Abstract
It is long known that a large portion of the lumbosacral plexus is located intra-abdominally, in the retroperitoneal space. However, most of literature descriptions of lesions on this plexus refer to its extra-abdominal parts whereas its intra-abdominal portions are often neglected. The objective of this review article is to describe the laparoscopic anatomy of intrapelvic nerve bundles, as well as the findings and advances already achieved by Neuropelveology practitioners.
INTRODUCTION
It is long known that a large portion of the lumbosacral plexus is located intra-abdominally, in the retroperitoneal space [1]. However, most of literature descriptions of lesions on this plexus refer to its extra-abdominal parts whereas its intra-abdominal portions are often neglected [2].
In 2007, Possover et al. [3] described the Laparoscopic Neuronavigation (LANN) technique, opening the doors to accessing the retroperitoneal portion of the lumbosacral plexus through a safe, minimally invasive and objective way. Since then, multiple causes of intrapelvic nerve entrapments have been described and a new field in Medicine—the Neuropelveology—was created.
The objective of this review article is to describe the laparoscopic anatomy of intrapelvic nerve bundles, as well as the findings and advances already achieved by Neuropelveology practitioners.
LAPAROSCOPIC ANATOMY OF THE INTRAPELVIC NERVES
Ilio-hypogastric, ilio-inguinalis and genito-femoralis nerves
These nerves are sensitive branches of the lumbar plexus and enter the retroperitoneal space emerging on the lateral border of the psoas muscle and follow anteriorly and distally to leave the abdomen through the femoral and inguinal canals. Their fibrotic entrapment is related to post-herniorrhaphy inguinodynia [4] (Fig. 1).
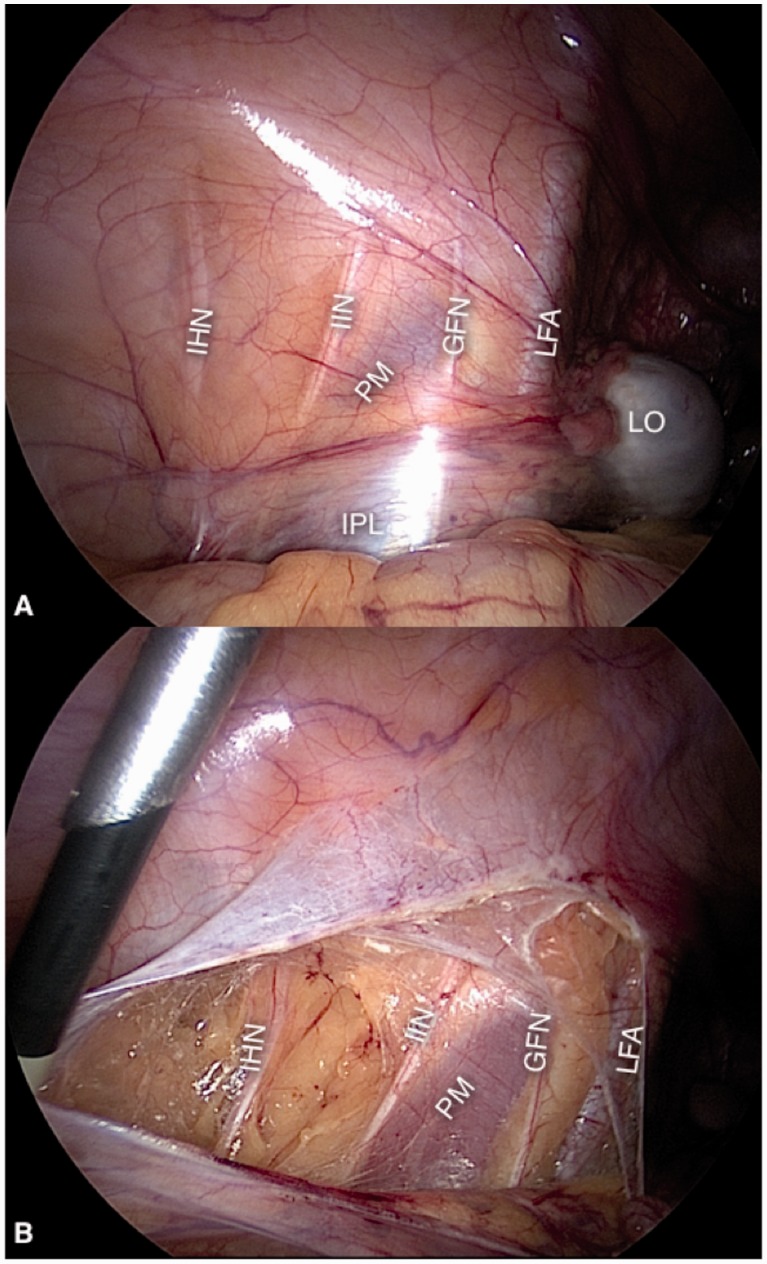
Fig. 1.
Ilio-hypogastric (IHN), ilio-inguinalis (IIN) and genito-femoralis (GFN) nerves, with the overlying peritoneum intact (A) and exposed (B). PM, psoas muscle; LO, left ovary; IPL, infundibulopelvic ligament; LFA, left femoral artery.
