Abstract
A variety of stressors including alcohol (EtOH) are known to induce collagen production and fibrotic diseases. Matrix metalloproteinases (MMP) play an important role in regulating fibrosis, but little is known regarding the relationship between EtOH and MMPs. In addition, the signaling cascades involved in this process have not been elucidated. We have identified the MMP Adamts1 as a target of EtOH regulation. To characterize the function of Adamts1, we examined EtOH-induced alterations in collagen I and elastin protein levels in C2C12 myocytes. Incubation of myocytes with 100 mM EtOH decreased elastin and increased collagen content, respectively, and these changes were associated with increased O-GLcNAc modification of Adamts1. Conversely, silencing of Adamts1 by siRNA blocked the adverse effects of EtOH on collagen and elastin levels. Similar results were obtained after treatment with a pharmacological inhibitor of MMP. Changes in collagen were due, at least in part, to a decreased interaction of Adamts1 with its endogenous inhibitor TIMP3. The AMPK inhibitor compound C blocked the EtOH-induced stimulation of collagen and O-GLcNAc Adamts1 protein. Changes in AMPK appear linked to FoxO1, since inhibition of FoxO1 blocked the effects of EtOH on AMPK phosphorylation and O-GLcNAc levels. These FoxO-dependent modifications were associated with an upregulation of the FoxO1 transcription target sestrin 3, as well as increased binding of sestrin 3 with AMPK. Collectively, these data indicate that EtOH regulates the collagen I and elastin content in an Adamts1-dependent manner in myocytes. Furthermore, Adamts1 appears to be controlled by the FoxO1-sestrin 3-AMPK signaling cascade.
Keywords: matrix metalloproteinases, alcohol, FoxO, AMPK, collagen, elastin
Excessive ethanol (EtOH) consumption is a major cause of myocardial fibrosis, liver cirrhosis and pancreatitis [Mello et al., 2008; Oruc and Whitcomb, 2004]. A common characteristic linking each of these fibrotic diseases is the abnormal production of extracellular matrix (ECM) components such as collagen [Bailey et al., 2012; Wynn, 2008]. Although the effects may vary, damage to the ECM can lead to disruption of homeostasis, as well as increased tissue stiffness and progressive organ dysfunction. Previous studies demonstrated that EtOH or its metabolite acetaldehyde induce collagen I synthesis in cardiac fibroblast and hepatic stellate cells [Casini et al., 1991; El Hajj et al., 2014; Law and Carver, 2013; Moshage et al., 1990]. Conversely, studies using skin fibroblasts have shown a decrease in collagen I, albeit in conjunction with an increase in collagen III [Ranzer et al., 2011]. In smooth muscle cells, on the other hand, chronic EtOH exposure to rats decreases elastic fiber and collagen IV [Yeşilli et al., 2006].
The proteolytic degradation of collagen is regulated by a group of protease families that include matrix metalloproteinases (MMPs) and membrane proteinases that contain disintegrin and metalloproteinase domains (ADAM) [Mochizuki and Okada, 2007; Nagase et al., 2006; Ra and Parks, 2007]. Other members of the ADAM family are responsible for the shedding of cytokines and their receptors which may participate in tissue remodeling [Perna et al., 2013]. ECM remodeling in the presence of EtOH is promoted by up-regulation of the collagenase MMP-2, as well as down regulation of fibrillary collagenase MMP-1 expression. Thus, this results in the substitution of normal ECM components with a sclerotic matrix [Casini et al., 1994]. Despite the importance of EtOH-induced fibrosis, the molecular mechanisms underlying the changes in ECM proteins in various organs are still largely unknown.
Adamts1 (a disintegrin and metalloproteases with thrombospondin type 1 motifs) belongs to a family of metalloproteinases involved in the processing of procollagen I, procollagen III, and proteoglycans [Apte, 2009; Mochizuki and Okada, 2007]. Previous studies demonstrated that Adamts 1cleaves ECM components such as aggrecan and versican [Kuno et al., 2000; Rodriguez-Manzaneque et al., 2002; Russell et al., 2003]. This protein also has type I collagen degrading activity as demonstrated in rat and human osteoblasts, ovarian follicles, and a murine model of chronic viral myocarditis [Brown et al., 2006; Guo et al., 2010; Rehn et al., 2007]. The activity of Adamts1 is controlled by a variety of factors. For example, Adamts 1 is up-regulated in liver fibrosis model, in association with hepatic stellate cell activation and elevated type I collagen [Bourd-Boittin et al., 2011]. In addition, this proteinase can be induced by a number of external stimuli such as lipopolysaccharides, chemical agents as well as under inflammatory conditions [Krampert et al., 2005; Kuno et al., 1997].
AMP-activated protein kinase (AMPK) is a central nutrient and cell energy sensing protein activated under unfavorable conditions (e.g., hypoxia, glucose starvation, EtOH) [Gwinn et al., 2008; Hong-Brown et al., 2010]. AMPK signals regulate the activity of a number of proteins associated with mammalian target of rapamycin complex 1 (mTORC1), including tuberous sclerosis protein complex 2 (TSC2) and raptor, thereby regulating the activity of mTORC1. The FoxO family of forkhead transcription factors also plays an important role in energy metabolism, tumor suppression and cell proliferation [Greer and Brunet, 2005; Gross et al., 2008; Matsumoto et al., 2007]. It is well established that FoxO and mTORC1 are regulated by Akt in response to a variety of stimuli. However, FoxO has also been shown to regulate mTORC1 and mTORC2 under stress conditions, thereby maintaining a regulatory balance between the two mTOR complexes. For example, FoxO1 stimulates mTORC2 activity by increasing the level of one of its subunits, i.e., the rictor protein. Conversely, FoxO1 appears to inhibit the activity of mTORC1 via transcriptional up regulation of sestrin 3 [Chen et al., 2010]. Taken together, the FoxO-sestrin-AMPK-mTOR axis appears to play an important role in mammalian cell signaling events [Hay, 2011]. At present, however, it is unknown whether EtOH affects FoxO activity, or if there is a regulatory interplay between FoxO1, sestrin3 and AMPK in the presence of this drug.
