Abstract
Robotic-assistance has the potential to improve the accuracy of bony resections, when performing femoral osteochondroplasty in the treatment of cam-type femoroacetabular impingement (FAI). The purpose of this study was to determine the accuracy of robotic-assisted femoral osteochondroplasty and compare this to a conventional open, freehand technique. We hypothesized that robotic-assistance would increase the accuracy of femoral head-neck offset correction in cam FAI. Sixteen identical sawbones models with a cam-type impingement deformity were resected by a single surgeon, simulating an open femoral osteochondroplasty. Eight procedures were performed using an open freehand technique and eight were performed using robotic-assistance, through the creation of a three-dimensional haptic volume. A desired arc of resection of 117.7° was determined pre-operatively using an anatomic plan. Post-resection, all 16 sawbones were laser scanned to measure the arc of resection, volume of bone removed and depth of resection. For each sawbone, these measurements were compared with the pre-operatively planned desired resection, to determine the resection error. Freehand resection resulted in a mean arc of resection error of 42.0 ± 8.5° compared with robotic-assisted resection which had a mean arc of resection error of 1.2 ± 0.7° (P < 0.0001). Over-resection occurred with every freehand resection with a mean volume error of 758.3 ± 477.1 mm3 compared with a mean robotic-assisted resection volume error of 31.3 ± 220.7 mm3 (P < 0.01). This study has shown that robotic-assisted femoral osteochondroplasty in the treatment of cam-type FAI is more accurate than a conventional, freehand technique, which are currently in widespread use.
INTRODUCTION
Cam-type femoroacetabular impingement (FAI) refers to bony collision between the proximal femoral neck and acetabular labrum due to an aspherical junction between the femoral head and neck and a resultant decrease in femoral head–neck offset [1]. Current treatment of cam impingement involves proximal femoral osteochondroplasty through either an open or arthroscopic approach with good to excellent clinical results reported in 65–85% patients with open techniques and 67–100% patients with arthroscopic techniques [2].
Irrespective of the approach, improving the clinical outcomes after FAI surgery is dependent upon accurate restoration of the femoral head–neck offset and removal of all sources of focal impingement [3–8]. This approach has been shown to improve the kinematics of the hip joint [9, 10], even in the setting of decreased femoral anteversion [11]. With the increasing popularity of less-invasive arthroscopic and mini-open techniques, the incidence of inadequate reshaping of the femoral head–neck junction has risen [12]. Under-resection has been shown to be a common cause for revision FAI surgery, accounting for 79% [13] to 92% [14] of failed arthroscopic cam FAI corrections. Conversely, over-resection is also a hazard and can lead to femoral neck fractures [1, 15, 16].
Computer-assisted surgery (CAS) has been proposed as a potential solution for the increasing incidence of inaccurate bony resections in cam FAI. CAS can provide an accurate pre-operative assessment of the volume of bone resection needed for FAI correction [17–19] and can also guide the surgeon to reliably reproduce the pre-operative plan [20–22]. Although several prototypes exist that have shown promising results, early indications show that navigation has yet to display superior results [21]. An alternative intra-operative solution to increase accuracy and consequently improve surgical outcomes is robotic-assisted surgery [23, 24]. Haptic robotics provides a tactile system that uses pre-operative computed tomography (CT) scans to generate a three-dimensional (3D) computerized model that allows the surgeon to create a pre-operative plan. Intra-operatively, the surgeon can view the pre-operative template to guide osseous resection, which is further facilitated by a robotic arm that provides visual and haptic feedback, limiting the surgeon to resection that remains within the confines of the pre-determined cutting zone [25]. Tactile robotic-assistance has demonstrated greater accuracy compared with conventional techniques in unicompartmental knee arthroplasty and total hip arthroplasty cup placement [23, 26], but its superiority has not been demonstrated in the treatment of FAI.
The purpose of the present study was to compare the accuracy of a robotic-assisted technique to a conventional open freehand technique in the treatment of cam FAI in a sawbones model. We hypothesized that robotic-assistance would be more accurate in restoring femoral head-neck offset in cam FAI compared with a freehand technique.
MATERIALS AND METHODS
Sixteen identical sawbones models of the proximal femur, with a cam-type impingement deformity were created for resection by a single surgeon. Eight femurs underwent manual resection of the cam deformity with an open freehand technique. The other eight femurs underwent cam decompression with a robotic-assisted technique.
