Abstract
Type 1 diabetes (T1D) is a chronic disease resulting from the destruction of pancreatic beta cells, due to a poorly understood combination of genetic, environmental, and immune factors. The JDRF Network for Pancreatic Organ donors with Diabetes (nPOD) program recovers transplantation quality pancreas from organ donors throughout the USA. In addition to recovery of donors with T1D, non-diabetic donors include those with islet autoantibodies. Donors with type 2 diabetes and other conditions are also recovered to aid investigations directed at the full spectrum of pathophysiological mechanisms affecting beta cells. One central processing laboratory conducts standardized procedures for sample processing, storage, and distribution, intended for current and future cutting edge investigations. Baseline histology characterizations are performed on the pancreatic samples, with images of the staining results provided though whole-slide digital scans. Uniquely, these high-grade biospecimens are provided without expense to investigators, working worldwide, seeking methods for disease prevention and reversal strategies. Collaborative working groups are highly encouraged, bringing together multiple investigators with different expertise to foster collaborations in several areas of critical need. This mini-review will provide some key histopathological findings emanating from the nPOD collection, including the heterogeneity of beta cell loss and islet inflammation (insulitis), beta cell mass, insulin-producing beta cells in chronic T1D, and pancreas weight reductions at disease onset. Analysis of variations in histopathology observed from these organ donors could provide for mechanistic differences related to etiological agents and serve an important function in terms of identifying the heterogeneity of T1D.
Keywords: beta cell, image analysis, insulin, insulitis, islet
The disorder ‘diabetes’ is clinically heterogeneous, with the majority of cases classified into two main categories: type 1 diabetes (T1D), which is characterized by absolute deficiency of insulin from loss of functional beta cell mass; or type 2 diabetes (T2D) resulting from a failure of beta cells secondary to peripheral tissue insulin resistance (reviewed in 1, 2). T1D remains the most common form of diabetes in children and young adults, with 3–5% average annual global increases in incidences (3). Unfortunately, T2D is no longer a rarity in adolescents due to the rise in childhood obesity rates. The complexity of etiological factors in T1D is well known and likely due to multiple genetic susceptibilities, environmental factors, and complexities of autoimmunity (2, 4). The purpose of this mini-review is to consider the rational for the use of organ donor specimens for T1D research and describe histopathological findings from these samples.
Organ donor pancreas for diabetes research
The basis for using organ donor samples for pancreatic research is, in large part, due to the inherent inability to directly examine beta cell mass or function in vivo. The human pancreas is anatomically inaccessible as major organs (e.g., liver, gastrointestinal tract, and spleen) and vessels are in direct contact on multiple surfaces. Most importantly, pancreatic islets composed of insulin-secreting beta cells amount to only 1–2% of the pancreatic mass. Radiographic examination of the pancreas is feasible; however, specific contrast agents for islet imaging are not currently available (5). Pancreatic biopsy can be conducted under guided radiography or laparoscopy, but biopsy carries a high risk of complications from exposure to pancreatic enzymes and/or hemorrhage resulting in acute pancreatitis (6, 7). Moreover, biopsy samples are limited in the amount of tissue and regions analyzed. Surgical samples from tumor pancreatectomy are useful, yet these are also limited in amount and region. Many histopathological studies of beta cells in human T1D have, therefore, relied upon autopsy-based pancreas samples. While autopsy collection affords access to the entire pancreas, variability in cold ischemia can result in autolysis placing limitations on morphological quality and stability of proteins or nucleic acids.
Given these difficulties, several research groups have begun to utilize whole or partial pancreas samples from organ donors as alternative sources of these biospecimens (8–11). The advantage of using pancreas organ donors include representation of the general population demographics, recovery of the pancreas in a sterile operating suite, and access to additional tissues such as spleen, pancreatic, and non-pancreatic lymph nodes, and duodenum (8). The Organ Procurement and Transplantation Network web site offers a large amount of data regarding numbers of organ recovered and transplanted by year (http://optn.transplant.hrsa.gov/). In the USA from 2010–2013, ~1500 decreased organ donors had their pancreas recovered, of which ~1100 were transplanted. The remaining ~380 transplantation-grade pancreata have the potential and, thankfully, were often used for islet isolation and/or research use when specific informed consent was provided for the latter. To be clear (and, overcoming a wide spread misunderstanding), patients with either T1D or T2D can become organ donors as well as the need for organ donations are so great.
Dedicated biorepository for diabetes research
The Network for Pancreatic Organ donors with Diabetes (nPOD) program was started in 2007 by JDRF to address the needs of researchers for high quality pancreas and immune cell samples. The nPOD organization and operating structure were recently described and the reader is directed to those reports for more detailed information (8, 12, 13). The numbers of donors recovered to date by nPOD is greater than 325 and includes 110 donors without diabetes (age range 0–75 yr old) and 162 donors with T1D (age range 4–93 yr, diabetes duration from 0 to 80 yr) (http://www.jdrfnpod.org/for-investigators/donor-groups/). Donors are also recovered who had T2D with varying durations (onset to chronic duration) and similar age ranges as for those with T1D. The diagnosis of diabetes or other conditions for each donor is based on medical review by a Pediatric Endocrinologist (Dr D. Schatz) using the terminal hospitalization record and laboratory data such as autoantibody status, C-peptide levels, and transplant human leukocyte antigen (HLA) test results. Finally, additional donor samples are obtained by direct donation to the program by other investigators. To gain approval to use nPOD samples or data, researchers submit an application that is reviewed by the nPOD Tissue Prioritization Committee for feasibility, suitability, and lack of duplication to existing projects as well as providing documentation of institutional research approval for their studies.
A high priority of the nPOD program is to obtain non-diabetic donors with islet-associated autoantibodies. The non-diabetic donors are identified by prospective screening for the presence of islet-associated autoantibodies through rapid antibody assays at Organ Procurement Organization (OPO) laboratories during their clinical work up for transplantation suitability (8, 14). Screening is performed for three major beta cell autoantibodies, namely glutamic acid decarboxylase-65 (GADA), islet antigen-2 or insulinoma antigen-2 (IA-2A), and zinc transporter 8 (ZnT8A). If a donor is identified as autoantibody positive and the pancreas is not transplanted, these donor pancreata may still be recovered for research purposes. Also, a serum sample is obtained from most of the nPOD donors at organ recovery for testing for autoantibodies to GADA, IA-2A, ZnT8A, and insulin (IAAs) by radioimmunoassay at the University of Denver Autoantibody Core (Dr Liping Yu, Denver, CO, USA) (8). As shown by other studies, incidences of islet autoantibodies in organ donors mirror that found in the general population (9–11, 15). IAAs are rare in adults and patients with diabetes receiving exogenous insulin will develop IAA after 5–7 d of therapy (16). Other donors recovered include those with cystic fibrosis-related diabetes, gestational diabetes, and other physiological and metabolic conditions of interest to researchers in T1D (e.g., pregnancy and postgastric bypass). The causes of donor organ demise vary with the majority due to situations variant to anoxia, trauma, and suicide, amongst others. In particular, some patients with T1D are recovered whose death is unrelated to diabetic ketoacidosis and its attendant cerebral edema in contrast to most autopsy-based studies.