Results from a microarray profile in our laboratory indicated that chronic EtOH consumption by rats increased Adamts1 gene expression in both skeletal and heart tissues. To further characterize this effect, we utilized a C2C12 myocyte model system that is routinely used in our laboratory and examined the role that Adamts1 plays in controlling ECM proteins in response to EtOH. In our previous studies, EtOH has been shown to reduce protein synthesis in C2C12 cells. Furthermore, this effect was associated with a decrease in mTORC1 function, albeit with a corresponding increase in mTORC2 activity. In each case, the effects of EtOH on mTOR appeared to be mediated by an AMPK-TSC2 dependent mechanism [Hong-Brown et al., 2012]. In the present study, we elucidated the regulatory signaling and connections between AMPK and Adamts1. EtOH was observed to increase collagen I and decrease elastin in C2C12 myocytes, with these changes being mediated by Adamts1. Accordingly, knockdown of Adamts1 blunted the adverse effects of EtOH on these two proteins. Finally, our results indicate that EtOH affects Adamts1 via an AMPK signaling cascade that appears dependent on the FoxO1-sestrin axis.
MATERIALS AND METHODS
Ethanol was purchased from Fisher Scientific Co. (Springfield, NJ). Antibodies against collagen I (Col1A1) and elastin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The Adamts1and scrambled siRNA were obtained from the same source. Antibody to Adamts1 was from R&D System, Inc (Minneapolis, MN), and TIMP1 was purchased from Abcam (Cambridge, MA). The sestrin 3 antibody was from Acris (San Diego, CA). Antibodies against phosphorylated (p)-FoxO1 (Ser256), p-FoxO1(T24) /Fox3a (T32), p-AMPKα (T172), TIMP3 and tubulin were obtained from Cell Signaling Technology (Beverly, MA), with the latter serving as a control for equal protein loading of samples. Compound C, the FoxO1 inhibitor AS1842856, and the MMP inhibitor GM6001 [Ilomastat] were from EMD Millipore Corporation (Temecula, CA). Phenylmethanesulfonyl fluoride (PMSF), protease, phosphatase I and II inhibitor cocktails were from Sigma Aldrich (St. Louis, MO). Cell culture media and fetal bovine serum (FBS) were from Mediatech, Inc (Manassas, VA).
CELL CULTURE AND TRANSFECTION
C2C12 mouse myoblasts (American Type Culture Collection; Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml) and amphotericin (25 μg/ml) at 37°C in 5% CO2. Cells were plated on 6-well or 10 cm dishes and grown to confluence. All studies were conducted using cells at the early passage of the myoblast stage unless otherwise indicated. Upon confluence, cells were switched to 1.5 % FBS media in the presence or absence of 100 mM EtOH for 18–24 h as previously described [Hong-Brown et al., 2013]. The presence of serum was necessary because the survival of C2C12 myocytes is decreased when cells are cultured for extended periods in serum-free media (SFM). A concentration of 100 mM EtOH was used because it produces an optimal effect without being cytotoxic to myocytes. During the incubation, plates were sealed with parafilm to minimize the evaporation of EtOH.
For transient expression, the siRNA target Adamts1 (sc41426) or scrambled sequences [Trimborn et al., 2004] were transfected into C2C12 myocytes using electroporation technology (Amaxa Biosystems nucleofector II, Germany) and the Amaxa cell line nucleofector kit V (Lonza Walkersville Inc, Walkersville, MD). All transfections were performed following the manufactures’ protocol. Experiments were carried out 24–36 h post-transfection, and cells were harvested 18–24 h thereafter for immunoblot analysis.
DRUG TREATMENTS
For studies using chemical inhibitors of AMPK, FoxO1 or MMP, cells were pre-treated as noted in the figure legends. The concentration and incubation times for reagents were chosen based on dose- and time-response curves derived from our preliminary studies and the literature [Hong-Brown et al., 2007; Tanaka et al., 2010]. Total protein was determined using the bicinchoninic acid reagent (BCA) protein assay kit with bovine serum albumin (BSA) as a standard (Pierce, Rockford, IL). The results were normalized and compared with the control group. Data were expressed as a percentage of the control value.
AGAROSE WHEAT GERM AGGLUTININ (WGA) PRECIPITATION, IMMUNO-PRECIPITATION AND WESTERN BLOT ANALYSIS
C2C12 myocytes were lysed in buffer containing 1% NP40, 50mM Tris-HCl (pH 8), 137 mM NaCl, 10% glycerol, PMSF and a cocktail of protease and phosphatase inhibitors as described by Kuo et al. (2008) with minor modification. Briefly, soluble fractions of cell extracts (250–500 μg of protein) were pre-cleared with sepharose CL4B (Sigma Aldrich) for 1h at 4°C. The supernatant was incubated with 30–40 μl of WGA beads (Vector Laboratories, Burlingame, CA) overnight at 4°C with rotation to capture O-GLcNAc modified proteins. The beads were washed and suspended in 2x Laemmli sample buffer (LSB). For immunoprecipitation (IP), cells were lysed with 0.3% CHAPS (3-[(3-cholamindopropyl) dimethylammonio]-1-propanesulfonate) buffer containing 40 mM Hepes (pH 7.5), 120 mM NaCl, 1 mM EDTA, PMSF and a cocktail of protease and phosphatase inhibitors. Primary antibody (1:60) was added to equal amounts of protein (300–500 μg) from cell lysates and rotated overnight at 4°C. A total of 40 μl of a 50% slurry of protein A sepharose (GE Healthcare, Piscataway, NJ) was then added for an additional 1 h. Immunoprecipitates were washed 3 times with lysis buffer and the precipitated proteins were denatured by the addition of 2x LSB.
For immunobloting, equal amounts of protein from IP or WGA precipitated lysates were electrophoresed on denaturing polyacrylamide gels and transferred to nitrocellulose. The resulting blots were blocked with 5% nonfat dry milk or 3–5 % BSA and incubated with the antibodies of interest. Unbound primary antibody was removed by washing with TBS containing 0.05% Tween-20 and blots were incubated with secondary antibody conjugated with horseradish peroxidase (HRP). Blots were briefly incubated with an enhanced chemiluminescent detection system (GE Healthcare, Buckinghamshire, UK) and exposed to X-ray blue film (Cole Parmer, Vernon Hills, IL) or the FluroChem M multifluor system was used for visualization (Proteinsimple, Santa Clara, CA) and quantified with Image J (NIH, Bethesda, MD).
QUANTITATIVE REAL-TIME PCR
Total RNA was isolated using Qiagen RNeasy mini kit (Qiagen, Valencia, CA). RNA (1 μg) was reverse-transcribed using Invitrogen Reverse Transcriptase superscript III. The quantitative PCR (qPCR) assays were performed following the manufactures’s protocol: an initial 10 min denaturation step at 95°C, followed by forty cycles at 95°C (15 sec) and 60°C (1 min) using an Applied Biosystem StepOne Plus Real-Time PCR instrument (Foster city, CA). The TapMan primer probe set Adamt1 (Mm00477355_m1) and RpL32 (Mm02528467_g1) were purchased from Applied Biosystems. Results were calculated using the 2 −Δ ΔCT relative quantification method normalized to endogenous control RpL32.