Pre-operative planning
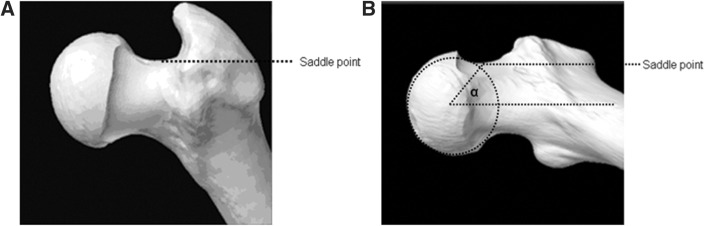
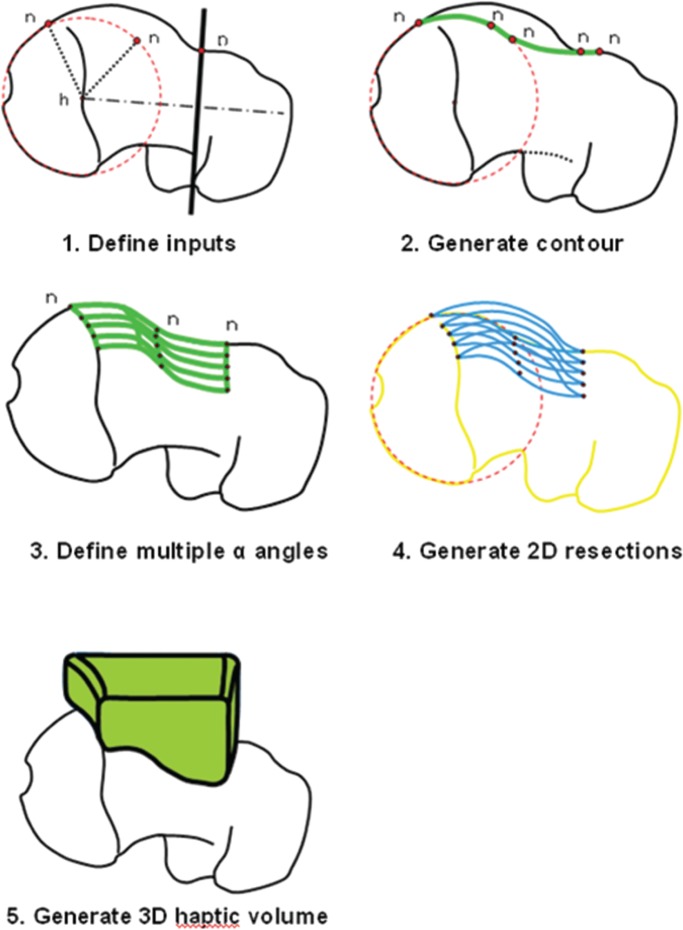
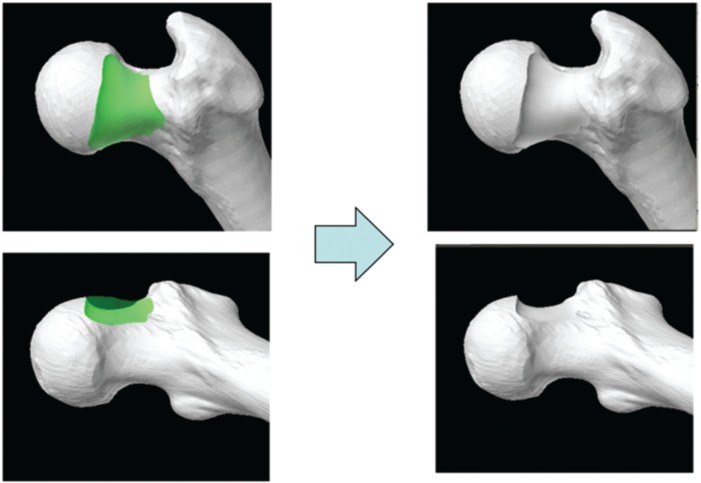
A desired arc of resection was determined pre-operatively using an anatomic plan generated from a CT scan of an uncut sawbone. By defining the ‘12 o’clock position’ on the femoral head as 0°, a map of pathologic alpha angles was generated in 0.9° increments from the 0° to the 200° position (Fig. 1). An anatomic correction was planned based on an absolute correction of the alpha angle to <50° or a maximum reduction of the alpha angle to the base of the femoral neck or saddle point (Fig. 2) [27]. The desired alpha angles were then used to generate a contour of resection in 2D and by extending this contour along the anterior circumference of the femoral head and distally along the femoral neck, a 3D resection volume was generated (Fig. 3). The proximal boundary was the physeal scar of the femoral head and the distally the resection was tapered to the femoral neck, ensuring not to violate the saddle point. The desired arc of resection created by this anatomic plan was 117.7° starting at −1.8° and ending at 115.9° and the desired volume of resection was 3373.0 mm3 (Fig. 4). The ideal final resected shape generated by this anatomic plan was the surgical goal in all 16 cases in this study (Fig. 5). A laser scan was obtained pre-operatively to compare to post-operative laser scans using a Roland LPX-600 Laser scanner (Roland DG, Japan). This scanner has a 1 mm plane scanning pitch and rotates in 0.9° increments.
