SUMMARY
Correction of faulty kinetochore-microtubule attachments is essential for faithful chromosome segregation and dictated by the opposing activities of Aurora B kinase and PP1 and PP2A phosphatases. How kinase and phosphatase activities are appropriately balanced is less clear. Here, we show that a centromeric pool of PP2A-B56 counteracts Aurora B T-loop phosphorylation and is recruited to centromeres through Shugoshin-1 (Sgo1). In non-transformed RPE-1 cells, Aurora B, Sgo1, and PP2A-B56 are enriched on centromeres and levels diminish as chromosomes establish bi-oriented attachments. Elevating Sgo1 levels at centromeres recruits excess PP2A-B56, and this counteracts Aurora B kinase activity, undermining efficient correction of kinetochore-microtubule attachment errors. Conversely, Sgo1-depleted cells display reduced centromeric localization of Aurora B, whereas the remaining kinase is hyperactive due to concomitant reduction of centromeric PP2A-B56. Our data suggest that Sgo1 can tune the stability of kinetochore-microtubule attachments through recruitment of PP2A-B56 that balances Aurora B activity at the centromere.
Graphical abstract
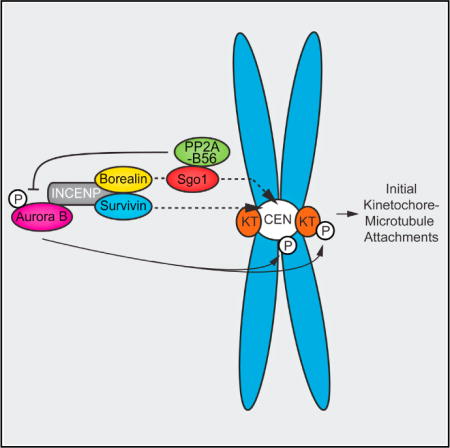
INTRODUCTION
Acquisition, correction, and stabilization of kinetochore-microtubule (k-MT) attachments are dictated by a tight balance of kinase and phosphatase activities at the centromere and kinetochore (Funabiki and Wynne, 2013). Aurora B kinase destabilizes k-MT attachments, allowing the correction of erroneous k-MT attachments, thereby preventing chromosome missegregations (van der Waal et al., 2012a). PP2A-B56 is thought to dampen Aurora B activity to allow the establishment of initial k-MT attachments in early mitosis (Foley et al., 2011), and the mitotic checkpoint protein BubR1 contributes to this inhibitory effect on Aurora B by recruiting PP2A-B56 to kinetochores (Kruse et al., 2013; Suijkerbuijk et al., 2012; Xu et al., 2013). Human cells express five different PP2A-B56 holoenzymes discriminated by their B56 regulatory subunit (B56 α, β, γ, δ, and ɛ; Bollen et al., 2009). Expression of HA-tagged B56 isoforms in HeLa cells revealed that B56γ and B56δ preferentially localize to kinetochores, whereas B56α, B56β, and B56ɛ appeared to localize to centromeres (Nijenhuis et al., 2014). Whether a centromere pool of PP2A-B56 can regulate Aurora B-dependent k-MT attachment stability is unknown. The shugoshin proteins (Sgo1 and Sgo2 in humans) are plausible recruiters of this centromeric pool of PP2A-B56 because they both interact with PP2A-B56 and localize to centromeres of unattached chromosomes (Huang et al., 2007; Tanno et al., 2010; Xu et al., 2009). However, whereas depletion of Sgo2 was shown to impair centromeric PP2A-B56 recruitment (Kitajima et al., 2006; Tanno et al., 2010), the role of Sgo1 in recruiting PP2A to centromeres remains unclear despite the evidence that Sgo1-PP2A is required to protect centromeric cohesin (Kitajima et al., 2006; Liu et al., 2013; Riedel et al., 2006; Tang et al., 2006; Tanno et al., 2010) and the recent observation in budding yeast where PP2A-Rts1 recruitment by Sgo1 is required for chromosome bi-orientation (Eshleman and Morgan, 2014). Here, we demonstrate that human Sgo1 recruits a pool of PP2A-B56 to centromeres that counteracts Aurora B kinase activity to appropriately tune the stability of k-MT attachments.
RESULTS
PP2A-B56 Colocalizes with Aurora B and Sgo1 at the Inner Centromere of Unattached Chromosomes
When we analyzed non-transformed RPE-1 cells with both bi-oriented and unaligned chromosomes (Figure 1A), we confirmed that the levels of Aurora B and endogenous PP2A-B56α were much higher on unattached chromosomes compared to attached chromosomes (Figures 1B, 1E, S1A, and S1B) (Foley et al., 2011; Salimian et al., 2011). In addition, we found that also PP2A-B56ɛ and Sgo1 were enriched on unattached chromosomes (Figures 1C and 1D). However, we failed to detect any B56δ (Figure 2B; note: the available antibodies only detect endogenous B56α, B56δ, and B56ɛ by immunofluorescence [IF]). On unattached chromosomes, Sgo1 colocalized with PP2A-B56 and Aurora B at the (inner)centromere (Figures 1E–1G and S1B) (Kitajima et al., 2006).