STATISTICAL ANALYSIS
For experimental protocols with more than two groups, statistical significance was determined using one-way ANOVA followed by the Dunnett’s test to compare all data to the appropriate control group. For experiments with only two groups, an unpaired Student’s t-test was performed. Data are presented as mean ± SE. A value of P < 0.05 was considered statistically significant.
RESULTS
EtOH DECREASES ELASTIN AND INCREASES COLLAGEN I CONTENT
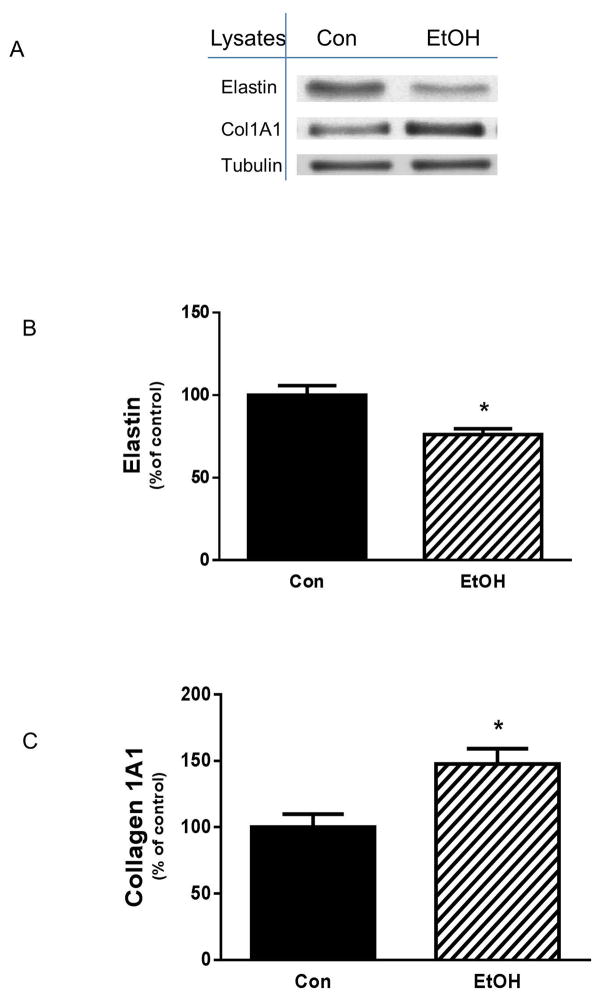
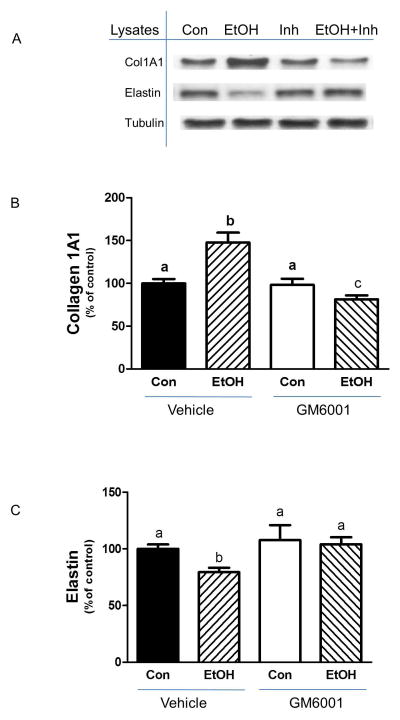
A variety of external insults have been reported to alter type I collagen and other components of the extracellular matrix [Bourd-Boittin et al., 2011; Yu et al., 2013]. Furthermore, these changes are associated with fibrosis in a number of cell types and tissues. A recent study found that EtOH increases the amount of collagen I and III in both cardiac fibroblasts and heart tissue [El Hajj et al., 2014]. However, at present, little is known regarding the effects of EtOH on collagen I and elastin in skeletal muscle. To address this issue, mouse C2C12 myocytes were treated with 100 mM of EtOH and examined for the effects on ECM components. EtOH decreased elastin level as compared to controls (Fig.1A&B). In contrast, an increase in collagen I A1content was observed following incubation with EtOH (Fig. 1A & C). This effect was not dependent on the state of muscle cell differentiation, as a similar enhancement was detected in C2C12 myotubes (data not shown).
Fig. 1. Effect of EtOH on elastin and collagen I contents.
C2C12 myocytes were incubated in the presence or absence of 100 mM EtOH for 18 h. Equal amounts of protein from cell lysates were analyzed via Western blotting using antibodies against the indicated proteins (panel A). The protein levels were quantified and plotted on a bar graph (panel B&C). Results were normalized to total protein and expressed as percentage of control levels. Data are means ± SE of 5 independent experiments consisting of 4 replicate samples per experiment. * P< 0.05 versus the control value.
EFFECTS OF EtOH ON ADAMTS 1
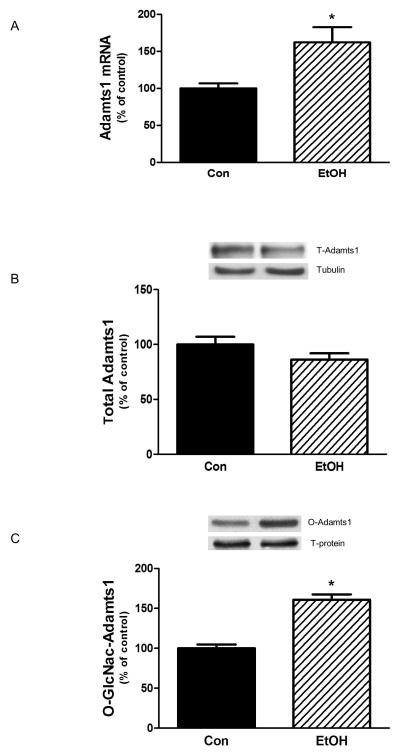
Adamts play a role in regulating various extracellular matrix proteins including collagen [Mochizuki and Okada, 2007]. While conducting a screen for EtOH-induced changes in gene expression, we identified Adamts1 as a target that was modified by EtOH. Hence, we undertook a characterization of the effects of EtOH on Adamts1 levels and function. As illustrated in Fig. 2A, incubation of myocytes with EtOH increased Adamt1 mRNA as compared to control cells, but this elevation did not change the overall level of Adamts1 protein (Fig. 2B).