Fig. 1.
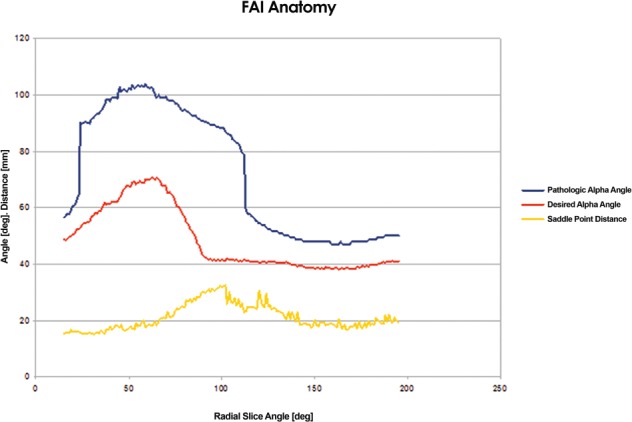
Pre-operative anatomic plan mapping the pathologic and desired alpha angles and saddle point distance from the 0° to 200° position.
Fig. 2.
(A) 3D CT image of the femoral neck indicating the saddle point and (B) post-operative alpha angle as per preoperative anatomic plan.
Fig. 3.
Steps followed in the generation of a 3D haptic resection volume.
Fig. 4.
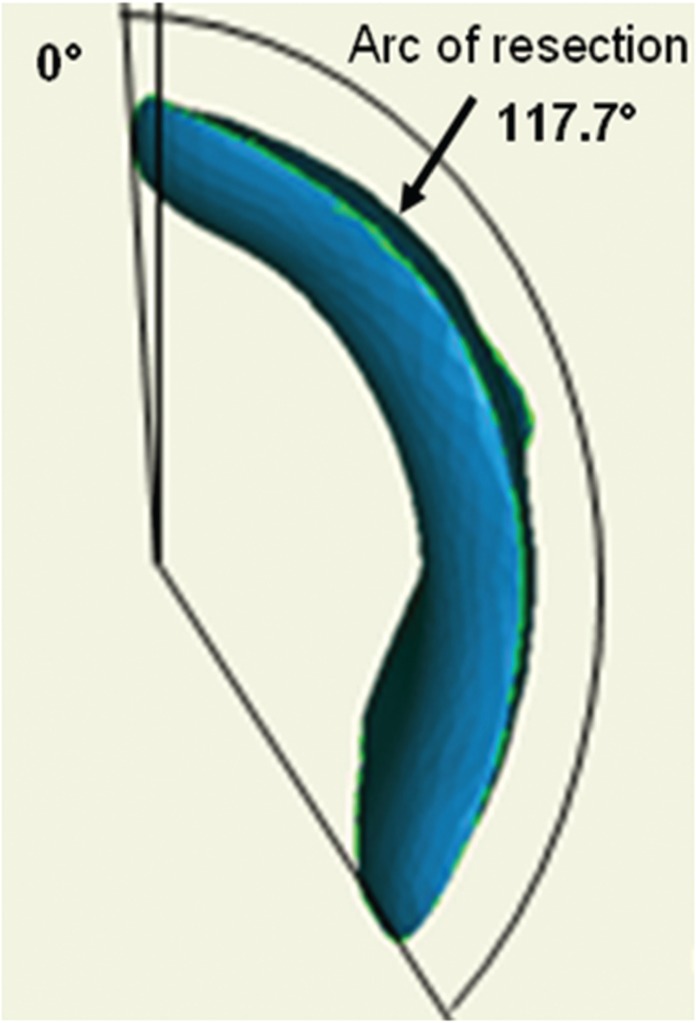
The desired arc of resection created by the preoperative anatomic plan.
Fig. 5.
CT-generated models of the ‘Ideal Final Resected Shape’. The green area represents the preoperatively planned desired resection volume.
Manual freehand procedure
Eight femurs were resected with an open, freehand technique. This procedure was optimized to represent the best-case scenario for open surgery, with the surgeon afforded maximal visualization, while performing the osseous resection with a high-speed burr or osteotomes.