Femoral nerve
The femoral nerve is the largest motoric and sensitive nerve of the lumbar plexus. It enters the abdomen by the postero-lateral aspect of the psoas muscle and leaves it through the femoral canal (Fig. 2) to innervate the quadriceps muscle and the skin covering the anterior thigh.
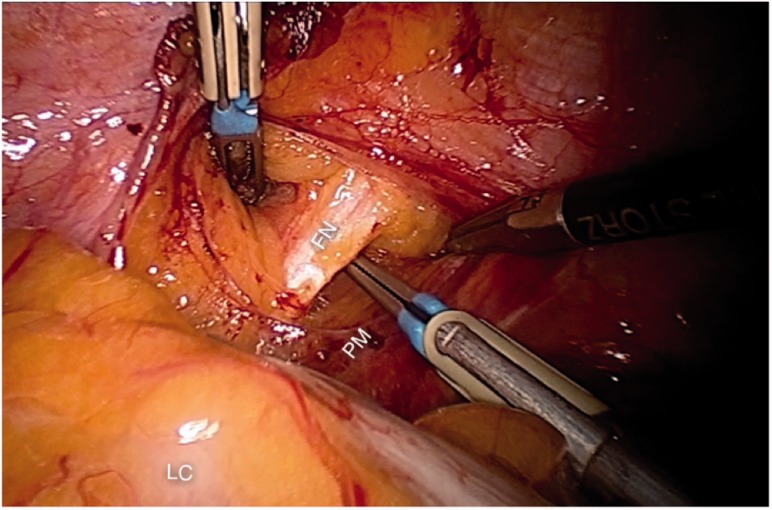
Fig. 2.
The left femoral nerve (FN) entering the retroperitoneal space on the posterolateral aspect of the psoas muscle (PM). LC, left colon.
Nerves of the obturator space
The obturator nerve enters the obturator space at the level of the pelvic rim and leaves it through the obturator canal. It gives sensitive branches to the skin of the medial thigh and motoric branches to the hip adductors (Fig. 3A).
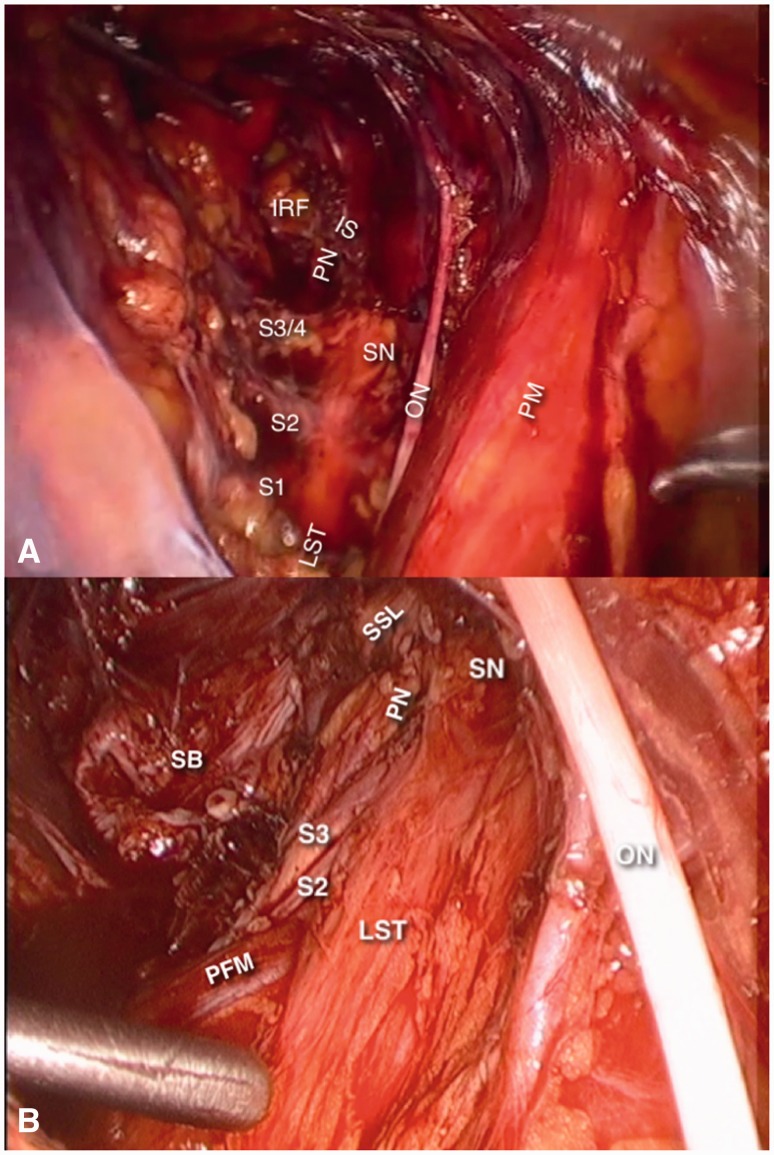
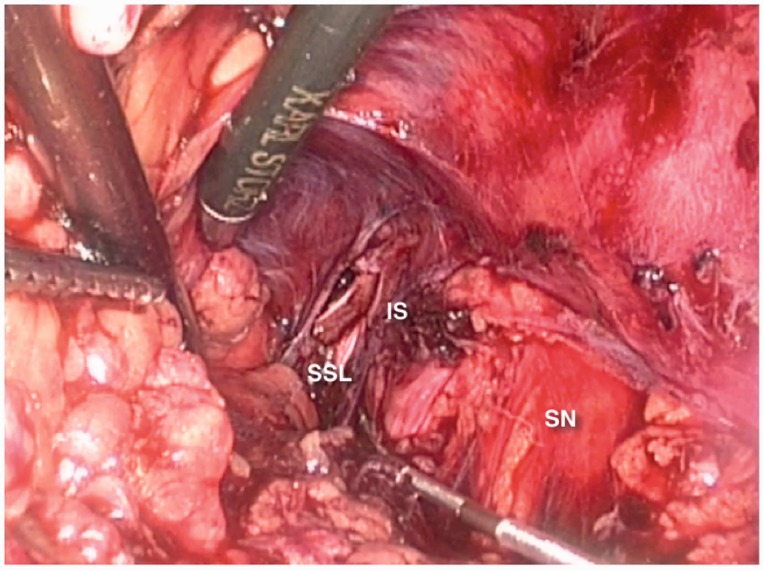
Fig. 3.