Fig. 2. EtOH increases gene expression and O-GLcNAc modification of Adamts1.
C2C12 myocytes were incubated with EtOH as described in Fig.1. mRNA levels were determined by quantitative real-time PCR as described in the Materials and Methods section. Changes in mRNA level were calculated and normalized to rpL32 mRNA (Panel A). Panel B, equal amounts of cell lysates were immunoblotted with antibodies against Adamts1. Panel C, Cytosolic extracts (250–400 μg of protein) from control and EtOH-treated cells were incubated with WGA-conjugated beads overnight and analyzed for Adamts1 O-GLcNAc-modification. Results were normalized to total protein and expressed as a percentage of control levels. Data are means ± SE of 4 independent experiments consisting of 4 replicate samples per experiment. * P< 0.05 versus the control value.
Because antibodies recognizing phosphorylated forms of Adamts1 were not available, we could not determine whether EtOH modified this parameter. However, studies have shown that addition of a single O-linked β-N acetylglucoamine (O-GlcNAc ) on either serine or threonine residues of a cytosol or nucleus protein can also affect the function of this modified protein [Wells et al., 2001]. Furthermore, in many cases, increased levels of O-GlcNAc are associated with a diminished level of protein phosphorylation [Slawson et al., 2006]. Therefore, as an alternative approach, we examined the effect of EtOH on the O-GlcNAcylation of Adamts1. Treatment of myocytes with EtOH increased the level of O-GlcNAc modification of Adamts1 (Fig. 2C). Thus, O-GlcNAc modification may be indicative of changes in the activity of Adamts1 towards downstream targets such as collagen and elastin.
ADAMTS 1 REGULATES EtOH-INDUCED CHANGES IN COLLAGEN I AND ELASTIN LEVELS
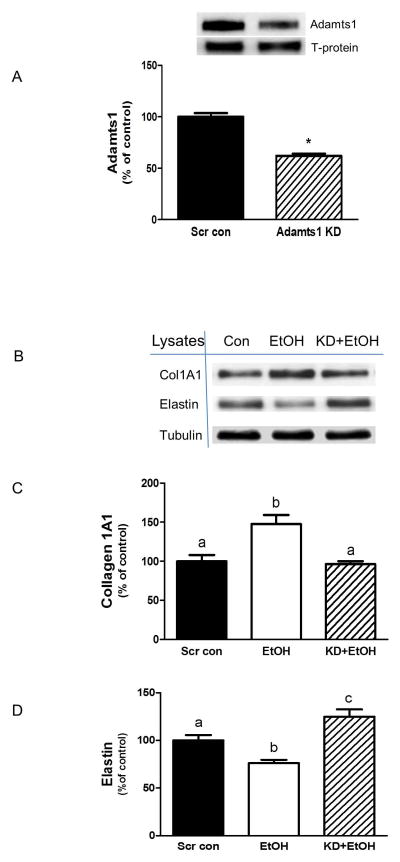
To assess the potential role of Adamts1 in regulating collagen and elastin, myocytes were subjected to Adamts1 silencing. Figure 3A shows that myocytes transfected with Adamts1 siRNA had decreased levels of this protein (35–40%) as compare to scrambled control cells. When these cells were incubated in the presence or absence of EtOH, the knockdown (KD) of Adamts1 blocked the EtOH effect on collagen I (Fig. 3B&C) and elastin protein (Fig. 3B&D). These data suggest that Adamts1 plays some role in directly modulating the levels of these proteins.
Fig. 3. Effect of Adamts1 knockdown (KD) on collagen I and elastin protein.
C2C12 myocytes were transfected with scrambled siRNA or an Adamts1 specific siRNA. Equal amounts of protein lysates from scrambled control and Adamts1 KD cells were analyzed via Western blots using antibody against Adamts1 (panel A), collagen I and elastin (panel B). Quantified data were plotted on a bar graph (panel C&D). Results were normalized to total cell protein and expressed as a percentage of scrambled control levels. Each bar graph represents mean ± SE of 3 independent experiments consisting of 3 replicate samples per experiment. * P< 0.05 versus the scrambled control value. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
EFFECTS OF EtOH ON TIMP1 AND TIMP3
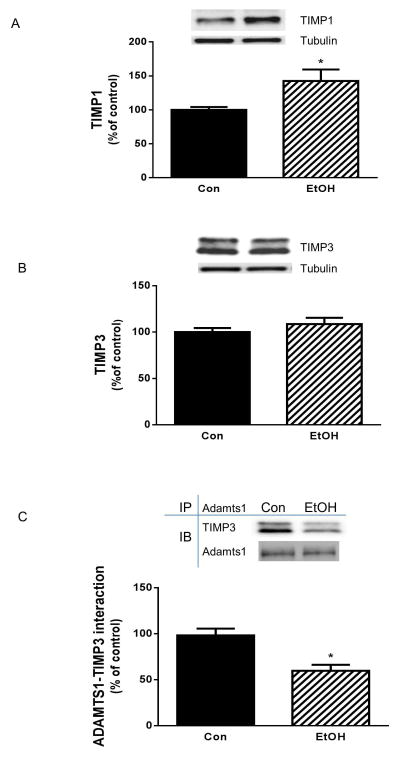
Tissue inhibitors of metalloproteinases (TIMPs) can complex with Adamts and irreversibly inhibit the activity of these proteins [Baker et al., 2002]. Furthermore, the levels of various TIMPs are elevated in response to EtOH in cardiac fibroblasts [El Hajj et al., 2014]. EtOH-induced changes in Adamts function may be subject to regulation by proteins of the TIMP family. Hence, we examined whether EtOH had any effect on these endogenous inhibitors. In the presence of EtOH there were elevated levels of TIMP1, both in myoblasts (Fig. 4A) and in myotubes (data not shown). In contrast, TIMP3 protein levels were not affected by EtOH (Fig. 4B). Because Adamts1 is reportedly affected by TIMP3 [Rodriguez-Manzaneque et al., 2002], we examined whether EtOH alters the association of these proteins. Fig. 4C illustrates that EtOH decreased the interaction between TIMP3 and Adamts1 compared to control values. This result suggests that the function of Adamts1 toward its downstream target collagen and elastin is due, at least in part, to changes in this protein-protein interaction.
Fig. 4. Effects of EtOH on TIMP1, TIMP3 and its binding with Adamts1.