Figure 1. Sgo1, Aurora B, and PP2A-B56 Colocalize at Centromeres of Unattached Chromosomes.
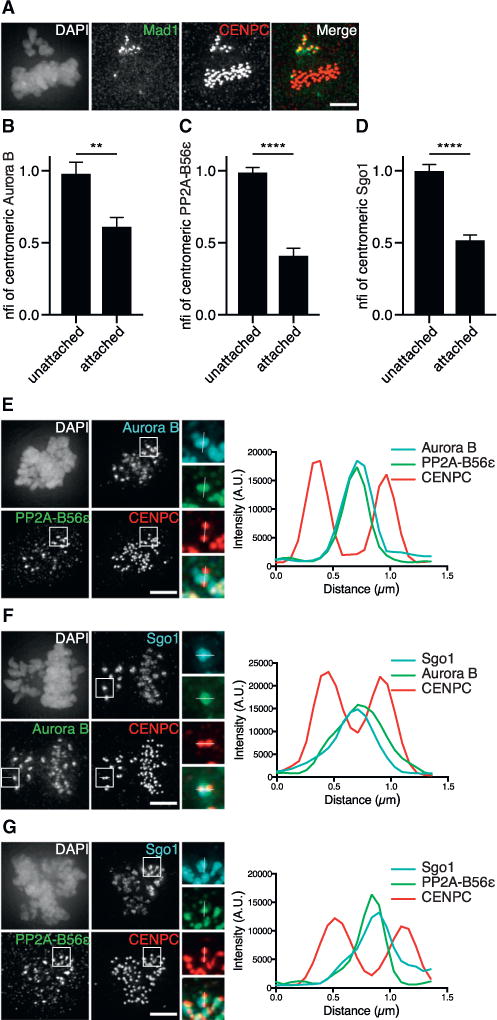
(A) IF of Mad1 and CENPC in RPE-1 cells, treated with a low dose (0.069 μM) of nocodazole for 14 hr to increase the frequency of cells with both bi-oriented (Mad1−) and unattached (Mad1+) chromosomes.
(B–D) Quantifications of centromeric fluorescence intensities (CFIs) of Aurora B, PP2A-B56ɛ, and Sgo1 on attached and unattached chromosomes of RPE-1 cells treated as in (A). CFIs were normalized for the unattached centromeres (nfi). Error bars are SEM between cells from two independent experiments (10–15 cells/experiment).
(E–G) IF of Aurora B and PP2A-B56ɛ, Sgo1 and Aurora B, or Sgo1 and PP2AB56ɛ of cells treated as in (A). Insets show kinetochore pairs of the boxed regions used for line plot analysis. nfi, normalized fluorescence intensity; A.U., arbitrary units. The scale bars represent 5 μm; **p < 0.01; ****p < 0.0001 (unpaired t test). See also Figure S1.
Figure 2. Sgo1 Overexpression Leads to Extra Recruitment of Centromeric PP2A-B56 and a Reduction of Aurora B Substrate Phosphorylation.
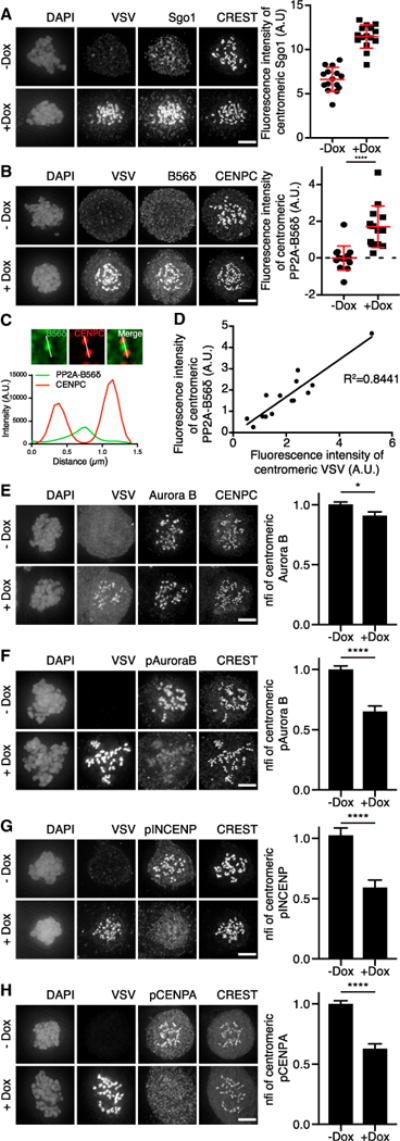
(A) IF and quantifications of VSV, Sgo1, and CREST of RPE-1 cells stably expressing inducible VSV-Sgo1, treated with a high dose (0.83 μM) of nocodazole and doxycycline where indicated. Absolute CFIs were measured. Each dot represents the intensity measured for all centromeres in one cell (n = 15–20 cells). Error bars represent SD.
