Abstract
Herbivory has been long considered an important component of plant-animal interactions that influences the success of invasive species in novel habitats. One of the most important hypotheses linking herbivory and invasion processes is the enemy-release hypothesis, in which exotic plants are hypothesized to suffer less herbivory and fitness-costs in their novel ranges as they leave behind their enemies in the original range. Most evidence, however, comes from studies on leaf herbivory, and the importance of flower herbivory for the invasion process remains largely unknown. Here we present the results of a meta-analysis of the impact of flower herbivory on plant reproductive success, using as moderators the type of damage caused by floral herbivores and the residence status of the plant species. We found 51 papers that fulfilled our criteria. We also included 60 records from unpublished data of the laboratory, gathering a total of 143 case studies. The effects of florivory and nectar robbing were both negative on plant fitness. The methodology employed in studies of flower herbivory influenced substantially the outcome of flower damage. Experiments using natural herbivory imposed a higher fitness cost than simulated herbivory, such as clipping and petal removal, indicating that studies using artificial herbivory as surrogates of natural herbivory underestimate the real fitness impact of flower herbivory. Although the fitness cost of floral herbivory was high both in native and exotic plant species, floral herbivores had a three-fold stronger fitness impact on exotic than native plants, contravening a critical element of the enemy-release hypothesis. Our results suggest a critical but largely unrecognized role of floral herbivores in preventing the spread of introduced species into newly colonized areas.
Introduction
Research on flower herbivory has grown remarkably over the past few decades, which has permitted to confirm its prevalence in a wide variety of flowering plant species and environments, and to develop new perspectives on its ecological, evolutionary and functional role in plant populations. Recent studies suggest flower herbivory needs to be conceptualized as different from leaf herbivory [1], as they represent ecological interactions that differ in important ways. For example, unlike leaf herbivores that indirectly affect plant reproduction through alteration of the photosynthetic capacity and water balance function [2,3], floral herbivores influence not only plant physiology but link most processes related with plant reproduction through damaging primary reproductive tissues such as pistils, anthers and ovules [4–6]. Likewise, by consuming accessory tissues such as petals, sepals, or bracts, flower herbivores change flower display and floral integration, which often discourage pollinators to visit damaged flowers and reduce substantially plant reproduction [1,7–11]. Even though flower herbivory can decrease plant fitness to degrees comparable with or exceeding leaf herbivory [12–16], relatively few studies have examined its importance for processes that occur beyond the scale of local populations [17], such as those involving colonization of new habitats and establishment in novel environments. This omission is unfortunate as the fate of invasive species in new habitats is determined, at least in part, by the biotic scenario and the balance between mutualistic and antagonistic interactions found in novel communities [18–20].
One of the most important frameworks linking antagonistic interactions and invasion processes is the enemy-release hypothesis (ERH hereafter). This hypothesis indicates that exotic plants may suffer less herbivory and fitness costs in their novel ranges compared to co-occurring native plants, because invaders leave behind natural enemies present in their original range [18]. In the absence of natural enemies, the hypothesis predicts that plants in novel habitats may benefit from reduced herbivore regulation, leading to increased densities that may result in population spread. While a large number of studies have been carried out to test this hypothesis [19–24], conclusions have provided mixed results [25,26], suggesting that the ERH may not be applicable to all cases. For instance, it has been reported that the ERH is a context-dependent hypothesis, where studies of herbivory at the local community level (i.e., comparing native and introduced species co-occurring in a community) rarely support the hypothesis in comparison to tests performed at a larger biogeographical scale (i.e., comparing the same plant species in its natural and introduced range) [27]. Likewise, results from a meta-analysis found that native species have better performance than invasive alien ones, suggesting that native species are more tolerant to damage [28]. To our knowledge, the only study addressing the ERH in the context of flower herbivory is that of Sowell and Wolfe (2010) on four Ipomoea species at the community level. Their main finding indicates that the intensity of floral herbivory was contingent upon the residence status of the plant species. The native Ipomoea species experienced higher florivory intensity and had a stronger reproductive impact than non-native species. In principle, this result would suggest that the ERH, first developed in the context of foliar herbivory, might also apply to studies of flower herbivory, as proposed by McCall and Irwin (2006). However, it is likely, at least in principle, that generalist flower herbivores found in novel habitats shift onto newly introduced plants, causing a stronger fitness cost than the observed original habitat. Unfortunately, no attempt has been made to quantitatively synthesize the existing evidence for floral herbivory at broad spatial scales, and in consequence, no generalization is possible regarding the specific effect of florivory for invasion processes.
In this study we present the results of a meta-analysis on the fitness impact of flower herbivory on native and exotic plant populations. While our primary emphasis is on the role of the provenance of plant species, we also examine the importance of additional moderators such as the type of damage inflicted to flowers and the ecological interaction responsible for flower damage. More specifically, in this study we will examine the magnitude and direction of overall florivory effects across studies, and will address the extent to which such effects depend on the methodology used in studies of flower herbivory (natural or simulated herbivory), the plant response to the type of flower herbivory (florivory or nectar robbing), and the plant residence status (native and exotic).
Materials and Methods
We searched the electronic databases ISI Web of Science (1981- August 2013) and Scopus (1960- August 2013) for the following keywords: “flower herbivory”, “floral herbivory”, “florivory”, “petal herbivory” and “nectar rob*”. In addition, we examined the reference list of narrative reviews [1,14,29,30]. To be included in the meta-analysis, the published study had to fulfill the following four criteria: 1) to describe the effect of the type of floral herbivory (florivory or nectar robbing) on plant fitness (e.g., seed set, fruit set, seed production per plant, fruit production per plant, pollen deposition on stigma, pollen removal and export); 2) to have at least two treatments, namely, control (undamaged flowers) and florivory or nectar robbing (natural or experimental flower damage); 3) to report the mean, sample size, and dispersion measure (standard deviation or standard error) of each treatment or the statistics of the test employed indicating the direction of the effect and its significance level. When information was presented in graphs only, we used Graph Click version 3.0 (available at: http://www.arizonasoftware.ch/graphclick/download.html) to extract the mean and dispersion measures, and 4) to present herbivory not performed by ungulates as they often browse and damage plants in a broader scale than flower units, which is the focus of this study. After inspection of 214 papers, we found 51 that satisfied the four criteria indicated above, gathering 83 records from them. We included more than one record per study only in cases where different plant species and/or populations were studied in the same research and when the study included female and male fitness estimations. When the same population was measured in different years, we computed a mean effect size across years to be included in the general analysis (see Table 1 for details). In addition to the 83 case studies extracted from the literature, we included 60 records corresponding to unpublished data (S1 Table). In total, we gathered 143 records from 41 families, 78 genera and 96 plant species.
Table 1. Major characteristics of studies included in the meta-analysis.