C2C12 myocytes were incubated with EtOH as described in Fig.1. Equal amounts of protein from cell lysates were analyzed via Western blotting using antibodies against TIMP1 (panel A) and TIMP3 (panel B). Adamts1 was immunoprecipitated from equal amount of cell extracts and immunoblotted with TIMP3 antibody (panel C). Results were normalized with immunoprecipitated (IP) Adamts1 that was assessed by immunoblotting. Data are means ± SE of 4 independent experiments consisting of 4 replicate samples per experiment. * P< 0.05 versus the control value.
To further confirm that Adamts1 plays a role in regulating collagen and elastin, we used a pharmacological inhibitor of MMP [Lim et al., 2014]. Incubation of cells with GM6001 alone did not affect collagen or elastin levels, as compared to control cells (Fig. 5A-C). However, this inhibitor attenuated the EtOH-induced increase in collagen, with levels remaining below those observed in control cells (Fig. 5A & B). Similarly, this agent prevented the suppressive effect of EtOH on elastin levels (Fig. 5A & C).
Fig. 5. MMP inhibition blocks the effect of EtOH on collagen I and elastin levels.
C2C12 myocytes were incubated with EtOH as described in Fig.1. Cells were pre-incubated with the MMP inhibitor GC6001 (15 μM) in SFM for 35 min. Thereafter, EtOH was added to the media for an additional 50 min in the presence or absence of inhibitor. Cells were collected and analyzed via Western blotting using antibodies against the indicated proteins (panel A). The protein levels were quantified on a bar graph (panel B&C). Results were normalized to total cell protein and expressed as a percentage of control values. Each bar graph represents mean ± SE of 4 independent experiments consisting of 3–4 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
EtOH ALTERS O-GlcNAc ADAMTS 1 IN AN AMPK-DEPENDENT MANNER
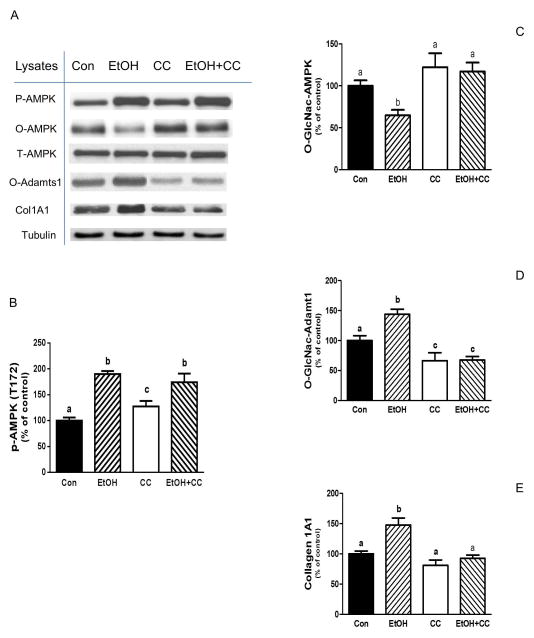
AMPK plays a key role in mediating the effects of EtOH on various signal transduction pathways. As such, EtOH increases both AMPK phosphorylation and its activity towards various downstream targets [Hong-Brown et al., 2012]. Therefore, we next investigated the connection between AMPK and Adamts1. Incubation of cells with the AMPK inhibitor compound C (CC) attenuated the effect of EtOH on O-GLcNAc Adamts1 levels, with these values remaining below controls (Fig.6A&D). Furthermore, the stimulatory effect of EtOH on collagen was also inhibited in the presence of CC (Fig 6A&E). An inverse relationship was observed between O-GlcNAcylation and phosphorylation for the AMPK protein. EtOH increased AMPK phosphorylation, whereas it decreased the level of O-GlCNAc modification by 35% relative to controls (Fig 6A-C). Collectively, these results demonstrate a link between AMPK and Adamts1, specifically in terms of collagen regulation in C2C12 myocytes exposed to EtOH.
Fig. 6. EtOH increases collagen I and O-GlcNAc-Adamts1 levels via an AMPK dependent pathway.
C2C12 myocytes were incubated with EtOH as described in Fig.1. Cells were pre-incubated for 1 h in the presence or absence of the AMPK inhibitor compound C (CC, 20 μM). Thereafter, EtOH was added to SFM for an additional 50 min in the presence or absence of inhibitor. Cells were collected and analyzed via Western blotting using antibodies against the indicated proteins (panel A). The protein levels were quantified and presented as a bar graph (panel B-E). Results were normalized to total cell protein and expressed as a percentage of control values. Each bar graph represents a mean ± SE of 4 independent experiments consisting of 4 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
EtOH INCREASES FOXO1 FUNCTION AND MODULATES AMPK ACTIVITY TOWARD COLLAGEN
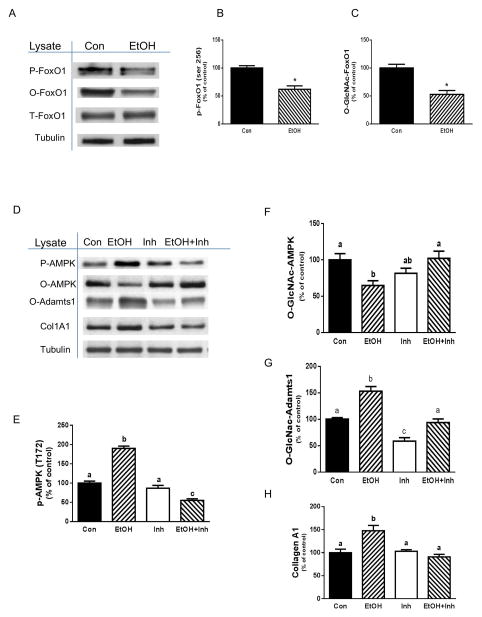
Forkhead transcription factors (FoxOs) such as FoxO1 have multiple cellular and biological roles, many of which modulate metabolism, stress resistance, longevity and apotosis [Greer et al., 2009; Gross et al., 2008]. FoxO is phosphorylated following anabolic stimulation and in the absence of these stimuli, there is an increase in the activity of the transcription factor [Greer and Brunet, 2005]. As FoxO integrates a wide range of external stimuli, we investigated whether EtOH affected the function of this protein, either through changes in its phosphorylation or O-GLcNAc status. Incubation of myocytes with EtOH decreased FoxO1 phosphorylation on both the Ser 256 (Fig. 7A&B) and Thr 24 residues (data not shown), although it did not affect the overall level of protein. Next, we examined whether levels of O-GLcNAc FoxO1 were affected by EtOH. Similar to the phosphorylation results, EtOH decreased levels of O-GLcNAc FoxO1 as compared to controls (Fig. 7A&C).
Fig. 7. EtOH increases collagen I via a FoxO1-AMPK-Adamts1 dependent pathway.