(B) IF and quantification of VSV, PP2A-B56δ, and CENPC of cells treated as in (A). Absolute CFIs were measured. One representative experiment out of two is shown. Error bars represent SD.
(C) Enlargements of cells treated as in (A). A single z plane was chosen and indicated kinetochore pairs were used for line plot analysis.
(D) Pearson correlation between absolute CFIs of VSV and PP2A-B56δ from (B).
(E–H) IF and quantifications of Aurora B, pAurora B, pINCENP, and pCENPA of cells, treated as in (A). CFIs were measured on centromeres and normalized for the non-induced condition (nfi). Error bars are SEM between cells from independent experiments (n = 5 for Aurora B; n = 3 for pAurora B and pCENPA; n = 2 for pINCENP; 15–30 cells/experiment). The scale bars represent 5 μm; *p < 0.05; ****p < 0.0001 (unpaired t test). See also Figure S2.
Sgo1 Overexpression Recruits Extra PP2A-B56 to Centromeres
To test whether the high levels of Sgo1 at centromeres of unattached chromosomes could be responsible for the high centromeric levels of PP2A-B56, we generated stable RPE-1 and U2OS cell lines with inducible expression of VSV-Sgo1. Addition of doxycycline resulted in an approximate 2- and 7-fold increase in centromeric Sgo1 levels in the RPE-1 and U2OS cell lines, respectively (Figures 2A and S2A). Strikingly, centromere levels of B56ɛ in RPE-1 cells and of B56α in U2OS cells increased upon overexpression of Sgo1 (Figures S2B and S2C). Moreover, endogenous B56δ, which was undetectable in non-induced RPE-1 cells, became clearly detectable at centromeres when Sgo1 levels were increased (Figures 2B and 2C). In fact, a linear relationship was found between the centromere levels of overexpressed Sgo1 and the centromere levels of PP2A-B56δ and PP2A-B56α in RPE-1 and U2OS cells, respectively (Figures 2D and S2D). Of note, in contrast to U2OS cells, the centromere levels of B56α were already high in RPE-1 cells and they were not significantly increased upon Sgo1 overexpression (Figures S2E, S3B, and S3L), most likely because of limited availability of B56α in these cells.
Excess Sgo1 Diminishes Aurora B Substrate Phosphorylation at Centromeres
Sgo1 is known to assist in the recruitment of Aurora B to centromeres by bridging Borealin with phosphorylated histone 2A (Kawashima et al., 2007; Tsukahara et al., 2010). Despite the increased centromeric levels of Sgo1 in RPE-1 cells, overexpression of Sgo1 did not enhance the overall levels of Aurora B at the inner centromere (Figure 2E). In contrast, phosphorylation of the Aurora B T-loop (T232; pAurora B; Yasui et al., 2004) and the Aurora B substrates INCENP (S893 and S894; pINCENP; Salimian et al., 2011) and CENPA (S7; pCENPA; Zeitlin et al., 2001) was reduced by 35%, 41%, and 37%, respectively, in nocodazole-treated RPE-1 cells (Figures 2F–2H). Total levels of INCENP and CENPA at centromeres did not change dramatically (Figures S2F and S2G). The higher Sgo1 overexpression levels in U2OS cells (Figure S2A) led to a more pronounced decrease in phosphorylation of CENPA (approx. 80% reduction; Figure S2H) compared to RPE-1 cells.
To test whether the excessive PP2A recruitment was responsible for the reduction in Aurora B substrate phosphorylation upon Sgo1 overexpression, we created a point mutant (N61I) of Sgo1 that perturbed its interaction with PP2A-B56 (Tang et al., 2006; Xu et al., 2009). As expected, this mutant no longer bound PP2A-B56, but in accordance with Tang et al. (2006), we also found that it localized less well to centromeres compared to wild-type Sgo1 (Figures S2I and S2J). To obtain equal levels of both proteins at the centromere, we fused Sgo1WT and Sgo1N61I to the centromere-targeting domain of CENPB (CB) (Liu et al., 2009; Ricke et al., 2012). Importantly, only the fusion protein that interacted with PP2A-B56 increased the quantity of B56δ and B56ɛ at centromeres (Figures 3A, S3A, and S3K). Similar to overexpression of Sgo1, overexpression of CB-Sgo1WT in RPE-1 cells also decreased the levels of pAurora B, pINCENP, and pCENPA; however, phosphorylation of these substrates did not change upon CB-Sgo1N61I overexpression (Figures 3B–3D and S3D–S3F). Expression of either fusion protein in RPE-1 cells slightly reduced total Aurora B levels at centromeres and resulted in a marginal change in the overall centromeric levels of INCENP and CENPA (Figures 3E, S3C, S3G, S3H, S3M, and S3N). The effect of CB-Sgo1WT overexpression on Aurora B substrate phosphorylation was more dramatic than of Sgo1 overexpression (a reduction of ±70% versus ±40%, respectively), most likely because, similar to Sgo1 overexpression in U2OS cells (Figures S2A, S2C, and S2D), we gained higher overall and centromeric Sgo1 levels as well as centromeric PP2A levels. Together, this shows that the decrease in Aurora B substrate phosphorylation due to overexpression of Sgo1 is dependent on the capacity of Sgo1 to bind and recruit PP2A-B56 to centromeres.
