SUMMARY
We examined the growth of tuberculosis (TB) genotype clusters during 2005–2010 in the United States, categorized by country of origin and ethnicity of the index case and geographic proximity to the US–Mexico border at the time of TB diagnosis. Nationwide, 38.9% of cases subsequent to Mexico-born index cases were US-born. Among clusters following US-born Hispanic and US-born non-Hispanic index cases, respectively 29.2% and 5.3% of subsequent cluster members were Mexico-born. In border areas, the majority of subsequent cases were Mexico-born following US-born Hispanic (56.4%) and US-born non-Hispanic (55.6%) index cases. These findings suggest that TB transmission commonly occurs between US-born and Mexico-born persons. Along the US–Mexico border, prioritizing TB genotype clusters following US-born index cases for investigation may prevent subsequent cases among both US-born and Mexico-born persons.
Keywords: tuberculosis transmission, epidemiology, emigrants and immigrants, United States epidemiology, genotype
IN 2010, 60% of reported tuberculosis (TB) cases in the United States occurred among foreign-born persons, including 21% in Mexico-born persons.1 Previous studies have noted high TB morbidity along the US–Mexico border.2,3 Studies of TB transmission dynamics (using TB genotyping data) between foreign-born and native-born persons in the United States vary by setting and by population studied.4–6 We examined the effect of the country of origin and ethnicity of the index case and geographic proximity to the US–Mexico border at the time of TB diagnosis on the demographic characteristics of subsequent TB genotype cluster members.
STUDY POPULATION AND METHODS
All cases reported to the US National Tuberculosis Genotyping Service with complete genotype results (spoligotyping and 12-locus mycobacterial interspersed repetitive unit–variable number of tandem repeats [MIRU-VNTR] typing) during the period from January 2005 to December 2010 were eligible for analysis.7 The most likely geographic cluster for each genotype was derived using spatial analysis and a Poisson probability model, SaTScan (Kulldorff, Boston, MA, USA), tested for statistical significance (P < 0.05) using 999 Monte Carlo replications.8,9 The maximum radius was set at 50 km based on the resident zip code centroid coordinates of each genotyped case within four overlapping 3-year window periods (2005–2007, 2006–2008, 2007–2009 and 2008–2010). A cluster from one 3-year period could be linked to a cluster in a subsequent, overlapping 3-year time period by means of at least one overlapping case. This methodology allowed us to identify likely chains of transmission across the entire study period. There was no duplicative case counting between window periods. Cases with missing or invalid zip code were excluded. A genotype cluster was defined as ≥2 cases with matching genotype results within the same geographic cluster. Cases with non-matching genotypes or who were not geographically clustered using SaTScan were considered non-clustered.
An index case was defined as the first TB case identified in a genotype cluster by case date (i.e., earliest of count date, treatment start date or report date). Clusters were excluded from the analysis if the presumptive index case occurred on or before 31 December 2005 to reduce misclassification of subsequent cases as index cases, and to eliminate clusters with long-standing transmission before the start of the study. As a result, most common genotypes were excluded from the analysis. We censored data 36 months after the index case (i.e., potential index cases had to occur during 2006–2008) to allow an equal chance of cluster growth.