Robotic-assisted procedure
Eight femurs were resected with robotic-assistance. The MAKO Robotic-arm Interactive Orthopedic System (RIO) (MAKO Surgical Corp, Fort Lauderdale, FL) was used in each case [28]. The system consists of three components: robotic arm, optical infra-red camera and user interface module [29]. The robotic arm is connected to a high-speed burr which is controlled by the surgeon. The desired volume of resection is pre-operatively saved on the system and this is used to define the boundaries of resection which are displayed during the case. The robot gives the surgeon visual and tactile feedback of bone being resected and haptic feedback (stiffness of the robotic arm), if rapid movement or excessive pressure is applied. The robot also immediately stops the cutting instrument if the surgeon unintentionally attempts to resect bone beyond the pre-operatively derived anatomic plan. In this fashion, a 3D haptic volume defined by the desired post-operative morphology is created which theoretically prevents inaccurate resection.
Post-resection analysis
Post-resection, all of the 16 sawbones were scanned using a Roland LPX-600 Laser scanner (Roland DG). Measurements included arc of resection, volume of bone removed and resection depth. These results were compared with the pre-operatively planned desired resection for each case, to determine the resection error.
Statistical analysis
All continuous variables were recorded as mean ± standard deviation (SD). Statistical comparisons between groups were performed with a 2-tailed unpaired Student’s t-test. P values of < 0.05 were considered significant. All data were collected and analysed using Microsoft Excel software (Microsoft Corporation, Redmond, WA).
RESULTS
Manual freehand technique
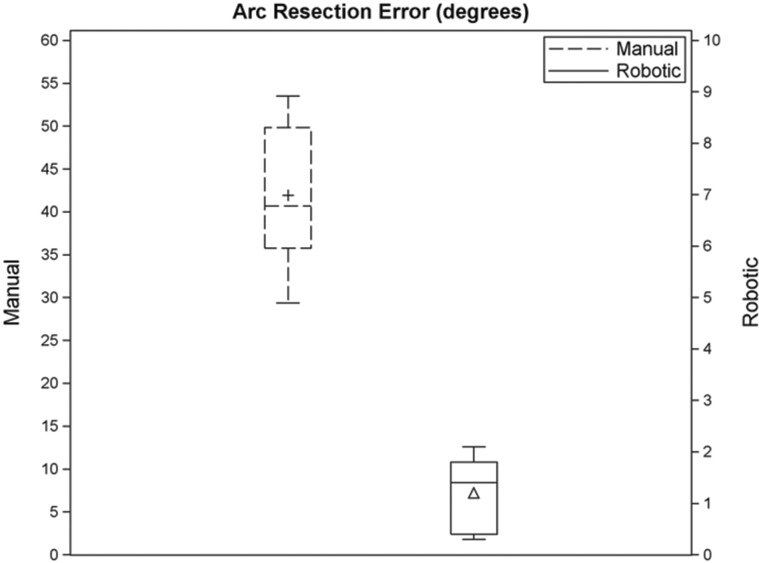
Manual freehand resection resulted in a mean (±SD) arc of resection error of 42.0 ± 8.5° (Fig. 5). The mean (±SD) start error was −18.1 ± 5.6° and the mean (±SD) end error was 23.9 ± 9.9°. Over-resection occurred with every manual resection with a mean (±SD) volume error of 758.3 ± 477.1 mm3. The mean (±SD) maximum resection depth was 6.5 ± 0.4 mm.
Robotic-assisted technique
Robotic-assisted resection resulted in a mean arc of resection error of 1.2 ± 0.7° (Fig. 6). The mean (±SD) start error was −1.1 ± 0.9° and the mean (±SD) end error was −0.1 ± 1.0°. Over-resection occurred in four cases and under-resection in four cases. The mean (±SD) robotic resection volume error was 31.3 ± 220.7 mm3. The mean (±SD) maximum resection depth was 6.2 ± 0.5 mm.
Fig. 6.
Box plot showing the spread of resection arc errors (degrees) for manual and robotic resection. Note that the scales for manual and robotic resection errors are different in order to clearly display the difference in distribution for manual and robotic resection errors.
Arc resection and volume errors for each freehand and robotic-assisted case are displayed in Tables I and II, respectively. A summary of these results with statistical comparisons between groups is outlined in Table III.
Table I.