Figure 3. Excessive Centromeric PP2A-B56 Recruitment by Sgo1 Impairs Aurora B Function.
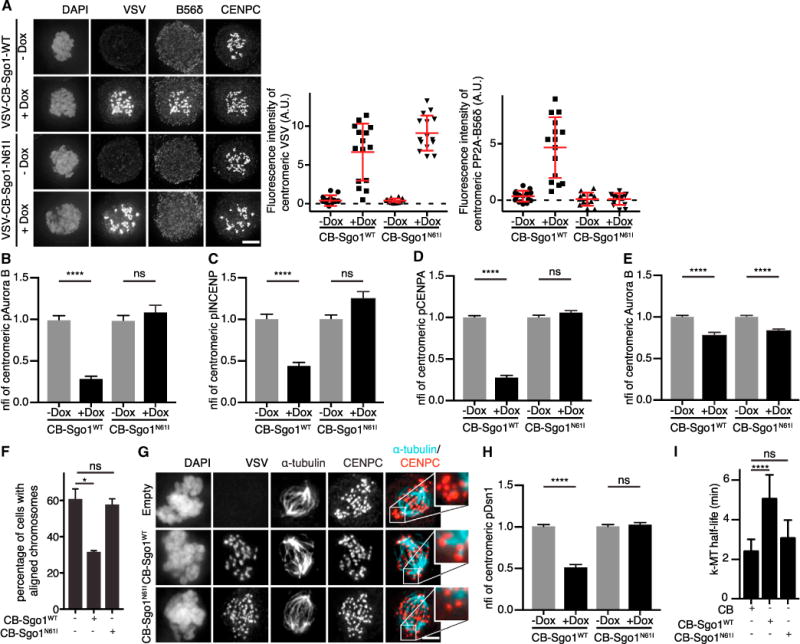
(A) IF and quantifications of PP2A-B56δ and VSV of RPE-1 cells stably expressing inducible VSV-CB-Sgo1WT or VSV-CB-Sgo1N61I, treated with 0.83 μM nocodazole and doxycycline where indicated. Absolute CFIs were measured. One representative experiment out of three is shown. Each dot represents a single cell (n = 15 cells). Error bars represent SD.
(B–E) Quantifications of pAurora B, pINCENP, pCENPA, and Aurora B of cells treated as in (A). Error bars represent the SEM between cells from independent experiments (n = 2 for pAuroraB and pINCENP; n = 3 for pCENPA and Aurora B; 15–30 cells/experiment).
(F) RPE-1 cells or RPE-1 cells expressing VSV-CB-Sgo1WT or VSV-CB-Sgo1N61I were released from a monastrol block into MG132 and fixed 60 min later. Percentage of cells with all chromosomes correctly aligned in metaphase was determined (n > 200 cells; two independent experiments). Error bars represent SD.
(G) IF of VSV, α-tubulin, and CENPC of cells treated as in (F) and subjected to cold-induced microtubule depolymerization. Insets are enlargements of boxed regions.
(H) Quantification of pDsn1 of cells treated as in (B)–(E). Error bars are SEM between cells from two independent experiments (15 cells/experiment).
(I) k-MT half-life calculated from the exponential decay curve (Figure S3P) of photoactivated fluorescence of GFP-tubulin (r2 > 0.99) in U2OS cells transiently transfected with the indicated plasmids (n ≥ 10 cells). Error bars represent SD. The scale bars represent 5 μm; ****p < 0.0001; *p < 0.05; ns, not significant (unpaired t test). See also Figures S2 and S3.
Excessive Centromeric PP2A-B56 Recruitment by Sgo1 Impairs Aurora B Function
To examine whether the Sgo1-PP2A-B56-dependent hypophosphorylation of Aurora B substrates translated into impaired Aurora B kinetochore function, we investigated the ability of cells to correct k-MT attachment errors induced after treatment with the Eg5 inhibitor monastrol. Upon wash-out of monastrol, incorrect k-MT attachments are destabilized by Aurora B, resulting in cells with fully aligned chromosomes in metaphase (Lampson et al., 2004). In RPE-1 cells overexpressing CB-Sgo1WT chromosome alignment was impaired, indicating that k-MT attachment error correction occurred less efficient (Figure 3F). α-tubulin staining, after monastrol release and cold treatment to destabilize non-k-MTs, revealed that the kinetochores of the remaining unaligned chromosomes were unattached in both control and CB-Sgo1N61I-expressing cells. However, in CB-Sgo1WT-expressing cells, the kinetochores of unaligned chromosomes were predominantly stably connected to spindle microtubules (Figure 3G). This correlated with reduced phosphorylation of the Aurora B kinetochore substrate Dsn1 (pDsn1; S109; Welburn et al., 2010) (Figures 3H and S3I), whereas the overall levels of Dsn1 were not decreased (Figures S3J and S3O). In addition, when we compared k-MT dynamics in control cells versus CB-Sgo1WT- and CB-Sgo1N61I-expressing U2OS cells (Bakhoum et al., 2009), we found that only overexpression of CB-Sgo1WT significantly increased the half-life of k-MTs at prometaphase (Figures 3I and S3P). This indicated that k-MTs became more stable in CB-Sgo1WT-overexpressing cells. Taken together, these data demonstrate that Sgo1 overexpression counteracts Aurora B activity through excessive recruitment of PP2A-B56, resulting in hyperstable k-MTs that disrupt efficient error correction.
