This meta-analysis showed that metformin was associated with better overall survival times and cancer-specific survival times in diabetic patients with breast cancer. Subgroup analysis revealed that metformin improved the overall survival by 65% after adjusting for hormone receptor expression. The findings of this study highlight the potential usage of metformin in diabetic patients with breast cancer.
Keywords: Metformin, Breast cancer, Diabetes mellitus, Survival, Meta-analysis
Abstract
Background.
Diabetic patients with breast cancer receiving metformin and neoadjuvant chemotherapy have a higher pathologic complete response rate than do diabetic patients not receiving metformin, but findings on salvage treatment have been inconsistent. We performed a meta-analysis to assess the effect of adding metformin to standard therapy on the prognosis of breast cancer patients with diabetes.
Methods.
We searched PubMed, Embase, Web of Science (Thomson Scientific), China Knowledge Resource Integrated Database, VIP journal integration platform, and Chinese BioMedical Literature Database from inception to January 10, 2015, without language restrictions, including references related to metformin, breast cancer, and prognosis. We performed the meta-analysis using a random-effects model, with hazard ratios (HRs) and 95% confidence intervals (95% CIs) as effect measures.
Results.
A total of 11 studies consisting of 5,464 breast cancer patients with diabetes were included, comprising 2,760 patients who had received metformin and 2,704 patients who had not. The meta-analysis showed that metformin was associated with better overall survival times (HR: 0.53; 95% CI: 0.39-0.71) and cancer-specific survival times (HR: 0.89; 95% CI: 0.79-1.00). Subgroup analysis revealed that metformin improved the overall survival by 65% after adjusting for hormone receptor expression (HR: 0.35; 95% CI: 0.15–0.84). Taking metformin after the diagnosis of breast cancer was still associated with prolonged overall survival.
Conclusion.
The use of metformin in standard cancer therapy might improve both overall and cancer-specific survivals of diabetic patients with breast cancer.
Implications for Practice:
Diabetic patients with breast cancer receiving metformin and neoadjuvant chemotherapy have a higher pathologic complete response rate than diabetic patients not receiving metformin, but findings on salvage treatment have been inconsistent. The meta-analysis showed that metformin was associated with better overall survival times and cancer-specific survival times. Subgroup analysis revealed that metformin improved the overall survival by 65% after adjusting for hormone receptor expression. Taking metformin after the diagnosis of breast cancer was still associated with prolonged overall survival. The findings of this study highlight the potential usage of metformin in diabetic patients with breast cancer.
Abstract
摘要
背景. 合并糖尿病的乳腺癌患者中,接受二甲双胍与新辅助化疗者的病理学完全缓解率优于未接受二甲双胍治疗者,但有关挽救性治疗的研究结果并不一致。我们开展了一项 meta 分析,目的是在合并糖尿病的乳腺癌患者中,评估标准治疗基础上添加二甲双胍治疗对预后的影响。
方法. 我们在 PubMed、Embase、Web of Science (汤普森科技)、中国知识资源总库、维普期刊资源整合服务平台和中国生物医学文献数据库中,对起始至 2015 年 1 月 10 日的数据进行了检索。检索对象包括与二甲双胍、乳腺癌和预后相关的参考文献,未设语种限制。我们的 meta 分析使用了随机效应模型,使用风险比 (HR) 和 95%可信区间 (CI) 进行效应测量。
结果. 共纳入 11 项研究,涉及 5 464 例合并糖尿病的乳腺癌患者,包括 2 760 例接受二甲双胍治疗的患者和 2 704 例未接受二甲双胍治疗的患者。Meta 分析显示二甲双胍治疗患者的总生存时间(HR: 0.53, 95%CI: 0.39 ∼ 0.71)和癌症相关生存时间较长 (HR: 0.89, 95%CI: 0.79 ∼ 1.00)。亚组分析显示,校正激素受体表达状态后,二甲双胍治疗使总生存时间延长了65% (HR: 0.35, 95%CI: 0.15 ∼ 0.84)。在诊断为乳腺癌之后才开始二甲双胍治疗仍然与总生存时间延长相关。
结论. 在标准抗癌治疗方案基础上加用二甲双胍,可改善合并糖尿病的乳腺癌患者的总生存及癌症相关生存。The Oncologist 2015;20:1236–1234
对临床实践的提示:合并糖尿病的乳腺癌患者中,接受二甲双胍与新辅助化疗的患者病理学完全缓解率高于未接受二甲双胍治疗的患者,但有关挽救性治疗的研究结果并不一致。本项 meta 分析显示二甲双胍与总生存时间和癌症相关生存时间较长相关。亚组分析显示,校正激素受体表达状态后,二甲双胍使总生存时间延长了 65%。在诊断为乳腺癌之后才开始二甲双胍治疗仍然与总生存时间延长相关。本研究的结果强调了二甲双胍在合并糖尿病的乳腺癌患者中的潜在应用。
Introduction
Epidemiologic and clinical evidence has linked diabetes mellitus and insulin resistance to the poor prognosis of many cancers including breast cancer, the most common cancer among women [1]. Antidiabetic medications such as metformin have received great attention in both cancer prevention and treatment. However, a cancer-preventive advantage associated with metformin does not necessarily imply effective therapeutic efficacy in those patients with diabetes and established cancers. It is unclear whether the use of metformin could also translate into better clinical outcomes for patients with cancer who also receive standard cancer therapy.