C2C12 myocytes were incubated with EtOH as described in Fig.1. Equal amounts of protein from cell lysates were analyzed via Western blotting using an antibody against p-FoxO1 (panel A). The protein levels were quantified on a bar graph (panel B). Cytosolic extracts from control and EtOH-treated cells were incubated with WGA-conjugated beads for analysis of O-GLcNAc-modification of FoxO1 (panel A&C). Panel D, cells were pre-incubated for 40 min in the presence or absence of a FoxO1 specific inhibitor (0.3 μM). Thereafter, EtOH was added to the media for an additional 50 min in the presence or absence of inhibitor. Cells were collected and analyzed via Western blotting using antibodies against the indicated proteins. Results were normalized to total cell protein and expressed as a percentage of control values (panels E-H). Each bar graph represents a mean ± SE of 4 independent experiments consisting of 3 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
Both FoxO1 and AMPK regulate energy metabolism. Furthermore, previous studies have shown a role for FoxO in the activation of AMPK [Hay, 2011]. Therefore, we examined whether the EtOH-induced changes in AMPK modification and activity were linked to FoxO1 activity. For these experiments, myocytes were incubated with a specific FoxO1 inhibitor in the absence or presence of EtOH. As illustrated in Fig. 7D&E, treatment of the cells with inhibitor alone did not alter AMPK phosphorylation. However, this inhibitor completely suppressed the EtOH-induced stimulation of AMPK phosphorylation, with levels remaining below control values. Likewise, this agent blocked the suppressive effect of EtOH on O-GLcNAc AMPK content (Fig. 7D&F). Finally, incubation of myocytes with the FoxO1 inhibitor blocked the EtOH-induced increase in O-GLcNAc Adamts1 (Fig 7D&G) and collagen content (Fig 7. D&H), indicating that Adamts1 and collagen are downstream targets of the FoxO1 pathway. Taken together, these results suggest that FoxO1 is an upstream regulator modulating the effects of EtOH on AMPK and Adamts1.
EtOH-ENHANCED FOXO1 ACTIVITY AFFECTS SESTRIN 3 AND AMPK INTERACTION
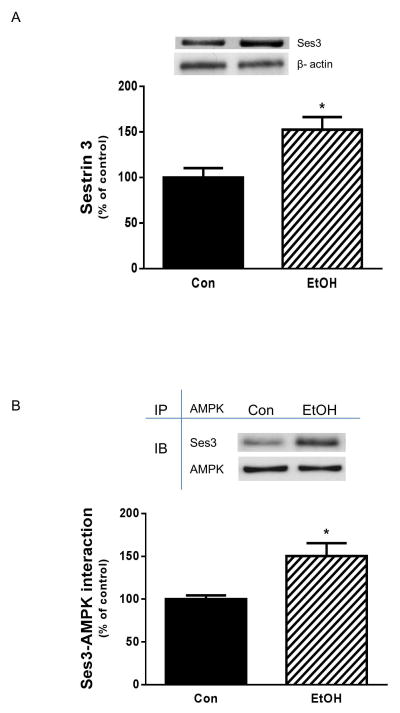
Activation of FoxO1 increases expression of the sestrin3 protein [Chen et al., 2010]. Furthermore, sestrin1/2 can activate AMPK, at least under oxidative stress conditions, by forming an interaction with this protein [Budanov and Karin, 2008]. As noted above, FoxO1 appears to modulate the effect of EtOH on AMPK phosphorylation. Thus, these findings suggest a linkage between FoxO1, sestrin and AMPK. Therefore, we examined whether enhanced AMPK function in the presence of EtOH is due to alternations in either the sestrin 3 levels or its interaction with AMPK. As demonstrated in Fig. 8A, treatment of myocytes with EtOH increased sestrin 3 content as compared to control cells. In addition, there was increased binding between sestrin3 and AMPK in the presence of EtOH (Fig. 8B). These data suggest that changes in sestsrin 3 level and protein-protein interaction may account for increased AMPK phosphorylation and activity in response to EtOH.
Fig. 8. EtOH elevates sestrin 3 and its interaction with AMPK.
C2C12 myocytes were incubated with EtOH as described in Fig.1. Equal amounts of protein from cell lysates were analyzed via Western blotting using antibody against sestrin3 (panel A). AMPK was immunoprecipitated from equal amount of cell extracts and immunoblotted with sestrin3 antibody. Results were normalized with immunoprecipitated (IP) AMPK that was assessed by immunoblotting (panel B). Data are means ± SE of 4 independent experiments consisting of 4 replicate samples per experiment. * P< 0.05 versus the control value.
DISCUSSION
A number of biochemical and mechanical factors have been implicated in promoting fibrotic diseases. Along these lines, an enhanced production of collagen I and/or III has been noted in EtOH-induced pathological conditions [El Hajj et al., 2014; Law and Carver, 2013; Roman, 2014]. In the present study, EtOH increased collagen type I A1 chain content in C2C12 myocytes. This finding was consistent with reports showing an involvement of EtOH in the stimulation of type I collagen protein and gene expression in cardiac fibroblasts and hepatic stellate cells, respectively [El Hajj et al., 2014; Mello et al., 2008]. However, this observation was in contrast to results from Ranzer et al, where acute EtOH exposure decreased collagen I while increasing collagen III levels in skin fibroblasts. The reason for this discrepancy is unclear, although it may be due to differences in cell type and/or the dose of EtOH used. In addition to changes in collagen, we detected a decrease in elastin content in response to ETOH. This finding is in agreement with published data whereby EtOH attenuated levels of elastic fibers in smooth muscle cells [Yeşilli et al., 2006]. Thus, the increase of fibrillar collagen I and/or III in combination with decreased elastic fibers may contribute to the characteristic syndrome of fibrotic diseases.
Collagen and elastic fiber levels are regulated by a group of metalloproteinases, including members of the ADAM family. Multiple lines of evidence have demonstrated that Adamts1 is essential for aggrecan and versican degradation, whereas it is not involved in the turnover of procollagen or collagen [Rodriguez-Manzaneque et al., 2002; Russell et al., 2003]. However, in the present study, we observed that silencing of Adamts1 by siRNA or chemical inhibition of this protein blocked the ability of EtOH to induce changes in collagen and elastin contents. Thus, this suggests that Adamts1 is involved in the regulation of these two proteins in C2C12 myocytes. As such, this is consistent with published reports suggesting that collagen I is a potential substrate for Adamts1[Guo et al., 2010; Rehn et al., 2007].