Asterisk indicates mean values across years for the same study, species, site and response variable.
| Ref # | Peer reviewed | Authors | Plant species | Family | Residence status | Type of damage | Response variable | Hedges'd | Variance | Total sample size |
|---|---|---|---|---|---|---|---|---|---|---|
| [4] | yes | Krupnick & Weis 1999 | Isomeris arborea | Capparaceae | Native | Florivory | Pollen grains per stamen | -0.531 | 0.030 | 139 |
| [5] | yes | Maron et al 2002 | Cirsium occidentale | Asteraceae | Native | Florivory | Viable seeds (old dune) | -1.881 | 0.115 | 50 |
| [5] | yes | Maron et al 2002 | Cirsium occidentale | Asteraceae | Native | Florivory | Viable seeds (new dune) | -0.732 | 0.089 | 48 |
| [15] | yes | Mothershead & Marquis 2000 | Oenothera macrocarpa | Onagraceae | Native | Florivory | Fruit set | -0.445 | 0.025 | 514 |
| [16]* | yes | Hendrix & Trapp 1989 | Pastinaca sativa | Apiaceae | Exotic | Florivory | Recruitment | -0.651 | 0.164 | 20 |
| [16] | yes | Hendrix & Trapp 1989 | Pastinaca sativa | Apiaceae | Exotic | Florivory | Pollen grains per stamen | 0.323 | 0.029 | 141 |
| [53] | yes | Caballero et al 2013 | Tristerix aphyllus | Loranthaceae | Native | Nectar robbery | Fruit set | 0.093 | 0.127 | 32 |
| [54] | yes | Hendrix 1984 | Heracleum lanatum | Apiaceae | Native | Florivory | Seeds per plant | -0.331 | 0.152 | 27 |
| [55] | yes | Ashman et al 2004 | Fragaria virginiana | Rosaceae | Native | Florivory | Fruit Number | -0.161 | 0.053 | 76 |
| [56] | yes | Hendrix & Trapp 1981 | Pastinaca sativa | Apiaceae | Exotic | Florivory | Seed production | 2.154 | 0.578 | 11 |
| [57] | yes | Krupnick & Weis 1998 | Isomeris arborea | Capparaceae | Native | Florivory | Viable seeds per fruit | 0.432 | 0.117 | 35 |
| [58]* | yes | Louda & Potvin 1995 | Cirsium canescens | Asteraceae | Native | Florivory | Viable undamaged seeds | -0.819 | 0.048 | 81 |
| [59] | yes | Burkle et al 2007 | Delphinium nuttallianum | Ranunculaceae | Native | Nectar robbery | Seeds per fruit | -0.396 | 0.138 | 38 |
| [59] | yes | Burkle et al 2007 | Linaria vulgaris | Scrophulariaceae | Exotic | Nectar robbery | Seeds per fruit | 1.538 | 0.259 | 20 |
| [60] | yes | Deng et al 2004 | Alpinia kwangsiensis | Zingiberaceae | Native | Nectar robbery | Fruit set | -1.930 | 0.977 | 6 |
| [61] | yes | Maloof 2001 | Corydalis caseana | Fumariaceae | Native | Nectar robbery | Seeds per fruit | -0.688 | 0.265 | 16 |
| [62] | yes | Navarro 2001 | Macleania bullata | Ericaceae | Native | Nectar robbery | Fruit set | -2.068 | 0.018 | 344 |
| [63] | yes | Richardson 2004 | Chilopsis linearis | Bignoniaceae | Native | Nectar robbery | Pollen tubes per style | 0.420 | 0.069 | 64 |
| [64] | yes | Traveset et al 1998 | Fuchsia magellanica | Onagraceae | Native | Nectar robbery | Fruit set | -1.947 | 0.295 | 20 |
| [65] | yes | Zhang et al 2009 | Corydalis tomentella | Fumariaceae | Native | Nectar robbery | Seed set | -0.257 | 0.022 | 191 |
| [65] | yes | Zhang et al 2009 | Corydalis incisa | Fumariaceae | Native | Nectar robbery | Seed set | 0.046 | 0.017 | 234 |
| [65] | yes | Zhang et al 2009 | Corydalis ternatifolia | Fumariaceae | Native | Nectar robbery | Seed set | -0.197 | 0.017 | 236 |
| [66] | yes | Amsberry & Maron 2006 | Balsamorhiza sagittata | Asteraceae | Native | Florivory | Seeds per plant (site 1) | -0.197 | 0.033 | 120 |
| [66] | yes | Amsberry & Maron 2006 | Balsamorhiza sagittata | Asteraceae | Native | Florivory | Seeds per plant (site 2) | -0.471 | 0.034 | 120 |
| [66] | yes | Amsberry & Maron 2006 | Balsamorhiza sagittata | Asteraceae | Native | Florivory | Seeds per plant (site 3) | -0.358 | 0.034 | 120 |
| [66] | yes | Amsberry & Maron 2006 | Balsamorhiza sagittata | Asteraceae | Native | Florivory | Seeds per plant (site 4) | 0.109 | 0.033 | 120 |
| [67] | yes | Valdivia & Niemeyer 2005 | Alstroemeria umbellata | Alstroemeriaceae | Native | Florivory | Seed set | -0.438 | 0.011 | 385 |
| [68] | yes | Fritz & Morse 1981 | Asclepias syriaca | Asclepiadaceae | Native | Nectar robbery | Pollinia insertions | -0.084 | 0.148 | 27 |
| [69] | yes | Navarro 2000 | Anthyllis vulneraria | Fabacecae | Native | Nectar robbery | Fruit set | 1.214 | 0.070 | 68 |
| [70] | yes | Temeles & Pan 2002 | Impatiens capensis | Balsaminaceae | Native | Nectar robbery | Pollen on stigmas | -0.031 | 0.051 | 79 |
| [71] | yes | Utelli & Roy 2001 | Aconitum lycoctonum | Ranunculaceae | Native | Nectar robbery | Seeds per fruit | -0.138 | 0.074 | 54 |
| [72] | yes | Zhang et al 2007 | Glechoma longituba | Lamiaceae | Native | Nectar robbery | Pollen in anthers | 0.112 | 0.050 | 80 |
| [72] | yes | Zhang et al 2007 | Glechoma longituba | Lamiaceae | Native | Nectar robbery | Fruit set | -0.227 | 0.003 | 1159 |
| [72] | yes | Zhang et al 2007 | Glechoma longituba | Lamiaceae | Native | Nectar robbery | Seed set | -0.632 | 0.420 | 10 |
| [73] | yes | de Waal et al 2012 | Babiana ringens | Iridaceae | Native | Florivory | Seed set | 0.096 | 0.067 | 60 |
| [74] | yes | Navarro et al 1993 | Petrocoptis grandiflora | Caryophyllaceae | Native | Nectar robbery | Fruit set | 1.673 | 0.100 | 54 |
| [75] | yes | Wise et al 2008 | Solanum carolinense | Solanaceae | Native | Florivory | Fruits per plant | -1.596 | 0.110 | 48 |
| - | no | Navarro, L. unpublished data | Centropogon granulosus | Campanulaceae | Native | Nectar robbery | Fruit set | -2.433 | 0.696 | 10 |
| - | no | Navarro, L. unpublished data | Barleria cristata | Acanthaceae | Exotic | Nectar robbery | Fruit set | -1.989 | 0.272 | 22 |
| - | no | Navarro, L. unpublished data | Asystasia gangetica | Acanthaceae | Exotic | Nectar robbery | Fruit set | -1.407 | 0.499 | 10 |
| - | no | Navarro, L. unpublished data | Alloplectus tetragonoides | Gesneriaceae | Native | Nectar robbery | Fruit set | -0.051 | 0.400 | 10 |
| - | no | Navarro, L. unpublished data | Aloe secundiflora | Xanthorrhoeaceae | Native | Nectar robbery | Fruit set | 0.708 | 0.213 | 20 |