Uniquely, the nPOD program performs baseline histopathological characterizations of each donor pancreas and shares such data as whole digital slide scans and clinical data through an online pathology database (http://www.jdrfnpod.org/for-investigators/online-pathology-information/) (17). Slide images include those with standard H&E stains for histopathology and double immunohistochemistry stains for endocrine cells (insulin and glucagon), cell proliferation (Ki67), and CD3+ T lymphocytes. This baseline screening is performed on blocks from each region (head, body, and tail). Islet morphology in the head region is also reviewed by staining with pancreatic polypeptide in the context of division into ventral and dorsal lobes. Islets in the ventral lobe contain primarily pancreatic polypeptide endocrine cells with fewer beta cells than dorsal lobe islets (~30% compared to ~60%, respectively) (18, 19). The collection of representative slides grows for each donor as additional slides are added when new blocks are sectioned for investigations. The online image database provides basic donor demographic and clinical data with additional data available through the online nPOD DataShare system (Dr. John Kaddis, City of Hope, CA, USA) (Table 1).
Table 1.
Demographic and HLA genotyping data for non-diabetic donors with islet-associated autoantibodies. Representative demographic and laboratory data available for donors to approved investigators through the online pathology or DataShare databases are shown
| Autoantibody number and type* |
Age (yr) | Sex | Race | High-resolution HLA genotyping* DRB1-DQA1-DQB1 |
Case ID |
|---|---|---|---|---|---|
| Single | |||||
| GADA+ | 4.30 | Female | Caucasian | 03:01–05:01–02:01/03:01–05:01–02:01 | 6171 |
| 22.00 | Male | Caucasian | 03:01–05:01–02:01/07:01–06:01–02:02 | 6303 | |
| 23.20 | Female | Caucasian | 08:01–04:01–04:02/11:01–05:01–03:01 | 6123 | |
| 23.80 | Female | Caucasian | 04:01–03:01–03:01/08:02–04:01–04:02 | 6147 | |
| 26.00 | Male | African Am | 11:01–05:01–03:19/13:04–01:02–06:02 | 6301 | |
| 30.00 | Male | Caucasian | 01:01–01:01–05:01/07:01–02:01–02:02 | 6151 | |
| 31.90 | Male | Caucasian | 04:01–03:01–03:02/01:01–01:01–05:01 | 6181 | |
| 40.00 | Male | Caucasian | 01:01–01:01–05:01/01:02–01:01–05:01 | 6156 | |
| 41.40 | Male | Hispanic/Latino | 01:02–01:01–05:01/15:01–01:02–06:02 | 6044 | |
| 47.50 | Female | Hispanic/Latino | 04:07–03:01–03:02/04:07–03:01–03:02 | 6184 | |
| 48.50 | Female | Caucasian | 09:01–03:01–03:03/15:01–01:02–06:03 | 6154 | |
| 64.80 | Male | Caucasian | 13:02–01:02–06:04/15:01–01:02–06:02 | 6101 | |
| mIAA+ | 66.00 | Male | Caucasian | 03:01/5–05:01–02:01/15:01/5–01:02/0–06:02/4 | 6023 |
| ZnT8A+ | 18.80 | Male | Caucasian | 03:01–05:01–02:01/15:01–01:02–06:02 | 6027 |
| Multiple | |||||
| GADA+ IA-2A+ | 22.00 | Male | African Am | 03:02–04:01–04:02/07:01–02:01–02:02 | 6197† |
| 23.00 | Female | Caucasian | 04:01–03:01–03:02/04:04–03:01–03:02 | 6267† | |
| GADA+ mIAA+ | 40.30 | Male | Caucasian | 04:01–03:01–03:01/13:02–01:02–06:04 | 6158 |
| 69.20 | Female | Caucasian | 04:01–03:01–03:01/01:01–01:01–05:01 | 6080 | |
| IA-2A+ ZnT8A+ | 37.00 | Male | Caucasian | 04:04–03:01–03:02/15:02–01:03–06:01 | 6167 |
GADA, glutamic acid decarboxylase-65 autoantibody; HLA, human leukocyte antigen; IA-2A, islet antigen-2 or insulinoma antigen-2; IAA, insulin autoantibody; ZnT8, zinc transporter 8 autoantibody.
Autoantibody status (number and type) and high-resolution HLA genotyping were determined as previously reported (8). HLA haplotypes are shown in bold or italics for risk or protective type 1 diabetes genotypes, respectively (20).
Donors with insulitis.
The nPOD program recovers matched non-diabetic donors (age and sex) to those with T1D, allowing the diversity of human pancreatic pathology to be evaluated from the neonate to elderly person. Minor pathological changes can be seen in the normal human exocrine and endocrine pancreas. Such changes may include acinar fatty infiltration, acute or chronic inflammatory infiltrates (pancreatitis), and periductal and acinar fibrosis particularly in aged patients (21). Chronic pancreatitis can occur in those with a history of moderate to marked alcohol consumption. Vascular congestion can be seen in sporadic islets underscoring the extensive microvascular system and in nPOD organ donors may be related to organ perfusion methods independent of diabetes status.
Limitations do exist for the use of donor pancreas samples for research. The pancreas is considered one of the most difficult tissues from which to recover high quality RNA due to the abundance of digestive enzymes including RNAses. Such caveats apply to pancreas samples obtained from surgery as well. A major difference between pancreas organ recovery and surgical specimens exists in that for the former, the pancreas is perfused and transported in cold specialized media as for transplantation. Conversely, surgical specimens can be rapidly processed within minutes under controlled biobanking protocols (reviewed in 22). Another critical factor relates to the donor themselves as each represents a single cross-sectional analysis. Limited information can be available regarding pertinent past medical history beyond that reported in the terminal hospitalization record. The durations of brain death and intensive care support can also vary and may affect pancreatic histopathology (23). Indeed, a study from In’t Veld et al. showed a significant effect of young donor age (≤25 yr) and long ICU duration (≥3 d) on increased beta cell proliferation rates and exocrine CD68+ and CD45+ infiltrates in organ donors (24). Another study using nPOD samples did not find any influence of ICU duration on exocrine CD8 cell counts in control donors or those with T1D or T2D (25).