Errors for each manual case
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| Arc resect error (degrees) | 51.1 | 48.6 | 29.4 | 40.3 | 37.9 | 53.5 | 33.6 | 41.06 |
| Start error (degrees) | 13.3 | 27.9 | 19.2 | 18.6 | 19.7 | 14.0 | 10.0 | 21.9 |
| End error (degrees) | 37.7 | 20.7 | 10.2 | 21.6 | 18.2 | 39.5 | 23.6 | 19.7 |
| Volume error (mm3) | 827.0 | 1139.0 | 1333.0 | 362.0 | 143.0 | 1195.0 | 865.0 | 153.0 |
| Cutting time (s) | 317.0 | 295.5 | 223.0 | 257.4 | 293.2 | 323.4 | 364.9 | 348.5 |
Table II.
Errors for each robotic case
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| Arc resect error (degrees) | 1.9 | 2.1 | 1.7 | 0.3 | 1.7 | 0.5 | 0.3 | 1.1 |
| Start error (degrees) | 1.0 | 2.2 | 0.2 | 1.9 | 1.7 | 1.0 | 1.1 | 0.6 |
| End error (degrees) | 0.9 | 0 | 1.5 | 1.5 | 0 | 0.5 | 0.8 | 0.5 |
| Volume error (mm3) | −168.0 | −188.0 | 134.0 | −132.0 | 260.0 | 91.0 | 393.0 | −140.0 |
| Cutting time (s) | 211.7 | 203.5 | 225 | 223.6 | 196.6 | 177.2 | 233.8 | 204.9 |
Table III.
Summary of study results
| Manual | Robotic | Factor | P-Value | |
|---|---|---|---|---|
| Arc resect error (degrees) | 42.0 ± 8.5° | 1.2 ± 0.7° | 35 | P < 0.0001 |
| Start error (degrees) | −18.1 ± 5.6° | −1.1 ± 0.9° | 16.5 | P < 0.0001 |
| End error (degrees) | 23.9 ± 9.9° | −0.1 ± 1.0° | 239 | P < 0.0001 |
| Average volume error (mm3) | 758.3 ± 477 | 31.3 ± 221 | 24.2 | P < 0.01 |
| Average cutting time (s) | 303 | 210 | 1.44 | P < 0.001 |
Cutting time
The mean cutting time for freehand and robotic-assisted resection was 303 and 210 s, respectively (P < 0.001).
DISCUSSION AND CONCLUSION
This study examined the potential for robotic-assisted surgery to enhance the accuracy of correcting cam-type FAI, in comparison to a conventional freehand technique. We found that robotic-assistance significantly improved the accuracy of bony resection of a cam lesion compared with a freehand technique. These results suggest that robotic-assistance may have an important role in reducing the number of revision hip arthroscopies that are currently being performed for suboptimal reshaping of the femoral head–neck junction in cam FAI [13, 14].
The clinical outcome of FAI surgery is largely dependent upon accurately identifying all pathologic sources of impingement and then meticulously removing them, thus improving femoral head–neck offset [5, 30]. With improvements in instrumentation and demonstration of satisfactory results with less invasive techniques, arthroscopic FAI surgery has dramatically increased in popularity, with surgeons performing femoral osteochondroplasties, acetabular rim resections and labral repairs routinely [2, 3, 6, 31]. Hip arthroscopy, however, remains a technically challenging procedure with a well-documented steep learning curve [32, 33]. Visualization and accessibility to some regions of the hip joint can be challenging arthroscopically and this difficulty, combined with the increasing popularity of arthroscopic FAI surgery, has unfortunately increased the burden of revision hip arthroscopy procedures secondary to inadequate removal of the offending lesions.
Philippon et al. [14] reported that of the 37 revision hip arthroscopies performed over a 1-year period, 22 cases were performed for previously unaddressed FAI and 12 for repeat treatment of FAI. Femoral osteochondroplasties were required in 28/37 (76%) cases. Further, Heyworth et al. [13] reviewed 24 revision hip arthroscopies and found unaddressed or undertreated impingement lesions in 19 cases (79%) and failed labral repair in 8 cases. Over-resection of cam lesions is also problematic and can lead to fractures. Finite-element models [15] and cadaveric studies [16] have suggested that neck resections >10 mm or up to 30% of the anterolateral quadrant of the head–neck junction can produce a fracture. Under and over-resection of pincer lesions on the acetabular side are also being reported. In a cadaveric study, Zumstein et al. [34] found that there was a significant risk of underestimating the arc of rim resection with focal pincer lesions, particularly when they are located posterosuperiorly. Over-resection of the rim, on the other hand, can cause iatrogenic acetabular dysplasia, hip instability and dislocation [35, 36].