Nerves of the obturator space (right side). Picture (A) is the final aspect of a laparoscopic approach to Alcock’s Canal Syndrome, where the sacrospinous ligament has been transected to expose the pudendal nerve (PN). In picture B, the sacrospinous ligament (SSL) is intact. In both pictures, the internal and external iliac vessels are retracted medially. ON, obturator nerve; PM, psoas muscle; SN, sciatic nerve; LST, lumbosacral trunk; PN, pudendal nerve; IRF, ischiorectal fossa; IS, ischial spine; SB, sacral bone; PFM, pyriformis muscle.
The lumbosacral trunk and the distal portions of the S1, S2, S3 and S4 nerve roots merge into the obturator space and form the sciatic and pudendal nerves (Fig. 3B).
The sciatic nerve is formed by the L4 and L5 fibers of the lumbosacral trunk and fibers from the S1, S2 and S3 nerve roots and leaves the pelvis through the sciatic notch. It gives out sensitive branches to the upper gluteal region, postero-lateral thigh, leg ankle and foot. It also controls the hip extensors, abductors and rotators, knee flexors and all the muscles for the ankle and foot.
The pudendal nerve is formed by fibers of the second, third and fourth nerve roots and leaves the pelvis through the pudendal (Alcock’s) canal. It gives out sensitive branches to the lower gluteal region and the perineal skin. It also sends motoric branches to the perineal muscles and the anterior fibers of the levator ani muscles.
Finally, there are direct motoric and sensitive nerves from the S3 and S4 nerve roots to the posterior fibers of the levator ani muscle.
Nerves of the presacral and pararectal spaces
The superior hypogastric plexus, which is formed by fibers from para-aortic sympathetic trunk and gives rise to the hypogastric nerves. The hypogastric nerves run over the hypogastric fascia in an anterior and distal direction. After crossing about two thirds of the distance between the sacrum and the uterine cervix or the prostate, its fibers spread to join the pelvic splanchnic nerves (described later) to form the inferior hypogastric plexus (Fig. 4). The hypogastric nerves carry the sympathetic signals to the internal urethral and anal sphincters, rectum and bladder, which cause detrusor relaxation and bladder contraction, thus promoting continence. They also carry proprioceptive and nociceptive afferent signals from the pelvic viscerae.
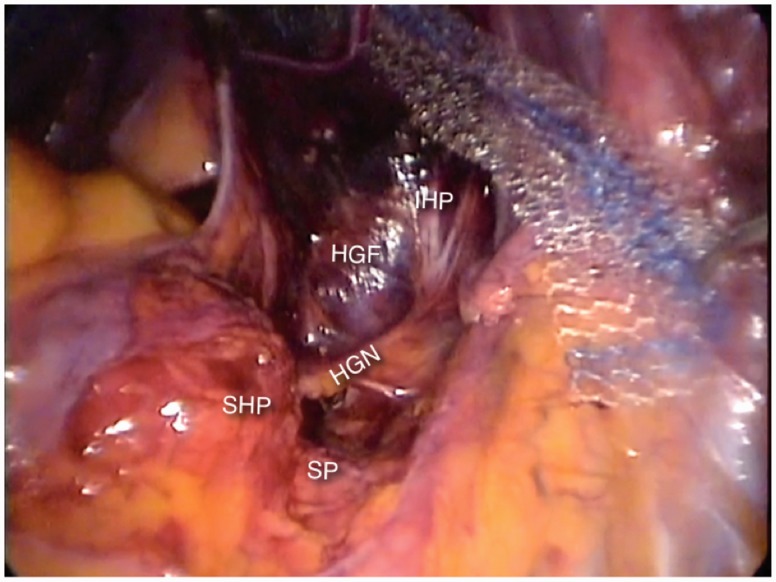
Fig. 4.