Insulitis in the natural history of T1D
Historically, histopathological findings of inflamed islets (insulitis) in T1D patients were based on fewer than 150 patient reports and investigator access to said samples was extremely limited due to their age and small sampling sizes (one to two blocks per patient) (reviewed in 26, 27). The current consensus definition of human insulitis requires only the presence of ≥ 15 CD45+ leukocytes/islet (alternatively ≥6 CD3+ lymphocytes) in three islets with the presence of pseudoatrophic (insulin-negative) islets (26). When determining insulitis rates, islets are often subtyped according to insulin immunopositivity (insulin+ and insulin−) and presence of insulitis (CD3+ or CD3−). Preliminary data from the nPOD program corroborate other studies and show that proportions of islets with insulitis (% insulitis) are low [%, range 3–18%, N= 18 donors with T1D, N = 2 non-diabetic donors with multiple autoantibodies (5–29 yr old)] (Campbell-Thompson et al., unpublished data) (12, 27). All donors with T1D and insulitic islets had some insulin + islets which also corroborates previous reports (reviewed in 27). Such data help provide morphological correlation to studies showing residual C-peptide secretion in patients with T1D decades after onset (28–31). The insulitis data from nPOD contrasts slightly with some reports wherein fewer than 5% of patients with T1D of chronic duration (>1 yr) had insulitic islets (27, 32–34). In the nPOD donors, insulitis was present in 35% of donors with T1D whose disease durations ranged between 1 and 20 yr (Campbell-Thompson et al., unpublished data). These differences could reflect changing environmental factors related to T1D initiation over the decades.
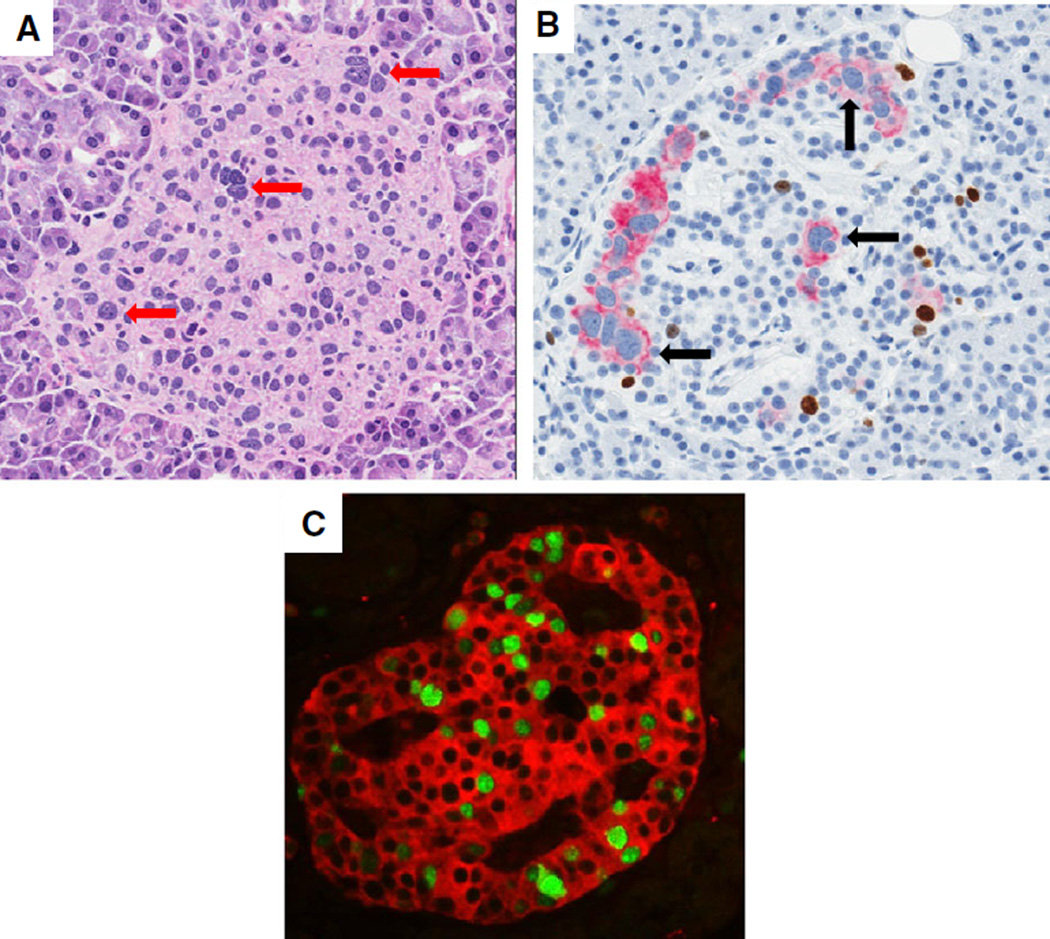
The insulitic islets in nPOD donor samples show heterogeneity in terms of insulin content (reviewed in 27, 35). Importantly, insulitis can be observed in islets with apparently normal insulin + beta cell numbers and proportions as well as islets with insulin-cells (Fig. 1). Second, the insulitis lesions are composed of variable numbers of B and T lymphocytes in a given donor and between donors, also irrespective of islet insulin immunopositivity (Figs 2 and 3).These insulitis islets may be restricted to a single lobule or may be scattered between several lobules of several regions (36, 37).
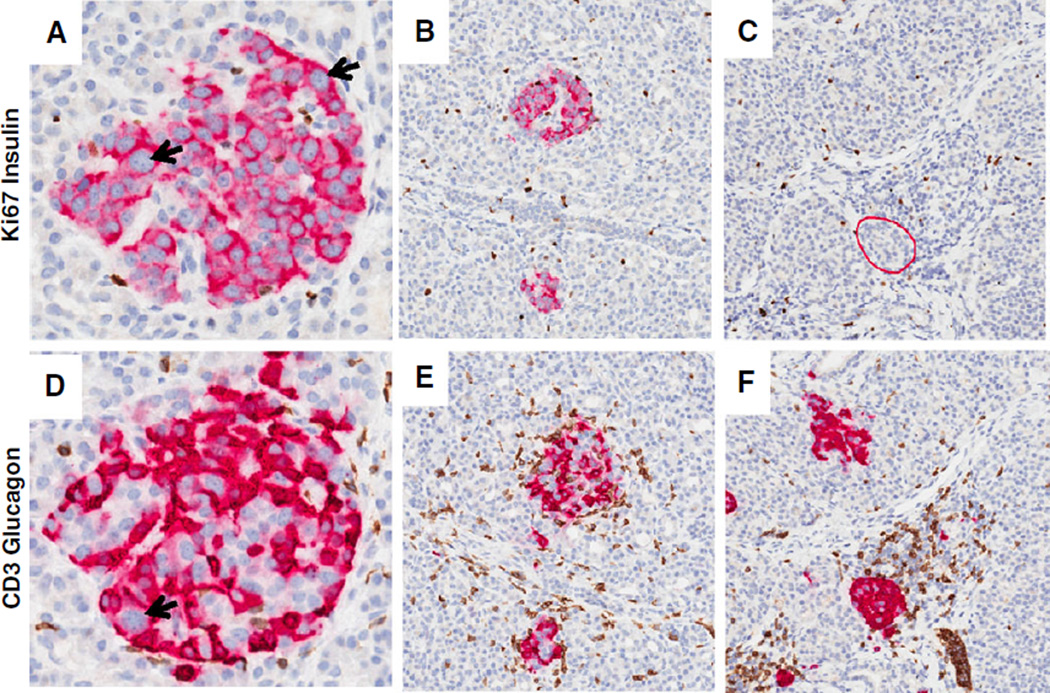
Fig. 1.