| - | no | Navarro, L. unpublished data | Aloe vera | Xanthorrhoeaceae | Exotic | Nectar robbery | Fruit set | -1.036 | 0.351 | 13 |
| - | no | Navarro, L. unpublished data | Alpinia purpurata | Zingiberaceae | Exotic | Nectar robbery | Fruit set | -2.902 | 0.684 | 12 |
| - | no | Navarro, L. unpublished data | Alpinia purpurata | Zingiberaceae | Exotic | Nectar robbery | Fruit set | -3.276 | 1.204 | 8 |
| - | no | Navarro, L. unpublished data | Anthirrinun majus | Plantaginaceae | Native | Nectar robbery | Fruit set | -0.516 | 0.258 | 16 |
| - | no | Navarro, L. unpublished data | Aquilegia vulgaris | Ranunculaceae | Native | Nectar robbery | Fruit set | -2.307 | 0.196 | 34 |
| - | no | Navarro, L. unpublished data | Capanea grandiflora affinis | Gesneriaceae | Native | Nectar robbery | Fruit set | -1.032 | 0.378 | 12 |
| - | no | Navarro, L. unpublished data | Castilleja angustifolia | Orobanchaceae | Native | Nectar robbery | Fruit set | -2.115 | 0.329 | 19 |
| - | no | Navarro, L. unpublished data | Castilleja sp2 | Orobanchaceae | Native | Nectar robbery | Fruit set | -1.849 | 0.571 | 10 |
| - | no | Navarro, L. unpublished data | Cavendishia grandifolia | Ericaceae | Native | Nectar robbery | Fruit set | -0.719 | 0.304 | 14 |
| - | no | Navarro, L. unpublished data | Ceratostema fasciculatum | Ericaceae | Native | Nectar robbery | Fruit set | -2.062 | 0.613 | 10 |
| - | no | Navarro, L. unpublished data | Columnea glabra | Gesneriaceae | Native | Nectar robbery | Fruit set | -2.979 | 0.796 | 11 |
| - | no | Navarro, L. unpublished data | Columnea minor | Gesneriaceae | Native | Nectar robbery | Fruit set | -2.549 | 0.604 | 12 |
| - | no | Navarro, L. unpublished data | Delphinium halteratum | Ranunculaceae | Native | Nectar robbery | Fruit set | -1.422 | 0.147 | 34 |
| - | no | Navarro, L. unpublished data | Disterigma stereophylla | Ericaceae | Native | Nectar robbery | Fruit set | -0.288 | 0.227 | 18 |
| - | no | Navarro, L. unpublished data | Drymonia coriacea | Gesneriaceae | Native | Nectar robbery | Fruit set | -2.257 | 0.468 | 14 |
| - | no | Navarro, L. unpublished data | Escallonia rubra | Escalloniaceae | Exotic | Nectar robbery | Fruit set | -5.984 | 1.564 | 14 |
| - | no | Navarro, L. unpublished data | Hamelia patens | Rubiaceae | Native | Nectar robbery | Fruit set | -0.550 | 0.380 | 11 |
| - | no | Navarro, L. unpublished data | Jasminum fruticans | Oleaceae | Native | Nectar robbery | Fruit set | -1.436 | 0.419 | 12 |
| - | no | Navarro, L. unpublished data | Justicia aurea | Acanthaceae | Native | Nectar robbery | Fruit set | -0.589 | 0.279 | 15 |
| - | no | Navarro, L. unpublished data | Justicia pectoralis | Acanthaceae | Native | Nectar robbery | Fruit set | -0.354 | 0.290 | 14 |
| - | no | Navarro, L. unpublished data | Kalanchoe pinnata | Crassulaceae | Exotic | Nectar robbery | Fruit set | -0.917 | 0.030 | 187 |
| - | no | Navarro, L. unpublished data | Kalanchoe pinnata | Crassulaceae | Exotic | Nectar robbery | Fruit set | -2.841 | 0.449 | 18 |
| - | no | Navarro, L. unpublished data | Kalanchoe pinnata | Crassulaceae | Exotic | Nectar robbery | Fruit set | -0.886 | 0.139 | 33 |
| - | no | Navarro, L. unpublished data | Kniphofia thomsonii | Xanthorrhoeaceae | Exotic | Nectar robbery | Fruit set | -0.740 | 0.480 | 9 |
| - | no | Navarro, L. unpublished data | Lamiun maculatum | Lamiaceae | Native | Nectar robbery | Fruit set | -1.248 | 0.154 | 31 |
| - | no | Navarro, L. unpublished data | Lantana camara | Verbenaceae | Exotic | Nectar robbery | Fruit set | -2.838 | 0.446 | 18 |
| - | no | Navarro, L. unpublished data | Lantana camara | Verbenaceae | Exotic | Nectar robbery | Fruit set | -4.188 | 0.912 | 14 |
| - | no | Navarro, L. unpublished data | Linaria triornitophora | Scrophulariaceae | Native | Nectar robbery | Fruit set | -0.151 | 0.251 | 16 |
| - | no | Navarro, L. unpublished data | Linaria vulgaris | Scrophulariaceae | Native | Nectar robbery | Fruit set | -1.069 | 0.290 | 16 |
| - | no | Navarro, L. unpublished data | Lithodora prostrata | Boraginaceae | Native | Nectar robbery | Fruit set | -0.988 | 0.077 | 59 |
| - | no | Navarro, L. unpublished data | Lonicera periclymenum | Caprifoliaceae | Native | Nectar robbery | Fruit set | -0.284 | 0.094 | 43 |
| - | no | Navarro, L. unpublished data | Macleania stricta | Ericaceae | Native | Nectar robbery | Fruit set | -2.276 | 0.275 | 24 |
| - | no | Navarro, L. unpublished data | Melampyrum nemorosum | Orobanchaceae | Native | Nectar robbery | Fruit set | -0.242 | 0.270 | 15 |
| - | no | Navarro, L. unpublished data | Melampyrum polonicum | Orobanchaceae | Native | Nectar robbery | Fruit set | -0.124 | 0.223 | 18 |
| - | no | Navarro, L. unpublished data | Melampyrum pratense | Orobanchaceae | Native | Nectar robbery | Fruit set | -0.738 | 0.225 | 19 |
| - | no | Arroyo, J. unpublished data | Narcissus papyraceus | Amaryllidaceae | Native | Nectar robbery | Fruit set | -1.344 | 0.111 | 44 |
| - | no | Navarro, L. unpublished data | Nicotiana glauca | Solanaceae | Exotic | Nectar robbery | Fruit set | -1.559 | 0.435 | 12 |
| - | no | Navarro, L. unpublished data | Odontonema strictum | Acanthaceae | Native | Nectar robbery | Fruit set | -2.526 | 0.402 | 18 |
| - | no | Navarro, L. unpublished data | Palicourea croceoides | Rubiaceae | Native | Nectar robbery | Fruit set | -0.451 | 0.205 | 20 |
| - | no | Navarro, L. unpublished data | Passiflora mixta | Passifloraceae | Native | Nectar robbery | Fruit set | -0.762 | 0.482 | 9 |
| - | no | Navarro, L. unpublished data | Pedicularis sylvatica | Scrophulariaceae | Native | Nectar robbery | Fruit set | -1.359 | 0.164 | 30 |
| - | no | Navarro, L. unpublished data | Ruellia tuberosa | Acanthaceae | Native | Nectar robbery | Fruit set | -0.244 | 0.270 | 15 |
| - | no | Navarro, L. unpublished data | Russelia equisetiformis | Scrophulariaceae | Exotic | Nectar robbery | Fruit set | -0.205 | 0.201 | 20 |
| - | no | Navarro, L. unpublished data | Salvia haenkei | Lamiaceae | Native | Nectar robbery | Fruit set | -0.713 | 0.425 | 10 |