Sgo1 Co-recruits Aurora B and PP2A-B56 to Centromeres
Our findings with overexpressed Sgo1 infer that PP2A-B56 recruited by Sgo1 can counteract Aurora B activity. To investigate whether the endogenous Sgo1-PP2A-B56 pool regulates Aurora B activity, we depleted endogenous Sgo1 in RPE-1 cells. In concordance with previous reports, Sgo1 depletion resulted in a reduction of centromere-localized Aurora B (Figure 4A) (Tsukahara et al., 2010; van der Waal et al., 2012b; Wang et al., 2010). In contrast to studies in HeLa cells (Kitajima et al., 2006; Tang et al., 2006), we observed a reduction in centromeric PP2A-B56α and B56ɛ upon knockdown of Sgo1 in RPE-1 cells (Figures 4B and S4A). Importantly, Sgo1 depletion did not affect the centromeric levels of Sgo2 (Figure S4B). Interestingly, we found that, in the Sgo1-depleted cells, the remaining PP2A-B56α and B56ɛ became visible at kinetochores, colocalizing with BubR1 (Figures 4C, S4A, and S4C), in line with the observation that kinetochore levels of BubR1 as well as Plk1-phosphorylated BubR1 (T680; pBubR1; Kruse et al., 2013; Suijkerbuijk et al., 2012; Xu et al., 2013) did not change upon knockdown of Sgo1 (Figures S4D and S4E). To show that this was not a consequence of loss of cohesion, we co-depleted Sgo1 with Wapl to prevent premature separation of the sister chromatids (Gandhi et al., 2006; Kueng et al., 2006) and observed a similar decrease in centromeric PP2A-B56α levels and detection of kinetochore-localized PP2A-B56α (Figures S4F–S4H), suggesting Sgo1 itself is capable of recruiting a pool of PP2A-B56 to centromeres. Remarkably, the reduction in total centromeric levels of Aurora B did not result in a reduction of the levels of its phosphorylated T-loop (Figures 4D, S4I, and S4J), similar to findings in X. laevis (Rivera et al., 2012). The consequential increase in pAurora B/Aurora B ratio at centromeres (Figures 4D and 4E) suggested that, in the absence of Sgo1, the remaining centromeric pool of Aurora B was more active, most likely due to the concomitant reduction in centromeric PP2A. In line with this idea, depletion of PP2A-B56 using a siRNA pool targeting all B56 isoforms (Foley et al., 2011) resulted in enhanced Aurora B T232 phosphorylation without affecting the overall levels of Aurora B at centromeres (Figures 4F–4I).
Figure 4. Sgo1 Co-recruits Aurora B and PP2A-B56 to Centromeres.
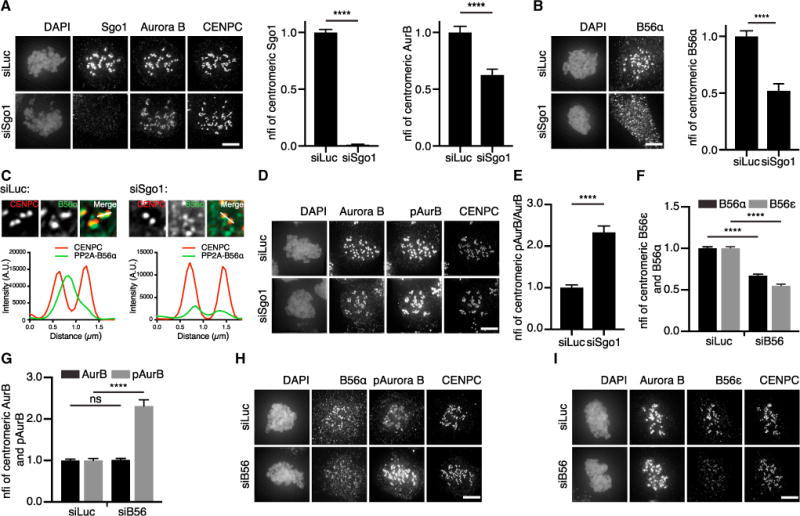
(A) IF and quantification of Sgo1 and Aurora B of RPE-1 cells transfected with siRNAs for Luciferase or Sgo1 and treated with 0.83 μM nocodazole. CFIs of Sgo1 were normalized for siLuc (nfi). Error bars are SEM between cells from three independent experiments (15 cells/experiment).
(B) IF and quantification of PP2A-B56α of cells treated as in (A).