The hypogastric nerve (HN) emerges from the superior hypogastric plexus (SHP) at the level of the sacral promontory (SP) and runs anteriorly and distally, juxta-laterally to the hypogastric fascia (HF), to merge with the pelvic splanchnic nerves to form the inferior hypogastric plexus (IHP).
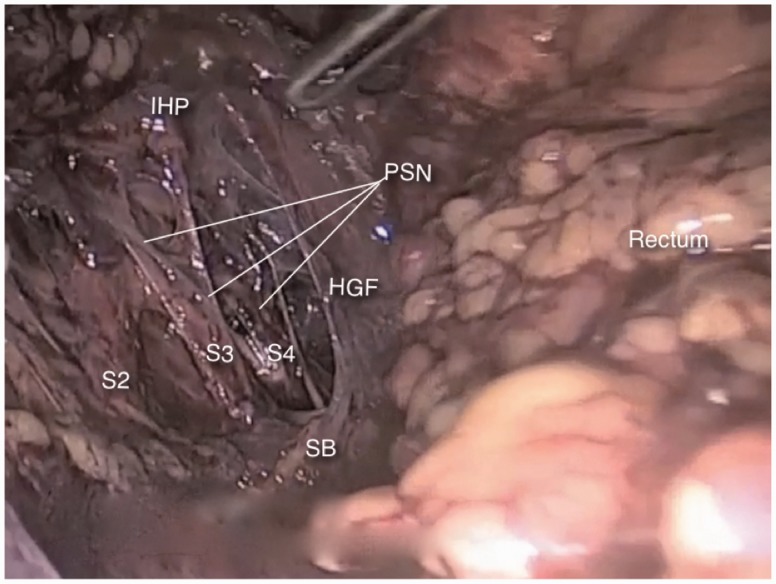
The lateral limit of the presacral space is the hypogastric fascia, which is the formed by the medial most fibers of the endopelvic fascia. The sacral nerve roots can be found juxta-laterally to this fascia (Fig. 5). They leave the sacral foramina and run anteriorly and distally, lying over the pyriformis muscle and crossing the internal iliac vessels laterally to them, to merge and form the nerves of the sacral plexus. Before crossing the internal iliac vessels, they give out the thin parasympathetic branches called pelvic splanchnic nerves, which promote detrusor contraction and provide extrinsic parasympathetic innervation to the colon descendens, sigmoid and rectum. They also carry nociceptive afferent signals from the pelvic viscerae. The pelvic splanchnic nerves join the hypogastric nerves to form the inferior hypogastric plexus in the pararectal fossae.
Fig. 5.
The sacral nerve roots (S2–S4) can be found juxta-laterally to the hypogastric fascia (HGF) and give origin to the pelvic splanchnic nerves (PSN), which run anteriorly and distally to merge the hypogastric nerve and form the inferior hypogastric plexus (IHP).
INTRAPELVIC NERVE ENTRAPMENT SYNDROME
Definition and symptoms
Nerve entrapment syndrome, or compression neuropathy, is a clinical condition caused by compression on a single nerve or nerve root. Its symptoms include pain, tingling, numbness and muscle weakness on the affected nerve’s dermatome [5]. Intrapelvic nerve entrapments are, therefore, entrapments of the intrapelvic portions of the nerves described in the previous sessions and will produce symptoms related to their dermatomes.
The above definition refers to the entrapment of somatic nerves. Autonomic nerve entrapment will produce visceral and vegetative symptoms, such as urinary frequency or urgency, dysuria, rectal pain, suprapubic and/or abdominal cramps and chills.
However, as described earlier, the sacral nerve roots give origin to both somatic and parasympathetic nerves. Therefore, entrapments of these roots will produce pain on their somatic dermatomes, together with urinary and bowel dysfunction.
In a concise manner, the main symptoms of intrapelvic nerve entrapments are:
– Sciatica associated with urinary symptoms (urgency, frequency, dysuria) or without any clear orthopedic cause;
– Gluteal pain associated with perineal, vaginal or penile pain;
– Dysuria and/or painful ejaculation;
– Refractory urinary symptoms;
– Refractory pelvic and perineal pain.
It is important to emphasize that, due to the distance between both plexuses, intrapelvic nerve entrapments will usually cause unilateral symptoms.
ETIOLOGY OF INTRAPELVIC ENTRAPMENTS
Endometriosis
The first report of intrapelvic nerve entrapment was made by Denton and Sherill [6], who described a case of cyclic sciatica due to endometriosis in 1955. After that, some other case reports and small series were published, until 2011, when Possover et al. [2] described the largest series so far, with 175 patients, all treated laparoscopically.
In endometriotic entrapments, the symptoms tend to be cyclic, worsening on the premenstrual and menstrual days and ameliorating or even disappearing on the post-menstrual period [2, 7, 8].
