Abstract
Objective
Baseline, persistent, incident, and remittent dipstick proteinuria have never been tested as predictors of mortality in an undeveloped country. The goal of this study was to determine which of these four types of proteinuria (if any) predict mortality.
Methods
Baseline data was collected from 2000–2002 in Bangladesh from 11,121 adults. Vital status was ascertained over 11–12 years. Cox models were used to evaluate proteinuria in relation to all-cause and cardiovascular disease (CVD) mortality. CVD mortality was evaluated only in those with baseline proteinuria. Persistent, remittent, and incident proteinuria were determined at the 2-year exam.
Results
Baseline proteinuria of 1+ or greater was significantly associated with all-cause (hazard ratio (HR) 2.87; 95% C.I., 1.71 – 4.80) and CVD mortality (HR: 3.55; 95% C.I., 1.81–6.95) compared to no proteinuria, adjusted for age, gender, arsenic well water concentration, education, hypertension, BMI, smoking, and diabetes mellitus. Persistent 1+ proteinuria had a stronger risk of death, 3.49 (1.64 – 7.41)-fold greater, than no proteinuria. Incident 1+ proteinuria had a 1.87 (0.92 – 3.78)-fold greater mortality over 9–10 years. Remittent proteinuria revealed no increased mortality.
Conclusions
Baseline, persistent, and incident dipstick proteinuria were predictors of all-cause mortality with persistent proteinuria having the greatest risk. In developing countries, those with 1+ dipstick proteinuria, particularly if persistent, should be targeted for definitive diagnosis and treatment. The two most common causes of proteinuria to search for are diabetes mellitus and hypertension.
Keywords: dipstick proteinuria and mortality, proteinuria and all-cause mortality, proteinuria and cardiovascular disease mortality, Bangladesh mortality, epidemiology
Introduction
Proteinuria is a known risk factor for cardiovascular and all-cause mortality in patients with diabetes mellitus and hypertension (Dineen et al., 1997, Agewall et al., 1997). Microalbuminuria has also been shown to be a predictor of cardiovascular and all-cause mortality in developed countries in several studies (Hillege et al., 2002, Klausen et al., 2006) independent of the presence of diabetes mellitus or hypertension. This would suggest that the simple, dipstick test for proteinuria would be an ideal test to screen populations to find individuals at high risk of death from all causes and cardiovascular disease. Although relevant in all venues, this would be particularly important in undeveloped countries in primary care settings with limited resources. Dipstick proteinuria testing is cost-effective (less than one half dollar) considering that almost 4 billion individual’s world-wide live in moderate poverty making less than five dollars per day(Hossein et al., 2008).
A number of cohort studies using dipstick proteinuria as an exposure have found evidence for an increase in total mortality in the middle aged (Wagener et al., 1994, Grimm et al., 1997, Tanihara et al., 2005, Ha et al., 2006) and elderly (Culleton et al., 2000, Casiglia et al., 1996). The studies varied in length from a minimum of 10 years (Grimm et al., 1997, Casiglia et al., 1996) up to 39 years (Kojima et al., 2014). All studies (Wagener et al., 1994, Grimm et al., 1997, Tanihara et al., 2005, Ha et al., 2006, Casiglia et al., 1996, Kojima et al., 2014) were from developed countries, used baseline proteinuria, and controlled for the two major risk factors for proteinuria, hypertension and diabetes mellitus, and still found a difference in mortality. However, incident and remittent proteinuria have never or rarely been well characterized from a mortality point of view in any study. This study is the only one evaluating all types of proteinuria: baseline, persistent, incident, and remittent. The objective was to evaluate dipstick proteinuria to determine which of these four types of proteinuria are clinically important (predict mortality). In developing countries, competing risks from other causes of mortality that occur at younger ages could theoretically result in no increase in mortality from proteinuria.
Methods
Study Participants/Approvals
The subjects were from the Health Effects of Arsenic Longitudinal Study (HEALS) which is an ongoing cohort study in Araihazar, Bangladesh (Ahsan et al., 2006). Details of study methodology have been reported elsewhere (Ahsan et al., 2006, Pesola et al., 2012). Briefly, 11,746 adult subjects were recruited to the study from a geographically defined 25 km2 area in Araihazar. Baseline recruitment occurred between October of 2000 to May of 2002. Women and men were sampled only if married (an eligibility criteria to keep loss to follow-up to a minimum), aged 18 to 75 (adults), residing in the study area at least 5 years (to be potentially exposed to well water that may or may not contain high arsenic levels), and who agreed to participate. A standardized questionnaire was used to gather information on demographics; height and weight were measured. Blood pressure was measured by trained clinicians as previously described (Chen et al., 2007). Diabetes mellitus was defined as presence of physician diagnosis and/or on medication for diabetes mellitus.