(C) Enlargements of cells treated as in (B). A single z plane was chosen, and indicated kinetochore pairs were used for line plot analysis. Of note, in the siSgo1 condition, levels of PP2A-B56α were enhanced in order to visualize staining.
(D) IF for pAurora B, Aurora B, and CENPC of cells treated as in (A).
(E) Quantification of Aurora B and pAurora B belonging to (D). CFIs of pAurora B and Aurora B were measured, and the CFI ratio of pAurora B/Aurora B was calculated and normalized for siLuc. Error bars are SEM between cells from two independent experiments (10–15 cells/experiment).
(F and G) Quantification of PP2A-B56α and PP2A-B56ɛ and Aurora B and pAurora B of cells transfected with siRNAs for Luciferase or a pool of siRNAs targeting all five PP2A-B56 isoforms (siB56) and treated with 0.83 μM nocodazole. Error bars are SEM between cells from three independent experiments (10–15 cells/experiment).
(H and I) Representative images belonging to (F) and (G). The scale bars represent 5 μm; ****p < 0.0001. See also Figure S4.
DISCUSSION
We here provide evidence that Sgo1 recruits a pool of PP2A-B56 to centromeres that counteracts Aurora B kinase activity, given its negative effect on Aurora B T-loop phosphorylation. Increased levels of Sgo1-PP2A at centromeres also diminished phosphorylation of INCENP-S893, S894, CENPA-S7, and Dsn1-S109. Whether these sites are direct substrates for Sgo1-PP2A or less well phosphorylated by Aurora B remains to be determined. The high levels of Sgo1, PP2A-B56, and Aurora B on centromeres of unattached chromosomes places Sgo1-PP2A-B56 in an excellent position to locally dampen Aurora B activity to allow initial establishment of k-MT attachments in early mitosis (Foley et al., 2011).
Sgo1-dependent PP2A recruitment is thought to be required for protection of centromeric cohesin during mitosis (Kitajima et al., 2006; Riedel et al., 2006), and a direct association between Sgo1 and PP2A-B56 was demonstrated (Tang et al., 2006; Xu et al., 2009). However, depletion of Sgo1 in HeLa cells did not impair centromeric PP2A-B56 levels (Huang et al., 2007; Kitajima et al., 2006). This suggested that, during vertebrate mitosis, Sgo1 could have cohesin-protective activities independent of PP2A or that mammalian Sgo1 might recruit a small subpopulation of PP2A-B56 specifically required for cohesin regulation (Hara et al., 2014; Tang et al., 2006; Xu et al., 2009). In marked contrast, depletion of Sgo2 results in a clear loss of PP2A from centromeres of HeLa cells (Huang et al., 2007; Kitajima et al., 2006; Tanno et al., 2010). Yet, Sgo2 depletion does not cause overt cohesin defects but instead gives rise to k-MT attachment errors and chromosome congression defects, which are associated with the loss of MCAK rather than the loss of PP2A from centromeres (Huang et al., 2007; Tanno et al., 2010). We here show that depletion of Sgo1 in non-transformed RPE-1 cells results in an approximate 40% reduction of centromeric PP2A-B56 levels and that elevating the levels of Sgo1 at centromeres increases the centromeric levels of PP2A-B56 in these cells. At present, we have no satisfactory explanation for this discrepancy with previous work in HeLa cells, other than the obvious difference in cell type used and/or efficiency of Sgo1 knockdown. Our work demonstrates that, in RPE-1 cells, Sgo1 plays a role in the recruitment of PP2A-B56 to centromeres and that this pool of PP2A regulates k-MT attachment stability by balancing Aurora B activity.
The function, regulation, and substrate specificity of the different PP2A-B56 holoenzymes is not well understood. Expression of HA-tagged B56 isoforms in HeLa cells showed that B56α, B56bβ, and B56ɛ localized to centromeres and B56gγ and B56δ to kinetochores (Nijenhuis et al., 2014). Due to lack of good antibodies that detect all five B56 isoforms by IF, it is presently unclear how this relates to the localization of the endogenous B56 isoforms. We found that endogenous B56α and B56ɛ were present at centromeres of unattached chromosomes in RPE-1 cells, similar to the HA-tagged variants in HeLa cells (Nijenhuis et al., 2014). Yet, we could detect B56α and B56ɛ at kinetochores of unattached chromosomes, when Sgo1 was depleted. Whether a particular PP2A-B56 holoenzyme localizes to the centromere or kinetochore might thus be explained by a higher binding affinity of B56γ and B56δ for BuBR1 and of B56α, B56β, and B56ɛ for Sgo1 (or Sgo2). However, despite the clear presence of BubR1 at kinetochores in RPE-1 cells, we were unable to detect B56δ. Only upon overexpression of Sgo1 did we perceive B56δ at centromeres. This could mean that the different B56 isoforms can bind to both BubR1 and Sgo1 and that the relative abundance of the B56 isoforms or of BubR1 and Sgo1 (or Sgo2) in a particular cell type may in the end determine which PP2A-B56 holoenzyme ends up at the centromere and which one at the kinetochore.