Insulitic islets at onset of T1D. Representative images of insulitic islets are shown from a 13-yr-old male Caucasian donor at T1D onset (GADA+ IA-2A+ ZnT8+; DR3/4; nPOD 6228). Serial paraffin sections were stained by two double immunohistochemistry stains (Ki67 and insulin, CD3 and glucagon) (17). Insulin + islets are shown in panels (A and B) and insulin-islets are shown in panel (C) (insulitic islet is circled in red). The islets in panels (D–F) show insulitis with varying numbers of CD3+ cells. CD3+ cells were found at the islet periphery and interior region. Panels (E) and (F) also display examples of the large numbers of CD3+ cells in the exocrine region. Islet endocrine cell nuclear pleomorphism is also shown in panels (A) and (D) (arrows). Additional donor information and whole slide images are available through the nPOD Online Pathology Database (http://www.jdrfnpod.org/for-investigators/online-pathology-information/). Original magnification: 20×. GADA, glutamic acid decarboxylase-65 autoantibody; IA-2A, islet antigen-2 or insulinoma antigen-2; nPOD, Network for Pancreatic Organ donors with Diabetes; T1D, type 1 diabetes; ZnT8, zinc transporter 8 autoantibody.
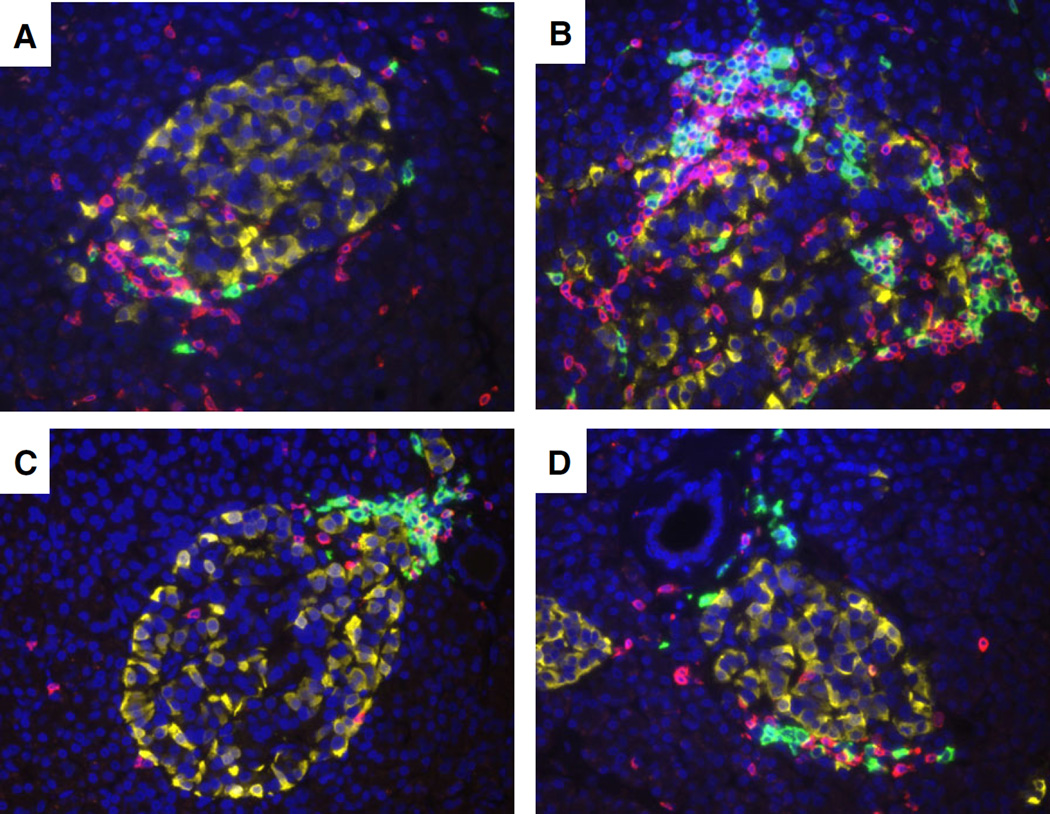
Fig. 2.
CD20+ and CD3+ lymphocyte numbers between insulitic islets in a young donor with recent onset. Representative images of four islets with insulitis are shown from a 12-yr-old male African American donor 1 yr after onset (IA-2A+, DR09/16 nPOD 6052). The paraffin section was stained by multiple immunofluorescence for CD20 (green), CD3 (yellow), and glucagon (red) using methods previously described (17). Total numbers of CD20+ and CD3+ cells varied by islet and their locations were at the islet periphery (A–D) and interior (B) Original magnification: 40×. IA-2A, islet antigen-2 or insulinoma antigen-2; nPOD, Network for Pancreatic Organ donors with Diabetes.
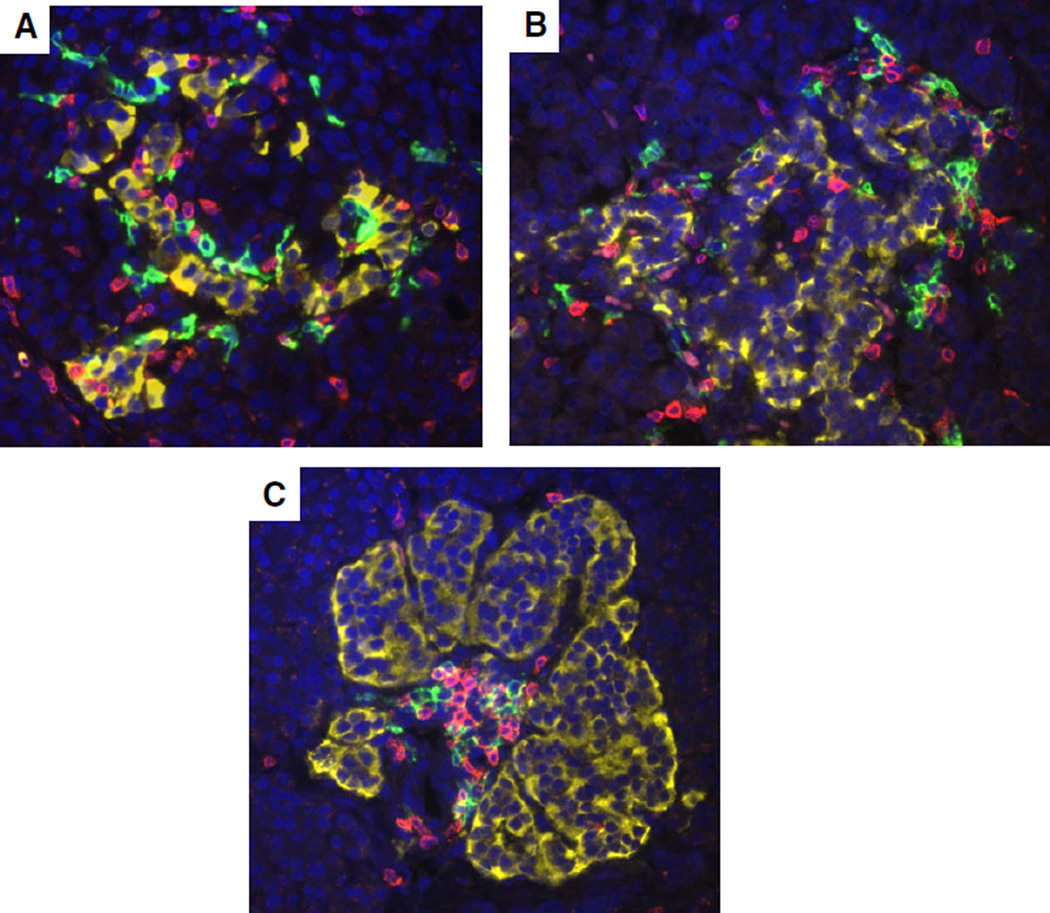
Fig. 3.
