Abstract
In the current study of Mycobacterium tuberculosis (MTB)-specific T and B cells, we found that MTB-specific peptides from early secreted antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10) induced the expression of IL-21 predominantly in CD4+ T cells. A fraction of IL-21-expressing CD4+ T cells simultaneously expressed Th1 cytokines but did not secrete Th2 or Th17 cytokines, suggesting that MTB-specific IL-21-expressing CD4+ T cells were different from Th1, Th2 and Th17 subpopulations. The majority of MTB-specific IL-21-expressing CD4+ T cells co-expressed IFN-γ and IL-21+IFN-γ+CD4+ T cells exhibited obviously polyfunctionality. In addition, MTB-specific IL-21-expressing CD4+ T cells displayed a CD45RO+CD62LlowCCR7lowCD40LhighICOShigh phenotype. Bcl-6-expression was significantly higher in IL-21-expressing CD4+ T cells than IL-21-CD4+ T cells. Moreover, IL-12 could up-regulate MTB-specific IL-21 expression, especially the frequency of IL-21+IFN-γ+CD4+ T cells. Taken together, our results demonstrated that MTB-specific IL-21+IFN-γ+CD4+ T cells from local sites of tuberculosis (TB) infection could be enhanced by IL-12, which have the features of both Tfh and Th1 cells and may have an important role in local immune responses against TB infection.
Introduction
Tuberculosis (TB) is one of the most ancient diseases of mankind and currently remains a leading cause of death from infectious disease worldwide [1–3]. The incidence of TB has increased over the past few years for reasons such as inadequate preventative efforts, incorrect or inappropriate medication, the emergence of drug-resistant strains of Mycobacterium tuberculosis (MTB) and the prevalence of human immunodeficiency virus (HIV) infection [4–6].
Cell-mediated immunity is known to be crucial for protection against TB and most studies have shown that CD4+ and CD8+ T cells are essential for protective immunity [7–10]. We have been committed to studying MTB-specific effector and memory CD4+ T cells, including Th1, Th17, and Th22 cells [11,12], and we have identified the epitopes, functions and regulation of CD8+ T cells against MTB infection [13,14]. Recently, we found that pleural fluid cells (PFCs) secrete IL-21 following stimulation with specific peptides.
IL-21, a potent immunomodulatory cytokine, has pleiotropic effects on both innate and adaptive immune responses [15–17]. Owing to the broad cellular distribution of the IL-21 receptor, IL-21 exerts pleiotropic effects on the immune system [16,18]. The role of IL-21 in sustaining and regulating T cell, B cell, and NK cell responses during autoimmune diseases, chronic infectious diseases and immunodeficiency diseases has recently come into focus [17,19,20].
It has been reported that follicular helper T (Tfh) cells, Th17 cells, NKT cells, Th1 cells and Th2 cells can produce IL-21, although Tfh cells have the closest relationship with IL-21 [21–24]. In addition, activated human dendritic cells have been shown to induce naïve CD4+ T cells to become IL-21-expressing Tfh-like cells through IL-12 [25]. Tfh cells in humans were initially described in 2000 and 2001, when several groups reported that a large proportion of CD4+ T cells in tonsils have a unique phenotype and express high levels of chemokine (C-X-C motif) receptor 5 (CXCR5) [24]. Currently, Tfh cells are considered to be a distinct CD4+ T cell type and they are important for protective immunity [24,26]. Those cells are characterized by expression of the transcription factor B-cell lymphoma 6 (Bcl-6), production of high amounts of the B-cell stimulatory cytokine IL-21, and increased levels of CXCR5, inducible costimulator (ICOS) and programmed death 1 (PD-1) [24,26,27].
In the current study, we tried to define the relationship between MTB-specific IL-21-expressing cells and Tfh cells. We conducted studies to determine the immunophenotypical characteristics, functional properties and regulatory factors of MTB-specific IL-21-expressing CD4+ T cells. Our data demonstrated that MTB-specific IL-21-expressing CD4+ T cells are present at local sites of infection in patients with tuberculous pleurisy (TBP) and these cells may play an important role in local cellular immunity against TB infection.
Results
MTB-specific peptides induce IL-21 production by PFCs
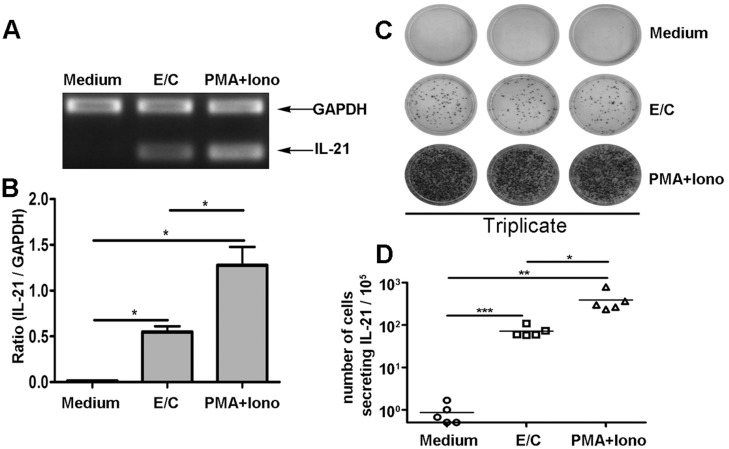
To determine whether the MTB-specific peptides ESAT-6 and CFP-10 (E/C) induce IL-21 production, PFCs were cultured in the presence of medium alone, E/C peptides, or PMA plus ionomycin. RT-PCR results revealed that E/C peptides induce markedly higher levels of IL-21 mRNA transcription than cultures with medium alone. As expected, PMA plus ionomycin also induced significantly high levels of IL-21 (Fig 1A and 1B). To further analyze the frequency of IL-21-producing cells, an enzyme-linked immunospot (ELISPOT) assay was conducted. IL-21+ spots were not detectable without stimulation. E/C peptides, however, elicited a strong antigen-specific T cell response with an average of 71 spot-forming cells (SFCs) (range, 57–108 SFCs), which was significantly higher than in medium alone (Fig 1C and 1D). PMA plus ionomycin induced a stronger response. Altogether, these results indicated that E/C peptides induced IL-21 production by PFCs at both mRNA and protein levels.
Fig 1. ESAT-6/CFP-10 (E/C) peptides induced IL-21 production at the levels of mRNA and protein by PFCs in tuberculous pleurisy.