Study procedures were approved by the ethical committee of the Bangladesh Medical Research Council (Dhaka, Bangladesh) and institutional review boards of Columbia University (New York) and University of Chicago (Chicago, IL).
Proteinuria including definition of proteinuria types
At the baseline visit dipstick urinalysis was performed on a fresh urine sample by physicians using the Chemstrip Micral Test Strips (Roche Diagnostics, USA) and visually read one minute after the dipstick. The urine test strip results were based on a color scale that quantified proteinuria as absent, trace, 1+, 2+, or 3+ proteinuria. This scale correlates with proteinuria of < 10 mg/dL, 10 – 20 mg/dL, ≥ 30 mg/dL, > 100 mg/dL, and > 500 mg/dl for protein in the urine.
Baseline proteinuria was defined as proteinuria present during the baseline collection period from 2000 to 2002. Persistent proteinuria was defined as proteinuria present during the baseline collection period and also present at the 2-year mark. Persistent proteinuria was also defined for incident proteinuria as proteinuria present for the first time at the 2-year mark and also present at the 4-year mark. Incident proteinuria was defined as proteinuria that was not present at baseline but did occur for the first time at the 2-year mark. Finally, remittent proteinuria was defined as proteinuria that was present during the baseline collection period but was not present at the 2-year mark.
Trace proteinuria as well as 1+ proteinuria were analyzed to determine if mortality differences could be found between groups.
Arsenic Exposure Measurements
At baseline, water samples from 5966 tube Wells were collected in 50 ml acid-washed tubes. Total arsenic concentrations were measured as previously described (Cheng et al., 2004). Arsenic was measured as part of the HEALS study (Smith et al., 2000, Zierold et al., 2004, Chen et al., 2011) since elevated baseline well arsenic has been associated with an increase in all-cause mortality (Argos et al., 2010) and incident proteinuria if increasing (Chen et al., 2011).
Mortality Classification and mortality rate
From 2000 to 2013, vital status was assessed by verbal autopsy questionnaire, as noted previously (Ronsmans et al., 1998, Argos et al., 2010). Mortality classification used the ICD-10 system. All heart disease mortality was ICD-I00 to ICD-I52. Stroke was defined as ICD-I60 to ICD-I69. Cardiovascular (CVD) mortality was combined as all heart disease and stroke mortality. Renal failure was defined as ICD-N00 to N19. Cancer mortality was defined as ICDC00 to C97 and D37 to D48.
The number of days between the baseline interview and date of death or, if alive, date of last interview or report of being alive was the calculated follow-up time. For all-cause mortality the event was death and for CVD mortality the event was a CVD death. Those without the event of interest were censored.
Statistical Analysis
Descriptive analysis were initially used to compare those with and without proteinuria using the nonpaired t-test for continuous data and the Chi-Square test for categorical data.
The Kaplan-Meier survival curve was used to compare mortality over time in those with and without proteinuria. The logrank test evaluated if there was a difference between groups.
A log minus log survival curve was plotted to check the Cox Proportional Hazards assumption of a constant hazard between groups.
The Cox proportional hazards (PH) model was used to estimate hazard ratios (HR) between those with proteinuria and those without proteinuria and all-cause and CVD mortality. Full models included as a minimum proteinuria, age, and gender, and further adjusted in multivariate analysis for the potential confounding variables education (continuous), hypertension, BMI (dichotomous with normal of 18.5 to 24.99 as referent), smoking (referent as nonsmoker), diabetes mellitus (dichotomous) and arsenic well water concentration (continuous).
The Pearson's Chi Square test evaluated whether there was a statistical difference between all heart disease, all strokes, all cancers, and all renal failure between those with 1+ proteinuria versus those without 1+ proteinuria.
A p value less than 0.05 was significant. Analysis was performed with SPSS 22 (SPSS Inc. Chicago, IL, USA).
Results
Of 11,746 original subjects, 11,121 had baseline dipstick data and were included. The prevalence of baseline dipstick proteinuria was 7.3% (809/11,121) with a mean age of 37.1 years (range: 17 – 75). The breakdown of proteinuria as persistent, remittent, or unknown as compared at 2 years to baseline was 1.19% persistent, 5.70% as remittent, and 0.38% as unknown secondary to death or no coding. Therefore, 133/809 original dipstick positive results were persistent (16.4%). Most were remittent or could not be classified at 2-years.
