of malignant refractory ascites by positioning of Denver peritoneovenous shunt. Tumori. 2002;88:123–127. doi: 10.1177/030089160208800208. [DOI] [PubMed] [Google Scholar]
- 7.Woopen H, Sehouli J. Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res. 2009;29:3353–3359. [PubMed] [Google Scholar]
- 8.Schmitt M, Schmitt A, Reinhardt P, et al. Opsonization with a trifunctional bispecific (alphaCD3 x alphaEpCAM) antibody results in efficient lysis in vitro and in vivo of EpCAM positive tumor cells by cytotoxic T lymphocytes. Int J Oncol. 2004;25:841–848. [PubMed] [Google Scholar]
- 9.Ruf P, Gires O, Jäger M, et al. Characterisation of the new EpCAM-specific antibody HO-3: Implications for trifunctional antibody immunotherapy of cancer. Br J Cancer. 2007;97:315–321. doi: 10.1038/sj.bjc.6603881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeidler R, Mysliwietz J, Csánady M, et al. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer. 2000;83:261–266. doi: 10.1054/bjoc.2000.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebastian M. Review of catumaxomab in the treatment of malignant ascites. Cancer Manag Res. 2010;2:283–286. doi: 10.2147/CMR.S14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heiss MM, Ströhlein MA, Jäger M, et al. Immunotherapy of malignant ascites with trifunctional antibodies. Int J Cancer. 2005;117:435–443. doi: 10.1002/ijc.21165. [DOI] [PubMed] [Google Scholar]
- 13.Burges A, Wimberger P, Kümper C, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: A phase I/II study. Clin Cancer Res. 2007;13:3899–3905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]
- 14.Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36:458–467. doi: 10.1016/j.ctrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Eskander RN, Tewari KS. Epithelial cell-adhesion molecule-directed trifunctional antibody immunotherapy for symptom management of advanced ovarian cancer. Clin Pharmacol. 2013;5(suppl 1):55–61. doi: 10.2147/CPAA.S45885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wimberger P, Gilet H, Gonschior A-K, et al. Deterioration in quality of life (QoL) in patients with malignant ascites: Results from a phase II/III study comparing paracentesis plus catumaxomab with paracentesis alone. Ann Oncol. 2012;23:1979–1985. doi: 10.1093/annonc/mds178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehouli J, Pietzner K, Wimberger P, et al. Catumaxomab with and without prednisolone premedication for the treatment of malignant ascites due to epithelial cancer: Results of the randomised phase IIIb CASIMAS study. Med Oncol. 2014;31:76. doi: 10.1007/s12032-014-0076-7. [DOI] [PubMed] [Google Scholar]
- 19.Ott MG, Marmé F, Moldenhauer G, et al. Humoral response to catumaxomab correlates with clinical outcome: Results of the pivotal phase II/III study in patients with malignant ascites. Int J Cancer. 2012;130:2195–2203. doi: 10.1002/ijc.26258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bokemeyer C, Heiss MM, Lordick F, et al. Pooled analysis of two phase III studies provides prognostic indicators for clinical out-come after catumaxomab treatment for malignant ascites. Eur J Cancer. 2013;49(suppl 2):1013a. [Google Scholar]
- 21.Chekerov R, Wimberger P, Vergote I, et al. Treatment with the trifunctional antibody catumaxomab followed by systemic chemotherapy: Results of a subgroup analysis from a phase III study in patients with relapsed ovarian cancer and malignant ascites. Eur J Cancer. 2013;49(suppl 2):3097a. [Google Scholar]
- 22.Heiss MM, Ströhlein MA, Bokemeyer C, et al. The role of relative lymphocyte count as a biomarker for the effect of catumaxomab on survival in malignant ascites patients: Results from a phase II/III study. Clin Cancer Res. 2014;20:3348–3357. doi: 10.1158/1078-0432.CCR-13-2351. [DOI] [PubMed] [Google Scholar]
- 23.Kurbacher CM, Sehouli J, Welslau M, et al. Results of the CARMA study to investigate catumaxomab therapy for malignant ascites related to peritoneal carcinomatosis in clinical practice. J Clin Oncol. 2013;31(suppl):TPS3119a. [Google Scholar]
- 24.Kurbacher C, Sehouli J, Welslau M, et al. The use of catumaxomab for treatment of malignant ascites in clinical practice: Results of an observational trial. Eur J Cancer. 2013;49(suppl 2):1371a. [Google Scholar]
- 25.Ammouri L, Prommer EE. Palliative treatment of malignant ascites: Profile of catumaxomab. Biologics. 2010;4:103–110. doi: 10.2147/btt.s6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seimetz D. Novel monoclonal antibodies for cancer treatment: The trifunctional antibody catumaxomab (removab) J Cancer. 2011;2:309–316. doi: 10.7150/jca.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cella D, Neubauer N, Thomas J, et al. The FACIT-AI, a new tool for assessing symptoms associated with malignant ascites. Gynecol Oncol. 2013;128:187–190. doi: 10.1016/j.ygyno.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Berek JS, Edwards RP, Parker LP, et al. Catumaxomab for the treatment of malignant ascites in patients with chemotherapy-refractory ovarian cancer: A phase II study. Int J Gynecol Cancer. 2014;24:1583–1589. doi: 10.1097/IGC.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 29.Petrelli F, Borgonovo K, Lonati V, et al. Regression of liver metastases after treatment with intraperitoneal catumaxomab for malignant ascites due to breast cancer. Target Oncol. 2013;8:291–294. doi: 10.1007/s11523-012-0240-y. [DOI] [PubMed] [Google Scholar]
- 30.Bezan A, Hohla F, Meissnitzer T, et al. Systemic effect of catumaxomab in a patient with metastasized colorectal cancer: A case report. BMC Cancer. 2013;13:618. doi: 10.1186/1471-2407-13-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jehn CF, Küpferling S, Oskay-Özcelik G, et al. A survey of treatment approaches of malignant ascites in Germany and Austria. Support Care Cancer. 2015;23:2073–2078. doi: 10.1007/s00520-014-2557-9. [DOI] [PubMed] [Google Scholar]