CD20+ and CD3+ lymphocytes in insulitic islets with varying disease durations. Representative islets are shown from a 5-yr-old female Caucasian donor with T1D for 3 months (IA-2A+ ZnT8A+, DR3/4, nPOD 6209, A), a 13-yr-old male Caucasian donor at onset (GADA+ IA-2A+ ZnT8+; DR3/4, nPOD 6228, B), and a 19-yr-old male Caucasian donor with T1D for 5 yr (GADA+ IA-2A+ ZnT8+, DR3/4, nPOD 6195, C). Sections were stained as in Fig. 2. Morphology of islets varied between donors and CD20+ and CD3+ cells were both present in insulitic islets. Original magnification: 40×. GADA, glutamic acid decarboxylase-65 autoantibody; IA-2A, islet antigen-2 or insulinoma antigen-2; T1D, type 1 diabetes; ZnT8, zinc transporter 8 autoantibody; nPOD, Network for Pancreatic Organ donors with Diabetes.
As indicated previously, a major aim of the nPOD program and other biobanks is to recover pancreata from non-diabetic donors with islet-associated autoantibodies to understand the types of autoimmune cells involved and their potential mechanisms of beta cell attack. A summary of 19 non-diabetic donors with autoantibodies is shown in Table 1. Insulitis was observed in two donors with similar multiple autoantibodies (GADA+ and IA2A+) (Table 1, Fig. 4). One of the two multiple autoantibody positive donors with insulitis had the high-risk HLA DR4/4 (20, 38). Interestingly, In’t Veld et al. also observed insulitis in organ donors with multiple autoantibody types (GADA+ and IA-2A+) and risk HLA genotypes, albeit they were older (46 and 59 yr respectively) (10).
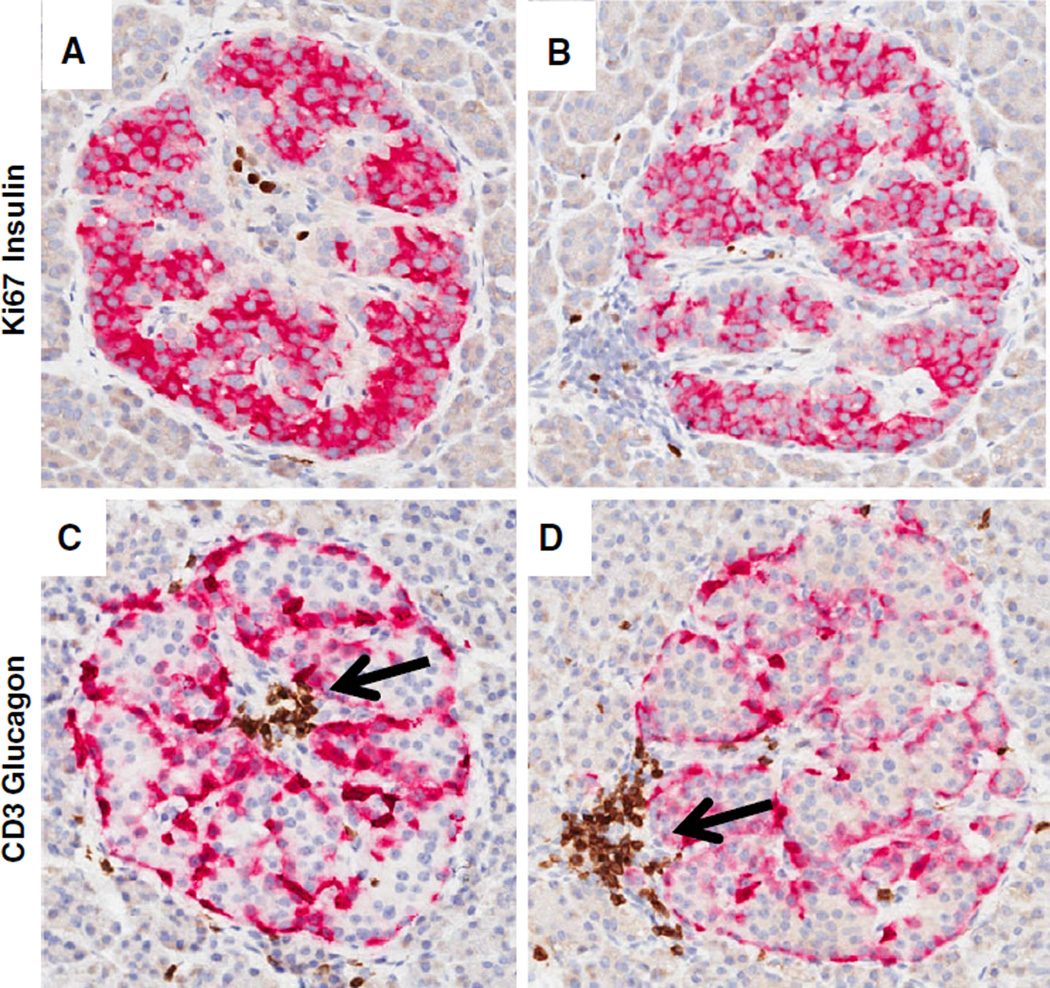
Fig. 4.
Early signs of insulitis. Representative images of two islets are shown from a 23-yr-old female Caucasian non-diabetic donor with islet autoantibodies (GADA+ IA-2A+, DR4/4, nPOD 6267). Serial sections were stained by double immunohistochemistry as in Fig. 1. The majority of islet endocrine cells were insulin+ with occasional Ki67+ cells found in the islet interior (A and B) or lymphocyte aggregate (B). Glucagon+ cells are located along the periphery of islet folds (C and D). Focal accumulations of CD3+ lymphocytes are in the interior region (C) or periphery (D). Original magnification: 20×. GADA, glutamic acid decarboxylase-65 autoantibody; IA-2A, islet antigen-2 or insulinoma antigen-2; nPOD, Network for Pancreatic Organ donors with Diabetes.