Changes in collagen and elastin contents are influenced by the expression and/or activity of proteinases. In the present study, Adamts1 gene expression was elevated by EtOH, although this did not affect the level of adamts1 protein. On the other hand, EtOH did cause a post-translation modification of Adamts1 which may account for a change in the function of this protein. Furthermore, this activity can be regulated by physiological inhibitors such as TIMPs which complex with proteinases to inhibit the function of these proteins [Baker et al., 2002].
Adamts1 activity is reportedly regulated by both TIMP2 and TIMP3 [Rodriguez-Manzaneque et al., 2002]. Our results indicated that protein levels of TIMP3 were unchanged following exposure to EtOH. This is in contrast to published data whereby EtOH increased TIMP3 content in cardiac fibroblasts [El Hajj et al., 2014]. On the other hand, the interaction between Adamts1 and TIMP3 was observed to decrease in the presence of EtOH. Thus, there may indeed be changes in Adamts1 activity with EtOH, even in the absence of elevated levels of Adamts1. We did note increased TIMP1 levels in C2C12 myocytes following EtOH exposure, which is in agreement with others [El Hajj et al., 2014]. At present, however, it is unclear whether TIMP1 plays a role in modulating Adamts1 activity. Taken together, our data suggest that the EtOH-induced increase in collagen I is mediated through changes in protein-protein interactions and the O-GLcNAcylation of Adamts1.
It is well established that a variety of factors increase the activity of AMPK. We previously showed that EtOH increases AMPK phosphorylation in myocytes as well as the phosphorylation of its downstream targets TSC2, raptor and eEF2. This, in turn, results in decreased mTORC1 activity and protein synthesis [Hong-Brown et al., 2012]. There is also a connection between mTOR and the synthesis of collagen. For example, studies with human fibroblasts showed that inhibition of mTOR activity by siRNA, or treatment with rapamycin decrease the expression levels of Col 1A1 and Col 1A2 by 50% [Shegogue and Trojanowska, 2004]. In the present study, we could not directly determine whether AMPK affected the phosphorylation state of Adamts1, due to the unavailability of phosphor-specific antibodies. However, we were able to utilize O-GLcNAc modification to examine the function of Adamts1. As noted previously, the addition of a single O-GLcNAc sugar to either Ser or Thr residues can affect the function of a protein and, in some cases, phosphorylation and O-GLcNAc are reciprocal modifications on the same Ser and Thr sites [Griffith and Schmitz, 1999; Slawson et al., 2006; Wells et al., 2001]. In addition, this process can occur rapidly and is inducible by a number of stimuli. Furthermore, the attachment and removal of O-GLcNAc to and from nucleocytoplasmic proteins shares a number of similarities with protein phosphorylation and dephosphorylation [Cheng et al., 2000]. In the present study, we observed an EtOH-induced increase in AMPK phosphorylation activity that was correlated with a decrease in O-GLcNAcylation. Furthermore, the EtOH-induced increases in O-GLcNAc-Adamts1 and collagen were blocked in the presence of the AMPK inhibitor compound C. Thus, this suggests a link between AMPK activity and O-GLcNAcylation of selected substrates.
The transcriptional mechanisms by which EtOH decreases mTORC1 activity and protein synthesis are not well characterized. Previously, we reported an important role for AMPK in mediating the effects of EtOH, and in the current study, we have added an additional link to FoxO1. FoxO transcription factors are involved in multiple signaling pathways and are closely correlated with metabolism, proliferation and apoptosis [Gross et al., 2008; Matsumoto et al., 2006]. Previous studies reported that FoxO is influenced by the energy status of cells, as AMPK has been shown to directly regulate FoxO3 factors [Greer et al., 2007]. On the other hand, FoxO can indirectly activate AMPK through the FoxO-sestrin-AMPK-mTORC1 axis which is conserved in mammalian cells [Hay, 2011].
Our results indicate that EtOH decreases both the phosphorylation and O-GLcNAcylation of FoxO1. Interestingly, a FoxO1 specific inhibitor blocked the EtOH-induced increase in AMPK activity. Likewise, there were significant effects on the downstream targets of AMPK, namely Adamts1 and collagen. Taken together, these results support a model in which FoxO1 plays an important role in regulating the activity of AMPK. FoxO1 binds to the promoter region of sestrin3, thereby causing elevated sestrin 3 expression, and subsequent activation of AMPK [Chen et al., 2010]. In our studies, EtOH appeared to increase FoxO1 activity, as indicated by elevated levels of sestrin 3. Although the precise mechanism by which sestrin3 regulates AMPK is not clear, an increased interaction between sestrin1 or sestrin2 and AMPK has been reported [Budanov and Karin, 2008]. Therefore, a similar mechanism may be responsible for the enhanced AMPK function under stressful conditions. Indeed, increased sestrin 3 and AMPK binding in the presence of EtOH may at least partially account for the elevated AMPK activity. In conclusion, we observed an EtOH-induced increase in collagen I levels in myocytes that was influenced by Adamts1. Furthermore, this process appears to be regulated via a FoxO1-sestrin3-AMPK signaling cascade.
Acknowledgments
We thank Drs. Abid A. Kazi and Jennifer L Steiner for their technical support. This study was supported by National Institute of Health grant R37 AA-011290.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest, financial or otherwise.