(A,B) PFCs were cultured in the presence of medium alone, with E/C peptides or with PMA plus ionomycin for 12 h, after which IL-21 and GAPDH mRNA levels were determined by RT-PCR (upper panel). The graph (lower panel) shows the ratio of IL-21 over GAPDH, calculated according to the relative intensities of the bands revealed under UV illumination with Bio-1D software. Data are representative of five separate experiments with similar results. (C) A representative data of ELISPOT results is shown. Data are representative of five separate experiments with similar results. (D) The frequency of IL-21-producing cells was enumerated by ELISPOT assay (n = 5). Each dot represents one donor. Horizontal bars represent the mean value. *p<0.05; **p<0.01; *** p<0.001.
CD4+ T cells from PFCs express IL-21 following stimulation with E/C peptides
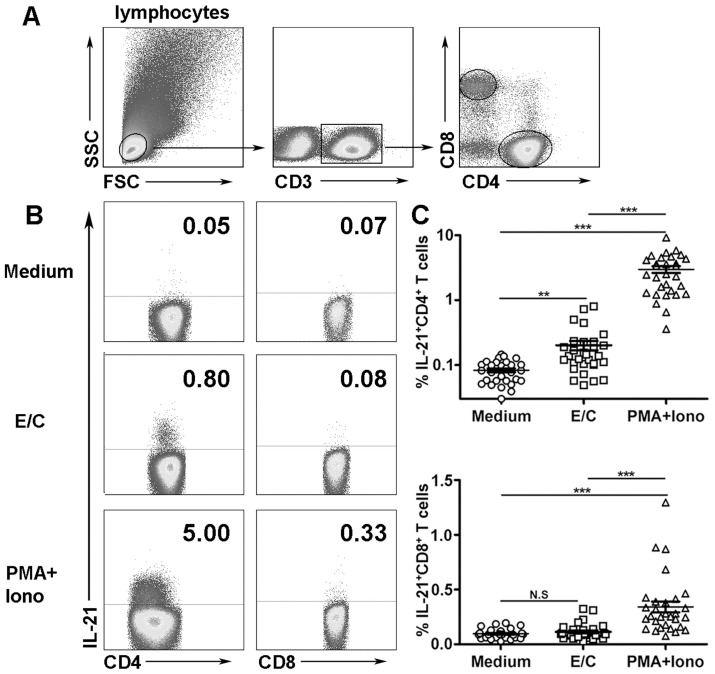
To further determine which subsets of cells produce IL-21, fluorescence-activated cell sorting (FACS) was conducted. The results showed a very low frequency of IL-21 expression by PFCs in the presence of medium alone. Following stimulation with E/C peptides, CD4+ T cells, but not CD8+ T cells, expressed IL-21 (Fig 2A and 2B). Notably, PMA plus ionomycin induced strong IL-21 expression in CD4+ T cells, which was consistent with the results from ELISPOT. Statistical analysis indicated that E/C peptides induced significantly higher percentages of IL-21-expressing CD4+ T cells than medium alone (Fig 2C, n = 30, p<0.01), but no significant difference was observed in CD8+ T cells. In addition, PMA plus ionomycin induced significantly high levels of IL-21 expression by CD4+ and CD8+ T cells.
Fig 2. E/C peptides induced IL-21 expression by CD4+ T cells from PFCs.
(A) Flow cytometric gating strategy used for analysis of lymphocytes from PFCs. (B) PFCs were stimulated in the presence of medium alone, with E/C peptides, or with PMA plus ionomycin. The expression of IL-21 was evaluated by FACS. Representative dot plots of thirty independent experiments are shown. (C) Statistical results of IL-21 expression by CD4+ and CD8+ T cells from PFCs are shown as the mean ± SEM. **p<0.01; *** p<0.001; N.S., not significant.
The subset of IL-21-expressing CD4+ T cells is different from Th1, Th2, and Th17 subpopulations
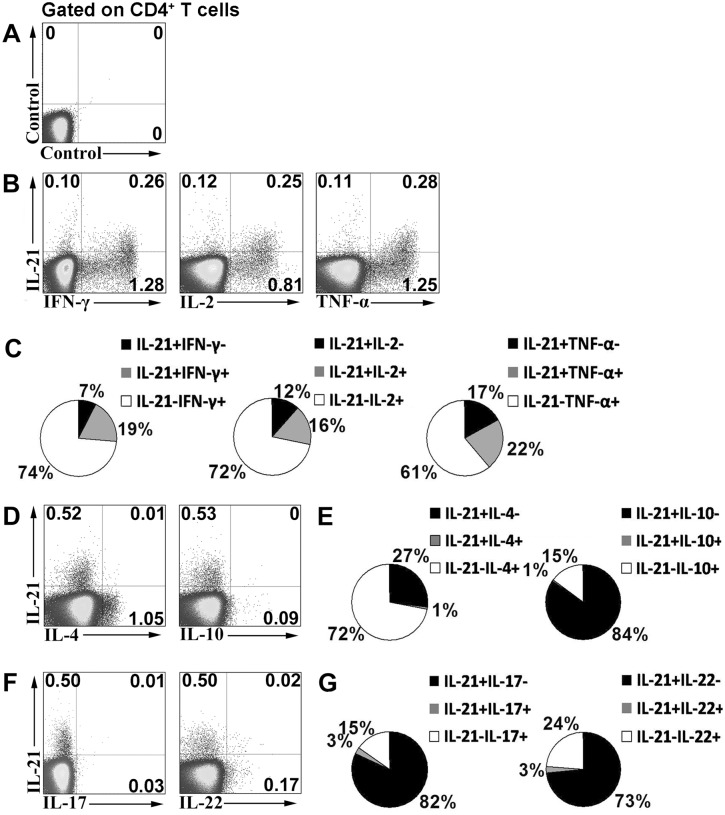
To determine whether MTB-specific IL-21-expressing cells are related to Th1, Th2, and Th17 cell populations, PFCs were stimulated with E/C peptides, and the expression of cytokines was analyzed. In the correlation between IL-21 and IFN-γ expression, cytokine-expressing cells could be clearly divided into three subsets: IFN-γ+IL-21-, IL-21+IFN-γ+, and IL-21+IFN-γ- cells. A fraction of IL-21-expressing cells simultaneously expressed IFN-γ. A similar correlation was observed between IL-21 and IL-2 or TNF-α (Fig 3A and 3B). Statistical analysis showed that IFN-γ single-positive cells had the largest proportion of cytokine-secreting cells, followed by IL-21+IFN-γ+ cells and IL-21 single-positive cells. Similar distributions were observed between IL-21 and IL-2 or TNF-α, as shown in the pie charts in Fig 3C. Interestingly, IL-21-expressing cells did not express IL-4 and IL-10 (Fig 3D). The pie charts in Fig 3E show the distributions between IL-21 and IL-4 or IL-10. Moreover, IL-21-expressing cells did not express IL-17 and IL-22 (Fig 3F). Corresponding statistical results are shown Fig 3G. Taken together, these results indicate that a subset of human IL-21-expressing CD4+ T cells co-expressed Th1 cytokines. Meanwhile, IL-21-expressing cells were distinct from Th2, Th17, and Th22 cells based on cytokine expression. To further evaluate the polyfunctionality of IL-21+IFN-γ+ cells, CD4+ T cells were divided into four subsets: IFN-γ+IL-21-, IL-21+IFN-γ+, IL-21+IFN-γ- and IL-21-IFN-γ- T cells according to the production of IL-21 and IFN-γ. The expression of IL-2 and TNF-α within these distinct cell subsets was further analyzed. The results indicated that IL-21+IFN-γ+ T cells co-expressed high levels of IL-2 and TNF-α compared to IL-21+IFN-γ- T cells, suggesting the obviously polyfunctionality of these cells (Fig 4).