Table 1 summarizes the baseline data between those with and without 1+ proteinuria. There were significant differences in blood pressure between groups.
Table 1.
Baseline characteristics of the Bangladesh cohort by 1+ proteinuria status collected from 2000 to 2002.
| Variables | Proteinuria | No Proteinuria | p value* |
|---|---|---|---|
| Number (11,121) | 81 | 11040 | |
| Age-years, mean (SD) | 38.7 (11.2) | 37.1 (10.1) | = 0.173 |
| Sex | |||
| Female (%) | 65.4 | 56.5 | = 0.108 |
| Male (%) | 34.6 | 43.5 | |
| Arsenic well water conc. (ug/L), mean (SD) | 86 (114) | 101 (116) | = 0.239 |
| Education-years, mean (SD) | 3.5 (3.9) | 3.9 (4.3) | = 0.300 |
| BMI-kg/m2, mean (SD) | 19.8 (3.2) | 20.4 (4.7) | = 0.069 |
| SBP in mm Hg, mean (SD) | 129.6 (28.5) | 114.6 (17.8) | < 0.001 |
| DBP in mm Hg mean (SD) | 83.8 (17.0) | 73.9 (11.8) | < 0.001 |
| Hypertension (percent) | 43.2 | 12.5 | < 0.001 |
| Smoking (%) | |||
| Never smoked | 69.1 | 64.1 | = 0.344 |
| Current/Ex-Smoker | 30.9 | 35.9 | |
| Diabetes (percent) | 3.7 | 1.4 | = 0.084 |
p value by nonpaired t-test (continuous) or Chi Square test (categorical).
BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure. % = percent.
Diabetes means diabetes mellitus.
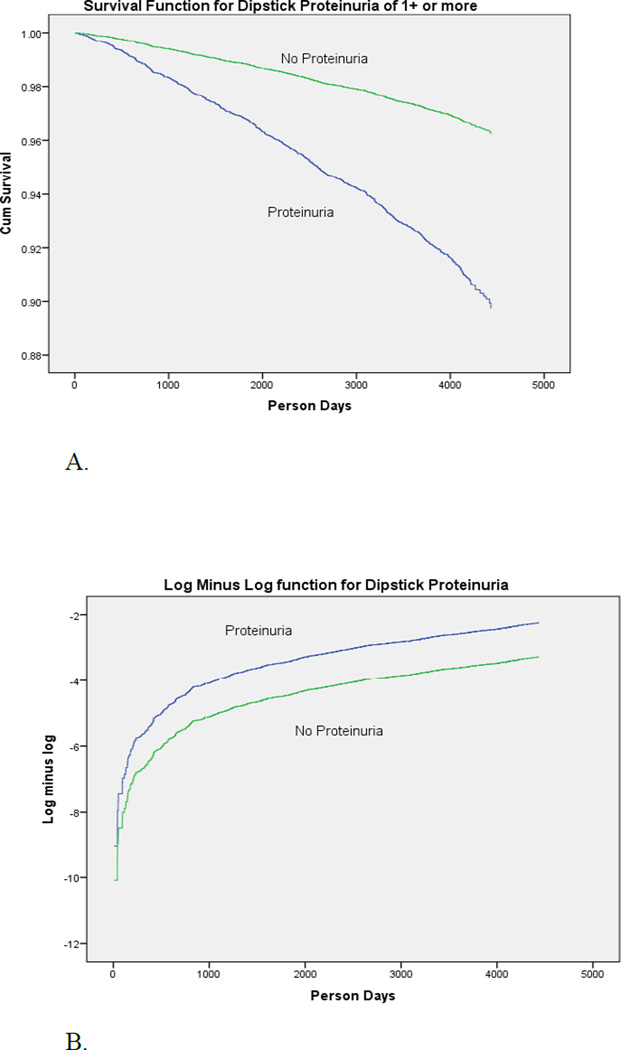
There was no difference in mortality using all proteinuria as the exposure and no proteinuria as the referent (data not shown) at baseline. Using baseline trace proteinuria only as the exposure versus no proteinuria also revealed no difference in mortality, either all-cause or cardiovascular disease between groups. With dipstick proteinuria at the 1+ and greater level, there was a significant difference in mortality. The Kaplan-Meier survival curve shows a difference in survival between those with 1+ or greater proteinuria at baseline and without proteinuria (trace or no proteinuria at baseline) (Figure 1A). The separation between curves was significant by the logrank test (p < 0.01). A log minus log plot of the same data (Figure 1B) revealed a constant separation between curves over 11–12 years consistent with the proportional hazards assumption.