| - | no | Navarro, L. unpublished data | Salvia verbenaca | Lamiaceae | Native | Nectar robbery | Fruit set | -0.817 | 0.207 | 21 |
| - | no | Navarro, L. unpublished data | Siphocampylus aureus | Campanulaceae | Native | Nectar robbery | Fruit set | -0.432 | 0.274 | 15 |
| - | no | Navarro, L. unpublished data | Siphocampylus aureus | Campanulaceae | Native | Nectar robbery | Fruit set | -0.339 | 0.422 | 10 |
| - | no | Navarro, L. unpublished data | Sphyrospermun sp. | Ericaceae | Native | Nectar robbery | Fruit set | -1.382 | 0.413 | 12 |
| - | no | Navarro, L. unpublished data | Stachytarpheta jamaicensis | Verbenaceae | Native | Nectar robbery | Fruit set | -0.269 | 0.150 | 27 |
| - | no | Navarro, L. unpublished data | Thunbergia grandiflora | Acanthaceae | Exotic | Nectar robbery | Fruit set | -0.132 | 0.401 | 10 |
| - | no | Navarro, L. unpublished data | Thunbergia grandiflora | Acanthaceae | Exotic | Nectar robbery | Fruit set | -0.617 | 0.349 | 12 |
| - | no | Navarro, L. unpublished data | Trifolium campestre | Fabacecae | Native | Nectar robbery | Fruit set | 0.199 | 0.096 | 42 |
| - | no | Navarro, L. unpublished data | Weigela florida | Caprifoliaceae | Exotic | Nectar robbery | Fruit set | -5.483 | 0.634 | 30 |
| - | no | Navarro, L. unpublished data | Wisteria sinensis | Fabacecae | Exotic | Nectar robbery | Fruit set | -8.033 | 1.648 | 22 |
| - | no | Navarro, L. unpublished data | Duranta erecta | Verbenaceae | Exotic | Nectar robbery | Fruit set | -2.334 | 0.306 | 22 |
* Values correspond to mean across years for the same study, species, site, and response variable
We calculated the Hedges unbiased standardized mean difference effect size for each data set to estimate the difference in the mean fitness of undamaged and damaged plants [31]. The effect size d was expressed as follows:
in which and are the sample means of the two groups (damaged and undamaged plants, respectively) and Spooled their pooled standard deviation, expressed as:
where n1 and n2 are the sample sizes and s1 and s2 are the standard deviations of the two groups corrected for sample size with the correction factor j [32]. The weighting factor J was calculated as:
In this study, a positive effect size indicates that plant fitness is lower in control plants (i.e., flowers not damaged) compared to treatment (damaged) plants, while a negative effect size implies a fitness cost for the damage plants as compared to the control.
We first performed a general analysis to describe the global effect of florivory on plant fitness, and then incorporated moderators. We evaluated the effect of three categorical variables, namely: 1) Design, including two levels: natural herbivory damage and simulated damage (clipping, floral and petal removal and simulated nectar robbing). The aim of this categorization was to determine whether artificial damage faithfully mimics the natural flower damage experienced by plants [33]; 2) Residence status, with two levels: native (a species that inhabits its natural range) and exotic (a species that has been introduced to novel habitats outside its natural range). The aim of this categorization was to assess whether the fitness impact of floral damage was contingent on the provenance of the plant species to the place where the study was performed. When residence status was not informed in the article, we looked for information about the native distribution of the species involved in other publications or data bases; 3) Type of damage, using two levels: florivory (damage to petals, sepals or any other floral attraction trait) and nectar robbery (damage at the corolla base to access the nectar chamber). Potential bias in the representation of cases among moderator levels was evaluated in a Fisher’s exact test (S2 Fig). To examine whether variation in effect size was attributable to differences between moderator levels, we calculated between-group homogeneity (QB) and tested it against the χ2 distribution with N (the number of levels) minus one degrees of freedom [34].
As most experimental reports on flower herbivory have been performed on a per species basis in one locality, often omitting information related to the community context, our meta-analysis was restricted to native and exotic plants that do not necessarily co-occur in local communities. Therefore we considered the effects of flower herbivory on plant species that could be clearly classified as native or exotic to the region where studies were conducted, regardless of community co-occurrence. We used a mixed-effect model for the analysis of moderators, assuming a random effect within moderator levels because measurements were recorded from a variety of plant species and environments, and a fixed effect to compare moderator levels based on the idea that we gathered all possible categories into two levels for each moderator rather than a random sample of the possible existing levels [35,36]. Additionally, in order to evaluate if our results were real or resulted from non-independent phylogeny effects, we performed a second analysis where “family” was incorporated as a random factor in the model. This analysis was carried out using the package metafor in R [37]. Publication bias was estimated using the Pearson’s correlation coefficient and the funnel plot method, which indicates that in the absence of bias, effect size should not correlate with sample size [38]. When the mean effect size significantly differed from zero we calculated Rosenthal’s fail-safe number, which represents the number of unpublished studies with zero effect needed to reverse the significant effect revealed in the meta-analysis [39]. When the fail-safe number was greater than 5n + 10 (where n is the number of records in the analysis), it may be concluded that the results were robust against publication bias [40]. All analyses were performed in Comprehensive Meta-Analysis v. 2.0 (Biostat Inc.)