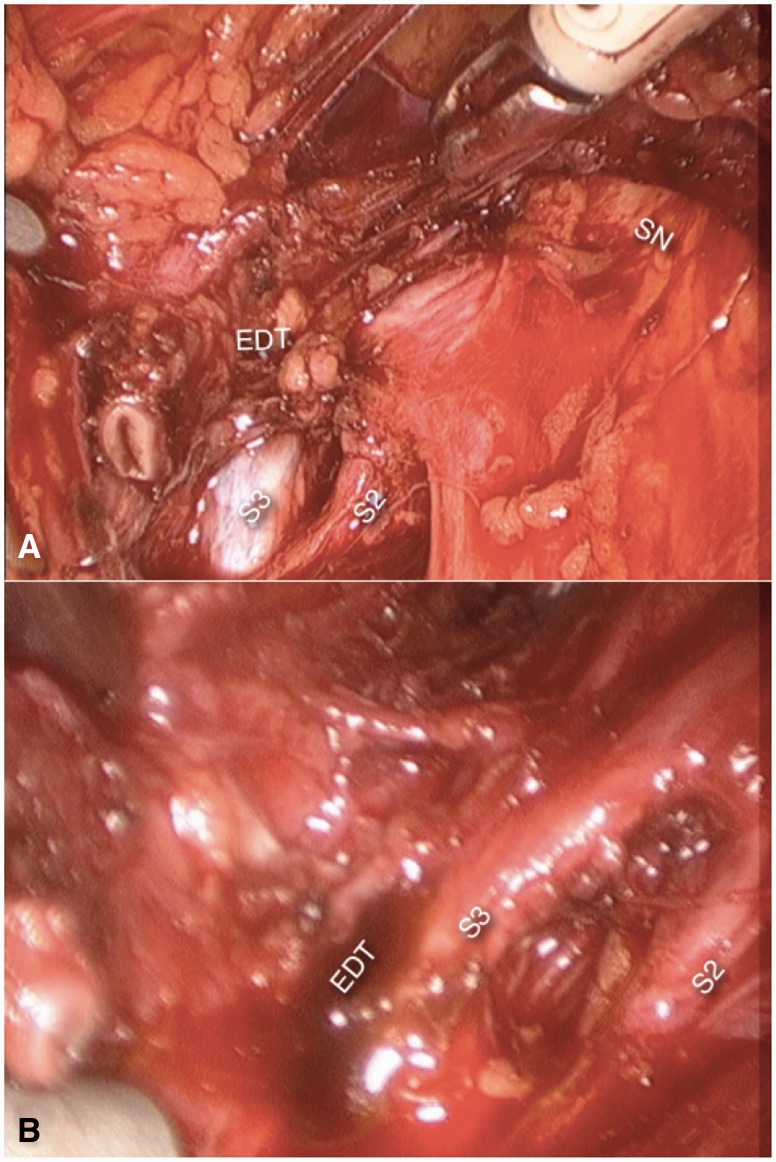
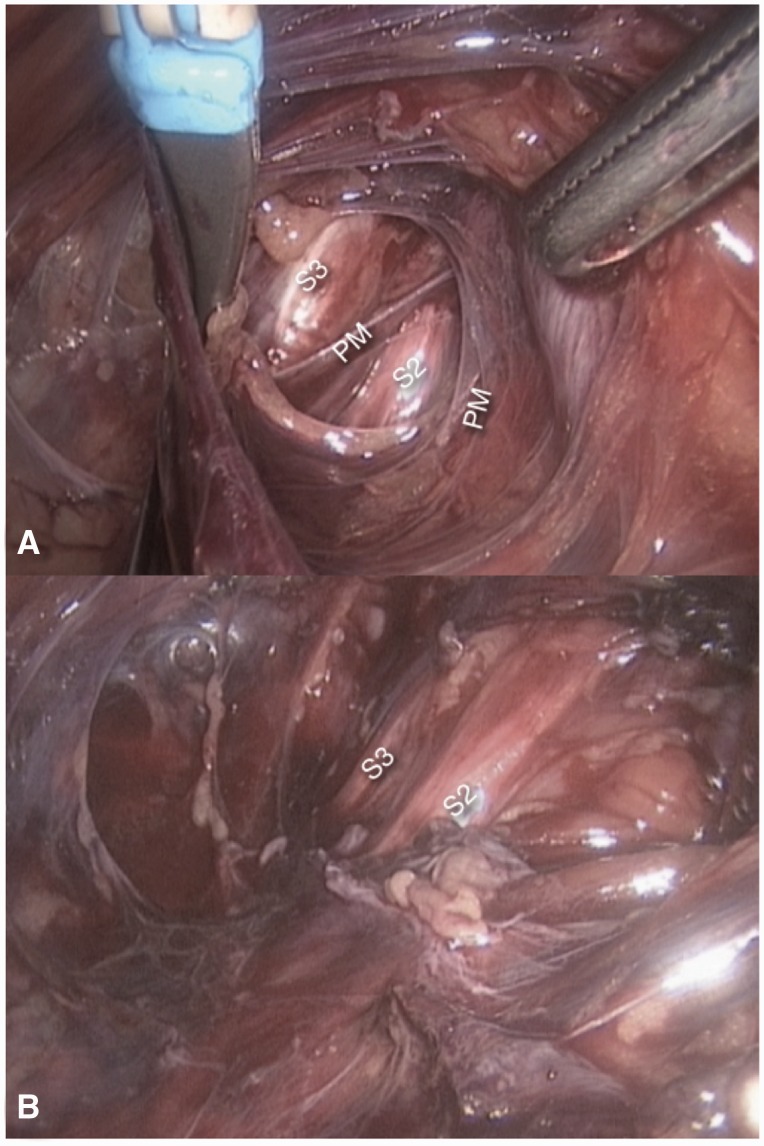
Treatment consists of preoperatively identifying the symptoms and determining the topographical localization of the lesions (by means, mainly, of anamnesis and neurological examination and, sometimes, by MRI) and laparoscopically exploring all suspect segments of the plexus, with radical removal of all endometriotic foci and fibrosis [2, 7, 8] (Fig. 6).