Beta cell mass
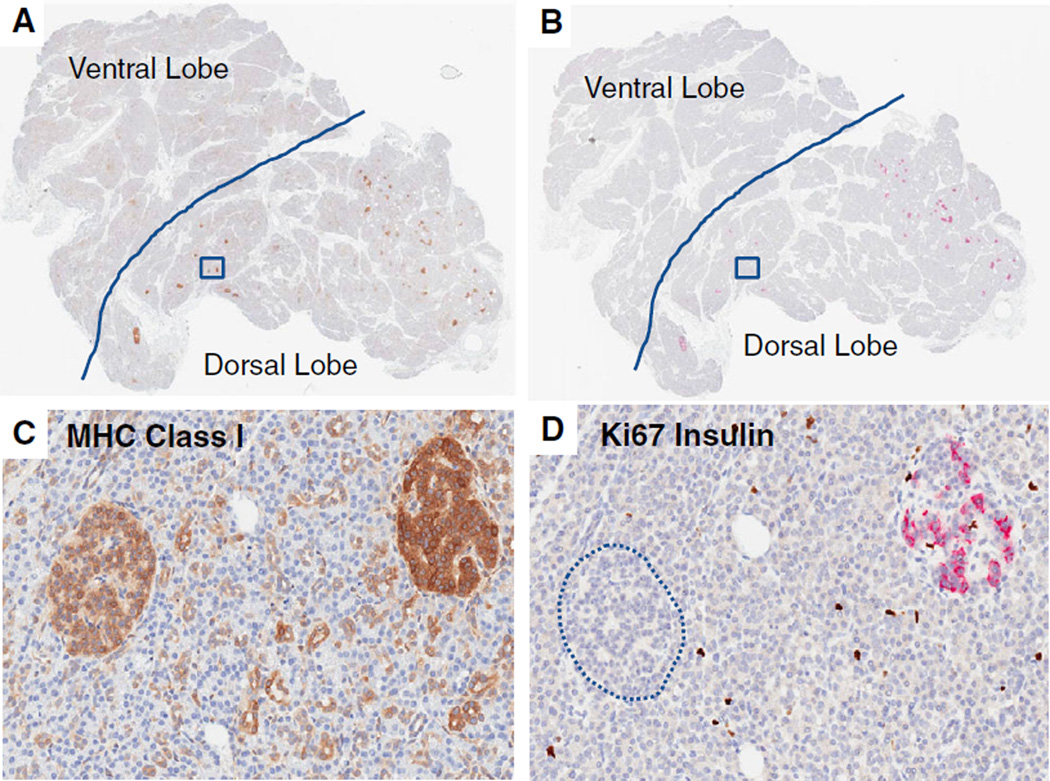
A large loss of functional beta cell mass is concomitant with clinical signs of T1D with estimates of loss ranging from 60 to 90% (39, 40). Numbers of single beta cells and those in small cell clusters show marked decreases with remaining islets showing a range of sizes. Normal islet sizes are highly variable with a mean diameter of ~135 µm, ranging from 6 to 10 cells depending on the study criteria, up to 250 µm (41, 42). The proportion of beta cells within a given islet is also variable between normal islets (30–80%) (42, 43). Hyperplastic islets (>250 µm) can also be observed in young donors with T1D as originally reported by Gepts (Fig. 5) (32). Nuclear pleomorphism is a feature of neuroendocrine cells with secretory functions and can be seen in donors with T1D; its potential relationship to beta cell regeneration is undefined (Fig. 5) (33, 44). Indeed, conflicting reports exist regarding beta cell proliferation rates in patients at disease onset and organ donors, with or without diabetes (24, 41, 45). Several studies are underway by nPOD investigators to address key questions related to age and disease status (46). Finally, another example of islet heterogeneity can be seen in the degree of major histocompatibility complex (MHC) class I molecules (HLA-A, B, and C) expression described by Bottazzo and others (47, 48). Such heterogeneity may be explained in part by higher expression in insulin + islets compared to insulin− islets (Fig. 6). High expression of MHC class I in islets from nPOD donors with T1D was also reported in association with infiltration by CD8+ and CD4+ T cells and studies in samples from other cohorts may also indicate a correlation of high MHC Class I expression with enterovirus (25, 47, 49).
Fig. 5.
Islet endocrine cell nuclear pleomorphism and proliferation. Representative islets are shown from three donors with T1D [12-yr-old female Caucasian with T1D for 3 yr, nPOD 6268 (A); 14-yr-old male Caucasian with T1D for 4 yr, nPOD 6084 (B);12-yr-old male African American with T1D for 1 yr, nPOD 6052 (C). (A) H&E stained islet (250 µm diameter) shows a wide range of nuclear morphologies and sizes, some with enlarged nuclei and prominent nucleoli (A, red arrows). Nuclear pleomorphism is also shown in an islet stained with Ki67 (brown) and insulin (red) showing that most of the insulin + beta cells with enlarged nuclei are not Ki67+ (black arrow) (B). An islet stained by multi-immunofluorescence using Ki67 (green nuclei) and synaptophysin (red) shows a very high number of proliferating endocrine cells (C). Original magnifications: 20× (A and B); 40× (C). nPOD, Network for Pancreatic Organ donors with Diabetes; T1D, type 1 diabetes.
Fig. 6.
MHC class I expression varies by islet insulin positivity. Representative whole slide scans from the pancreas head region of two serial sections are shown from a 13-yr-old male Caucasian with T1D for 5 yr (nPOD 6243). Sections were stained for MHC class I (A and C) or Ki67 and insulin (B and D). Another serial section stained with pancreatic polypeptide defined the ventral lobe boundaries (denoted by solid line, images are available through the nPOD Online Pathology Database). Note the absence of insulin staining in islets in the ventral lobe and inter-and intra-lobular variability in insulin staining in dorsal lobe islets (B and D). A higher magnification of two islets (boxed regions in A and B) for each stain is shown with higher MHC class I expression in the insulin + islet compared to the insulin-islet (circled) (C and D). Original magnification: 20×. nPOD, Network for Pancreatic Organ donors with Diabetes; T1D, type 1 diabetes.
The time course of functional beta cell loss in T1D is of great interest, with biomarkers being sought for better discrimination of disease progression. Pancreatic beta cell mass is estimated from the product of relative insulin area (%, proportion insulin stained area to total tissue area) multiplied by pancreas weight (g) (50). Several-fold variations in beta cell mass for normal subjects have been reported that could result from variability in insulin area and/or pancreas weight. Relative insulin area ranges from 1 to 2% in most studies with beta cell mass of 0.25–1.5 g (51, 52). Islet density in the tail region is generally considered higher than head or body regions, although the literature has some conflicting findings (43, 51–54).
Pancreas size in diabetes
Variability in pancreas weights was described with ranges from 38.9 to 170 g in adults (15–81 yr) (reviewed in 55, 56). Examinations of pancreas weight at autopsy and from the nPOD program showed reduced weights in patients with new onset T1D as well as in those with longer durations (32, 55, 57, 53, 58). These findings have also been shown by assessment of pancreatic volume in situ using radiology such as ultrasound, computerized tomography, or magnetic resonance imaging (reviewed in 59, 60). Taken collectively, autopsy and clinical imaging studies show that pancreatic weights or volumes are reduced by 20–50% in patients with T1D compared to non-diabetic controls. The potential mechanisms underlying this finding are not known and could be due to impaired pancreatic growth, atrophy, or combinations of both. Genetic factors influencing pancreas organ size are also not known. Longitudinal imaging studies in living subjects may provide information to identify different pathways involved in reduced pancreas size at disease onset.