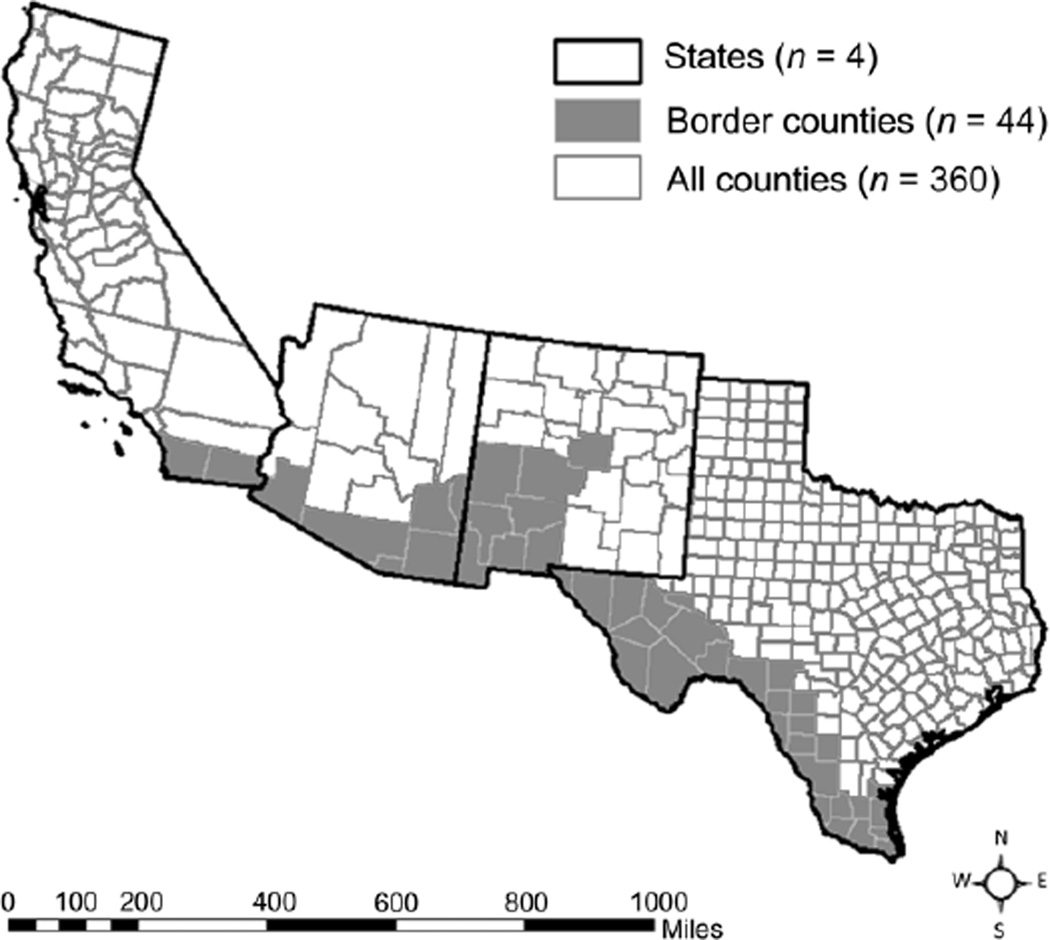
Cases were categorized by country of origin and ethnicity as Mexico-born, US-born Hispanic, US-born non-Hispanic or foreign-born from a country other than Mexico (other foreign-born). Cases for whom information on country of origin was missing were excluded. Border proximity was defined as three mutually exclusive categories: non-border states (i.e., all states except for California, Texas, Arizona and New Mexico), border areas within US–Mexico border states (defined by Public Use Microdata Areas used by the American Community Survey; Figure), and non-border areas within border states.10 Trends were analyzed using the Cochran-Armitage test.
Figure.
Border areas defined by PUMAs. A map of the four US states bordering Mexico (from left to right: California, Arizona, New Mexico and Texas). Dark lines represent state borders, grey lines represent county borders. PUMAs are composed of one or more counties; counties with fewer than 100 000 inhabitants are combined for American Community Survey population estimates. Shaded areas indicate PUMAs that share a geographic border with Mexico (considered ‘border areas’ for this analysis). PUMA = Public Use Microdata Area.
As the data collected were part of routine TB surveillance, this project was determined by the US Centers for Disease Control and Prevention not to be research involving human subjects.
RESULTS
Among 76 710 cases reported in the United States during 2005–2010, 45 573 (59.4%) culture-positive genotyped cases were eligible for inclusion in the study. Cases for whom zip code (n = 623) and place of birth (n = 34) were missing were excluded. A total of 14 142 cases meeting the inclusion criteria were studied, including 831 index cases and 2049 subsequent clustered cases; 11 262 cases were not in a TB genotype cluster.