Fig. 6.
Endometriosis (EDT) involving nerve Roots S2 and S3 and the sciatic nerve (SN). Further dissection (B) revealed an endometriotic cyst (EDT) in S3.
The true incidence of endometriosis involving the sacral plexus is unknown, as this presentation of the disease is often neglected. In average, patients undergo four surgical procedures seeking to treat the pain before getting the right diagnosis [2]. Moreover, ∼40% of women with endometriosis refer unilateral pain on the inferior limb [9] and, in 30% of patients with endometriosis, leg pain was demonstrated to be neuropathic [10], which leads to the conclusion that endometriotic involvement of the lumbosacral plexus is probably underdiagnosed and much more frequent than reported.
Fibrosis
This is one of the most frequent causes of intrapelvic nerve entrapments and possibly the most well-known etiology, since Amarenco [11] described the pudendal neuralgia in cyclists, in whom the pain is a consequence of fibrotic entrapment due to continued trauma.
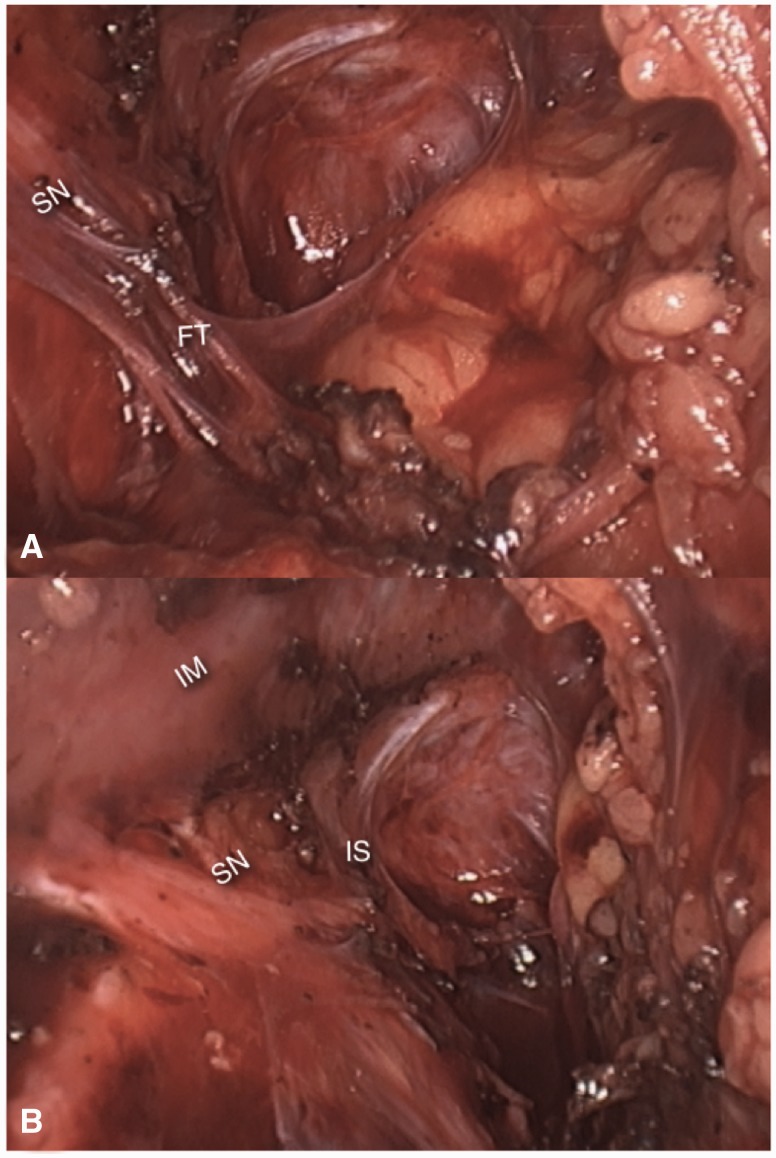
Despite the historical aspect, however, surgical manipulation seems to be the most frequent cause of fibrosis over the sacral plexus (Fig. 7). Among the surgeries with higher risks of inducing such kinds of entrapments are the pelvic reconstructive procedures [12].
Fig. 7.
(A) Dense fibrotic tissue (FT) entrapping the left sciatic nerve (SN) against the pyriformis muscle. (B) final aspect after neurolysis. IM, iliac muscle; IS, ischial spine).
Vascular entrapment
Pelvic congestion syndrome is a well-known cause of cyclic pelvic pain. Patients commonly present with pelvic pain without evidence of inflammatory disease. The pain is worse during the premenstrual period and pregnancy, and is exacerbated by fatigue and standing [13].