Conclusions
Effective strategies to prevent and treat T1D will be aided by a better understanding of the histopathology of the disease in combination with clinical studies. Understanding the histopathology of T1D is predicated on understanding the natural heterogeneity of islets in normal pancreata representing at-risk age groups. The nPOD program is opening the door to such knowledge through recovery efforts of pancreata from non-diabetic organ donors with islet autoantibodies and those with diabetes. The program performs basic donor and pancreata histopathological characterizations and said data are openly shared with the research community to maximize access to rare samples. Investigators have access to multiple biospecimens with a user agreement to share their findings back with the community. This system allows sharing of each donor’s samples with multiple investigators studying different aspects of beta cell physiology, pathology, immunology, genetics, and other key areas.
Several key findings from nPOD studies were recently reviewed by others (12, 13, 35). Some of these key findings related to histopathology include the following:
-
(i)
Heterogeneity of beta cell loss and degree of insulitis was observed in donors with T1D, both at onset and with chronic duration (36, 37).
-
(ii)
Autoimmune-related phenomenon in islets and exocrine regions continue to be defined including detection of antigen-specific CD8+ T cells in insulitis, increased numbers of CD8+, CD4+, and CD11c + cells in exocrine infiltrates, detection of CXCL10 expression, and complement C4d deposition (25, 37, 61–63).
-
(iv)
Transdifferentiation or dedifferentiation potential of adult beta cells was shown by colocalization of multiple endocrine hormones in donors with T1D or T2D (64, 65).
-
(v)
Beta cells from donors with T1D showed a partial endoplasmic reticulum stress response, with evidence of the induction of some components of the unfolded protein response (66).
-
(vi)
Coxsackie viral protein VP1 was detected in beta cells, particularly in T1D donors, and included those with disease of long duration (reviewed in 67).
In addition to ongoing investigations, there are numerous novel questions that nPOD studies are helping to address. Studies directed at islet alterations during the preclinical phase of T1D will be particularly crucial to better understand mechanisms of beta cell loss as well as genotype–phenotype effects. New working groups continue to form that bring together research expertise with the latest technologies. Understanding the key factors that alter beta cell mass will aid in deciphering the complex genetic, immunologic, and environmental factors related to T1D disease initiation and progression. These factors will also be critical for protecting residual beta cells after disease onset or when beta cells are transplanted from organ donors and stem cells.
Acknowledgments
This study was funded by JDRF 6-2006-1140 and 25-2013-268 and NIH (DP3-DK101120-01 and UC4 DK 104155 01).
The author thanks Dr. Mark A. Atkinson for thoughtful suggestions, Clive Wasserfall for autoantibody screening, Dr. Alberto Pugliese for HLA risk interpretations, and Dr. Des Schatz and all members of the JDRF nPOD program for their invaluable efforts on behalf of the donors and investigators. This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative T1D research project sponsored by JDRF. OPOs partnering with nPOD to provide research resources are listed at (http://www.jdrfnpod.org/for-partners/npod-partners/).
Footnotes
Conflict of interest
The author declares no conflict of interest.
References
- 1.Craig ME, Jefferies C, Dabelea D, Balde N, Seth A, Donaghue KC. Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2014;15(Suppl. 20):4–17. doi: 10.1111/pedi.12186. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran F, Stone M, Huang CY, et al. Population-based incidence of diabetes in Australian youth aged 10–18 yr: increase in type 1 diabetes but not type 2 diabetes. Pediatr Diabetes. 2014;15:585–590. doi: 10.1111/pedi.12131. [DOI] [PubMed] [Google Scholar]
- 4.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Malaisse WJ, Maedler K. Imaging of the β-cells of the islets of Langerhans. Diabetes Res Clin Pract. 2012;98:11–18. doi: 10.1016/j.diabres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Imagawa A, Hanafusa T, Tamura S, et al. Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomena in type 1 diabetes: close correlation between serological markers and histological evidence of cellular autoimmunity. Diabetes. 2001;50:1269–1273. doi: 10.2337/diabetes.50.6.1269. [DOI] [PubMed] [Google Scholar]
- 7.Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. 2014;57:841–843. doi: 10.1007/s00125-013-3155-y. [DOI] [PubMed] [Google Scholar]
- 8.Campbell-Thompson M, Wasserfall C, Kaddis J, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28:608–617. doi: 10.1002/dmrr.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianani R, Putnam A, Still T, et al. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J Clin Endocrinol Metab. 2006;91:1855–1861. doi: 10.1210/jc.2005-1171. [DOI] [PubMed] [Google Scholar]
- 10.In’t Veld P, Lievens D, De Grijse J, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56:2400–2404. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- 11.Tauriainen S, Salmela K, Rantala I, Knip M, Hyöty H. Collecting high-quality pancreatic tissue for experimental study from organ donors with signs of β-cell autoimmunity. Diabetes Metab Res Rev. 2010;26:585–592. doi: 10.1002/dmrr.1129. [DOI] [PubMed] [Google Scholar]
- 12.Pugliese A, Yang M, Kusmarteva I, et al. The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: goals, operational model and emerging findings. Pediatr Diabetes. 2014;15:1–9. doi: 10.1111/pedi.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugliese A, Vendrame F, Reijonen H, Atkinson MA, Campbell-Thompson M, Burke GW. New insight on human type 1 diabetes biology: nPOD and nPOD-transplantation. Curr Diab Rep. 2014;14:530. doi: 10.1007/s11892-014-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maniatis AK, Yu L, Miao D, Nelson K, Eisenbarth GS. Rapid assays for detection of anti-islet autoantibodies: implications for organ donor screening. J Autoimmun. 2001;16:71–76. doi: 10.1006/jaut.2000.0457. [DOI] [PubMed] [Google Scholar]
- 15.Diamantopoulos S, Allende G, Ferreira J, Ciancio G, Burke G, Pugliese A. Retrospective assessment of islet cell autoantibodies in pancreas organ donors. Diabetes Care. 2008;31:1741–1742. doi: 10.2337/dc08-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter WE, Harris N, Schatz D. Type 1 diabetes islet autoantibody markers. Diabetes Technol Ther. 2002;4:817–839. doi: 10.1089/152091502321118838. [DOI] [PubMed] [Google Scholar]
- 17.Campbell-Thompson ML, Heiple T, Montgomery E, Zhang L, Schneider L. Staining protocols for human pancreatic islets. J Vis Exp. 2012;63:4068. doi: 10.3791/4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bommer G, Friedl U, Heitz PU, Klöppel G. Pancreatic PP cell distribution and hyperplasia. Immunocytochemical morphology in the normal human pancreas, in chronic pancreatitis and pancreatic carcinoma. Virchows Arch A Pathol Anat Histol. 1980;387:319–331. doi: 10.1007/BF00454835. [DOI] [PubMed] [Google Scholar]