Among clusters that followed the diagnosis of US-born non-Hispanic index cases, 82.1% of the subsequent cases were also US-born non-Hispanic, while 5.3% were Mexico-born (Table). In border areas, 22.2% of the subsequent cases were US-born non-Hispanic, while 55.6% of the subsequent cases were Mexico-born. Among clusters that followed the diagnosis of US-born Hispanic index cases, 33.2% of the subsequent cases were US-born Hispanic, 32.7% were US-born non-Hispanic and 29.2% were Mexico-born. In border areas, 56.4% of the subsequent cases were Mexico-born. Among the 27 US-born index cases in border areas, 81.5% were US-born Hispanic, including 18.2% aged <14 years.
Table.
Origin of subsequently clustered cases by origin of index case and border proximity, United States, 2005–2010
| Other foreign-born | Mexico-born | US-born Hispanic | US-born non-Hispanic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Border states | Border states | Border states | Border states | |||||||||||||
| Non- border states |
Non- border states |
Border areas |
All areas |
Non- border states |
Non- border areas |
Border areas |
All areas |
Non- border states |
Non- border areas |
Border areas |
All areas |
Non- border states |
Non- border areas |
Border areas |
All areas |
|
| Index cases, n* | 212 | 89 | 8 | 309 | 50 | 44 | 26 | 120 | 18 | 29 | 22 | 69 | 259 | 69 | 5 | 333 |
| Subsequent cases, n | 414 | 243 | 22 | 679 | 81 | 117 | 61 | 259 | 40 | 131 | 55 | 226 | 623 | 253 | 9 | 885 |
| Origin of subsequent cases, col % | ||||||||||||||||
| Other foreign-born | 70.5 | 77.0 | 86.4 | 73.3 | 12.3 | 2.6 | 3.3 | 5.8 | 15.0 | 3.8 | 0.0 | 4.9 | 8.5 | 8.7 | 0.0 | 8.5 |
| Mexico-born | 5.1 | 6.6 | 4.5 | 5.6 | 48.1 | 56.4 | 62.3 | 55.2 | 22.5 | 19.8 | 56.4 | 29.2 | 2.2 | 11.1 | 55.6 | 5.3 |
| US-born Hispanic | 5.6 | 2.5 | 4.5 | 4.4 | 16.0 | 22.2 | 24.6 | 20.8 | 25.0 | 35.1 | 34.5 | 33.2 | 2.4 | 7.5 | 22.2 | 4.1 |
| US-born non-Hispanic | 18.8 | 14.0 | 4.5 | 16.6 | 23.5 | 18.8 | 9.8 | 18.1 | 37.5 | 41.2 | 9.1 | 32.7 | 86.8 | 72.7 | 22.2 | 82.1 |
The number of index cases is equivalent to the number of clusters.
Mexico-born index cases had respectively 55.2% Mexico-born, 20.8% US-born Hispanic, and 18.1% US-born non-Hispanic subsequent cluster members. Among the 54 US-born Hispanic cluster members subsequent to Mexico-born index cases, 25.9% were aged <14 years. Clusters subsequent to Mexico-born index cases became more homogeneous moving from non-border states (48.1% Mexico-born), to non-border areas of border states (56.4%), to border areas (62.3%, P < 0.05).
DISCUSSION
This study revealed that the country of origin and ethnicity of the index case and US–Mexico border proximity were associated with the demographic characteristics of subsequent cluster members. We found that the majority of clusters subsequent to US-born non-Hispanic index cases were homogeneous; nationwide, 82.1% of subsequent cases were also US-born non-Hispanic. Meanwhile, clusters subsequent to US-born Hispanic index cases demonstrated a more heterogeneous picture: only 33.2% of sub sequent cases were US-born Hispanic, while 32.7% were US-born Hispanic and 29.2% were Mexico-born. In border areas, the majority of the cases that followed US-born Hispanic (56.4%) and US-born non-Hispanic (55.6%) index cases were Mexico-born. These findings suggest a novel concept: border-area clusters following US-born index cases (both Hispanic and non-Hispanic) may contribute to TB transmission to Mexico-born persons and to the higher case rates seen in this latter group. Among clusters following Mexico-born index cases, 20.8% of subsequent cases were US-born Hispanic (of which 25.9% were children), suggesting that the proportion of TB transmission occurring between Mexico-born and US-born persons was larger than previously reported.4,5
Of note, when compared with the population demographics of non-border states, border areas had an eight-fold increase in the proportion of the population that was US-born Hispanic and a nine-fold increase in the proportion that was Mexico-born.10 However, clusters following other foreign-born index cases did not have a larger proportion of subsequent US-born Hispanic or Mexico-born cases in border states or border areas, suggesting that demographic changes in border areas do not alone account for our findings.