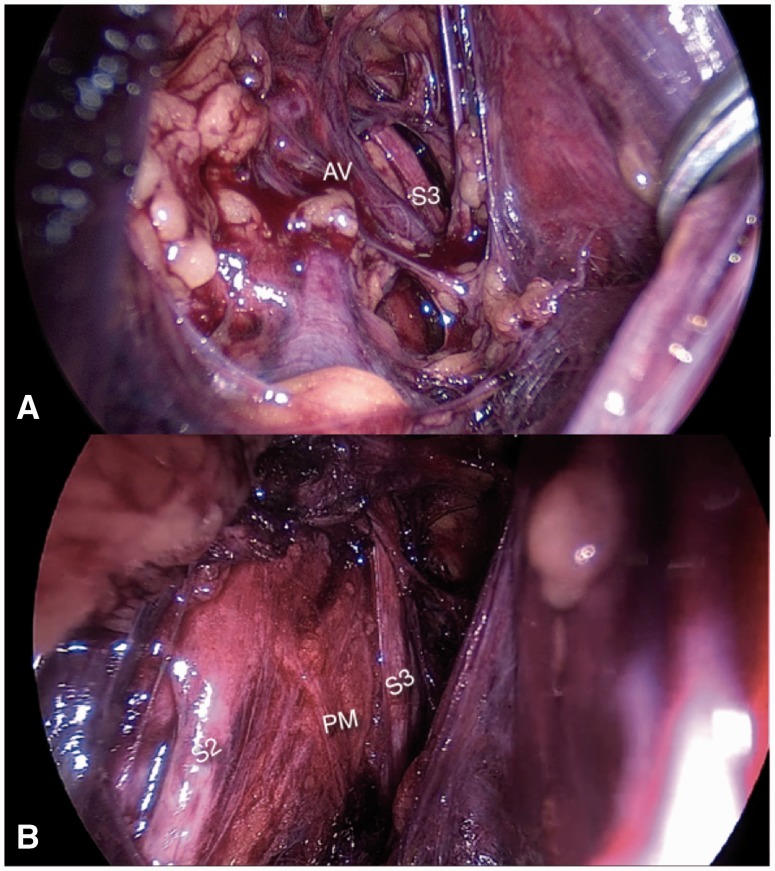
However, what is much less known is the fact that dilated or malformed branches of the internal or external iliac vessels can entrap the nerves of the sacral plexus against the pelvic sidewalls, producing symptoms such as sciatica, or refractory urinary and anorectal dysfunction [2, 14] (Fig. 8).
Fig. 8.
(A) Abnormal varicose vein (AV) entrapping S2 and S3 nerve roots against the pyriformis muscle (PM).
Pyriformis syndrome
Numerous malformations of the pyriformis muscle have been described in the deep gluteal space that can entrap branches of the sciatic nerve. The laparoscopic approach has revealed that the intrapelvic fibers of this muscle can also entrap the sacral nerve roots [15]. Usually, these fibers originate from the sacral bone, laterally to the sacral foramina; some people present with some of the pyriformis fibers originating medially to the sacral foramina and involving the sacral nerve roots (Fig. 9). Differentiating intrapelvic from extrapelvic pyriformis syndrome can be very challenging. Bowel and urinary symptoms are a good indication that the entrapment is intrapelvic, but these are not always present.
Fig. 9.
S2 and S3 nerve roots entrapped in between the pyriformis muscle (PM) fibers.
Primary neuropathic pain, nerve transection and secondary neuropathic pain
All the previously described causes of intrapelvic neuropathies have extrinsic entrapment as the etiology of pain. Intrapelvic radiculopathies can also result from intrinsic dysfunctions of the nerves themselves.
Nerve transections can occur during surgery or trauma and can induce neuroma formation, resulting in phantom pain and anesthesia of the affected nerve dermatome. The pictorial example of this is the phantom pain secondary to amputations, where branches of the sciatic and femoral nerves are transected. In the same fashion, pudendal transection will induce perineal pain and perineal anaesthesia, as well as unilateral atrophy of perineal muscles, usually resulting in urinary and fecal incontinence.
In entrapment syndromes chronic ischemia induces cytoarchitectural changes to the neuron, which do not heal properly after detrapment, resulting in neuropathic pain. The latter the detrapment is performed, the higher the risk of neuropathic pain.
Neuropathic pain can also result from metabolic disturbances of the neuron, infectious agents, chronic exposure to neurotoxic substances or a myriad of other causes.
Finally, in many cases, although the topography of the lesion is determined, its etiology cannot be identified.
In all of these situations, the laparoscopic implantation of neuromodulation can be used to specifically modulate the affected nerve, producing very encouraging results when compared with the more commonly available epidural neuromodulation [4,15].
The laparoscopic implantation of neuroprosthesis—the LION procedure—was first reported by Possover in 2009 as a rescue procedure in patients with local complications of a Brindley procedure [16]. Due to its successful results and lesser invasiveness, it was then used as a primary procedure in spinal cord-injured patients, aiming to improve locomotion and bladder function [17]. Long-term data has shown improvement in voluntary motor function and sensitivity, suggesting positive effects on neuroplasticity [18] (Fig. 10).
Fig. 10.
LION electrode placed on right sciatic and pudendal nerves. PM, psoas muscle; IS, ischial spine; SN, sciatic nerve; SSL, sacrospinous ligament.