- 19.Uchida T, Takada T, Ammori BJ, Suda K, Takahashi T. Three-dimensional reconstruction of the ventral and dorsal pancreas: a new insight into anatomy and embryonic development. J Hepatobiliary Pancreat Surg. 1999;6:176–180. doi: 10.1007/s005340050102. [DOI] [PubMed] [Google Scholar]
- 20.Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007732. doi: 10.1101/cshperspect.a007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol. 1984;15:677–683. doi: 10.1016/s0046-8177(84)80294-4. [DOI] [PubMed] [Google Scholar]
- 22.Rudloff U, Bhanot U, Gerald W, et al. Biobanking of human pancreas cancer tissue: impact of ex-vivo procurement times on RNA quality. Ann Surg Oncol. 2010;17:2229–2236. doi: 10.1245/s10434-010-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rech TH, Crispim D, Rheinheimer J, et al. Brain death-induced inflammatory activity in human pancreatic tissue: a case-control study. Transplantation. 2014;97:212–219. doi: 10.1097/TP.0b013e3182a949fa. [DOI] [PubMed] [Google Scholar]
- 24.In’t Veld P, De Munck N, Van Belle K, et al. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes. 2010;59:1702–1708. doi: 10.2337/db09-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;38:476–481. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013;56:2541–2543. doi: 10.1007/s00125-013-3043-5. [DOI] [PubMed] [Google Scholar]
- 27.In’t Veld P. Insulitis in human type 1 diabetes: the quest for an elusive lesion. Islets. 2011;3:131–138. doi: 10.4161/isl.3.4.15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oram RA, McDonald TJ, Shields BM, et al. Most people with long-duration type 1 diabetes in a large population-based study are insulin microsecretors. Diabetes Care. 2014;38:323–328. doi: 10.2337/dc14-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis AK, DuBose SN, Haller MJ, et al. Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2014;38:476–481. doi: 10.2337/dc14-1952. [DOI] [PubMed] [Google Scholar]
- 30.Liu EH, Digon BJ, Hirshberg B, et al. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia. 2009;52:1369–1380. doi: 10.1007/s00125-009-1342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 33.Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia. 1986;29:267–274. doi: 10.1007/BF00452061. [DOI] [PubMed] [Google Scholar]
- 34.Kloppel G, Clemens A. Insulin-dependent diabetes mellitus: islet changes in relation to etiology and pathogenesis. Endocr Pathol. 1997;8:273–282. doi: 10.1007/BF02739929. [DOI] [PubMed] [Google Scholar]
- 35.Battaglia M, Atkinson MA. The streetlight effect in type 1 diabetes. Diabetes. 2015;64:1081–1090. doi: 10.2337/db14-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianani R, Campbell-Thompson M, Sarkar SA, et al. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia. 2010;53:690–698. doi: 10.1007/s00125-009-1642-y. [DOI] [PubMed] [Google Scholar]
- 37.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Galarza FF, Christmas S, Middleton D, Jones AR. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2011;39:D913–D919. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pipeleers D, In’t Veld P, Pipeleers-Marichal M, Gorus F. The beta cell population in type 1 diabetes. Novartis Found Symp. 2008;292:19–24. doi: 10.1002/9780470697405.ch3. discussion 24–31, 122–129, 202–203. [DOI] [PubMed] [Google Scholar]
- 40.Löhr M, Klöppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia. 1987;30:757–762. doi: 10.1007/BF00275740. [DOI] [PubMed] [Google Scholar]
- 41.Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kilimnik G, Jo J, Periwal V, Zielinski MC, Hara M. Quantification of islet size and architecture. Islets. 2012;4:167–172. doi: 10.4161/isl.19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 44.Ehrie MG, Swartz FJ. Diploid, tetraploid and octaploid beta cells in the islets of Langerhans of the normal human pancreas. Diabetes. 1974;23:583–588. doi: 10.2337/diab.23.7.583. [DOI] [PubMed] [Google Scholar]
- 45.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50:2323–2331. doi: 10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- 46.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Evidence of increased islet cell proliferation in patients with recent-onset type 1 diabetes. Diabetologia. 2010;53:2020–2028. doi: 10.1007/s00125-010-1817-6. [DOI] [PubMed] [Google Scholar]
- 47.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313:353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 48.Foulis AK, Farquharson MA. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986;35:1215–1224. doi: 10.2337/diab.35.11.1215. [DOI] [PubMed] [Google Scholar]
- 49.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 50.Bonner-Weir S. Beta-cell turnover: its assessment and implications. Diabetes. 2001;50(Suppl. 1):S20–S24. doi: 10.2337/diabetes.50.2007.s20. [DOI] [PubMed] [Google Scholar]
- 51.Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54:1720–1725. doi: 10.1007/s00125-011-2118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111–117. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 54.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl. 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 55.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308:2337–2339. doi: 10.1001/jama.2012.15008. [DOI] [PubMed] [Google Scholar]
- 56.Kin T, Murdoch TB, Shapiro AM, Lakey JR. Estimation of pancreas weight from donor variables. Cell Transplant. 2006;15:181–185. doi: 10.3727/000000006783982133. [DOI] [PubMed] [Google Scholar]
- 57.MacLean N, Ogilvie RF. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes. 1955;4:367–376. doi: 10.2337/diab.4.5.367. [DOI] [PubMed] [Google Scholar]
- 58.Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984;26:456–461. doi: 10.1007/BF00262221. [DOI] [PubMed] [Google Scholar]
- 59.Williams AJ, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;62:2595–2604. doi: 10.1210/jc.2012-1815. [DOI] [PubMed] [Google Scholar]
- 60.Gaglia JL, Guimaraes AR, Harisinghani M, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442–445. doi: 10.1172/JCI44339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowe P, Wasserfall C, Croker B, et al. Increased complement activation in human type 1 diabetes pancreata. Diabetes Care. 2013;36:3815–3817. doi: 10.2337/dc13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarkar SA, Lee CE, Victorino F, et al. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes. 2012;61:436–446. doi: 10.2337/db11-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reddy S, Zeng N, Al-Diery H. Analysis of peri-islet CD45-positive leucocytic infiltrates in longstanding type 1 diabetic patients. Diabetologia. 2015;58:1024–1035. doi: 10.1007/s00125-015-3519-6. [DOI] [PubMed] [Google Scholar]
- 64.Piran R, Lee SH, Li CR, Charbono A, Bradley LM, Levine F. Pharmacological induction of pancreatic islet cell transdifferentiation: relevance to type I diabetes. Cell Death Dis. 2014;5:e1357. doi: 10.1038/cddis.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;58:1024–1035. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55:2417–2420. doi: 10.1007/s00125-012-2604-3. [DOI] [PubMed] [Google Scholar]
- 67.Morgan NG, Richardson SJ. Enteroviruses as causative agents in type 1 diabetes: loose ends or lost cause? Trends Endocrinol Metab. 2014;25:611–619. doi: 10.1016/j.tem.2014.08.002. [DOI] [PubMed] [Google Scholar]