Figure 1.
A. Kaplan-Meier survival curve for all subjects (n = 11,121) for 1+ dipstick proteinuria compared to no proteinuria over 11–12 years in rural Bangladesh. Baseline data collected from 2000 to 2002.
B. The log minus log plot reveals a constant difference (hazard) between groups over 11–12 years consistent with the proportional hazards assumption for 1+ baseline dipstick proteinuria. The graph includes all subjects (n = 11,121).
Types of Proteinuria – Baseline, Persistent, Incident, Remittent
Table 2A summarizes the Cox proportional hazards (PH) model revealing a significant crude and adjusted hazard ratio (HR) for baseline 1+ proteinuria all-cause mortality when followed over 11–12 years (referent is no proteinuria).
Table 2.
Cox regression model with the primary exposure baseline 1+ dipstick proteinuria and primary outcome all-cause mortality 2A. Table 2B is the same except for 1+ persistent proteinuria. Crude, age adjusted, and fully adjusted models. Bangladesh data 2000 to 2013.
| A | HR (95% C.I.) | P value |
|---|---|---|
| Baseline Proteinuria | ||
| Crude | 3.12 (1.87 – 5.21) | < 0.001 |
| Age Adjusted | 2.80 (1.68 – 4.66) | < 0.001 |
| Age | 1.115 (1.106 – 1.123) | < 0.001 |
| Proteinuria | ||
| Adjusted | 2.87 (1.71 – 4.80) | < 0.001 |
| Age | 1.092 (1.083 – 1.101) | < 0.001 |
| Sex | 1.32 (1.05 – 1.67) | < 0.025 |
| Arsenic Well Water | 1.001 (1.000 – 1.001) | < 0.050 |
| Education | 0.98 (0.96 – 1.00) | < 0.050 |
| Hypertension | 1.73 (1.45 – 2.06) | < 0.001 |
| BMI | 1.41 (1.21 – 1.64) | < 0.001 |
| Smoking | 1.66 (1.33 – 2.08) | < 0.001 |
| Diabetes | 1.81 (1.26 – 2.61) | < 0.010 |
| B | HR (95% C.I.) | P value |
| Persistent Proteinuria | ||
| Crude | 6.10 (2.90 – 12.86) | < 0.001 |
| Age Adjusted | 3.20 (1.52 – 6.75) | < 0.010 |
| Age | 1.116 (1.107 – 1.125) | < 0.001 |
| Proteinuria | ||
| Adjusted | 3.49 (1.64 – 7.41) | < 0.001 |
| Age | 1.093 (1.083 – 1.103) | < 0.001 |
| Sex | 1.40 (1.08 – 1.80) | < 0.020 |
| Arsenic Well Water | 1.000 (1.000 – 1.001) | = 0.173 |
| Education | 0.98 (0.96 – 1.00) | < 0.050 |
| Hypertension | 1.72 (1.42 – 2.08) | < 0.001 |
| BMI | 1.49 (1.26 – 1.76) | < 0.001 |
| Smoking | 1.59 (1.25 – 2.03) | < 0.001 |
| Diabetes | 2.26 (1.55 – 3.30) | < 0.001 |
HR = hazard ratio, Hypertension = blood pressure systolic of at least 140 and/or blood pressure diastolic of at least 90, dichotomous variable, BMI = body mass index.
Female is referent for sex. Smoking was ever/never. Arsenic well water concentration is a continuous variable.
Diabetes means diabetes mellitus.
Table 2B is a Cox PH model evaluating only those with persistent 1+ or greater proteinuria at baseline and also at 2 years. Analysis of trace persistent proteinuria revealed an adjusted HR of 1.31 (95% C.I.; 0.79–2.17)-fold greater risk of mortality over 9–10 years. However, with 1+ persistent proteinuria, there was a significant 3.4-fold greater mortality risk (referent no proteinuria). Thus, a dose-response for persistent proteinuria from none to trace to 1+ proteinuria was present.
Incident proteinuria determined from baseline to the 2-year evaluation is summarized in table 1 (Online appendix). Tables 1A and 1B reveal adjusted HR of 1.21 (0.91 – 1.61) and a 1.87 (0.92–3.78)-fold greater risk of mortality using trace and 1+ proteinuria as exposure at 9–10 years, relative to no proteinuria, respectively. Therefore, a dose-response from referent to trace to 1+ proteinuria was present. Tables 1C and 1D eliminate remittent incident proteinuria found at year-4 and evaluate incident (year 2) persistent (determined at year 4) proteinuria over 9–10 years. The data was limited but does show adjusted HR of 1.42 (0.59 – 3.44) and 2.30 (0.32 – 16.42) respectively, for trace and 1+ proteinuria. Here, a dose-response with increased magnitude of effect but no statistical power due to small numbers was found.