With the increasing rate of complications, it is becoming clear that intra-operative surgical judgement with uniplanar fluoroscopy, particularly in the case of the inexperienced surgeon, is not acceptable when treating young patients with complex hip problems. To address this issue, we investigated the use of robotic-assistance in improving the accuracy of FAI surgery. The key principle behind robotic-assistance is the translation of quantitative pre-operative assessments produced by navigation software into an automated, precise mechanical action which remains under the control of the surgeon. Robotic systems have been used in orthopaedic surgery since 1992 [25], but their utility in FAI surgery has not been investigated. ‘Haptic’ or tactile systems have recently become very popular in orthopaedic surgery and have dramatically improved the accuracy of component alignment in unicompartmental knee replacement [23, 26, 28, 29]. Haptic technology is referred to as semi-active because it affords the surgeon active control over the robot but prevents bony resection outside the boundaries of a pre-operatively defined resection volume. Kather et al. [37] were one of the first groups to investigate the applicability of robotic-assistance in hip arthroscopy. They used the remotely controlled ‘da Vinci’ tele-robotic platform [38] and found that they were successfully able to introduce and manipulate instruments in cadaveric hip joints. They did not, however, perform any robotic-assisted FAI surgery.
Brunner et al. [21] performed a prospective study looking at the clinical outcomes and head–neck offset correction in patients with cam impingement using a 3D-CT-based navigation system which uploads a preoperative CT scan of the pelvis and cross-matches this with intra-operative fluoroscopy. The software (BrainLAB AG, Feldkirchen, Germany) was initially designed for total hip arthroplasty, but later modified for hip arthroscopy. This system gives the surgeon real time information about the position of surgical instruments in relation to the femoral neck but does not allow for preoperative planning, nor highlights the zone of impingement, nor displays the amount of bone resected as the surgery progresses. The study found that their navigation system did not improve the accuracy of femoral offset restoration with 24% of subjects in both navigated and non-navigated groups having an inadequate correction of the alpha angle.
Ecker et al. [39] attempted to address the problem of inaccurate FAI surgery through the use of a system which has a pre-operative planning module and a computer-assisted surgical milling device which provides visual feedback to the surgeon. In their study, two surgeons performed femoral osteochondroplasties on nine identical sawbones each, using a color-coded distance map which guided the surgeon towards a pre-operatively determined resection goal. All femurs were post-operatively laser scanned to determine resection accuracy. They found that the mean difference between planned and actual reaming was 0.41 mm at the femoral neck with all 18 sawbone operations consistently showing a discrepancy of <1 mm between actual and planned reaming. This narrow range of discrepancy is in concordance with our data. We found that in each of our eight robotic-assisted cases, the arc of resection error was not >2°. Ecker et al. concluded that navigated osteochondroplasty is highly accurate and reproducible. A notable limitation of their study was that it was conducted in a non-arthroscopic environment, much like our study. The significance of performing our manual resections in an open environment was to create a best-case scenario for the freehand arm of the study, with maximal visualization, thereby giving the surgeon the highest likelihood of performing the best possible resection, and therefore comparing the robotic-assisted resections to an accepted gold-standard. Further limitations of their study were that it was not controlled, did not give any information on surgical time, and was not performed with aid of haptic barriers, which may prove invaluable in a tight space like the hip joint. We found that robotic-assistance resulted in significantly faster bony resection compared with a freehand technique (210 versus 303 s). This is likely due to that fact that less time is spent carefully contouring the femoral head-neck junction at the boundaries of the deformity, as the haptic barrier compensates for the need to be extra-vigilant as normal bony anatomy is reached.
Our study is not without limitations. First, our experiments simulated an open femoral osteochondroplasty. Our aim is to eventually test this technology in the arthroscopic setting. Our results have, however, allowed us to verify the ability of our system to ream a complex spherical structure, without causing implant failure or damage to surrounding bone. Additionally, since the femur was fully exposed in both cohorts, registration quality was optimized. Although registration was not in the scope of this study, the goal is to eventually test the registration capability of the robotic system in an arthroscopic setting. Second, with only one surgeon performing all procedures, the reproducibility of our results cannot be validated. Considering this is novel technology that has not been used for FAI surgery before, we feel the results of our study can be applied to other users, new to this system. Third, the pre-operative plans used in this study were anatomic. The benefits of formulating a kinematically derived resection volume are clear but we did not feel this was necessary in this study, for the purposes of addressing our hypothesis. Finally, our study showed that robotic-assisted surgery was more precise than the conventional freehand technique in reproducing the preoperative plan. The ideal preoperative plan is still highly debatable and comparing pre-operative plans was not the goal of this study.