Fig 3. Correlation of IL-21 expression with Th1, Th2 or Th17 cytokines following E/C peptides stimulation.
PFCs were stimulated with E/C peptides. The expression of cytokines was determined by FACS. (A) An isotype control is shown. (B) Expression of IL-21 and the Th1 cytokines, IFN-γ, IL-2, and TNF-α was assessed by FACS. Numbers in quadrants indicate percentages of cells in each population. (C) Data are quantified and presented in a pie chart; each slice of the pie represents the fraction of the mean value of a given quadrant. Independent experiments were repeated at least eight times. (D) Expression of IL-21 and the Th2 cytokines, IL-4 and IL-10, was assessed by FACS. Data are representative of seven separate experiments. (E) Corresponding statistical results are shown in pie charts. (F) IL-21, IL-17 and IL-22 levels were assessed by FACS. Data are representative of seven separate experiments. (G) Corresponding pie charts are shown.
Fig 4. Polyfunctional IL-21+IFN-γ+CD4+ T cells following E/C peptides stimulation.
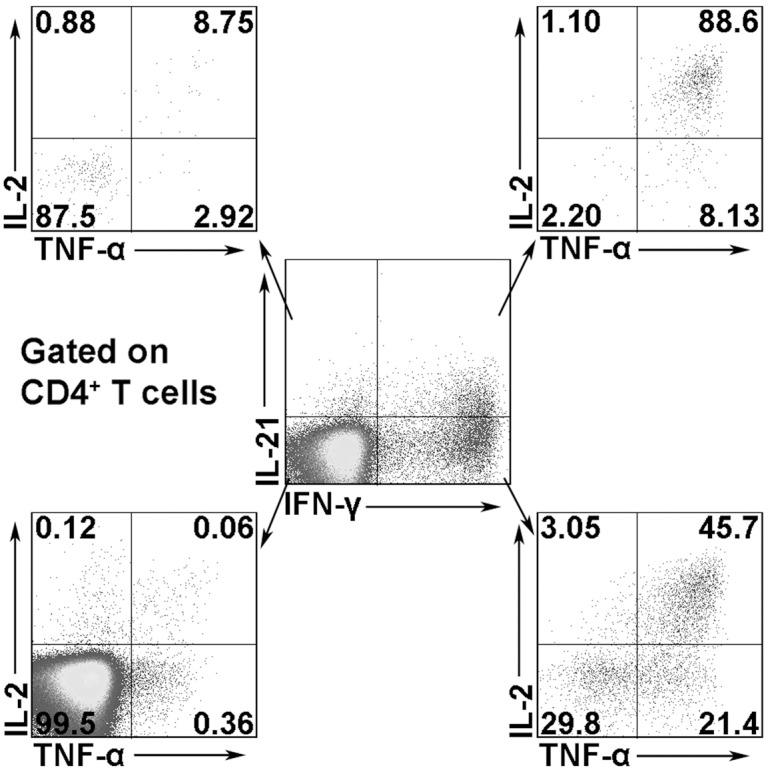
PFCs were stimulated with E/C peptides. CD4+ T cells were gated in lymphocytes from PFCs. The expression of IL-21 and IFN-γ was detected by FACS. The expression of IL-2 and TNF-α within each cell subset was analyzed. Representative data of six independent experiments are shown.
CD4+IL-21+ T cells are effector or effector memory T cells
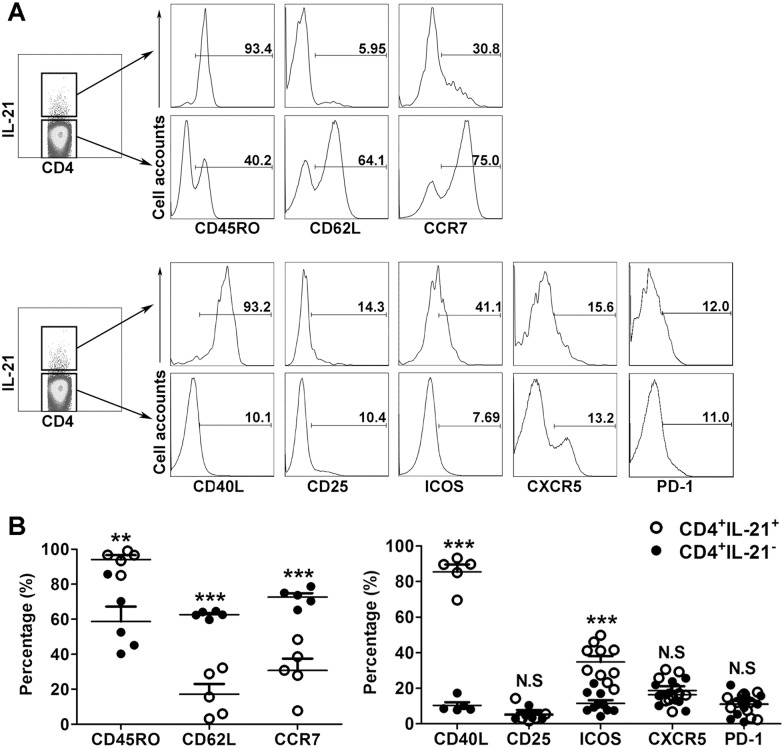
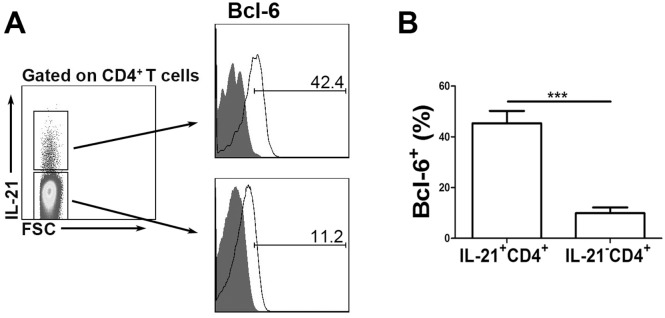
We next investigated the phenotype of the CD4+IL-21+ T cells with respect to memory and activation markers. As shown in Fig 5A, the CD4+IL-21+ T cells were found to be predominantly CD45RO+, thereby exhibiting a memory cell phenotype. In contrast, the CD4+IL-21- T cells consisted of a significantly low percentage of CD45RO+ cells. We also found that CD4+IL-21+ T cells expressed significantly lower levels of CD62L and CCR7 than CD4+IL-21- T cells. We also examined the expression of activation markers, such as CD40L, CD25 and ICOS. Significantly higher levels of CD40L and ICOS expression were detected on CD4+IL-21+ T cells compared to CD4+IL-21- T cells. However, no significant difference was observed in terms of CD25 expression by CD4+IL-21+ and CD4+IL-21- cells (Fig 5B). CXCR5 and PD-1 were reported to be important markers for Tfh cells. However, we did not find any differences in terms of CXCR5 and PD-1 expression between CD4+IL-21+ and CD4+IL-21- cells. Bcl-6 is the key transcription factor for Tfh cell differentiation and function. Therefore, we also assessed the expression of Bcl-6. The results indicated that CD4+IL-21+ cells had significantly higher expression of Bcl-6 than CD4+IL-21- cells (Fig 6).