Results
We performed a general analysis including the 143 records to evaluate if natural and artificial damage had different effects on plant reproductive success. Floral damage had a significant cost on plant reproductive success (d = -0.535, N = 143, p < 0.001; heterogeneity, Q = 1033.9, df = 142, p<0.001). Additionally, our results indicated that the impact of natural floral herbivory on plant fitness was greater than the artificial damage (Fig 1, Natural: d = -0.780, N = 97, p < 0.001; Artificial: d = -0.309, N = 46, p < 0.001) and this difference was statistically significant (QB = 22.1, df = 1, p < 0.001), indicating that natural and artificial damage differ in the magnitude of effects. Therefore, only data from studies evaluating natural damage were included in subsequent analyses. The original dataset was reduced by 32% and included 97 reports from 29 publications, corresponding to 81 different plant species from 66 genera and 35 families (Table 1).
Fig 1. Mean effect size of flower herbivory on plant fitness in studies using natural and artificial herbivory.
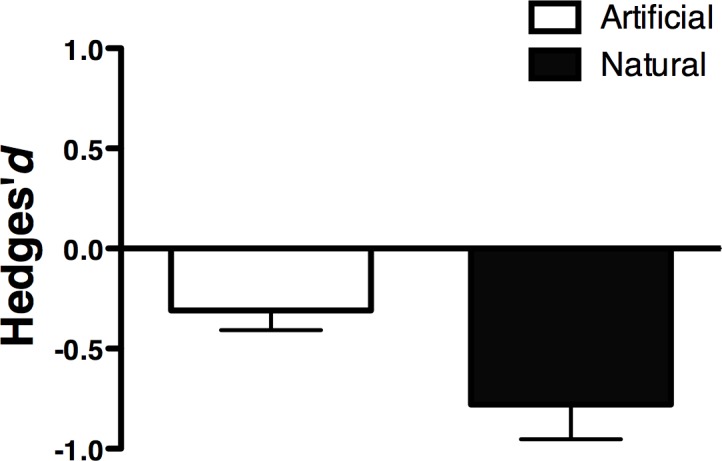
Bars around means indicate 95% confidence intervals. Numbers indicate the number of records.
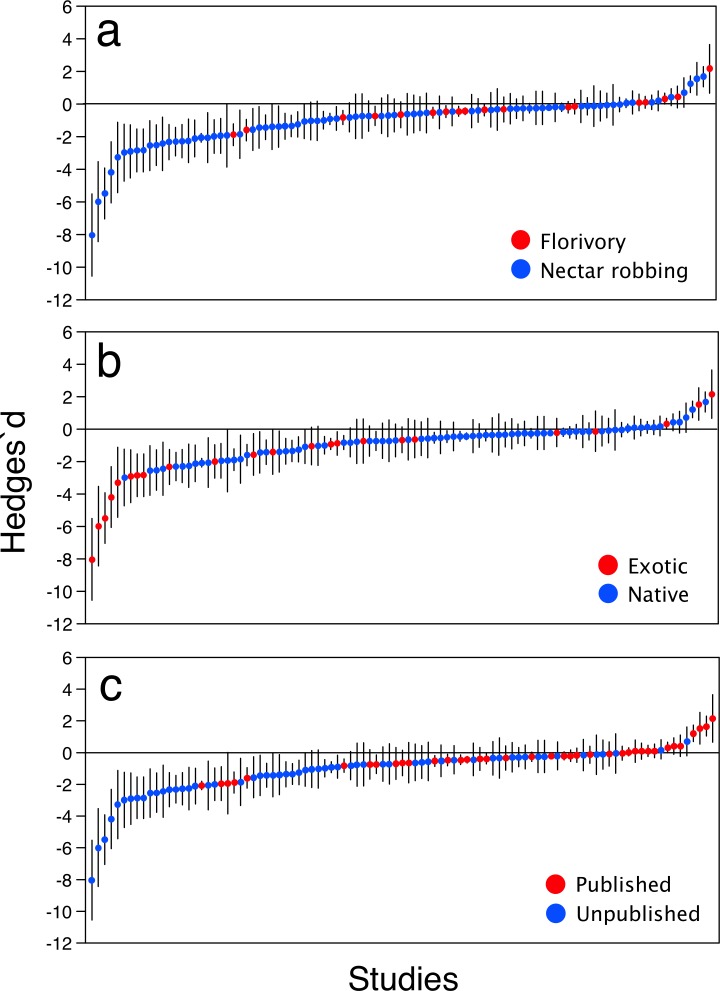
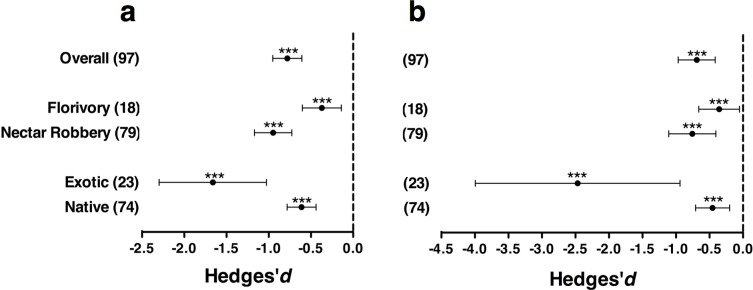
The distribution of Hedges’ d values revealed a predominance of negative (44.3%) and neutral effects (51.6%), with a minor contribution of positive effects (4.1%) (Fig 2). Among significant effects there was a predominance of negative ones (85.5% of cases). When examined across all studies, the mean effect size was significantly less than zero (Fig 3A). Regarding the type of damage, florivory and nectar robbery imposed a significant cost to plant reproduction (d = -0.37, N = 18, p = 0.002; d = -0.95, N = 79, p < 0.001, respectively) but differed in the magnitude of their effects (QB = 12.5 df = 1, p < 0.001); the impact of nectar robbing was greater than that of florivory (Fig 3A). The inclusion of the residence status as moderator revealed that floral herbivory had a significant fitness cost on native and exotic plants, and a significant heterogeneity in the magnitude of effects between levels (QB = 9.8, df = 1, p = 0.002). The mean fitness impact of flower herbivory upon exotics was three-fold stronger than on native plants (d = -1.66, N = 23, p < 0.001 versus d = -0.61, N = 74, p < 0.001, respectively, Fig 3A). Exotic plants had more variable effects than native plants (Bartlett's K-squared = 37.9, df = 1, p < 0.001). When data were analyzed incorporating “Family” as a random factor, the results showed the same tendency as the first analysis (Fig 3B). The presence of natural floral damage strongly reduced plant fitness (d = -0.61, N = 97, p < 0.001) and the separate effects of nectar robbing and florivory upon plant fitness were also negative (d = -0.76, N = 79, p <0.0001; d = -0.36, N = 18, p = 0.02, respectively), and as in the first analysis, nectar robbers and florivores differed in their effects upon plant fitness (QB = 4.16, df = 1, p = 0.04). Regarding residence status, flower herbivores reduced the fitness of exotic and native plants (exotics: d = -2.47, N = 23, p = 0.002; native: d = -0.45, N = 74, p = 0.0005), and such impact was stronger on exotic than native plants (QB = 13.3, df = 1, p = 0.0003)
Fig 2. Distribution of Hedges’d effects of flower herbivory on plant fitness arranged in increasing order for (a) type of damage, (b) origin, and (c) data source.