Remittent proteinuria determined at 2-years revealed no increased risk of mortality over 11–12 years (data not shown, log rank test p = 0.261 for no difference between groups). The referent for remittent proteinuria was no proteinuria at baseline.
Proteinuria – Gender and Cardiovascular Disease
Tables 2A and 2B (Online appendix) are gender specific models for baseline proteinuria and all-cause mortality for females and males, respectively. For females, the risk of all-cause mortality was 3.48-fold (95% C.I.; 1.53 – 7.90) greater with 1+ proteinuria at baseline relative to no proteinuria (referent no proteinuria at baseline) after adjustment. Similar results were found for males with a 2.47-fold (95% C.I.; 1.27 – 4.80) greater risk of all-cause mortality in men with proteinuria.
Table 3 evaluates all cardiovascular disease (CVD) mortality that includes both heart disease and stroke. There was a 3.5 (95% C.I.; 1.81 – 6.95)-fold greater CVD mortality in those with 1+ proteinuria at baseline, when followed for 11–12 years, compared to those without proteinuria (referent no proteinuria at baseline).
Table 3.
Cox regression model with the primary exposure variable baseline 1+ dipstick proteinuria and the primary outcome variable CVD mortality. Crude, age adjusted, and fully adjusted models. Bangladesh data 2000 to 2013.
| HR (95% C.I.) | P value | |
|---|---|---|
| Proteinuria | ||
| Crude | 4.62 (2.38 – 8.97) | < 0.001 |
| Proteinuria | ||
| Age Adjusted | 4.03 (2.08 – 7.83) | < 0.001 |
| Age | 1.135 (1.122 – 1.149) | < 0.001 |
| Proteinuria | ||
| Adjusted | 3.55 (1.81 – 6.95) | < 0.001 |
| Age | 1.108 (1.094 – 1.123) | < 0.001 |
| Sex | 1.58 (1.09 – 2.31) | < 0.025 |
| Arsenic Well Water | 1.001 (1.000 – 1.002) | = 0.131 |
| Education | 0.99 (0.97 – 1.03) | = 0.751 |
| Hypertension | 2.39 (1.88 – 3.08) | < 0.001 |
| BMI | 1.30 (1.03 – 1.65) | < 0.025 |
| Smoking | 1.39 (0.99 – 1.94) | = 0.055 |
| Diabetes | 2.47 (1.57 – 3.89) | < 0.001 |
HR = hazard ratio, BP = blood pressure, dichotomous, BMI = body mass index. Smoking combines ex-smokers and current smokers with referent never smokers. BMI referent is 18.5 – 24.99. Sex referent is female. Arsenic well water concentration is a continuous variable. CVD = cardiovascular disease.
Diabetes means diabetes mellitus.
There was no difference in cancer or renal failure mortality between proteinuria and no proteinuria groups.
There was also no evidence of biological interaction between proteinuria and; arsenic, smoking, BMI, sex, or hypertension.
Discussion
This is the first study to find baseline dipstick proteinuria to be a predictor of all-cause mortality or CVD mortality in an undeveloped country. At least three studies have found dipstick proteinuria at the 1+ or greater level to be a predictor of all-cause mortality (Grimm et al., 1997, Ha et al., 2006, Culleton et al., 2000). Our study was comparable with a 2.87-fold (95% C.I.; 1.71 – 4.80) greater risk of mortality with 1+ dipstick proteinuria versus less over 11–12 years, with results similar by gender. For women, the risk of mortality was the greatest with a 3.48-fold (95% C.I.; 1.53 – 7.90) greater risk with 1+ dipstick proteinuria at baseline compared to other. For men there was a 2.47 fold (95% C.I.; 1.27 – 4.80) greater risk of mortality over 11–12 years compared to referent (table 2B, online appendix). Therefore, the force of mortality for proteinuria is gender independent.
Our study was unable to find an increased all-cause mortality using dipstick proteinuria at only trace level and above in contrast to others (Wagener et al., 1994, Tanihara et al., 2005, Casiglia et al., 2000, Kojima et al, 2014). Two studies (Wagener et al., 1994, Tanihara et al., 2005) were consistent with the current study revealing a similar to higher risk of mortality in women compared to men. It is unclear why our study found no exposure-mortality relationship using either trace proteinuria as the exposure only or trace and above proteinuria as the exposure. One possibility is that heat, dehydration, and exercise can all cause a transient reversible proteinuria of no consequence. In tropical Bangladesh these conditions are common and may have resulted in false positive reversible proteinuria. In contrast, 1+ and higher proteinuria in all populations signifies a much higher level of proteinuria which is more likely to signify disease.