There remain numerous areas of FAI surgery that could be addressed by haptic-guided robotic FAI surgery. We have studied only one facet of FAI, namely cam deformity. Pincer and mixed deformities form a large proportion of the pathology in FAI and robotic-guidance for bony resection of these lesions will undoubtedly be helpful. Aside from bony resection, portal and anchor placement is paramount in arthroscopic hip surgery, and there is a potential role for haptic guidance in this area to prevent iatrogenic complications including neurovascular and chondral damage. Finally, computer guidance may also be indicated to quantify hip stability and thereby determine the extent of capsular release that can be safely performed in FAI surgery.
To our knowledge, we are the first group to investigate the use of robotic haptic technology in improving the accuracy of FAI surgery. The complexity of FAI necessitates both an accurate pre-operative assessment and intra-operative execution. Computer-assisted solutions can potentially overcome the limitations of open and arthroscopic approaches to FAI correction. The robotic-assisted haptic system used in this study is significantly more accurate than an optimized, conventional freehand technique at performing a femoral osteochondroplasty according to a pre-operative plan. The next step is to develop this technology further for use in a cadaveric hip arthroscopy model followed by use in vivo. Ultimately, the aim is to achieve highly accurate arthroscopic FAI correction with a view to reducing the burden of revision hip arthroscopy secondary to technical error.
FUNDING
Funding was received from MAKO Surgical Corp., Fort Lauderdale, FL.
CONFLICT OF INTEREST STATEMENT
One of the authors (A.S.R) is a consultant for Stryker/MAKO Surgical Corp., Fort Lauderdale, FL and received funding for this study from MAKO Surgical Corp., Fort Lauderdale, FL.
REFERENCES
- 1.Ayeni OR, Bedi A, Lorich DG, Kelly BT. Femoral neck fracture after arthroscopic management of femoroacetabular impingement: a case report. J Bone Joint Surg Am 2011; 93: e47. [DOI] [PubMed] [Google Scholar]
- 2.Bedi A, Chen N, Robertson W, Kelly BT. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy 2008; 24: 1135–45. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JW, Jones KS. Arthroscopic femoroplasty in the management of cam-type femoroacetabular impingement. Clin Orthop Relat Res 2009; 467: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford JR, Villar RN. Current concepts in the management of femoroacetabular impingement. J Bone Joint Surg Br 2005; 87: 1459–62. [DOI] [PubMed] [Google Scholar]
- 5.Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res 2008; 466: 264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy 2008; 24: 540–46. [DOI] [PubMed] [Google Scholar]
- 7.Lincoln M, Johnston K, Muldoon M, Santore R. Combined arthroscopic and modified open approach for cam femoroacetabular impingement: a preliminary experience. Arthroscopy 2009; 25: 392–99. [DOI] [PubMed] [Google Scholar]
- 8.Peters CL, Erickson JA. Treatment of femoro-acetabular impingement with surgical dislocation and debridgement in young adults. J Bone Joint Surg Am 2006; 88: 1735–41. [DOI] [PubMed] [Google Scholar]
- 9.Bedi A, Dolan M, Hetsroni I, et al. Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med 2011; 39: 43–49. [DOI] [PubMed] [Google Scholar]
- 10.Bedi A, Dolan M, Magennis E, et al. Computer-assisted modeling of osseous impingement and resection in femoroacetabular impingement. Arthroscopy 2012; 28: 204–10. [DOI] [PubMed] [Google Scholar]
- 11.Kelly BT, Bedi A, Robertson CM, et al. Alterations in internal rotation and alpha angles are associated with arthroscopic cam decompression in the hip. Am J Sports Med 2012; 40: 1107–12. [DOI] [PubMed] [Google Scholar]
- 12.Ilizaliturri VM., Jr Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res 2009; 467: 760–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyworth BE, Shindle MK, Voos JE, et al. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy 2007; 12: 1295–302. [DOI] [PubMed] [Google Scholar]
- 14.Philippon MJ, Schenker ML, Briggs KK, et al. Revision hip arthroscopy. Am J Sports Med 2007; 35: 1918–21. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Rasgado T, Jimenez-Cruz D, Bailey CG, et al. Changes in the stress in the femoral head neck junction after osteochondroplasty for hip impingement: a finite element study. J Orthop Res 2012; 30: 1999–2006. [DOI] [PubMed] [Google Scholar]