CONCLUSION
The laparoscopic approach to the intrapelvic bundles of the lumbosacral nerves opened a myriad of possibilities to assess and treat this neglected portion of this nerve plexus, by means of nerve decompression or selective neuromodulation.
When facing sciatica, gluteal or perineal pain without any obvious spinal or deep gluteal causes, the examiner should always remember that the entrapment could be in the intrapelvic portions, manly when urinary symptoms are present.
CONFLICT OF INTEREST STATEMENT
Nucelio Lemos received research grants from Medtronic Inc. and Laborie Inc. and travel and proctorship grants from Medtronic Inc. None of these grants are, however, directly related to the current publication.
REFERENCES
- 1.Gray H. Anatomy of the Human Body. IX. Neurology. 6d. The Lumbosacral Plexus. Philadelphia: Lea & Febiger, 1918. [Google Scholar]
- 2.Possover M, Schneider T, Henle KP. Laparoscopic therapy for endometriosis and vascular entrapment of sacral plexus. Fertil Steril 2011; 95: 756–8. [DOI] [PubMed] [Google Scholar]
- 3.Possover M, Chiantera V, Baekelandt J. Anatomy of the sacral roots and the pelvic splanchnic nerves in women using the LANN technique. Surg Laparosc Endosc Percutan Tech 2007; 17: 508–10. [DOI] [PubMed] [Google Scholar]
- 4.Possover M. Use of the LION procedure on the sensitive branches of the lumbar plexus for the treatment of intractable postherniorrhaphy neuropathic inguinodynia. Hernia 2013; 17: 333–7. [DOI] [PubMed] [Google Scholar]
- 5.Bouche P. Compression and entrapment neuropathies. Handb Clin Neurol 2013; 115: 311–66. [DOI] [PubMed] [Google Scholar]
- 6.Denton RO, Sherrill JD. Sciatic syndrome due to endometriosis of sciatic nerve. South Med J 1955; 48: 1027–31. [DOI] [PubMed] [Google Scholar]
- 7.Lemos N, Kamergorodsky G, Ploger C, et al. Sacral nerve infiltrative endometriosis presenting as perimenstrual right-sided sciatica and bladder atonia: case report and description of surgical technique. J Minim Invasive Gynecol 2012; 19: 396–400. [DOI] [PubMed] [Google Scholar]
- 8.Lemos N, Damico N, Marques RM, et al. Recognition and treatment of endometriosis involving the sacral nerve roots. In: 44th Annual Meeting of the International Continence Society (ICS), 2014, Rio de Janeiro. Neurourology and Urodynamics. Hoboken, NJ: Willey, 2014, vol. 33 997. [Google Scholar]
- 9.Missmer SA, Bove GM. A pilot study of the prevalence of leg pain among women with endometriosis. J Body Mov Ther 2011; 15: 304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacchiarotti A, Milazzo GN, Biasiotta A, et al. Pain in the upper anterior-lateral part of the thigh in women affected by endometriosis: study of sensitive neuropathy. Fertil Steril 2013; 100: 122–6. [DOI] [PubMed] [Google Scholar]
- 11.Amarenco G, Lanoe Y, Perrigot M, et al. [A new canal syndrome: compression of the pudendal nerve in Alcock's canal or perinal paralysis of cyclists]. Presse Med 1987; 16: 399. [PubMed] [Google Scholar]
- 12.Possover M, Lemos N. Risks, symptoms, and management of pelvic nerve damage secondary to surgery for pelvic organ prolapse: a report of 95 cases. Int Urogynecol J 2011; 22: 1485–90. [DOI] [PubMed] [Google Scholar]
- 13.Ganeshan A, Upponi S, Hon LQ, et al. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol 2007; 30: 1105–11. [DOI] [PubMed] [Google Scholar]
- 14.Lemos N, Marques RM, Kamergorodsky G, et al. Vascular entrapment of the sciatic plexus causing catamenial sciatica and urinary symptoms. In: 44th Annual Meeting of the International Continence Society (ICS), 2014, Rio de Janeiro. Neurourology and Urodynamics. Hoboken, NJ: Willey, 2014, vol. 33 999–1000. [Google Scholar]
- 15.Possover M. Laparoscopic management of endopelvic etiologies of pudendal pain in 134 consecutive patients. J Urol 2009; 181: 1732–6. [DOI] [PubMed] [Google Scholar]
- 16.Possover M. The sacral LION procedure for recovery of bladder/rectum/sexual functions in paraplegic patients after explantation of a previous Finetech-Brindley controller. J Minim Invasive Gynecol 2009; 16: 98–101. [DOI] [PubMed] [Google Scholar]
- 17.Possover M, Schurch B, Henle K. New strategies of pelvic nerves stimulation for recovery of pelvic visceral functions and locomotion in paraplegics. Neurourol Urodyn 2010; 29: 1433–8. [DOI] [PubMed] [Google Scholar]
- 18.Possover M. Recovery of sensory and supraspinal control of leg movement in people with chronic paraplegia: a case series. Arch Phys Med Rehabil 2014; 95: 610–4. [DOI] [PubMed] [Google Scholar]