Preclinical work has demonstrated a beneficial effect of metformin in breast cancer [2] through indirect (insulin-mediated) effects, or it may directly affect cell proliferation and apoptosis of cancer cells [3, 4]. Jiralerspong et al. reported a threefold greater pathologic complete response rate in patients with diabetes and breast cancer who received metformin during neoadjuvant chemotherapy compared with those who did not receive metformin (odds ratio: 2.95; 95% confidence interval [CI]: 1.07–8.17) [5]. However, despite the increase in the pathologic complete response, metformin did not significantly improve the estimated 3-year relapse-free survival rate in this study. Several studies showed a better survival in patients who received metformin than in those who did not receive metformin [6]. Not much evidence was accumulated on the topic. A recent meta-analysis [7] demonstrated that the administration of metformin to patients with cancer and type 2 diabetes was associated with a 34% reduction of death risk compared with those who did not receive metformin (hazard ratio [HR]: 0.66; 95% CI: 0.55–0.79). In stratified analyses by cancer type, a survival benefit was not observed in patients with breast cancer, which might be due to the small sample size. Understanding the efficacy of metformin in breast cancer treatment may lead to better clinical management of patients with this disease. We therefore conducted a meta-analysis to illustrate the association of metformin with overall and cancer-specific survivals in breast cancer patients.
Methods
Data Sources and Searches
We searched PubMed, Embase, Web of Science (Thomson Scientific), China Knowledge Resource Integrated Database, VIP journal integration platform, and Chinese BioMedical Literature databases from their inception to January 10, 2015, for articles and abstracts evaluating the association between metformin and outcome in breast cancer, including survival, stage at diagnosis, and treatment choice. Our overall search strategy included terms for metformin, breast cancer (e.g., “cancer,” “carcinoma,” “adenocarcinoma,” and “breast”), and prognosis (e.g., “prognosis,” “survival,” and “mortality”). We also searched the references of included articles. No language or publication-type restrictions were imposed (supplemental online Table 1).
Study Selection
Our overall search targeted articles describing studies that (a) evaluated any prognostic outcome by metformin use, (b) evaluated a breast cancer patient population, and (c) contained original data analysis. To avoid overlapping patient populations, we compared data on recruitment years, data source, and geographic location. Publications with duplicate data sets were triaged by keeping the most recent one, the one with the larger study population, or the one with multivariate-adjusted estimates. Articles that met the above three criteria and reported all-cause or cancer-specific mortality or overall survival were retained. To be included in our meta-analysis, articles had to report a risk estimate (e.g., HR) relating metformin use to subsequent death by using survival analysis regression models, with an estimate of precision such as SE or 95% CI. Articles with missing risk estimates were also included in the meta-analysis if the risk estimates were generated by author contact.
Data Extraction and Quality Assessment
Each article was abstracted by one author and reviewed by a second author for accuracy. Any disagreement was resolved by consensus. The data extracted from eligible articles included publication data (the first author’s last name, year of publication, and country of study population), study design (clinic-based or population-based cohort studies), sample size, data source, study recruitment years, study eligibility criteria, length of follow-up, outcome assessed, risk estimates with their corresponding CIs, and variables controlled for by matching or in the multivariable model. If several estimates were reported in the same article, we chose the most fully adjusted estimate (i.e., multivariate regression was selected over univariate regression). If an article reported multiple estimates by subgroup only, these estimates were entered separately into our relevant meta-analysis data set.