- 16.Mardones RM, Gonzalez C, Chen Q, et al. Surgical treatment of femoroacetabular impingement: evaluation of the effect of the size of the resection. J Bone Joint Surg Am 2005; 87: 273–9. [DOI] [PubMed] [Google Scholar]
- 17.Kubiak-Langer M, Tannast M, Murphy SB, et al. Range of motion in anterior femoroacetabular impingement. Clin Orthop Relat Res 2007; 458: 117–24. [DOI] [PubMed] [Google Scholar]
- 18.Puls M, Ecker TM, Tannast M, et al. The equidistant method—a novel hip joint simulation algorithm for detection of femoroacetabular impingement. Comput Aided Surg 2010; 15: 75–82. [DOI] [PubMed] [Google Scholar]
- 19.Tannast M, Kubiak-Langer M, Langlotz F, et al. Noninvasive three-dimensional assessment of femoroacetabular impingement. J Orthop Res 2007; 25: 122–31. [DOI] [PubMed] [Google Scholar]
- 20.Almoussa S, Barton C, Speris AD, et al. Computer-assisted correction of cam-type femoroacetabular impingement: a sawbones study. J Bone Joint Surg Am 2011; 93: 70–5. [DOI] [PubMed] [Google Scholar]
- 21.Brunner A, Horisberger M, Herzog RF. Evaluation of a computed tomography-based navigation system prototype for hip arthroscopy in the treatment of femoroacetabular cam impingement. Arthroscopy 2009; 25: 382–91. [DOI] [PubMed] [Google Scholar]
- 22.Monahan E, Shimada K. Computer-aided navigation for arthroscopic hip surgery using encoder linkages for position tracking. Int J Med Robot 2006; 2: 271–78. [DOI] [PubMed] [Google Scholar]
- 23.Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomized controlled study of the Acrobot system. J Bone Joint Surg Br 2006; 88: 188–97. [DOI] [PubMed] [Google Scholar]
- 24.Pearle AD, Kendoff D, Musahl V. Perspective on computer-assisted orthopaedic surgery: movement toward quantitative orthopaedic surgery. J Bone Joint Surg Am 2009; 91: 7–12. [DOI] [PubMed] [Google Scholar]
- 25.Lang JE, Mannava S, Floyd AJ, et al. Robotic system in orthopaedic surgery. J Bone Joint Surg Br 2011; 93: 1296–9. [DOI] [PubMed] [Google Scholar]
- 26.Dunbar NJ, Roche MW, Park BH, et al. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty 2012; 27: 803–8. [DOI] [PubMed] [Google Scholar]
- 27.Stahelin L, Stahelin T, Jolles BM, Herzog RF. Arthroscopic offset restoration in femoroacetabular cam impingement: accuracy and early clinical outcome. Arthroscopy 2008; 24: 51–7. [DOI] [PubMed] [Google Scholar]
- 28.Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am 2009; 91: 63–8. [DOI] [PubMed] [Google Scholar]
- 29.Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty 2010; 25: 230–7. [DOI] [PubMed] [Google Scholar]
- 30.Ganz R, Parvizi J, Beck M, et al. Femoroacetabular impingement: a cause of osteoarthritis of the hip. Clin Orthop Relat Res 2003; 417: 112–20. [DOI] [PubMed] [Google Scholar]
- 31.Ilizaliturri VM, Jr, Orozco-Rodriguez L, Acosta-Rodriguez E, Camacho-Galindo J. Arthroscopic treatment of cam-type femoroacetabular impingement: preliminary report at 2 years minimum follow-up. J Arthroplasty 2008; 23: 226–34. [DOI] [PubMed] [Google Scholar]
- 32.Botser IB, Smith TW, Jr, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy 2011; 27: 270–78. [DOI] [PubMed] [Google Scholar]
- 33.Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am 2011; 93: 52–6. [DOI] [PubMed] [Google Scholar]
- 34.Zumstein M, Hahn F, Sukthankar A, et al. How accurately can the acetabular rim be trimmed in hip arthroscopy for pincer-type femoral acetabular impingement: a cadaveric investigation. Arthroscopy 2009; 25: 164–68. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy 2009; 25: 400–4. [DOI] [PubMed] [Google Scholar]
- 36.Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip: A case report. J Bone Joint Surg Am 2009; 91: 192–7. [DOI] [PubMed] [Google Scholar]
- 37.Kather J, Hagen ME, Morel P, et al. Robotic hip arthroscopy in human anatomy. Int J Med Robot 2010; 6: 301–5. [DOI] [PubMed] [Google Scholar]
- 38.Advincula AP. Surgical techniques: robot-assisted laparoscopic hysterectomy with the da Vinci surgical system. Int J Med Robot 2006; 2: 305–11. [DOI] [PubMed] [Google Scholar]
- 39.Ecker TM, Puls M, Steppacher SD, et al. Computer-assisted femoral head-neck osteochondroplasty using a surgical milling device—An in vitro accuracy study. J Arthroplasty 2012; 27: 310–16. [DOI] [PubMed] [Google Scholar]