References
- Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–7. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JR, Bland PW, Tarlton JF, Peters I, Moorghen M, Sylvester PA, Probert CS, Whiting CV. IL-13 promotes collagen accumulation in Crohn’s disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? PLoS One. 2012;7:e52332. doi: 10.1371/journal.pone.0052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–27. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- Bourd-Boittin K, Bonnier D, Leyme A, Mari B, Tuffery P, Samson M, Ezan F, Baffet G, Theret N. Protease profiling of liver fibrosis reveals the ADAM metallopeptidase with thrombospondin type 1 motif, 1 as a central activator of transforming growth factor beta. Hepatology. 2011;54:2173–84. doi: 10.1002/hep.24598. [DOI] [PubMed] [Google Scholar]
- Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol. 2006;300:699–709. doi: 10.1016/j.ydbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–60. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini A, Ceni E, Salzano R, Milani S, Schuppan D, Surrenti C. Acetaldehyde regulates the gene expression of matrix-metalloproteinase-1 and -2 in human fat-storing cells. Life Sci. 1994;55:1311–6. doi: 10.1016/0024-3205(94)00763-2. [DOI] [PubMed] [Google Scholar]
- Casini A, Cunningham M, Rojkind M, Lieber CS. Acetaldehyde increases procollagen type I and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology. 1991;13:758–65. [PubMed] [Google Scholar]
- Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Cole RN, Zaia J, Hart GW. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry. 2000;39:11609–20. doi: 10.1021/bi000755i. [DOI] [PubMed] [Google Scholar]
- El Hajj EC, El Hajj MC, Voloshenyuk TG, Mouton AJ, Khoutorova E, Molina PE, Gilpin NW, Gardner JD. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res. 2014;38:448–56. doi: 10.1111/acer.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Banko MR, Brunet A. AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Ann N Y Acad Sci. 2009;1170:688–92. doi: 10.1111/j.1749-6632.2009.04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–19. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Griffith LS, Schmitz B. O-linked N-acetylglucosamine levels in cerebellar neurons respond reciprocally to pertubations of phosphorylation. Eur J Biochem. 1999;262:824–31. doi: 10.1046/j.1432-1327.1999.00439.x. [DOI] [PubMed] [Google Scholar]
- Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–36. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- Guo C, Wang Y, Liang H, Zhang J. ADAMTS-1 contributes to the antifibrotic effect of captopril by accelerating the degradation of type I collagen in chronic viral myocarditis. Eur J Pharmacol. 2010;629:104–10. doi: 10.1016/j.ejphar.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta. 2011;1813:1965–70. doi: 10.1016/j.bbamcr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol regulates eukaryotic elongation factor 2 phosphorylation via an AMP-activated protein kinase-dependent mechanism in C2C12 skeletal myocytes. J Biol Chem. 2007;282:3702–12. doi: 10.1074/jbc.M606593200. [DOI] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Kazi AA, Huber DS, Pruznak AM, Lang CH. Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction. J Cell Biochem. 2010;109:1172–84. doi: 10.1002/jcb.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol. 2012;302:C1557–65. doi: 10.1152/ajpcell.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Navaratnarajah M, Lang CH. Activation of AMPK/TSC2/PLD by alcohol regulates mTORC1 and mTORC2 assembly in C2C12 myocytes. Alcohol Clin Exp Res. 2013;37:1849–61. doi: 10.1111/acer.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampert M, Kuenzle S, Thai SN, Lee N, Iruela-Arispe ML, Werner S. ADAMTS1 proteinase is up-regulated in wounded skin and regulates migration of fibroblasts and endothelial cells. J Biol Chem. 2005;280:23844–52. doi: 10.1074/jbc.M412212200. [DOI] [PubMed] [Google Scholar]
- Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556–62. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, Matsushima K. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 2000;478:241–5. doi: 10.1016/s0014-5793(00)01854-8. [DOI] [PubMed] [Google Scholar]
- Law BA, Carver WE. Activation of cardiac fibroblasts by ethanol is blocked by TGF-β inhibition. Alcohol Clin Exp Res. 2013;37:1286–94. doi: 10.1111/acer.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim NH, Meinjohanns E, Bou-Gharios G, Gompels LL, Nuti E, Rossello A, Devel L, Dive V, Meldal M, Nagase H. In vivo imaging of matrix metalloproteinase 12 and matrix metalloproteinase 13 activities in the mouse model of collagen-induced arthritis. Arthritis Rheumatol. 2014;66:589–98. doi: 10.1002/art.38295. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–72. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–16. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Mello T, Ceni E, Surrenti C, Galli A. Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol Aspects Med. 2008;29:17–21. doi: 10.1016/j.mam.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007;98:621–8. doi: 10.1111/j.1349-7006.2007.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshage H, Casini A, Lieber CS. Acetaldehyde selectively stimulates collagen production in cultured rat liver fat-storing cells but not in hepatocytes. Hepatology. 1990;12:511–8. doi: 10.1002/hep.1840120311. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Oruc N, Whitcomb DC. Theories, mechanisms, and models of alcoholic chronic pancreatitis. Gastroenterol Clin North Am. 2004;33:733–50. v–vi. doi: 10.1016/j.gtc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Perna AF, Sepe I, Lanza D, Capasso R, Zappavigna S, Capasso G, Caraglia M, Ingrosso D. Hydrogen sulfide reduces cell adhesion and relevant inflammatory triggering by preventing ADAM17-dependent TNF-alpha activation. J Cell Biochem. 2013;114:1536–48. doi: 10.1002/jcb.24495. [DOI] [PubMed] [Google Scholar]
- Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–96. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzer MJ, Chen L, DiPietro LA. Fibroblast function and wound breaking strength is impaired by acute ethanol intoxication. Alcohol Clin Exp Res. 2011;35:83–90. doi: 10.1111/j.1530-0277.2010.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn AP, Birch MA, Karlström E, Wendel M, Lind T. ADAMTS-1 increases the three-dimensional growth of osteoblasts through type I collagen processing. Bone. 2007;41:231–8. doi: 10.1016/j.bone.2007.04.187. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501–8. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Roman J. Chronic alcohol ingestion and predisposition to lung “cirrhosis”. Alcohol Clin Exp Res. 2014;38:312–5. doi: 10.1111/acer.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–9. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- Shegogue D, Trojanowska M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem. 2004;279:23166–75. doi: 10.1074/jbc.M401238200. [DOI] [PubMed] [Google Scholar]
- Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Nagashima T, Shimaya A, Urano Y, Shimokawa T, Shibasaki M. Effects of the novel Foxo1 inhibitor AS1708727 on plasma glucose and triglyceride levels in diabetic db/db mice. Eur J Pharmacol. 2010;645:185–91. doi: 10.1016/j.ejphar.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Trimborn M, Bell SM, Felix C, Rashid Y, Jafri H, Griffiths PD, Neumann LM, Krebs A, Reis A, Sperling K, Neitzel H, Jackson AP. Mutations in microcephalin cause aberrant regulation of chromosome condensation. Am J Hum Genet. 2004;75:261–6. doi: 10.1086/422855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–8. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeşilli C, Mungan G, Seçkiner I, Akduman B, Numanoğlu G, Mungan A. Effects of ethanol on intracorporeal structures of the rat. Int Urol Nephrol. 2006;38:129–32. doi: 10.1007/s11255-005-3150-4. [DOI] [PubMed] [Google Scholar]
- Yu BC, Lee DS, Bae SM, Jung WK, Chun JH, Urm SH, Lee DY, Heo SJ, Park SG, Seo SK, Yang JW, Choi JS, Park WS, Choi IW. The effect of cilostazol on the expression of matrix metalloproteinase-1 and type I procollagen in ultraviolet-irradiated human dermal fibroblasts. Life Sci. 2013;92:282–8. doi: 10.1016/j.lfs.2012.12.011. [DOI] [PubMed] [Google Scholar]