Bars indicate 95% CI. The zero line is presented for reference of statistical significance.
Fig 3. Plots of mean effect sizes for levels of both moderators at the (a) species level (97 reports, 81 species) and (b) species level, but including “family” as a random factor.
As some families are represented in the two levels of the moderators, sample size exceeds the overall sample size in the family-level analysis (N = 35). Bars around means denote 95% confidence intervals. Parentheses indicate sample size. *** p < 0.001; ** p < 0.01; * p < 0.05
Effect size was not associated with sample size (Pearson’s product-moment correlation coefficient, r = 0.139, N = 97, p = 0.174, indicating absence of publication bias. Visual inspection of the funnel plot suggests potential selection against small-sample studies that demonstrate positive effects of flower herbivory on plant fitness (S1 Fig). Rosenthal’s fail-safe number indicates that 8569 unpublished studies with zero effect would be necessary to reverse the significance of effects. As this number exceeds by far the expected value for absence of publication bias (5 x 97 + 10 = 495), we conclude that our results were robust to publication omission.
Discussion
The distribution of Hedges’ d values revealed a predominance of negative and neutral effects, which is consistent with previous conclusions from narrative reviews indicating that the effect of flower herbivory may vary from negative to neutral, and only rarely may benefit plant reproductive success [1]. As expected, the mean effect size was negative, corroborating the overall detrimental impact of flower herbivores on plant fitness. When effects were analyzed in the context of natural and artificial flower herbivory, both methodologies imposed an important cost to plant reproductive success, albeit the fitness cost of natural herbivory was two-fold stronger than that of artificial damage, cautioning the longstanding assumption that artificial flower damage can be used as a legitimate surrogate for the natural damage imposed by flower herbivores. This result alerts experimental studies using simulated flower herbivory, such as clipping or petal removal, as they do not completely mimic the plant response to natural flower herbivory and may underestimate the real fitness impact of herbivores (see also [41,42]). It is likely that plant responses following natural flower damage increase the susceptibility to subsequent antagonistic interactions such as foliar herbivory and seed predation [43,44], especially if the new consumers are able to detect chemical signals associated with floral damage such as volatiles released by the corolla tissues. Similarly, assuming the same concepts of resistance and tolerance can be extended to understand how plants and flowers cope with damage by florivores [1], induced defenses that deter florivores may also deter pollinators or simply impose a higher fitness cost related with the production and mobilization of such defenses [1,45]. Such a hypothesis clearly requires experimental investigation.
A previous meta-analysis performed on a broad review of the invasion literature including plants, invertebrates and vertebrate species examined whether exotics really have a low diversity of enemies in the new habitats, as predicted by the ERH [27]. The authors performed tests that compared the enemy species diversity between exotic and native populations of the same species (biogeographical level), and between exotic and native species co-occurring within the same community (community level). Their results supported the enemy release hypothesis at the biogeographical level only, indicating that the phenomenon seems to be contingent on the scale at which studies are performed. In consequence, in spite of the overemphasis received in the literature of invasion, the ERH seems to be insufficient to account for the inherent complexity of the invasion process. In our meta-analysis, the paucity of studies using the same plant species in native and novel habitats as well as the limited number of studies at the community level precluded examination of the importance of the residence status at the resolution levels suggested by Colautti et al. (2004). Notwithstanding, the effects of floral herbivory on plant fitness were clear and significantly modulated by plant origin and stronger on exotic than native species (Fig 3). It is likely that the ample variation in the effect size of exotics results from the limited number of studies in this category (23) in comparison to native species (74). The stronger effects on exotics however, is intriguing and may be explained, at least in part, if exotic plants are more susceptible to enemies in novel habitats and/or herbivores in novel habitats converge to the introduced plant. The evidence is mixed in this regard. On one hand, Cappuccino and Carpenter (2005) analyzed the importance of leaf herbivory on 18 exotic plant species divided into invasive and non-invasive depending on their spread in the novel habitat. Their results indicate that invasive plants suffered 96% less leaf damage than non-invasive exotic species. In the same line, Sugiura (2010) examined the incidence of herbivorous insect species on invasive and native plant species. The results indicated that herbivorous insects were mainly associated with native and indigenous species, hence confirming a critical element of the ERH. On the other hand, recent reviews indicate that exotics are not necessarily devoid of enemies in new habitats, which translate into similar levels of herbivory in coexisting invasive and native plants [28,46]. This effect has been attributed to the high susceptibility of exotic plants to new enemies and to the presence of enemies already present in their original habitat. Under this situation, previous types and levels of defense evolved in original habitats may be less efficient against new natural enemies after arrival [47–49], especially if generalist herbivores shift onto newly introduced plants. The mechanism involved in the greater susceptibility of exotics has been named the “increased susceptibility hypothesis” by Colautti et al. (2004) to denote the effect of invasion bottlenecks that reduce the genetic diversity of polymorphic defenses of exotics, leading to increased susceptibility to the native and introduced enemies found in new habitats. Under such circumstances novel instances of attack may impose high fitness costs on exotic plants in comparison to the more genetically diverse native species. The extent to which a similar situation occurs in studies of flower herbivory needs to be examined in future studies.
Regarding the damage inflicted to flowers, the fitness impact of flower herbivory was significantly modulated by the interaction involved in the herbivory process. Even though both florivores and nectar robbers had significant negative impacts on plant fitness (Fig 3A), nectar robbers had a stronger negative effect on plant fitness than flower consumers. This result is surprising, as unlike florivores that often suppress completely flower reproduction, nectar robbers do not damage reproductive organs but usually restrict their damage to tissues that encompass the nectar reward concealed at the base of floral tubes. There is, however, an important methodological consideration that needs to be taken into account. The effects of florivory and nectar robbing on plant reproduction considered in this meta-analysis were mostly compiled from studies that analyzed florivory and nectar robbing separately but not together. The only study examining potential interaction effects concluded that the exclusion of nectar robbing ants increased the activity of herbivorous beetles on flowers, leading to decreased female fitness [50]. In this way the impact of herbivorous beetles seemed to be contingent on the presence or absence of ants, illustrating the way non-additive effects determine the final outcome of flower herbivory through complex pathways of fitness impact. This is consistent with the increasing experimental evidence indicating that plant-animal interactions often impact plant fitness in non-additive ways, suggesting greater community complexity than previously thought [11,14,51,52] (but see [44,53]). While our meta-analysis revealed broad negative effects of florivory and nectar robbing on plant fitness, the extent to which such effects are canceled when interactions are examined in combination needs to be examined in future studies.