Fig 5. Phenotype of CD4+IL-21+ and CD4+IL-21- T cells.
PFCs were stimulated with E/C peptides. IL-21 and surface markers expressed by CD4+ T cells were evaluated by FACS. CD4+IL-21+ and CD4+IL-21- T cells were gated. (A) The expression of the typical memory T cell markers, CD45R0, CD62L and CCR7, and the activation markers, CD40L, CD25, ICOS, CXCR5 and PD-1, by CD4+IL-21+ and CD4+IL-21- T cells are shown. Representative flow cytometric graphs are shown. (B) Summary data of the frequency of CD45R0, CD62L, CCR7, CD40L, CD25, ICOS, CXCR5 and PD-1 expression by CD4+IL-21+ and CD4+IL-21- T cells. Data shown are the mean ± SEM from five to ten independent experiments. **p<0.01; *** p<0.001; N.S., not significant.
Fig 6. Bcl-6 expression was significantly higher on CD4+IL-21+ T cell subset than CD4+IL-21- T cells.
PFCs were stimulated with E/C peptides. IL-21 and Bcl-6 expression by CD4+ T cells was evaluated by FACS. (A) CD4+IL-21+ and CD4+IL-21- T cells were gated. The expression of Bcl-6 on CD4+IL-21+ and CD4+IL-21- T cells is shown. Data are representative of five independent experiments. (B) Statistical results of Bcl-6 expression on IL-21+ and IL-21- CD4+ T cells. *** p<0.001.
IL-12 can up-regulate IL-21+IFN-γ+CD4+ T cells induced by MTB-specific peptides
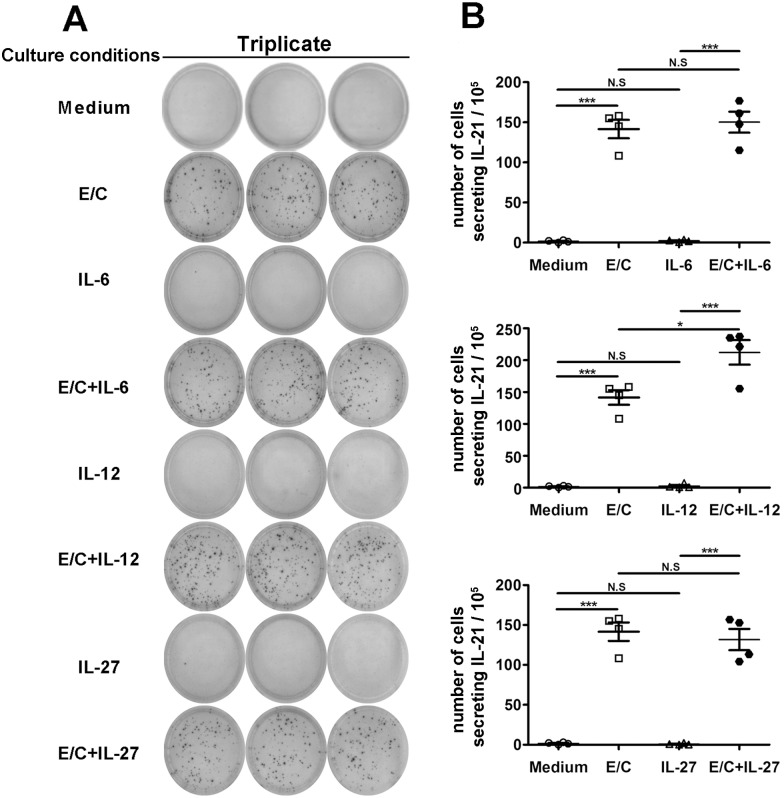
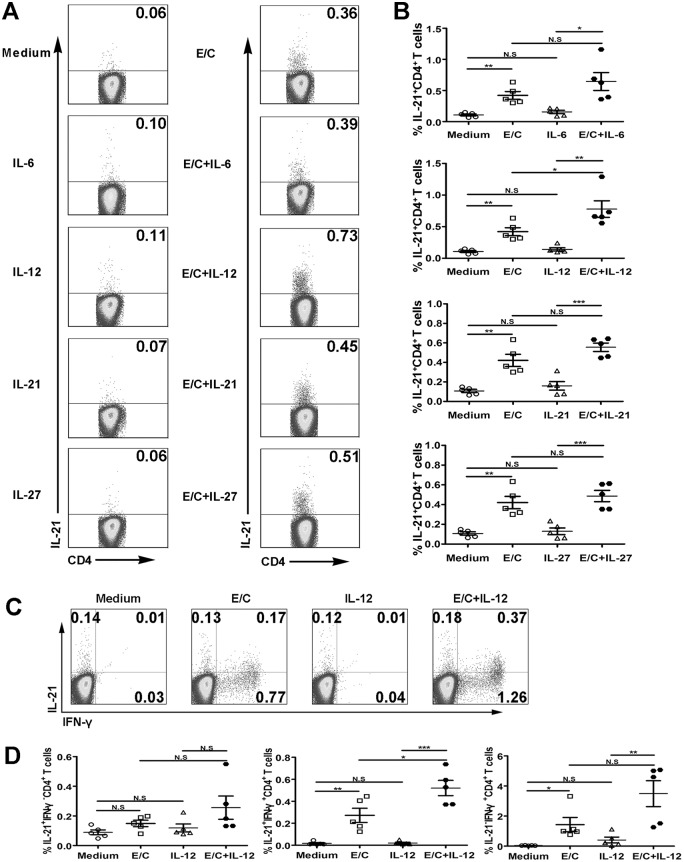
To further determine whether cytokines regulate MTB-specific IL-21 production by PFCs, an ELISPOT assay was conducted. No or few IL-21+ spots were formed without stimulation or in the presence of cytokine alone. However, E/C peptides or E/C peptides plus cytokine elicited strong antigen-specific IL-21 production (Fig 7A). Importantly, more IL-21+ spots were formed following E/C peptides plus IL-12 stimulation than stimulation with E/C peptides alone. However, IL-6 and IL-27 did not have an effect (Fig 7B). To confirm these results, we conducted FACS. Consistent with the ELISPOT results, medium and cytokine alone did not induce IL-21 production. However, both the E/C peptides and the E/C peptide plus cytokine conditions induced IL-21 production (Fig 8A). Significantly higher levels of IL-21 were induced by the combination of IL-12 and E/C peptides when compared with E/C peptides alone (Fig 8B). To specifically analyze which cell subset was regulated, the expression of IL-21 and IFN-γ was analyzed on the CD4+ T cell gate (Fig 8C). Analysis of cytokine-expressing CD4+ T cells indicated that the subset of IL-21+IFN-γ+ cells was markedly enhanced following the addition of IL-12 compared with E/C peptides alone, but no significant difference was observed with respect to the subset of IL-21+IFN-γ- or IL-21-IFN-γ+ cells (Fig 8D). Taken together, these results indicated that IL-12, but not IL-6, IL-27, or IL-21, promotes MTB-specific IL-21 production by PFCs.
Fig 7. IL-12 promoted E/C peptide-induced IL-21 production.
(A) PFCs were cultured in the presence of medium; with IL-6, IL-12 or IL-27 alone; with E/C peptides; or with E/C peptides plus cytokines. The number of IL-21-producing cells was determined by ELISPOT. A representative result is shown. (B) Statistical results from four independent experiments are shown. *p<0.05; *** p<0.001; N.S., not significant.