We also extracted information on key indicators of study quality with the use of Meta-analysis of Observational Studies in Epidemiology (MOOSE) standards [8] for reporting of meta-analyses of observational studies. From each study, we chose the risk estimates that represented the greatest degree of control for potential confounders. Quality was assessed by using elements of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [9]. To judge quality, we abstracted information on the characteristics of patients (including their source, inclusion, and exclusion criteria), the methods of diagnosing diabetes mellitus, the definition of metformin exposure, methods for ascertaining outcome, whether metformin was the primary exposure variable or was one of a group of prognostic variables, and statistical adjustment for confounders [10].
Data Analyses
For the meta-analysis, p values quoted at less than the specified threshold were assumed to be at the threshold, resulting in a conservative estimate of the significance level. I2 and Cochran Q estimates were performed in a heterogeneity assessment [11]. I2 values of >50% or p values of less than 0.1 represented significant heterogeneity. A DerSimonian-Laird random-effects model was used to calculate the pooled HR. Otherwise, an inverse variance fixed-effects model was selected. The meta-analysis was performed using Stata version 12.0 software (StataCorp, College Station, TX, http://www.stata.com). We used the “metan” command to pool the lnHR across studies. Forest plots were used to visually assess the HR estimates and corresponding 95% CIs across studies.
To assess the effect of study quality, we conducted a sensitivity analysis that omitted lower-quality studies. We considered studies to be of higher quality and calculated separate pooled HRs if they were with estimates adjusted for age (n = 10), body mass index (BMI) (n = 4), cancer stage (n = 6), types of antidiabetic medications (n = 4), or metformin use evaluated as the primary exposure variable (n = 10). Publication bias was evaluated by using Begg’s funnel plot [12] and the Egger’s test [13]. We performed the Duval and Tweedie nonparametric trim and fill procedure [14] to further assess potential effects of publication bias. This method considers the possibility of hypothetical missing studies, imputes their HRs, and recalculates a pooled estimate. For all tests, a p value (two-sided) of less than .05 was considered statistically significant.
Results
Of the 2,751 titles identified, 234 abstracts and 65 resulting full articles were reviewed to determine their eligibility. Of the 17 articles addressing the effect of metformin on breast cancer outcome, 4 were excluded for overlapping [15, 16] or lack of estimates [17, 18]. Two studies [19, 20] were excluded because their comparison of survival between metformin users and nonmetformin users included both patients with and those without diabetes. Overall, 11 full articles with 14 estimates were included in the meta-analysis (Fig. 1) [21–31]. Descriptive data for studies included in our meta-analysis are listed in Table 1 and supplemental online Table 2 according to the publication date.