One plus persistent proteinuria, determined at the 2-year follow-up, was the strongest predictor of mortality with a 3.5-fold greater risk of all-cause mortality over 9–10 years with a dose-response found with trace persistent proteinuria. This finding is more robust than several other studies using as exposure trace (Kojima et al., 2014) or 1+ proteinuria as the exposure (Grimm et al., 1997, Ha et al., 2006). These studies found a 1.7 to 2.2-fold greater all-cause mortality.
Incident dipstick proteinuria and mortality of any kind have never been documented, although incident proteinuria has been associated with cardiovascular risk factors such as diabetes mellitus, hypertension, increased BMI, smoking, and increased cholesterol in some studies (Grimm et al, 1997, Jee et al., 2005) and increasing arsenic exposure in one study (Chen et al., 2011). Theoretically, incident proteinuria should be associated with the highest mortality since it includes all detectable cases (no bias, Neyman, 1955) and not just prevalent cases. Our study found a clear-cut dose-response relationship with incident proteinuria and no proteinuria, trace proteinuria, 1+ proteinuria with HRs of 1.0, 1.21, and 1.87, respectively (table 1A and 1B, online appendix). In addition, on follow-up over 4 years (2 years after incident proteinuria was detected), remittent proteinuria was removed and only incident persistent proteinuria was analyzed (tables 1C and 1D, online appendix). Clearly, the adjusted risks for incident persistent trace and 1+ proteinuria increased compared to all incident proteinuria (table 1C and 1D), albeit there was no statistical power to detect significant differences. Therefore, more incident proteinuria data is needed to confirm these findings. However, the nonsignificant 2.3-fold greater risk of mortality found with 1+ incident persistent proteinuria is very clinically meaningful and makes biologic sense (Vaughan, 2007, Rothman, 2012). In addition, incident “persistent” proteinuria would be expected to have a higher mortality compared to all incident proteinuria that includes false positive values (as seen in this study).
Cardiovascular disease mortality was increased in those with 1+ proteinuria at baseline compared to those without proteinuria. There was a 3.55 (95% C.I.; 1.81 – 6.95)-fold greater risk of dying of CVD (table 3) over 11–12 years with proteinuria than without which has been seen before in developed countries (Wagener et al., 1994, Grimm et al., 1997, Tanihara et al., 2005, Ha et al., 2006).
Strengths and Limitations
Study strengths include a large general population-based homogeneous sample with complete ascertainment of the hard outcome, total mortality. The four basic types of proteinuria were all detected and studied in one population, improving comparisons. Incident 1+ proteinuria, the unbiased form of proteinuria, was detected and found to have an approximate 1.87-fold greater mortality risk compared to no proteinuria, (a novel finding). A dose-response with both persistent and incident proteinuria and mortality was found, markedly strengthening study findings. The study was also relatively long at 11–12 years making it more likely a mortality difference would be found.
Study limitations include using a urine dipstick screening method that can lead to exposure misclassification. This occurred since persistent proteinuria at the 2- year follow-up was the most powerful predictor of all-cause mortality (table 2B). The misclassification weakness turned into a strength since our study clearly demonstrated the usefulness of repeating the dipstick and following persistent proteinuria “not” remittent proteinuria. A second weakness was the observational nature of the study; it is always possible unknown confounding occurred. We think this is less likely since 1+ baseline proteinuria has been found to result in all-cause (Grimm et al., 1997, Ha et al., 2006) and CVD mortality (Wagener et al., 1994, Grimm et al., 1997, Tanihara et al., 2005, Ha et al., 2006) in previous studies in developed countries. Finally, the best test to determine true sensitivity/specificity and accuracy for proteinuria/albuminuria is the 24-hour urine collection that can never be done in large populations. Therefore, the true exposed/unexposed groups were only approximated. Despite this weakness, mortality differences were still found and were undoubtedly markedly underestimated.
Mechanistically, the relationship between proteinuria and mortality is unknown. A leading hypothesis is that proteinuria reflects vascular damage at the glomerular level that is also a reflection of generalized atherosclerosis, the Steno hypothesis (Deckert et al., 1989). The generalized atherosclerosis would then promote increased all-cause and cardiovascular mortality. Reduction of proteinuria has been well documented to reduce progression to renal disease which in and of itself increases mortality (Jafar et al., 2001, Tonelli et al., 2006). Proteinuria promotes renal disease progression through tubular toxicity from filtered proteins, tubular overload, and direct mesangial toxicity as well as induction of inflammatory molecules (Burton et al., 1996, Wang et al., 1999).