Fig 8. IL-12 promoted E/C peptide-induced CD4+IL-21+IFN-γ+ T cells.
(A) PFCs were cultured in the presence of medium alone, with IL-6, IL-12, IL-21, IL-27 and E/C peptides, or with E/C peptides plus cytokines. The expression of IL-21 by CD4+ T cells was evaluated by FACS. Representative dot plots are shown. (B) Statistical results of IL-21 expression by CD4+ T cells are shown (n = 5). (C) One representative experiment showing the expression of IL-21 and IFN-γ by CD4+ T cells. The numbers in each quadrant represent the percentages of positive cells within CD4+ T cell populations. (D) Statistical results of the proportions of IL-21+IFN-γ-, IL-21+IFN-γ+ and IL-21-IFN-γ+ within CD4+ T cells (n = 5). *p<0.05; **p<0.01; *** p<0.001; N.S., not significant.
Discussion
It is well known that MTB-specific CD4+ T cells are critical for protection against TB [28]. In the past few years, we published our efforts in determining the phenotype, function, and regulation of MTB-specific CD4+ T cells. Currently, we found that IL-21 was induced by PFCs at both the mRNA and protein level after stimulation with dominant peptides of E/C. The further studies showed that E/C peptides induced significantly higher levels of IL-21 expression in CD4+ T cells than in CD8+ T cells. In concordance with previous work [21], our study demonstrated that IL-21 was predominantly expressed by CD4+ T cells. There is evidence that other cell subsets can synthesize IL-21 under specific circumstances. Elevated frequencies of IL-21-compent CD8+ T cells have been described in HIV-1 infection and autoimmunity [29,30,31]. Our findings here were consistent with our earlier studies in nasal polyps that IL-21 could be produced by CD8+ T cells after PMA plus ionomycin stimulation [32]. Instead, Williams et al. have indicated that IL-21 production by CD8+ T cells was associated with high levels of activation [33]. Moreover, other studies have found that IL-21 is produced by NKT cells [22]. Recently, our studies have also demonstrated for the first time that IL-21 could be produced by NKT cells in TB infection following stimulation with MTB-specific antigens [34]. However, little data exists characterizing the production and regulation of IL-21-expressing CD4+ T cells during tuberculosis. These discrepancies of cell sources of IL-21 production might be due to differences in species, stimuli, or stimulation conditions.
Next, we examined the correlation of IL-21 with other cytokines, and we found that the subset of MTB-specific IL-21-expressing CD4+ T cells was different from the Th1, Th2, and Th17 subpopulations. A fraction of IL-21-expressing cells simultaneously produced Th1 cytokines, but not Th2 or Th17 cytokines. In support of these results, environmental factors that change the ratio of Bcl-6 to T-bet in Th1 cells may cause variability in Th1 and Tfh-like gene-expression patterns [35,36]. After stimulation with peptides, CD4+ T cells could simultaneously produce IFN-γ, IL-2, TNF-α and IL-21. Furthermore, IL-21+IFN-γ+CD4+ T cells exhibited obviously polyfunctionality than IL-21 single-expressing CD4+T cells.