Figure 1.
Flow chart of study selection.
Abbreviations: CBM, Chinese BioMedical Literature Database; CNKI, China Knowledge Resource Integrated Database; TS, Thomson Scientific; VIP, VIP journal integration platform.
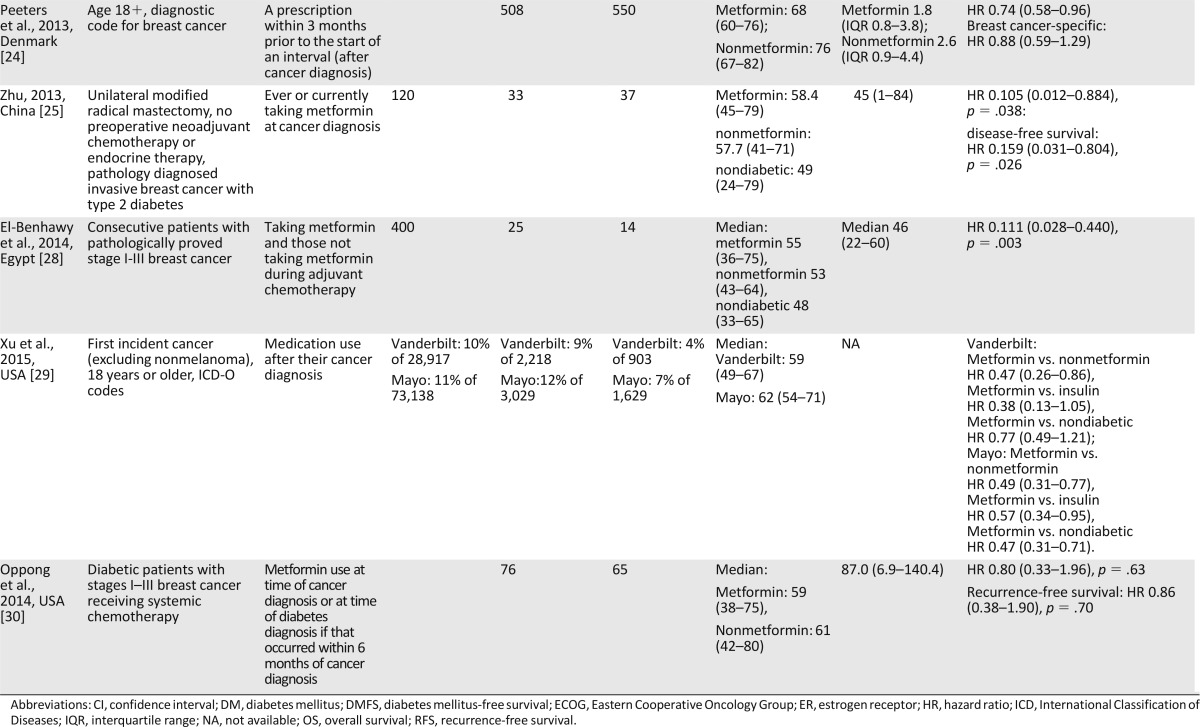
Table 1.
Characteristics of the 11 studies included in the meta-analysis of the effect of metformin on breast cancer mortality
Description of Studies
The 11 observational studies in our meta-analysis of overall survival included 5,464 breast cancer patients with diabetes, including 2,760 patients who took metformin and 2,704 who did not. The studies had been conducted in the United States (n = 5), China (n = 3), Denmark (n = 1), Canada (n = 1), and Egypt (n = 1). Sample sizes ranged from 39 to 2,361 patients, with a median of 141. The overall proportion of metformin use was 50.5% (range, 18.6%–79.0%). Reporting of age and follow-up time varied across studies. The time origin for survival analysis was generally the time of cancer diagnosis, except in the case of treatment or surgical cohorts, for which the time of origin was the beginning of treatment or the date of tumor resection. The metformin exposure was defined as ever or currently taking metformin at the diagnosis of cancer (n = 6) or taking metformin after cancer diagnosis (n = 5). Nine studies had a clinic-based design, and two studies were population-based cohorts. All studies ascertained diabetes mellitus by either blood tests and/or medical records. One study did not state the definition of metformin exposure. Eight studies used death registries, medical records, and/or interviews to ascertain vital status; the remaining studies did not report the method of outcome ascertainment. Ten studies investigated metformin as the primary exposure of interest, whereas the remaining study evaluated metformin among other prognostic variables (supplemental online Table 3).
Meta-Analysis on Metformin and All-Cause Mortality
The 11 studies with 14 estimates in the meta-analysis reported both risk (HR) and precision (95% CI). The descriptive data, adjustment or restriction variables, and major findings from each study are described in Table 1. The results of the meta-analysis are shown in Figure 2. Metformin use was associated with a 47% decreased risk of death from all causes in breast cancer patients with diabetes (HR: 0.53; 95% CI: 0.39–0.71). Considering the large variations in the study, we performed Begg’s funnel plot (p = .827) and Egger’s test (p < .001), which suggested the possible presence of publication bias. Using a “trim and fill” method to make an adjusted estimation of meta-analysis, we found that no trimming was needed and that metformin use was still associated with a reduced death risk in the adjusted analyses (HR: 0.92; 95% CI: 0.88–0.97 by fixed effects, and HR: 0.53; 95% CI: 0.39–0.70 by random effects) (supplemental online Figs. 1–3).
Figure 2.