Ample evidence indicates that Sgo1 is needed to recruit the CPC to centromeres (Tsukahara et al., 2010; van der Waal et al., 2012b; Wang et al., 2010), and this recruitment likely relies on phosphorylation of histone 2A (Yamagishi et al., 2010). It was therefore remarkable that we failed to detect an increase in Aurora B at centromeres in cells where Sgo1 overexpression led to increases in the deposition of Sgo1 on centromeres. However, this result is in line with data from Ricke et al. (2012), where expression of a CB-Sgo1 fusion protein failed to restore centromere localization of Aurora B in mouse embryonic fibroblasts of transgenic animals lacking phosphorylated H2A due to mutation of Bub1 kinase. Moreover, a similar pattern was observed for B56α in RPE-1 cells: centromere levels of PP2A-B56α were reduced by Sgo1 depletion but unchanged by Sgo1 overexpression (Figures 4B, S2B, and S3B). It suggests that the protein levels of certain components of the Aurora B centromere recruitment network may be limiting and that increasing the levels of just one recruitment factor may therefore be insufficient.
Interestingly, certain tumors seem to overexpress Sgo1 (Iwaizumi et al., 2009; Liu et al., 2012). We show that, when Sgo1 is overexpressed, excess PP2A is recruited to centromeres, without the concomitant extra recruitment of Aurora B. This appears to tip the phosphatase-kinase balance toward dephosphorylation, resulting in diminished Aurora B function and the consequent premature hyperstabilization of k-MT attachments. Because hyperstable k-MTs are associated with a high rate of chromosome missegregations (a.k.a. chromosomal instability; Bakhoum et al., 2009), it may be that, in tumors expressing high levels of Sgo1, Aurora B activity is imbalanced.
EXPERIMENTAL PROCEDURES
RPE-1 and U2OS cells were cultured in DMEM-F12 (Gibco) with 10% FCS (Lonza) and DMEM with 6% FCS, respectively. RPE-1 Trex/FlpIn cells (gift from J. Pines) were co-transfected with pOG44 (Invitrogen) and pcDNA5/FRT/TO plasmids (J. Pines), encoding VSV-Sgo1, VSV-CB-Sgo1WT, and VSV-CB-Sgo1N61I, and selected with 800 mg/ml G418. U2OS cells stably expressing TetR were transfected with pcDNA4/TO vectors (Invitrogen), encoding VSV-Sgo1WT and VSV-Sgo1N61I, and selected with 350 μg/ml Zeocin (Invitrogen). Protein expression was induced with 1 μg/ml doxycycline (Sigma-Aldrich). Although all stable cell lines were derived from single clones, differences in expression levels per cell were still observed. For IF experiments, only cells with equal VSV expression levels were compared and analyzed.
Supplementary Material
Highlights.
Sgo1 co-recruits Aurora B and PP2A to centromeres of unattached chromosomes
Sgo1 overexpression recruits excess centromeric PP2A that counteracts Aurora B
Sgo1 promotes chromosome bi-orientation by balancing Aurora B activity at centromeres
Acknowledgments
We thank J. Pines, W.C. Hahn, M. Lampson, A. Losada, A. Musacchio, I. Cheeseman, and G. Kops for reagents and the S.M.A.L., Kops, and Rowland labs for helpful discussions. This work was supported by grants from the Netherlands Organization of Scientific Research (NWO-Vici 918.10.036) and Utrecht University to S.M.A.L. and from NIH to D.A.C. (GM051542) and L.K. (GM008704).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.03.052.
AUTHOR CONTRIBUTIONS
L.K. did experiments for Figures 3I and S3P. M.J.M.V. generated CB-Sgo1. All other experiments were performed by A.M. Research design and data analysis were mainly conducted by A.M. and S.M.A.L. A.M., D.A.C., and S.M.A.L. wrote the paper.