Conclusions
This is the first study in a developing country to demonstrate that dipstick proteinuria results in an increase in mortality. Theoretically, developing countries such as Bangladesh with higher mortalities at younger ages may prevent detection of mortality differences secondary to competing risks (Chiang, 1991). We assume competing risks occurred with renal failure in this study where no differences were found between proteinuria groups. However, as has been shown in developed countries, there can be a greater than 10-fold increased risk of renal failure in those with dipstick proteinuria (Ha et al., 2006).
We conclude that baseline 1+ dipstick proteinuria is a gender independent6,8 predictor for allcause mortality and a predictor of CVD mortality (Wagener et al., 1994, Grimm et al., 1997, Tanihara et al., 2005, Ha et al., 2006) in rural Bangladesh. In addition, persistent 1+ proteinuria has the highest mortality risk. From a public health standpoint, our study suggests that repeat dipstick proteinuria testing is a better method for proteinuria screening in contrast to single testing where false positives will occur. One plus proteinuria of any type (baseline, persistent, or incident) predicts increased mortality with persistent 1+ proteinuria of greatest concern with a more than 3.4-fold risk of mortality over a decade. In developing as well as developed countries, repetitive dipstick proteinuria testing is cost effective, rapid (takes one minute) and in developing countries should be a preferred method both to detect disease and guide treatment. The two most common causes of proteinuria to search for first are diabetes mellitus and hypertension. In addition, smoking, elevated cholesterol, and obesity (typical CVD risk factors) have been associated with proteinuria and should be considered (Grimm et al, 1997, Jee et al, 2005, Pinto-Sietstma SJ et al, 2003).
Supplementary Material
Highlights.
Proteinuria as predictor of mortality has never been evaluated in an undeveloped country.
All four types of proteinuria have never been evaluated simultaneously in one study.
Three types of proteinuria were predictors of all-cause mortality in Bangladesh.
Persistent 1+ proteinuria was the strongest mortality predictor.
Baseline dipstick proteinuria was a predictor of cardiovascular disease mortality.
Acknowledgments
Funding: This work was supported by the National Institutes of Health grants P42ES010349, R01CA107431, and R01CA102484.
Abbreviations
- CVD
cardiovascular disease
- HR
hazard ratio
- HEALS
health effects of arsenic longitudinal study
- ICD
international classification of diseases
- PH
proportional hazards
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
No conflicts of interest are reported by any of the thirteen authors of this paper.
Presented in part at the Society of Epidemiologic Research 48th meeting June 16–19, 2015, Denver, CO with Professor James Tielsch from GWU as moderater for Global Health.
References
- 1.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin dependent diabetes mellitus. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 2.Agewall S, Wikstrand J, Ljungman S, Fagerberg B for the risk factor intervention study group. Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Am J Cardiol. 1997;80:164–169. doi: 10.1016/s0002-9149(97)00312-3. [DOI] [PubMed] [Google Scholar]
- 3.Hillege HL, Fidler V, Diercks GFH, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in a general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 4.Klausen KP, Scharling H, Jensen JS. Very low level of microalbuminuria is associated with increased risk of death in subjects with cardiovascular or cerebrovascular diseases. J Internal Med. 2006;260:231–37. doi: 10.1111/j.1365-2796.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- 5.Hossain MP, Goyder EC, Rigby JE, Nahas ME. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2008;53:166–174. doi: 10.1053/j.ajkd.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Wagener DK, Harris T, Madans JH. Proteinuria as a biomarker: risk of subsequent morbidity and mortality. Environmental Research. 1994;66:160–172. doi: 10.1006/enrs.1994.1052. [DOI] [PubMed] [Google Scholar]
- 7.Grimm RH, Svedsen KH, Kasiske B, Keane WF, Wahi MM. Proteinuria is a risk factor for mortality over 10 years follow-up. Kidney International. 1997;52(Suppl 63):S10–S14. [PubMed] [Google Scholar]
- 8.Tanihara S, Hayakawa T, Oki I, et al. Proteinuria is a prognostic marker for cardiovascular mortality: NIPPON DATA 80, 1980–1999. J Epidemiol. 2005;15:146–153. doi: 10.2188/jea.15.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha K, Kim HC, Kang DR, Nam CM, Vogue S, Suh AH. Dipstick urine protein as a predictor of cardiovascular mortality in Korean men: Korea medical insurance corporation study. J Prev Med Public Health. 2006;39:427–432. [PubMed] [Google Scholar]