Meta-analysis and pooled hazard ratio of long-term, all-cause mortality in 11 studies (14 estimates) comparing breast cancer patients with and without metformin. Xiao (1), Xiao (2), and Xiao (3) indicate Luminal A, Luminal B (high Ki-67), and Luminal B (HER2+) subgroups, respectively. Xu (1) and Xu (2) indicate Vanderbilt and Mayo Clinic subgroups, respectively. Weights are from random effects analysis. Data markers are proportional to study sample sizes. Squares indicate relative risk in each study. The square size is proportional to the weight of the corresponding study in the meta-analysis; the lengths of the horizontal lines represent the 95% CI. The unshaded diamond indicates the pooled relative risk and 95% CI.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Sensitivity Analyses
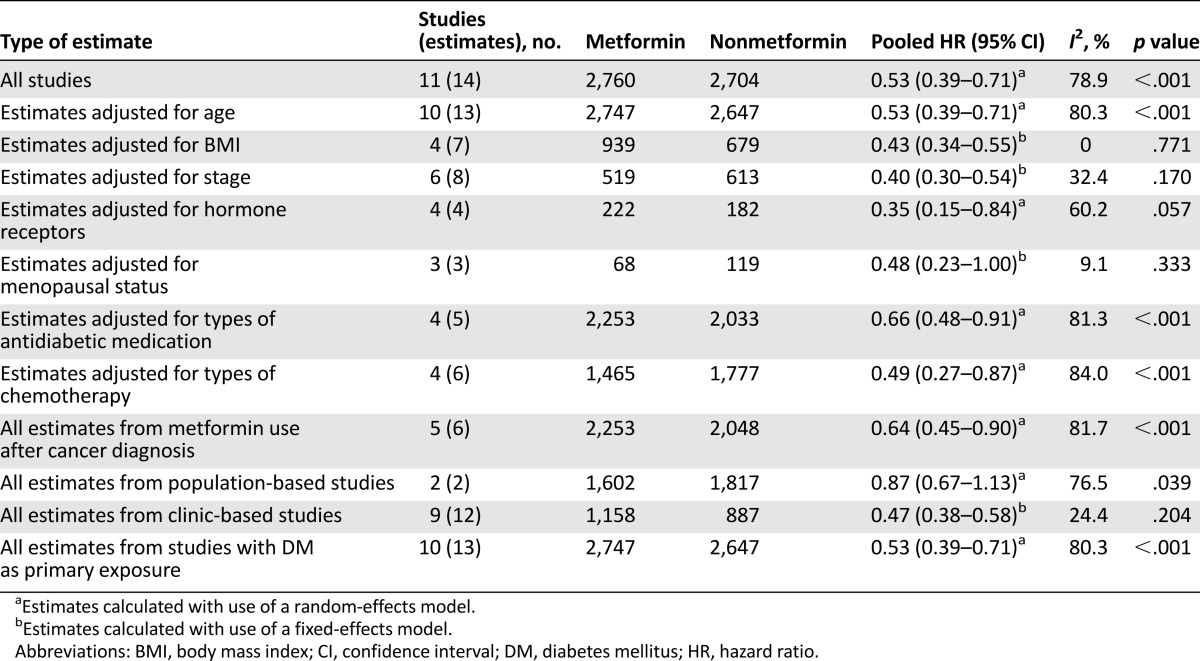
Considering the large variations in the covariates included in each study, we conducted a sensitivity analysis to confirm robustness (Table 2). The association between metformin use and better overall survival in breast cancer patients with diabetes was not changed after adjusting for confounders. More specifically, the above pooled HRs (95% CI) after adjusting for age, BMI, stage, menopausal status, antidiabetic medication, and types of chemotherapy were 0.53 (0.39–0.71), 0.43 (0.34–0.55), 0.40 (0.30–0.54), 0.48 (0.23–1.00), 0.66 (0.48–0.91), and 0.49 (0.27–0.87), respectively. Metformin use was associated with the biggest decrease of risk of death (65%) after adjusting for hormone receptor expression in cancer tissue (HR: 0.35; 95% CI: 0.15–0.84). Studies with metformin use after cancer diagnosis had a pooled HR (95% CI) of 0.64 (0.45–0.90).
Table 2.
Pooled hazard ratios of all-cause mortality in diabetic breast cancer patients with and without metformin
Analysis of influence revealed that the risk of all-cause mortality among patients with breast cancer and metformin use remained significant with the omission of each study in turn. Omission of the study by Lega et al. [23] resulted in the lowest pooled estimate (HR: 0.50; 95% CI: 0.39–0.64); omission of the study by Xiao et al. [31] resulted in the highest pooled estimate (HR: 0.61; 95% CI: 0.46–0.81).