It is well known that IL-21 is a marker for Tfh cells [24,26]. However, IL-21-producing cells cannot be classified as Tfh cells because other cell types can express substantial amounts of IL-21. In addition to the expression of IL-21, some important markers, such as ICOS, CXCR5, PD-1, as well as Bcl-6, are also critical in the identification of Tfh cells [24,26,27]. Therefore, we examined the phenotype of CD4+IL-21+ T cells. Our study showed that, in concordance with Tfh cells, MTB-specific IL-21-expressing cells expressed significantly lower levels of CD62L and CCR7, suggesting that they might migrate from secondary lymphoid organs. In addition, CD4+IL-21+ T cells expressed high levels of CD45RO, displaying the phenotype of effector vs. effector memory T cells. Although data in humans suggests that resting memory Tfh cells exist in the peripheral blood as CD45RO+CXCR5+ cells, this remains a contentious issue that requires more examination [37,38]. Recently, decreased frequencies of CD4+CXCR5+ T helper cells were reported in the blood of active pulmonary TB patients when compared with the blood of latent TB patients [39]. Moreover, Slight et al. have shown that CD4+CXCR5+ T cells play a protective role in the immune response against TB [40]. Nevertheless, the expression of CXCR5 is not an essential marker to define “Tfh-like” cells. For example, CD4+ T cells primed with IL-12 can induce B cells to produce Igs, which is dependent on IL-21 and ICOS. Thus, these cells shared fundamental characteristics with Tfh cells and can be called “IL-21-expressing Tfh-like cells”. Moreover, expression of high levels of CXCR5 leads to the migration of Tfh cells to CXCL13+ B-cell follicles [37,41]. Therefore, though the expression of CXCR5 was similar between IL-21+ and IL-21-CD4+ T cell subsets in our study, we speculate that the expression level of CXCR5 changes during cell migration to local sites. PD-1, another marker of Tfh cells, is induced by extended TCR signaling and is also high on most activated CD4+ and CD8+ T cells [24]. That might explain why PD-1 was similar between IL-21+ and IL-21-CD4+ T cell subsets. In addition, MTB-specific IL-21-expressing cells expressed high levels of CD40L and ICOS, suggesting that they were in an activating state, although the expression of CD25 was not that high. Taken together, our results indicated that our CD4+IL-21+ cell population corresponded to a CD45RO+CD62LlowCCR7lowCD40Lhigh ICOShigh cell population.
Bcl-6 is the most important transcription factor for Tfh cells [24]. Therefore, we examined the expression of Bcl-6. In concordance with the distinguishing feature of Tfh cells, Bcl-6 expression was significantly higher in CD4+IL-21+ cell population than CD4+IL-21- T cells. Altogether, the findings indicated that MTB-specific IL-21-expressing CD4+ T cells having the features of Tfh cells.
Previous work has suggested that IL-21, IL-6, IL-12 and IL-27 participate in Tfh cell development and IL-21 expression [24,42,43]. Few reports have studied the regulation of antigen-specific IL-21 production by memory CD4+ T cells. Therefore, we examined the regulation of MTB-specific IL-21 expression. The results indicated that IL-12 up-regulated IL-21 expression induced by MTB-specific peptides. IL-21, IL-6, and IL-27 had no effect on the production of IL-21. Similarly, two independent studies have shown that IL-12 induces the generation of human Tfh-like cells from naive CD4+ T cells and triggers a significant increase in IL-21 expression in vitro [25,44]. Moreover, IL-12, which signals via STAT4, can be an early inducer of the Tfh cell phenotype, while both IL-21 and IL-6, which signal via STAT3, are insufficient to drive Tfh differentiation [45,46]. Hence, the regulation of MTB-specific IL-21-expressing cells was similar to that of Tfh cells to some extent.
IL-21, produced by Tfh cells, plays a critical role in B-cell proliferation, class switching and Ig production [24,26]. Our study showed that IL-21 up-regulated the secretion of Ig by activated PFCs (data not shown). Therefore, we speculated that CD4+IL-21+ cells shared functional properties with Tfh cells from secondary lymphoid organs. In concordance with Tfh cells, the cells required activation to provide help to B cells and induce their differentiation into Ig-producing cells.
In summary, our studies provided important data concerning the phenotype, regulation and functional capacity of MTB-specific IL-21-expressing CD4+ T cells in PFCs from TB pleurisy. These findings showed that MTB-specific IL-21-expressing CD4+ T cells displayed a CD45RO+CD62LlowCCR7lowCD40LhighICOShigh phenotype and expressed high levels of Bcl-6. All of the above characteristics are distinguishing features of Tfh cells. However, in contrast to Tfh cells, these cells did not localize within B-cell follicles [47], so it is still challenging to address whether MTB-specific IL-21-expressing cells are circulating Tfh cells or “Tfh-like” cells. Nevertheless, evaluation of MTB-specific IL-21-expressing CD4+ T cells could help us understand cellular and humoral immune responses to MTB.
Materials and Methods
Ethics statement
Informed written consent was obtained from all patients. The study protocol was approved by the Ethics Committee of the Zhongshan School of Medicine, Sun Yat-sen University (Guangzhou, China) and the Chest Hospital of Guangzhou (Guangzhou, China).
Study participants
Thirty patients infected with Mycobacterium tuberculosis (12 females and 18 males, age 23 to 74 years old) were recruited into the study. All patients were newly diagnosed with TB pleurisy at the Chest Hospital of Guangzhou, China. Diagnosis of the patients was confirmed by the following criteria: positive cultures for MTB in cultures of pleural biopsy specimens or pleural fluid smear, histological evidence in biopsy specimens of pleural tissue, and positive staining for MTB. Patients who had been previously diagnosed with HIV, hepatitis B, or hepatitis C, or who had a history of autoimmune diseases, were excluded from the study. All pleurisy samples were collected before the initiation of anti-tuberculosis treatment.
Antigens and mAbs
We selected six highly immunogenic peptides, which are largely human leukocyte antigen (HLA)-DR-restricted. Four peptides were derived from ESAT-6 and two were from the CFP-10 protein of MTB. Synthetic peptides were obtained from Shenzhen Hanyu manufacture, Shenzhen, China. The amino acid sequences of the peptides were previously described [11,12]. Lyophilized peptides were reconstituted in DMSO and stored at -80°C. Anti-CD28 (clone CD28.2) and anti-CD49d (clone 9F10) mAbs were purchased from BD Biosciences Pharmingen (San Jose, CA). The following mAbs were purchased from BD Pharmingen (San Jose, CA, USA) and were used for phenotypic, intracellular cytokine, and transcription factor analysis: CD3-PE-cy7 (SK7), CD3-PE-CF594 (UCHT1), CD4-APC-Cy7 (RPA-T4), CD4-APC (RPA-T4), CD4-FITC (RPA-T4), CD8-APC (RPA-T8), CD8-FITC (RPA-T8), IFN-γ-PE-cy7 (4S.B3), IL-21-PE (3A3-N2.1), TNF-α-PE-Cy7 (MAb11), IL-2-APC (MQ1-17H12), IL-4-PerCP-cy5.5 (8D4-8), IL-4-APC (8D4-8), IL-10-APC (JES3-19F1), CD45RO-FITC (UCHL1), CD62L-PE (Dreg56), CCR7-PE-cy7 (3D12), CD40L-PE (TRAP1), CD25-FITC (M-A251), CXCR5-Alexa Fluor647 (RF8B2), CXCR5-Alexa Fluor488 (RF8B2), PD-1-PE-cy7 (EH12.1), Bcl-6-Alexa Fluor647 (K112-91) and Bcl-6-PE-CF594 (K112-91). IL-21- Alexa Fluor647 (3A3-N2), IL-17-FITC (eBio64DEC17) and ICOS-APC (ISA-3) were purchased from eBioscience (San Diego, CA, USA). IFN-γ-FITC (45.15) was purchased from Beckman Coulter (Fullerton, CA), and IL-22-APC (142928) was purchased from R&D Systems (Minneapolis, MN, USA).