In conclusion, our results revealed that floral herbivores impose a significant cost to plant fitness, which is significantly modulated by the type of damage and plant origin. More specifically, flower herbivores had a higher fitness impact on exotic than on native species, which is not consistent with predictions of the enemy release hypothesis. In consequence, our conclusions point out the limited utility of the ERH to account for the complexity of the invasion process in species subject to flower herbivory. Our results suggest that floral herbivores may play an important but largely unrecognized role in preventing the spread of introduced species in newly colonized areas. More experimental studies evaluating the fitness impact of flower herbivores at biogeographic and community levels are badly needed to extract useful generalizations on the importance of flower herbivory for the invasion process.
Supporting Information
Each dot corresponds to a report. The horizontal line indicates the mean effect size of the global analysis.
(JPG)
(JPG)
(DOCX)
Acknowledgments
We thank Carezza Botto-Mahan, Ramiro Bustamante, Gastón Carvallo, and two anonymous reviewers for important comments on a previous version of this manuscript. This research was supported by Fondo Nacional de Ciencia y Tecnologia, Grants 1120155 and 1150112 to RM, http://www.fondecyt.cl. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
Data are all contained within the paper and its Supporting information files.
Funding Statement
This research was supported by Fondo Nacional de Ciencia y Tecnologia, Grants 1120155 and 1150112, to RM (http://www.fondecyt.cl). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McCall AC, Irwin RE (2006) Florivory: the intersection of pollination and herbivory. Ecol Lett 9: 1351–1365. [DOI] [PubMed] [Google Scholar]
- 2.Nabity PD, Zavala JA, DeLucia EH (2009) Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Ann Bot-London 103: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron M, Flexas J, DeLucia EH (2012) Photosynthesis responses to biotic stress In: Flexas J, Loreta F, Medrano H, editors. Terrestrial photosynthesis in a changing environment: a molecular, physiological and ecological approach. UK: Cambridge University Press; pp. 331–350. [Google Scholar]
- 4.Krupnick GA, Weis AE (1999) The effect of floral herbivory on male and female reproductive success in Isomeris arborea. Ecology 80: 135–149. [Google Scholar]
- 5.Maron JL, Combs JK, Louda SM (2002) Convergent demographic effects of insect attack on related thistles in coastal vs. continental dunes. Ecology 83: 3382–3392. [Google Scholar]
- 6.Riba-Hernandez P, Stoner KE (2005) Massive destruction of Symphonia globulifera (Clusiaceae) flowers by Central American spider monkeys (Ateles geoffroyi). Biotropica 37: 274–278. [Google Scholar]
- 7.Karban R, Strauss SY (1993) Effects of herbivores on growth and reproduction of their perennial host, Erigeron glaucus. Ecology 74: 39–46. [Google Scholar]
- 8.Krupnick GA, Weis AE, Campbell DR (1999) The consequences of floral herbivory for pollinator service to Isomeris arborea. Ecology 80: 125–134. [Google Scholar]
- 9.Cardel YJ, Koptur S (2010) Effects of florivory on the pollination of flowers: An experimental field study with a perennial plant. Int J Plant Sci 171: 283–292. [Google Scholar]
- 10.Botto-Mahan C, Ramirez PA, Ossa CG, Medel R, Ojeda-Camacho M, et al. (2011) Floral herbivory affects female reproductive success and pollinator visitation in the perennial herb Alstroemeria Ligtu (Alstroemeriaceae). Int J Plant Sci 172: 1130–1136. [Google Scholar]
- 11.Pohl N, Carvallo G, Botto-Mahan C, Medel R (2006) Nonadditive effects of flower damage and hummingbird pollination on the fecundity of Mimulus luteus. Oecologia 149: 648–655. [DOI] [PubMed] [Google Scholar]
- 12.Garcia MB, Ehrlen J (2002) Reproductive effort and herbivory timing in a perennial herb: Fitness components at the individual and population levels. Am J Bot 89: 1295–1302. 10.3732/ajb.89.8.1295 [DOI] [PubMed] [Google Scholar]
- 13.Strauss SY, Irwin RE (2004) Ecological and evolutionary consequences of multispecies plant-animal interactions. Annu Rev Ecol Evol S 35: 435–466. [Google Scholar]
- 14.Strauss SY, Conner JK, Rush SL (1996) Foliar herbivory affects floral characters and plant attractiveness to pollinators: Implications for male and female plant fitness. Am Nat 147: 1098–1107. [Google Scholar]
- 15.Mothershead K, Marquis RJ (2000) Fitness impacts of herbivory through indirect effects on plant-pollinator interactions in Oenothera macrocarpa. Ecology 81: 30–40. [Google Scholar]
- 16.Hendrix SD, Trapp EJ (1989) Floral herbivory in Pastinaca sativa: do compensatory responses offset reductions in fitness? Evolution 43: 891–895. [DOI] [PubMed] [Google Scholar]
- 17.Sowell DR, Wolfe LM (2010) Pattern and consequences of floral herbivory in four sympatric Ipomoea species. Am Midl Nat 163: 173–185. [Google Scholar]
- 18.Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17: 164–170. [Google Scholar]
- 19.Maron JL, Vila M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95: 361–373. [Google Scholar]
- 20.Cappuccino N, Carpenter D (2005) Invasive exotic plants suffer less herbivory than non-invasive exotic plants. Biol Lett-Uk 1: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill SB, Kotanen PM (2010) Phylogenetically structured damage to Asteraceae: susceptibility of native and exotic species to foliar herbivores. Biol Invasions 12: 3333–3342. [Google Scholar]
- 22.Sugiura S (2010) Associations of leaf miners and leaf gallers with island plants of different residency histories. J Biogeogr 37: 237–244. [Google Scholar]
- 23.Agrawal AA, Kotanen PM (2003) Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecol Lett 6: 712–715. [Google Scholar]
- 24.Mitchell CE, Power AG (2003) Release of invasive plants from fungal and viral pathogens. Nature 421: 625–627. [DOI] [PubMed] [Google Scholar]
- 25.Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7: 975–989. [Google Scholar]
- 26.Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions 8: 1535–1545. [Google Scholar]
- 27.Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7: 721–733. [Google Scholar]
- 28.Chun YJ, van Kleunen M, Dawson W (2010) The role of enemy release, tolerance and resistance in plant invasions: linking damage to performance. Ecol Lett 13: 937–946. 10.1111/j.1461-0248.2010.01498.x [DOI] [PubMed] [Google Scholar]
- 29.Mutikainen P, Delph LF (1996) Effects of herbivory on male reproductive success in plants. Oikos 75: 353–358. [Google Scholar]
