Abstract
Stones made of bacterial aggregates can be found in chronically inflamed lymphoid tissue such as hypertrophied tonsils. Although it is common to find tonsilloliths in cryptic tonsils, it is rare to find stones in adenoid tissue. Here we present an interesting case of a patient who underwent adenoidectomy for adenoid hypertrophy, recurrent malaise and upper respiratory infections. Intraoperatively we found numerous bright green stones in the crypts of the adenoid tissue, reminiscent of tonsilloliths in tonsillar crypts. Pathology revealed polymicrobial bacterial aggregates surrounded by neutrophils. Our findings suggest that the pathophysiology is similar to that of tonsillolith formation. Thus, we should at least consider the presence of adenoid stones and consider adenoidectomy for symptoms often attributed to tonsilloliths. We have coined the term “adenoliths” to describe this interesting finding and present it as a potential source of recurrent infection.
Introduction
The finding of calcified stones within the cryptic folds of chronically inflamed hypertrophied tonsils is not a rare event. However, stones are not commonly found in other lymphoid tissues such as adenoids or lingual tonsils. A literature search in PubMed reveals no publications with the exception of one French, radiology abstract reporting radio-opaque stones in the pharynx as incidental findings in 6% of head/neck CTs [1]. Tonsilloliths are usually a result of chronic inflammation and have been described as biofilms of aerobic and anaerobic bacteria which calcify over time, are difficult to treat with antibiotics and serve as a nidus for recurrent infection [2]. Any bacteria or fungi causing tonsillitis can initiate the calcification process, which is attributed to the stasis of saliva [3]. They are often associated with halitosis, which is believed to be due to volatile sulfur molecules [4.5]. Stoodley et al. [2] performed histological examination and observed a layer of fibrinous debris and inflammatory cells between the tonsillolith and the crypt epithelium. They also observed that tonsilloliths are composed of densely packed polymicrobial bacteria, held together by extracellular matrix. Tsuenishi et al. [4] used PCR analysis of 16S ribosomal DNA of tonsilloliths and determined that the aggregates are polymicrobial and that one microbe in particular, F. nucleatum, is known to produce volatile sulfur compounds which can cause foul odor.
Case Report
We present an interesting case of a 30 year old healthy female whose chief complaint was nasal congestion, fatigue and recurrent upper respiratory infections. She had a history of prolonged Epstein - Barr virus (EBV) infection over 10 years prior, but has an otherwise unremarkable medical history and no prior adenotonsillectomy. Physical exam revealed 2+ palatine tonsils that appeared normal while fiber optic nasophyaryngoscopy demonstrated significant adenoid hypertrophy. The patient had frequent upper respiratory type infections and malaise as well as chronic nasal congestion, attributed to the hypertrophied adenoids. She did not complain of halitosis. A sinus CT scan showed normal sinuses, enlarged but otherwise non-malignant appearing adenoids and no radiopacities in her adenoid bed. A decision was made to proceed with adenoidectomy to alleviate her symptoms.
The patient was taken to the operating room for an endoscopic transnasal adenoidectomy. Intraoperatively, we found that the adenoid tissue was cryptic and as we suctioned the adenoid mass, small 1–2 mm sized bright lime-green colored stones were delivered (Figure 1). There were approximately 5–10 stones of similar size. These stones were sent to microbiology and pathology. A small biopsy of the adenoid was also sent for pathology and the adenoidectomy was performed using suction bovie electrocautery. The patient was seen for a follow-up visit several weeks later with improved nasal breathing. Long-term follow up by phone revealed that her nasal breathing was significantly improved and she had noticeably less fatigue and fewer upper airway infections.
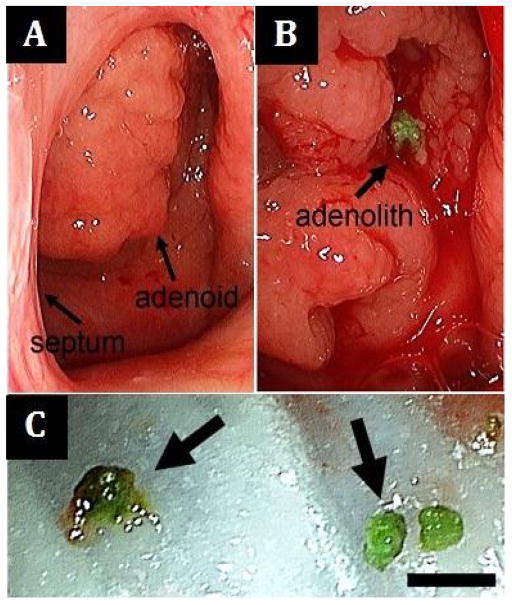
Figure 1.