There are several limitations to the study. First, index cases may not represent the source of localized transmission. However, examining index cases, as defined in this analysis, simulates the conditions that a local health department might experience before cluster growth (i.e., a single, as yet non-clustered, case of TB disease). Second, 24-locus MIRU-VNTR was not routinely performed during the study period; using 12-locus MIRU-VNTR may overestimate clustering, a surrogate marker for TB transmission. Third, Mycobacterium bovis clusters were included in the analysis, which may not reflect person-to-person transmission; however, a sensitivity analysis excluding M. bovis clusters did not impact the results of the study. Finally, cases who were missing zip code or data on origin, and were excluded from the analysis, may have been part of a chain of transmission.
CONCLUSIONS
Overall, we found that clusters subsequent to Mexico-born index cases had more US-born cases than previously reported. In addition, clusters subsequent to US-born index cases in border areas commonly included Mexico-born cases. Along the US–Mexico border, prioritizing TB clusters occurring after the diagnosis of US-born index cases for investigation may help prevent subsequent cases among both US-born and Mexico-born persons.
Acknowledgments
The authors gratefully acknowledge the staff of the National TB Genotyping Service contract laboratories, local and state public health laboratories and local and state health departments who collected data included for these analyses. They also thank S Althomsons, A France, J Grant, S Kammerer, T Navin and J Tobias for their contributions to this work.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Footnotes
Conflict of interest: none declared.
References
- 1.US Centers for Disease Control and Prevention. Trends in tuberculosis—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:181–185. [PubMed] [Google Scholar]
- 2.Schneider E, Laserson KF, Wells CD, Moore M. Tuberculosis along the United States–Mexico border, 1993–2001. Rev Panam Salud Publica. 2004;16:23–34. doi: 10.1590/s1020-49892004000700004. [DOI] [PubMed] [Google Scholar]
- 3.Wells CD, Ocaña M, Moser K, Bergmire-Sweat D, Mohle-Boetani JC, Binkin NJ. A study of tuberculosis among foreign-born Hispanic persons in the US States bordering Mexico. Am J Respir Crit Care Med. 1999;159:834–837. doi: 10.1164/ajrccm.159.3.9712122. [DOI] [PubMed] [Google Scholar]
- 4.Jasmer RM, Ponce de Leon A, Hopewell PC, et al. Tuberculosis in Mexican-born persons in San Francisco: reactivation, acquired infection and transmission. Int J Tuberc Lung Dis. 1997;1:536–541. [PubMed] [Google Scholar]
- 5.Chin DP, DeRiemer K, Small PM, et al. Differences in contributing factors to tuberculosis incidence in U.S.-born and foreign-born persons. Am J Respir Crit Care Med. 1998;158:1797–1803. doi: 10.1164/ajrccm.158.6.9804029. [DOI] [PubMed] [Google Scholar]
- 6.Driver CR, Macaraig M, McElroy PD, et al. Which patients’ factors predict the rate of growth of Mycobacterium tuberculosis clusters in an urban community? Am J Epidemiol. 2006;164:21–31. doi: 10.1093/aje/kwj153. [DOI] [PubMed] [Google Scholar]
- 7.Cowan LS, Diem L, Monson T, et al. Evaluation of a two-step approach for large-scale, prospective genotyping of Mycobacterium tuberculosis isolates in the United States. J Clin Microbiol. 2005;43:688–695. doi: 10.1128/JCM.43.2.688-695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulldorff M. A spatial scan statistic. Comm Statist Theory Methods. 1997;26:1481–1496. [Google Scholar]
- 9.Moonan PK, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent. Mycobacterium tuberculosis transmission, United States. Emerg Infect Dis. 2012;18:458–465. doi: 10.3201/eid1803.111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Census Bureau. Washington DC, USA: US Census Bureau; 2013. [Accessed December 2013]. American Community Survey. http://www.census.gov/acs/www/ [Google Scholar]