References
- Bakhoum SF, Genovese G, Compton DA. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M, Gerlich DW, Lesage B. Mitotic phosphatases: from entry guards to exit guides. Trends Cell Biol. 2009;19:531–541. doi: 10.1016/j.tcb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Eshleman HD, Morgan DO. Sgo1 recruits PP2A to chromosomes to ensure sister chromatid bi-orientation during mitosis. J Cell Sci. 2014;127:4974–4983. doi: 10.1242/jcs.161273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley EA, Maldonado M, Kapoor TM. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat Cell Biol. 2011;13:1265–1271. doi: 10.1038/ncb2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Wynne DJ. Making an effective switch at the kinetochore by phosphorylation and dephosphorylation. Chromosoma. 2013;122:135–158. doi: 10.1007/s00412-013-0401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi R, Gillespie PJ, Hirano T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol. 2006;16:2406–2417. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Zheng G, Qu Q, Liu H, Ouyang Z, Chen Z, Tomchick DR, Yu H. Structure of cohesin subcomplex pinpoints direct shugoshin-Wapl antagonism in centromeric cohesion. Nat Struct Mol Biol. 2014;21:864–870. doi: 10.1038/nsmb.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Feng J, Famulski J, Rattner JB, Liu ST, Kao GD, Muschel R, Chan GK, Yen TJ. Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J Cell Biol. 2007;177:413–424. doi: 10.1083/jcb.200701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaizumi M, Shinmura K, Mori H, Yamada H, Suzuki M, Kitayama Y, Igarashi H, Nakamura T, Suzuki H, Watanabe Y, et al. Human Sgo1 downregulation leads to chromosomal instability in colorectal cancer. Gut. 2009;58:249–260. doi: 10.1136/gut.2008.149468. [DOI] [PubMed] [Google Scholar]
- Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS, Watanabe Y. Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 2007;21:420–435. doi: 10.1101/gad.1497307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441:46–52. doi: 10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- Kruse T, Zhang G, Larsen MS, Lischetti T, Streicher W, Kragh Nielsen T, Bjørn SP, Nilsson J. Direct binding between BubR1 and B56-PP2A phosphatase complexes regulate mitotic progression. J Cell Sci. 2013;126:1086–1092. doi: 10.1242/jcs.122481. [DOI] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters JM. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang N, Liu J, Min J, Ma N, Liu N, Liu Y, Zhang H. Lentivirus-mediated siRNA interference targeting SGO-1 inhibits human NSCLC cell growth. Tumour Biol. 2012;33:515–521. doi: 10.1007/s13277-011-0284-0. [DOI] [PubMed] [Google Scholar]
- Liu H, Jia L, Yu H. Phospho-H2A and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr Biol. 2013;23:1927–1933. doi: 10.1016/j.cub.2013.07.078. [DOI] [PubMed] [Google Scholar]
- Nijenhuis W, Vallardi G, Teixeira A, Kops GJ, Saurin AT. Negative feedback at kinetochores underlies a responsive spindle checkpoint signal. Nat Cell Biol. 2014;16:1257–1264. doi: 10.1038/ncb3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke RM, Jeganathan KB, Malureanu L, Harrison AM, van Deursen JM. Bub1 kinase activity drives error correction and mitotic checkpoint control but not tumor suppression. J Cell Biol. 2012;199:931–949. doi: 10.1083/jcb.201205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, Gálová M, Petronczki M, Gregan J, Cetin B, et al. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature. 2006;441:53–61. doi: 10.1038/nature04664. [DOI] [PubMed] [Google Scholar]
- Rivera T, Ghenoiu C, Rodríguez-Corsino M, Mochida S, Funabiki H, Losada A. Xenopus Shugoshin 2 regulates the spindle assembly pathway mediated by the chromosomal passenger complex. EMBO J. 2012;31:1467–1479. doi: 10.1038/emboj.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimian KJ, Ballister ER, Smoak EM, Wood S, Panchenko T, Lampson MA, Black BE. Feedback control in sensing chromosome biorientation by the Aurora B kinase. Curr Biol. 2011;21:1158–1165. doi: 10.1016/j.cub.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suijkerbuijk SJ, Vleugel M, Teixeira A, Kops GJ. Integration of kinase and phosphatase activities by BUBR1 ensures formation of stable kinetochore-microtubule attachments. Dev Cell. 2012;23:745–755. doi: 10.1016/j.devcel.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell. 2006;10:575–585. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Tanno Y, Kitajima TS, Honda T, Ando Y, Ishiguro K, Watanabe Y. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 2010;24:2169–2179. doi: 10.1101/gad.1945310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467:719–723. doi: 10.1038/nature09390. [DOI] [PubMed] [Google Scholar]
- van der Waal MS, Hengeveld RC, van der Horst A, Lens SM. Cell division control by the Chromosomal Passenger Complex. Exp Cell Res. 2012a;318:1407–1420. doi: 10.1016/j.yexcr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- van der Waal MS, Saurin AT, Vromans MJ, Vleugel M, Wurzenberger C, Gerlich DW, Medema RH, Kops GJ, Lens SM. Mps1 promotes rapid centromere accumulation of Aurora B. EMBO Rep. 2012b;13:847–854. doi: 10.1038/embor.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JM. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;330:231–235. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn JP, Vleugel M, Liu D, Yates JR, 3rd, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Cetin B, Anger M, Cho US, Helmhart W, Nasmyth K, Xu W. Structure and function of the PP2A-shugoshin interaction. Mol Cell. 2009;35:426–441. doi: 10.1016/j.molcel.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Raetz EA, Kitagawa M, Virshup DM, Lee SH. BUBR1 recruits PP2A via the B56 family of targeting subunits to promote chromosome congression. Biol Open. 2013;2:479–486. doi: 10.1242/bio.20134051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330:239–243. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Urano T, Kawajiri A, Nagata K, Tatsuka M, Saya H, Furukawa K, Takahashi T, Izawa I, Inagaki M. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- Zeitlin SG, Barber CM, Allis CD, Sullivan KF. Differential regulation of CENP-A and histone H3 phosphorylation in G2/M. J Cell Sci. 2001;114:653–661. doi: 10.1242/jcs.114.4.653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