Preparation of PFCs
Pleural fluid was obtained by thoracocentesis from tuberculosis patients. PFCs were isolated by lysing erythrocytes with an ammonium chloride solution and resuspended to a final concentration of 2×106 cells/mL in complete RPMI-1640 medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS; Sijiqing, Hangzhou, China), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 50 μM 2-mercaptoethanol (all from Gibco BRL).
Cell culture conditions
PFCs were resuspended in complete RPMI-1640 medium, stimulated with 2 μg/mL peptides plus 1 μg/mL anti-CD28, 1 μg/mL anti-CD49d mAbs, or PMA (20 ng/ml; Sigma Aldrich, Saint Louis, MO, USA) plus ionomycin (1 μg/mL; Sigma-Aldrich). PFCs were cultured in the presence of medium alone, IL-6 (30 ng/mL; Peprotech), IL-12 (5 ng/ml; eBioscience, Santiago, Chile), IL-21 (50 ng/mL; Peprotech), IL-27 (40 ng/ml; eBioscience) alone, E/C peptides, or E/C peptides plus cytokines.
PCR for IL-21
PFCs were stimulated as described above for 12 h. Total RNA was isolated using an RNeasy mini kit (Qiagen, Valencia, CA), and residual DNA was removed using RNase-free DNAse (Qiagen). Reverse transcription of total RNA to cDNA was performed at 37°C using a Reaction Ready-First Strand cDNA Synthesis kit (Promega). Amplification of cDNA was conducted in a DNA thermal cycler (Biometra, Germany) using the following conditions: 95°C for 45 s, 55°C for 45 s, and 72°C for 45 s for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 95°C for 45 s, 51°C for 45 s, and 72°C for 45 s for IL-21. PCR was repeated for 35 cycles for both GAPDH and IL-21. Primer sequences were as follows: IL-21 sense 5’- GAG-TGG-TCA-GCT-TTT-TCC-TGT-T-3’, IL-21 anti-sense 5’-AGG-AAT-TCT-TTG-GGT-GGT-TTT-T-3’, GAPDH sense 5’-GCA-TGG-CCT-TCC-GTG-TCC-3’, and GAPDH anti-sense 5’-TGA-GTG-TGG-CAG-GGA-CTC-3’.
Cytokine ELISPOT assay
The frequency of IL-21-producing cells was quantified by ELISPOT using a commercially available set (Mabtech). Briefly, PFCs were stimulated as described above and added to microwells in triplicate and incubated for 24 h at 37°C in a 5% CO2 incubator. Spot-forming cells (SFC) were enumerated using an ELISPOT image analysis system (Champspot II, Sage Creation, Beijing, China). The average of spots in triplicate wells was calculated as SFC/ 1×105 PFCs.
Flow cytometry
PFCs were stimulated with peptides as described above for 12 h in the presence of brefeldin A (BFA, 10 μg /mL; Sigma-Aldrich, St Louis, MO) for the final 8 h, and PFCs were stimulated with PMA plus ionomycin in the presence of BFA for 6 h. After stimulation, the cells were washed with PBS buffer containing 0.1% BSA and 0.05% sodium azide (FACS buffer). The cells were incubated with mAbs at 4°C for surface staining, washed twice, and fixed with 4% paraformaldehyde. The cells were then permeabilized with PBS containing 0.1% saponin and stained for intracellular cytokines. Flow cytometry was performed using BD FACSCalibur (BD Biosciences, San Jose, CA, USA) or FACSAria II (BD Biosciences, San Jose, CA, USA), and the experimental data were analyzed using FlowJo software (TreeStar, San Carlos, CA, USA).
Statistical analysis
Data are presented as the mean ± SEM. Statistical tests were performed with GraphPad Prism software, version 5. Comparison between groups was performed by the Wilcoxon matched-pairs test (two-tailed). A value of p<0.05 was considered statistically significant.
Accession numbers of genes mentioned in the manuscript
IL-21 GenBank accession no.: AF254069.
Acknowledgments
We would like to thank all of the participants for their involvement in this study.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by grants from Guangdong Recruitment Program of Creative Research Groups (no. 2009010058), the National Natural Science Foundation of China (no. 31270942), the Natural Science Foundation of Guangdong Province (no. S2012010009159), Hubei Provincial Natural Science Foundation of China (no. 2015CFB255), and the Program of China during the Twelfth Five-Year Plan Period (no. 2013ZX10003007-002-003).