Metformin and Cancer-Specific Mortality
Three studies provided adjusted HRs of cancer-specific death in patients [22–24]. These three studies showed that breast cancer patients with metformin use had a significantly lower risk of cancer-specific mortality than did their counterparts (HR: 0.89; 95% CI: 0.79–1.00) (supplemental online Fig. 4). Post hoc power calculations indicated that the power was 52.3%. No publication bias was detected by Begg’s funnel plot (p = .296) or Egger’s test (p = .399).
All-Cause Mortality Between Diabetic Patients With Metformin and Nondiabetic Patients
Three studies compared all-cause mortality between diabetic patients with metformin use and nondiabetic patients [21, 29, 31]. These studies showed that breast cancer patients with metformin use had a significantly lower risk of all-cause mortality compared with their nondiabetic counterparts (HR: 0.63; 95% CI: 0.51–0.78) (supplemental online Fig. 5). No publication bias was detected by Begg’s funnel plot (p = 1.000) or Egger’s test (p = .712).
Discussion
Our meta-analyses of observational studies demonstrated that the addition of metformin to standard therapy in breast cancer patients with diabetes, compared with their counterparts who did not receive metformin, was associated with decreased risks of all-cause and cancer-specific mortalities. The risk of all-cause mortality in patients with metformin use was lower than for those patients who did not take metformin in studies adjusting for age, BMI, stage, menopausal status, antidiabetic medication, types of chemotherapy, and hormone receptor expression. Furthermore, taking metformin after the diagnosis of breast cancer still prolonged the overall survival. These observations could not be explained by confounding factors, publication bias, or undue influence by a single study.
To our knowledge, our meta-analysis is the first exclusive study of the association between metformin use and breast cancer outcome, though this topic has been investigated by many individual studies. Our results are not in accordance with a previous meta-analysis of mortality in cancer patients with concurrent diabetes by Yin et al. [7], who found that metformin use was associated with decreased risk of mortality in all cancers (HR: 0.66; 95% CI: 0.55–0.79) but not specifically in breast cancer (HR: 0.64; 95% CI: 0.37–1.12). Moreover, breast cancer-specific survival was miscalculated by adopting recurrence-free survival as cancer-specific survival from the study of Bayraktar et al. [21]. In another study [32] of metformin and cancer survival, metformin use was associated with better survival in the breast cancer subgroup (HR: 0.70; 95% CI: 0.55–0.88). However, the same patient population was calculated twice, which might overestimate the benefit of metformin. These two studies both included nondiabetic patients in the nonmetformin group. As is known, diabetes is a poor prognostic factor for breast cancer. Thus, this inclusion is problematic, because it would underestimate the survival benefit. In the current larger-scale meta-analysis, we conducted post hoc power calculations and found that our study had 98.6% power in demonstrating the association between metformin use and overall cancer mortality. These observations support the hypothesis that the previous inconsistent findings between individual studies might be partially explained by small patient populations.
The strengths of this study include a comprehensive review of the literature by multidisciplinary members including specialists in oncology and epidemiology, with each article reviewed by two persons. We used a broad search strategy and inclusion criteria to extract as much information from the literature as possible, including information from any type of publication and any language. Although two relevant articles were excluded from our meta-analysis for lack of information on risk estimates, findings from these articles were generally consistent with those in the pooled meta-analysis [17, 18].
There were several limitations in the literature and thus in our meta-analysis. First, studies varied in their inclusion criteria, study population, and adjustment for confounding variables, which may have led to both overestimations and underestimations of risks. Nevertheless, our sensitivity analyses, excluding studies that did not adjust for potential confounders, did not materially change the results. Residual or unknown confounding is still possible after adjusting for most relevant confounding factors. In addition, the association may not necessarily be causal, particularly in the observational studies [33].
The second limitation was that some of the articles did not report the types of anticancer or antidiabetic therapies used (other than metformin) and their effects on outcomes. This is important because studies have shown that some therapies (e.g., surgery, adjuvant chemotherapy, and the antidiabetic drug insulin) have a more positive effect than others on cancer outcome [34, 35]. Moreover, diabetes is a chronic metabolic disease, and thus its occurrence, development, and treatment may have different effects on prognosis, which may affect the final results. Finally, there seemed to be significant publication bias in the literature, as suggested by Egger’s test. However, this may be the small-study effect rather than true publication bias, especially in the presence of significant between-study heterogeneity [36]. We did attempt to adjust our quantitative analyses by including the missing studies. The trim and fill method is a statistical method used in meta-analysis that can underestimate the true positive effect when there is no publication bias or can give less biased estimates when publication bias is present [37]. Using this conservative method, we found that the association between metformin and increased survival still remained.