(A) Preoperative view of the hypertrophic adenoid tissue through the left choana, septum to the left; (B) Unroofing of the hypertrophic adenoid tissue reveals a bright green stone; (C) Multiple stones were removed (scale bar 4mm).
Pathology of the adenoid tissue revealed adenoid hypertrophy and no malignancy. Pathology of the stones revealed dense bacterial aggregates (including filamentous forms) surrounded by neutrophils (Figure 2), consistent with what is observed in tonsilloliths. Cultures grew: 4+ fastidious gram negative rods not identifiable by conventional biochemical methodology, 4+ Streptococcus milleri group (anginosus/constellatus/intermedius), lactose fermenting gram negative rods: isolated from broth only, and 4+ presumptive mixed anaerobic flora including anaerobic gram negative rods and anaerobic gram positive rods. The growth of gram-negative rods and finding of filamentous structures on histology are suggestive of Actinomyces sulfur granules often seen in tonsillar calculi [3].
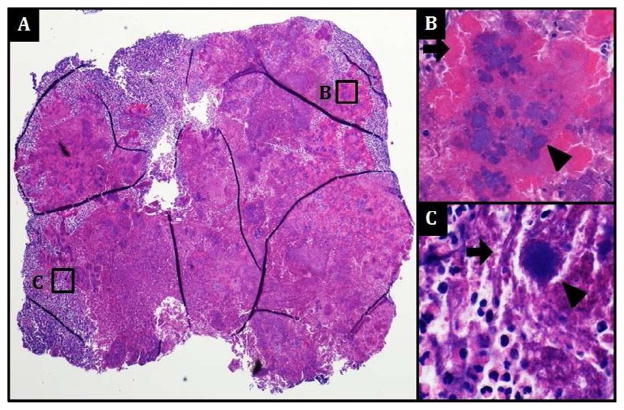
Figure 2.
(A) Photomicrographs of an H & E-stained section of the entire stone demonstrate predominantly necrotic tissue with numerous dense basophilic bacterial aggregates (B & C, arrowheads) surrounded by filamentous (C, arrow, 600x) structures and/or radiating amorphous eosinophilic material (B, arrow, 400x) reminiscent of granulomas. Abundant neutrophils surround the bacterial aggregates. No viable adenoid tissue or obvious calcifications are seen within the stone.
Discussion
Here we have described a rare case of adenoid stones that were removed from hypertrophied adenoid tissue in an otherwise healthy patient. Tonsilloliths appear to form in chronically inflamed tonsils and are composed of polymicrobial bacterial aggregates surrounded by fibrinous debris and inflammatory cells. Similar to prior reports on tonsilloliths, the stones we collected from the adenoid on pathological exam also contained polymicrobial bacterial aggregates surrounded by neutrophils. Our patient’s hypertrophied adenoids were much like a typical adenoid hypertrophy and were very cryptic, creating potential spaces for bacterial aggregates and biofilm formation. We assume the mechanism is similar to tonsillolith formation in tonsillar crypts with biofilm and inflammatory cell infiltration. This does raise the question of whether lymphoid stones could serve as a nidus for infection or if they are merely a result of ongoing chronic infection. For example, there is a recent study which suggests that the presence of adenoid biofilm, rather than the size of adenoid is correlated with chronic otitis media [6].
We expect that if stones can form in the adenoids, they are also just as likely to be a source of halitosis, should they contain sulfur producing bacteria found in oral flora. Fortunately our patient had no complaint of halitosis and, consistent with this, the cultures did not grow F. nucleatum. However, evaluation for adenoid stones perhaps should be a consideration for complaint of halitosis.