References
- 1.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362(9387):887–99. 10.1016/S0140-6736(03)14333-4 [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SH. Tuberculosis: back on the immunologists' agenda. Immunity. 2006;24(4):351–7. 10.1016/j.immuni.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 3.Cummings KJ. Tuberculosis control: challenges of an ancient and ongoing epidemic. Public Health Rep. 2007;122(5):683–92. PMCID: PMC1936956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harries AD, Hargreaves NJ, Kemp J, Jindani A, Enarson DA, Maher D, et al. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet. 2001;357(9267):1519–23. 10.1016/S0140-6736(00)04639-0 [DOI] [PubMed] [Google Scholar]
- 5.Harries AD, Zachariah R, Corbett EL, Lawn SD, Santos-Filho ET, Chimzizi R, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375(9729):1906–19. 10.1016/S0140-6736(10)60409-6 [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378(9785):57–72. 10.1016/S0140-6736(10)62173-3 [DOI] [PubMed] [Google Scholar]
- 7.Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, et al. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180(6):2069–73. 10.1086/315114 [DOI] [PubMed] [Google Scholar]
- 8.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol. 2001;167(5):2734–42. [DOI] [PubMed] [Google Scholar]
- 9.Lazarevic V, Flynn J. CD8+ T cells in tuberculosis. Am J Respir Crit Care Med. 2002;166(8):1116–21. 10.1164/rccm.2204027 [DOI] [PubMed] [Google Scholar]
- 10.Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am J Respir Crit Care Med. 2003;168(11):1346–52. 10.1164/rccm.200306-837OC [DOI] [PubMed] [Google Scholar]
- 11.Li L, Qiao D, Fu X, Lao S, Zhang X, Wu C. Identification of Mycobacterium tuberculosis-specific Th1, Th17 and Th22 cells using the expression of CD40L in tuberculous pleurisy. PLoS ONE. 2011;6(5):e20165 10.1371/journal.pone.0020165 PMCID: PMC3097245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Qiao D, Fu X, Lao S, Zhang X, Wu C. Identification of M. tuberculosis-specific Th1 cells expressing CD69 generated in vivo in pleural fluid cells from patients with tuberculous pleurisy. PLoS ONE. 2011;6(8):e23700 10.1371/journal.pone.0023700 PMCID: PMC3161751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao D, Li L, Guo J, Lao S, Zhang X, Zhang J, et al. Mycobacterium tuberculosis culture filtrate protein 10-specific effector/memory CD4(+) and CD8(+) T cells in tubercular pleural fluid, with biased usage of T cell receptor Vbeta chains. Infect Immun. 2011;79(8):3358–65. 10.1128/IAI.00014-11 PMCID: PMC3147558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Yang B, Yu S, Zhang X, Lao S, Wu C. Human CD8+ T cells from TB pleurisy respond to four immunodominant epitopes in Mtb CFP10 restricted by HLA-B alleles. PLoS ONE. 2013;8(12):e82196 10.1371/journal.pone.0082196 PMCID: PMC3861325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habib T, Nelson A, Kaushansky K. IL-21: a novel IL-2-family lymphokine that modulates B, T, and natural killer cell responses. J Allergy Clin Immunol. 2003;112(6):1033–45. 10.1016/j.jaci.2003.08.039 [DOI] [PubMed] [Google Scholar]
- 16.Mehta DS, Wurster AL, Grusby MJ. Biology of IL-21 and the IL-21 receptor. Immunol Rev. 2004;202:84–95. 10.1111/j.0105-2896.2004.00201.x [DOI] [PubMed] [Google Scholar]
- 17.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. 10.1146/annurev.immunol.26.021607.090316 [DOI] [PubMed] [Google Scholar]
- 18.Collins M, Whitters MJ, Young DA. IL-21 and IL-21 receptor: a new cytokine pathway modulates innate and adaptive immunity. Immunol Res. 2003;28(2):131–40. 10.1385/IR:28:2:131 [DOI] [PubMed] [Google Scholar]
- 19.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5(9):688–98. 10.1038/nri1688 [DOI] [PubMed] [Google Scholar]
- 20.Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010;184(1):114–26. 10.4049/jimmunol.0901967 [DOI] [PubMed] [Google Scholar]
- 21.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408(6808):57–63. 10.1038/35040504 [DOI] [PubMed] [Google Scholar]
- 22.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178(5):2827–34. [DOI] [PubMed] [Google Scholar]
- 23.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–3. 10.1038/nature05969 [DOI] [PubMed] [Google Scholar]
- 24.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–63. 10.1146/annurev-immunol-031210-101400 [DOI] [PubMed] [Google Scholar]
- 25.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31(1):158–69. 10.1016/j.immuni.2009.04.016 PMCID: PMC2731623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M, Guo Z, Ju W, Ryffel B, He X, Zheng SG. The development and function of follicular helper T cells in immune responses. Cell Mol Immunol. 2012;9(5):375–9. 10.1038/cmi.2012.18 PMCID: PMC4000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nurieva RI, Chung Y. Understanding the development and function of T follicular helper cells. Cell Mol Immunol. 2010;7(3):190–7. 10.1038/cmi.2010.24 PMCID: PMC4002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. 10.1146/annurev-immunol-032712-095939 [DOI] [PubMed] [Google Scholar]
- 29.Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol. 2011;85(5):2316–24. 10.1128/JVI.01476-10 PMCID: PMC3067790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X, Ma D, Zhang J, Peng J, Qu X, Ji C, et al. Elevated interleukin-21 correlated to Th17 and Th1 cells in patients with immune thrombocytopenia. J Clin Immunol. 2010;30(2):253–9. 10.1007/s10875-009-9353-1 [DOI] [PubMed] [Google Scholar]
- 31.Dolff S, Abdulahad WH, Westra J, Doornbos-van der Meer B, Limburg PC, Kallenberg CG, et al. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 2011;13(5):R157 10.1186/ar3474 PMCID: PMC3308088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao L, Jia L, Zhang Y, Yu S, Wu X, Yang B, et al. Human IL-21+IFN-gamma+CD4+ T cells in nasal polyps are regulated by IL-12. Sci Rep. 2015;5:12781 10.1038/srep12781 PMCID: PMC4523938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams LD, Amatya N, Bansal A, Sabbaj S, Heath SL, Sereti I, et al. Immune activation is associated with CD8 T cell interleukin-21 production in HIV-1-infected individuals. J Virol. 2014;88(17):10259–63. 10.1128/JVI.00764-14 PMCID: PMC4136345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Li Z, Fu X, Yu S, Lao S, Yang B. Antigen-specific human NKT cells from tuberculosis patients produce IL-21 to help B cells for the production of immunoglobulins. Oncotarget. 2015;6(30):28633–45. 10.18632/oncotarget.5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol. 2012;13(4):405–11. 10.1038/ni.2242 PMCID: PMC3561768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinmann AS. Regulatory mechanisms that control T-follicular helper and T-helper 1 cell flexibility. Immunol Cell Biol. 2014;92(1):34–9. 10.1038/icb.2013.49 [DOI] [PubMed] [Google Scholar]
- 37.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193(12):1373–81. PMCID: PMC2193300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192(11):1545–52. PMCID: PMC2193094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar NP, Sridhar R, Hanna LE, Banurekha VV, Nutman TB, Babu S. Decreased frequencies of circulating CD4(+) T follicular helper cells associated with diminished plasma IL-21 in active pulmonary tuberculosis. PLoS ONE. 2014;9(10):e111098 10.1371/journal.pone.0111098 PMCID: PMC4208798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, et al. CXCR5(+) T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123(2):712–26. 10.1172/JCI65728 PMCID: PMC3561804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190(8):1123–34 PMCID: PMC2195660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannons JL, Lu KT, Schwartzberg PL. T follicular helper cell diversity and plasticity. Trends Immunol. 2013;34(5):200–7. 10.1016/j.it.2013.01.001 PMCID: PMC3646926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207(13):2895–906. 10.1084/jem.20100064 PMCID: PMC3005229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87(8):590–600. 10.1038/icb.2009.64 [DOI] [PubMed] [Google Scholar]
- 45.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011;35(6):919–31. 10.1016/j.immuni.2011.11.012 PMCID: PMC3244883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE. 2011;6(3):e17739 10.1371/journal.pone.0017739 PMCID: PMC3056724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192(11):1553–62. PMCID: PMC2193097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.