There are several potential explanations for the observed association between metformin use and increased survival time in breast cancer patients with diabetes. First, metformin, a widely prescribed oral medication used as front-line therapy for type 2 diabetes, has been shown to inhibit the growth of cancer cell lines, including breast cancer lines, in both in vitro and in vivo tumor models. Population and retrospective studies have shown that metformin decreases the incidence of cancer and cancer-related mortality and increases the response to neoadjuvant chemotherapy in diabetic patients. Metformin induces AMPK activation, which decreases insulin levels and leads to inhibition of protein synthesis pathways, decreasing cancer cell proliferation and growth. Hence, metformin is being investigated as a therapeutic agent in different clinical settings for all breast cancer subtypes [38]. Second, breast cancer patients with diabetes, especially those with severe diabetes, may be given less vigorous anticancer regimens because they generally have more contradictions to surgery, chemotherapy, and other treatments [39]. The metabolic abnormalities associated with diabetes may have an adverse effect on the response to cancer treatment [40]. However, a survival advantage of metformin use in diabetic patients was still found compared with their nondiabetic counterparts. Finally, the high mortality rate observed among patients who did not take metformin may partially be due to noncancerous factors [20], such as complications of long-term diabetes. However, the benefit of metformin on cancer-specific mortality was also observed in a meta-analysis of 3,573 breast cancer patients.
Conclusion
The main implication of our meta-analyses on observational studies is that metformin use is significantly associated with favorable outcome in breast cancer. Taking metformin after the diagnosis of breast cancer still prolonged the overall survival. Our results reveal the need for further prospective studies to confirm metformin use as a prognostic factor and to assess the possibility of an antidiabetic regimen in the treatment of breast cancer.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank Michael Worley at the Department of Scientific Publications for copyediting the manuscript. This work was supported by National Natural Science Foundation of China Grants 30901765 (to Yixiang Mao) and 81272542 (to Min Tao) and funds from the China Scholarship Council (to Yixiang Mao) and the Science and Education for Health Foundation of Suzhou for Youth (to Yixiang Mao).
Footnotes
For Further Reading: Ming Yin, Jie Zhou, Edward J. Gorak et al. Metformin Is Associated With Survival Benefit in Cancer Patients With Concurrent Type 2 Diabetes: A Systematic Review and Meta-Analysis. The Oncologist 2013;18:1248-1255.
Implications for Practice: Patients with type 2 diabetes have increased cancer risk and cancer-related mortality, which can be reduced by metformin treatment. However, it is unclear whether metformin can also modulate clinical outcomes in patients with cancer and concurrent type 2 diabetes. This meta-analysis provided evidence that there was a relative survival benefit associated with metformin treatment compared with treatment with other glucose-lowering medications. These results suggest that metformin is the drug of choice in the treatment of patients with cancer and concurrent type 2 diabetes.
Author Contributions
Conception/Design: Kai Chen, Xiaoyan Jia, Min Tao, Yixiang Mao
Provision of study material or patients: Yixiang Mao
Collection and/or assembly of data: Yali Tian, Yun Dai, Jing Xie, Yixiang Mao
Data analysis and interpretation: Hong Xu, Xiaoyan Jia, Dapeng Li, Min Tao, Yixiang Mao
Manuscript writing: Hong Xu, Kai Chen, Xiaoyan Jia, Jing Xie, Min Tao, Yixiang Mao
Final approval of manuscript: Hong Xu, Kai Chen, Xiaoyan Jia, Yali Tian, Yun Dai, Dapeng Li, Jing Xie, Min Tao, Yixiang Mao
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin PJ. Insulin in the adjuvant breast cancer setting: A novel therapeutic target for lifestyle and pharmacologic interventions? J Clin Oncol. 2008;26:833–834. doi: 10.1200/JCO.2007.14.7132. [DOI] [PubMed] [Google Scholar]
- 4.Hadad SM, Fleming S, Thompson AM. Targeting AMPK: A new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol. 2008;67:1–7. doi: 10.1016/j.critrevonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Kim TI, Jeon SM, et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131:752–759. doi: 10.1002/ijc.26421. [DOI] [PubMed] [Google Scholar]
- 7.Yin M, Zhou J, Gorak EJ, et al. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: A systematic review and meta-analysis. The Oncologist. 2013;18:1248–1255. doi: 10.1634/theoncologist.2013-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 15.Sun B, Hou G, Zhang X, et al. Clinicopathologic characteristics and prognostic analysis of luminal B breast cancer patients with diabetes. Chinese J Clin Oncol. 2013;40:1042–1046. [Google Scholar]