We were also intrigued by the lack of publications of adenoid stones. This is the first case the authors have encountered such findings. Yet there is one abstract that reports incidental radiographic finding of stones in the nasopharynx in 6% of head and neck CTs [1]. The sensitivity of finding tonsil stones on CT is 46.1% of stones measuring at least 3 mm [7], therefore we would expect to find greater than 12% of patients to have stones somewhere in the nasopharynx. Why we have not encountered more stones in adenoids is unclear. Perhaps we are not looking for them. Our case patient did not yield radiographically visible stones. There are several explanations for this. One possibility is that the stones we collected do not contain gross calcifications. Another possibility is that the stones we collected are quite small, measuring 1–2 mm, and may be too small to detect on CT. From reports, the sensitivity appears to be limited to stones measuring at least 2–3 mm.
The patient did have a prolonged EBV infection. Although EBV has been suggested to cause adenotonsillar hypertrophy, such as in post-transplant lymphoproliferative disorders and mononucleosis, limited studies suggest that there is no association between recurrent tonsillitis and the presence of EBV [8]. We might conclude that there is likely to be no correlation between EBV and adenoid infections.
One may argue that to call an adenoid concretion an “adenolith” (i.e., stone) would require that it contain radio-opaque calcifications. Although we did not identify gross calcifications in the specimens we evaluated, we cannot rule out microcalcifications. More importantly, our findings suggest that the microbiological process of these adenoid stones is too strikingly similar to that of tonsil stones. Thus, we can only expect that grossly calcified adenoliths should similarly be expected to form if given enough time.
Conclusion
In summary we present a rare and interesting case of stones in the adenoid tissues. Although it is common to find stones within crypts in the palatine tonsils, it is uncommon to find them in other lymphoid tissues of the pharynx. Here we report that stones in the adenoids can indeed occur in much the same way as in the tonsils and we have coined the term “adenoliths” to describe them. Our findings suggest that these aggregates of bacterial biofilm can also form in adenoid tissue. This raises the possibility that adenoliths serve as nidus for chronic infection and source of halitosis and warrant removal of adenoids.
Acknowledgments
The first author is supported by a grant (NIDCD Research Training in Otolaryngology, DC000018).
References
- 1.Ben Salem D, Guiu B, Duvillard C, Couaillier JF, Ricolfi F. Nasopharyngeal tonsillolith: a report of 31 cases. J Radiol. 2007;88:259–262. doi: 10.1016/s0221-0363(07)89812-x. [DOI] [PubMed] [Google Scholar]
- 2.Stoodley P, Debeer D, Longwell M, Nistico L, Hall-Stoodley L, et al. Tonsillolith: not just a stone but a living biofilm. Otolaryngol Head Neck Surg. 2009;141:316–321. doi: 10.1016/j.otohns.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Cooper MM, Steinberg JJ, Lastra M, Antopol S. Tonsillar Calculi. Report of a case and review of the literature. Oral Surgery. 1983;55:239–243. doi: 10.1016/0030-4220(83)90320-1. [DOI] [PubMed] [Google Scholar]
- 4.Tsuneishi M, Yamamoto T, Kokeguchi S, Tamaki N, Fukui K, et al. Composition of the bacterial flora in tonsilloliths. Microbes Infect. 2006;8:2384–2389. doi: 10.1016/j.micinf.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Krespi YP, Shrime MG, Kacker A. The relationship between oral malodor and volatile sulfur compound-producing bacteria. Otolaryngol Head Neck Surg. 2006;135:671–676. doi: 10.1016/j.otohns.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Oda M, Kito S, Tanaka T, Nishida I, Awano S, et al. Prevalence and imaging characteristics of detectable tonsilloliths on 482 pairs of consecutive CT and panoramic radiographs. BMC Oral Health. 2013;13:54. doi: 10.1186/1472-6831-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saafan ME, Ibrahim WS, Tomoum MO. Role of adenoid biofilm in chronic otitis media with effusion in children. Eur Arch Otorhinolaryngol. 2013;270:2417–2425. doi: 10.1007/s00405-012-2259-1. [DOI] [PubMed] [Google Scholar]
- 8.Endo LH, Ferreira D, Montenegro MC, Pinto GA, Altemani A, et al. Detection of Epstein-Barr virus in tonsillar tissue of children and the relationship with recurrent tonsillitis. Int J Pediatr Otorhinolaryngol. 2001;58:9–15. doi: 10.1016/s0165-5876(00)00446-8. [DOI] [PubMed] [Google Scholar]