- 10.Culleton BF, Larson MG, Parfrey PS, Kannel WB, Levy D. Proteinuria as a risk factor for cardiovascular disease and mortality in older people: a prospective study. Am J Med. 2000;109:1–8. doi: 10.1016/s0002-9343(00)00444-7. [DOI] [PubMed] [Google Scholar]
- 11.Casiglia E, Pauletto P, Mazza A, et al. Impaired glucose tolerance and its co-variates among 2079 non-diabetic elderly subjects: ten-year mortality in the CASTEL study. Acta Diabetol. 1996;33:284–290. doi: 10.1007/BF00571566. [DOI] [PubMed] [Google Scholar]
- 12.Kojima G, Sonoda K, Bell CL, et al. Proteinuria in midlife and 39-year mortality: the Honolulu heart program. Ann Epidem. 2014;24:407–409. doi: 10.1016/j.annepidem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahsan H, Chen Y, Parvez F, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 14.Pesola GR, Parvez F, Chen Y, Ahmed A, Hasan R, Ahsan H. Arsenic exposure from drinking water and dyspnea risk in Araihazar, Bangladesh: a population-based study. Eur Respir J. 2012;39:1076–1083. doi: 10.1183/09031936.00042611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Factor-Litvak P, Howe GR, et al. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol. 2007;165:541–552. doi: 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Z, Zheng Y, Mortlock R, Van Geen A. Rapid multi-element analysis of ground water by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379:512–518. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 17.Smith AH, Lingas EO, Rahman M. Contamination of drinking water by arsenic in Bangladesh: a public health emergency. Bull World Health Org. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 18.Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health. 2004;94:1936–37. doi: 10.2105/ajph.94.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Parvez F, Liu M, et al. Association between arsenic exposure from drinking water and proteinuria: results from the Health Effects of Arsenic Longitudinal Study. Int J Epidemiol. 2011;40:828–835. doi: 10.1093/ije/dyr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argos M, Kaira T, Rathouz PJ, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376:252–258. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronsmans C, Vanneste AM, Chakraborty J, van Ginneken J. A comparison of three verbal autopsy methods to ascertain levels and causes of maternal deaths in Matlab, Bangladesh. Int J Epidemiol. 1998;27:660–666. doi: 10.1093/ije/27.4.660. [DOI] [PubMed] [Google Scholar]
- 22.Jee SH, Boulware LE, Guallar E, Suh I, Appel LJ, Miller ER., 3rd Direct, progressive association of cardiovascular risk factors with incident proteinuria. Arch Intern Med. 2005;165:2299–2304. doi: 10.1001/archinte.165.19.2299. [DOI] [PubMed] [Google Scholar]
- 23.Neyman J. Statistics: servant of all sciences. Science. 1955;122:401–406. doi: 10.1126/science.122.3166.401. [DOI] [PubMed] [Google Scholar]
- 24.Vaughan RD. The importance of meaning. Am J Public Health. 2007;97:592–593. doi: 10.2105/AJPH.2006.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothman KJ. Epidemiology: An Introduction. 2nd. Chapt. 8. New York, NY: Oxford University Press; 2012. Random error and the role of statistics. [Google Scholar]
- 26.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32(4):219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 27.Jafar TH, Stark PC, Schmid CH, et al. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney International. 2001;60:1131–1140. doi: 10.1046/j.1523-1755.2001.0600031131.x. [DOI] [PubMed] [Google Scholar]
- 28.Tonelli N, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol. 2006;17:2034–2037. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 29.Burton C, Harris KP. The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis. 1996;27:765–775. [Google Scholar]
- 30.Wang Y, Rangan GK, Tay YC, Harris DC. Induction of monocyte chemoattractant protein-1 by albumin is mediated by nuclear factor kappaB in proximal tubular cells. J Am Soc Nephrol. 1999;10:1204–1213. doi: 10.1681/ASN.V1061204. [DOI] [PubMed] [Google Scholar]
- 31.Chiang CL. Competing risks in mortality analysis. Annu Rev Publ Health. 1991;12:281–307. doi: 10.1146/annurev.pu.12.050191.001433. [DOI] [PubMed] [Google Scholar]
- 32.Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE PREVEND Study Group. A central body fat distribution is related to renal function impairments, even in lean subjects. Am J Kidney Dis. 2003;4(4):733–741. doi: 10.1016/s0272-6386(03)00020-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.