- 16.Hou G, Zhang S, Zhang X, et al. Clinical pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes. Breast Cancer Res Treat. 2013;137:807–816. doi: 10.1007/s10549-012-2404-y. [DOI] [PubMed] [Google Scholar]
- 17.Aksoy S, Sendur MAN, Altundag K. Demographic and clinico-pathological characteristics in patients with invasive breast cancer receiving metformin. Med Oncol. 2013;30:590. doi: 10.1007/s12032-013-0590-z. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Ahn SH. Impact of metformin in luminal subtype breast cancer. Eur J Cancer. 2013;49:S467. [Google Scholar]
- 19.Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: Impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, Virnig B, Hendryx M, et al. Diabetes, diabetes treatment and breast cancer prognosis. Breast Cancer Res Treat. 2014;148:153–162. doi: 10.1007/s10549-014-3146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayraktar S, Hernadez-Aya LF, Lei X, et al. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer. 2012;118:1202–1211. doi: 10.1002/cncr.26439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X, Esteva FJ, Ensor J, et al. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23:1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lega IC, Austin PC, Gruneir A, et al. Association between metformin therapy and mortality after breast cancer: A population-based study. Diabetes Care. 2013;36:3018–3026. doi: 10.2337/dc12-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeters PJHL, Bazelier MT, Vestergaard P, et al. Use of metformin and survival of diabetic women with breast cancer. Curr Drug Saf. 2013;8:357–363. doi: 10.2174/15680266113136660069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W. Effect of mefformin on prognosis of breast cancer patients complicated with type 2 diabetes mellitus [master’s thesis]. Jinan, China: Shandong University; 2013.
- 26.Zhao J. Effect of metformin on survival outcomes in estrogen receptor positive breast cancer patients with diabetes [master’s thesis]. Hangzhou, China: Zhejiang University; 2012.
- 27.Cleveland RJ, North KE, Stevens J, et al. The association of diabetes with breast cancer incidence and mortality in the Long Island Breast Cancer Study Project. Cancer Causes Control. 2012;23:1193–1203. doi: 10.1007/s10552-012-9989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Benhawy SA, El-Sheredy HG. Metformin and survival in diabetic patients with breast cancer. J Egypt Public Health Assoc. 2014;89:148–153. doi: 10.1097/01.EPX.0000456620.00173.c0. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Aldrich MC, Chen Q, et al. Validating drug repurposing signals using electronic health records: A case study of metformin associated with reduced cancer mortality. J Am Med Inform Assoc. 2015;22:179–191. doi: 10.1136/amiajnl-2014-002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oppong BA, Pharmer LA, Oskar S, et al. The effect of metformin on breast cancer outcomes in patients with type 2 diabetes. Cancer Med. 2014;3:1025–1034. doi: 10.1002/cam4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Y, Zhang S, Hou G, et al. Clinical pathological characteristics and prognostic analysis of diabetic women with luminal subtype breast cancer. Tumour Biol. 2014;35:2035–2045. doi: 10.1007/s13277-013-1270-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:707–710. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 33.Johnson JA, Gale EA. Diabetes, insulin use, and cancer risk: Are observational studies part of the solution-or part of the problem? Diabetes. 2010;59:1129–1131. doi: 10.2337/db10-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowker SL, Yasui Y, Veugelers P, et al. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: Assessing effects of time-varying exposure. Diabetologia. 2010;53:1631–1637. doi: 10.1007/s00125-010-1750-8. [DOI] [PubMed] [Google Scholar]
- 35.Sadeghi N, Abbruzzese JL, Yeung SCJ, et al. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terrin N, Schmid CH, Lau J, et al. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113–2126. doi: 10.1002/sim.1461. [DOI] [PubMed] [Google Scholar]
- 37.Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26:4544–4562. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: A therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16:1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiralerspong S, Kim ES, Dong W, et al. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24:2506–2514. doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson LC, Pollack LA. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.