- 30.Irwin RE, Bronstein JL, Manson JS, Richardson L (2010) Nectar robbing: Ecological and evolutionary perspectives. Annual Review of Ecology, Evolution, and Systematics, Vol 41 41: 271–292. [Google Scholar]
- 31.Hedges LV, Olkin I (1985) Statistical methods for meta-analysis Orlando: Academic Press. [Google Scholar]
- 32.Gurevitch J, Curtis PS, Jones MH (2001) Meta-analysis in ecology. Adv Ecol Res 32: 199–247. [Google Scholar]
- 33.McCall AC (2006) Natural and artificial floral damage induces resistance in Nemophila menziesii (Hydrophyllaceae) flowers. Oikos 112: 660–666. [Google Scholar]
- 34.Gurevitch J, Hedges LV (2001) Meta-analysis: combining the results of independent experiments In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments: Oxford University Press; pp. 347–369. [Google Scholar]
- 35.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to Meta-analysis. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 36.Gurevitch J, Hedges LV (1999) Statistical issues in ecological meta-analyses. Ecology 80: 1142–1149. [Google Scholar]
- 37.R Development Core Team (2014) R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 38.Moller AP, Jennions MD (2001) Testing and adjusting for publication bias. Trends Ecol Evol 16: 580–586. [Google Scholar]
- 39.Hillebrand H (2008) Meta-analysis in ecology Encyclopedia of Life Science (ELS). Chichester, UK: John Wiley & Sons. [Google Scholar]
- 40.Rosenthal R (1979) The “fail-drawer problem” and tolerance for null results. Psychological Bulletin 86: 638–641. [Google Scholar]
- 41.Heil M (2010) Plastic defence expression in plants. Evol Ecol 24: 555–569. [Google Scholar]
- 42.Quentin AG, Pinkard EA, Beadle CL, Wardlaw TJ, O'Grady AP, et al. (2010) Do artificial and natural defoliation have similar effects on physiology of Eucalyptus globulus Labill. seedlings? Ann Forest Sci 67. [Google Scholar]
- 43.Hufbauer RA, Root RB (2002) Interactive effects of different types of herbivore damage: Trirhabda beetle larvae and Philaenus spittlebugs on goldenrod (Solidago altissima). Am Midl Nat 147: 204–213. [Google Scholar]
- 44.Irwin RE, Brody AK (2011) Additive effects of herbivory, nectar robbing and seed predation on male and female fitness estimates of the host plant Ipomopsis aggregata. Oecologia 166: 681–692. 10.1007/s00442-010-1898-4 [DOI] [PubMed] [Google Scholar]
- 45.Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17: 278–285. [Google Scholar]
- 46.van Kleunen M, Fischer M (2009) Release from foliar and floral fungal pathogen species does not explain the geographic spread of naturalized North American plants in Europe. J Ecol 97. [Google Scholar]
- 47.Mitchell CE, Agrawal AA, Bever JD, Gilbert GS, Hufbauer RA, et al. (2006) Biotic interactions and plant invasions. Ecol Lett 9: 726–740. [DOI] [PubMed] [Google Scholar]
- 48.Ashton IW, Lerdau MT (2008) Tolerance to herbivory, and not resistance, may explain differential success of invasive, naturalized, and native North American temperate vines. Divers Distrib 14: 169–178. [Google Scholar]
- 49.Fornoni J (2011) Ecological and evolutionary implications of plant tolerance to herbivory. Funct Ecol 25: 399–407. [Google Scholar]
- 50.Newman DA, Thomson JD (2005) Interactions among nectar robbing, floral herbivory, and ant protection in Linaria vulgaris. Oikos 110: 497–506. [Google Scholar]
- 51.Herrera CM (2000) Measuring the effects of pollinators and herbivores: Evidence for non-additivity in a perennial herb. Ecology 81: 2170–2176. [Google Scholar]
- 52.Gomez JM (2005) Non-additive effects of herbivores and pollinators on Erysimum mediohispanicum (Cruciferae) fitness. Oecologia 143: 412–418. [DOI] [PubMed] [Google Scholar]
- 53.Caballero P, Ossa CG, Gonzales WL, Gonzalez-Browne C, Astorga G, et al. (2013) Testing non-additive effects of nectar-robbing ants and hummingbird pollination on the reproductive success of a parasitic plant. Plant Ecol 214: 633–640. [Google Scholar]
- 54.Hendrix SD (1984) Reactions of Heracleum lanatum to floral herbivory by Depressaria pastinacella. Ecology 65: 191–197. [Google Scholar]
- 55.Ashman TL, Cole DH, Bradburn M (2004) Sex-differential resistance and tolerance to herbivory in a gynodioecious wild strawberry. Ecology 85: 2550–2559. [Google Scholar]
- 56.Hendrix SD, Trapp EJ (1981) Plant-herbivory interactions: insect induced changes in host plant sex expression and fecundity. Oecologia 49: 119–122. [DOI] [PubMed] [Google Scholar]
- 57.Krupnick GA, Weis AE (1998) Floral herbivore effect on the sex expression of an andromonoecious plant, Isomeris arborea (Capparaceae). Plant Ecol 134: 151–162. [Google Scholar]
- 58.Louda SM, Potvin MA (1995) Effect of Inflorescence-Feeding Insects on the Demography and Lifetime Fitness of a Native Plant. Ecology 76: 229–245. [Google Scholar]
- 59.Burkle LA, Irwin RE, Newman DA (2007) Predicting the effects of nectar robbing on plant reproduction: Implications of pollen limitation and plant mating system. Am J Bot 94: 1935–1943. 10.3732/ajb.94.12.1935 [DOI] [PubMed] [Google Scholar]
- 60.Deng XB, Ren PY, Gao JY, Li QJ (2004) The striped squirrel (Tamiops swinhoei hainanus) as a nectar robber of ginger (Alpinia kwangsiensis). Biotropica 36: 633–636. [Google Scholar]
- 61.Maloof JE (2001) The effects of a bumble bee nectar robber on plant reproductive success and pollinator behavior. Am J Bot 88: 1960–1965. [PubMed] [Google Scholar]
- 62.Navarro L (2001) Reproductive biology and effect of nectar robbing on fruit production in Macleania bullata (Ericaceae). Plant Ecol 152: 59–65. [Google Scholar]
- 63.Richardson SC (2004) Are nectar-robbers mutualists or antagonists? Oecologia 139: 246–254. [DOI] [PubMed] [Google Scholar]
- 64.Traveset A, Willson MF, Sabag C (1998) Effect of nectar-robbing birds on fruit set of Fuchsia magellanica in Tierra del Fuego: a disrupted mutualism. Funct Ecol 12: 459–464. [Google Scholar]
- 65.Zhang YW, Yu Q, Zhao JM, Guo YH (2009) Differential effects of nectar robbing by the same bumble-bee species on three sympatric Corydalis species with varied mating systems. Ann Bot-London 104: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amsberry LK, Maron JL (2006) Effects of herbivore identity on plant fecundity. Plant Ecol 187: 39–48. [Google Scholar]
- 67.Valdivia CE, Niemeyer HM (2005) Reduced maternal fecundity of the high Andean perennial herb Alstroemeria umbellata (Alstroemeriaceae) by aphid herbivory. New Zeal J Ecol 29: 321–324. [Google Scholar]
- 68.Fritz RS, Morse DH (1981) Nectar parasitism of Asclepias syriaca by ants: effect on nectar levels, pollinia insertion, pollinaria removal and pod production. Oecologia 50: 316–319. [DOI] [PubMed] [Google Scholar]
- 69.Navarro L (2000) Pollination ecology of Anthyllis vulneraria subsp vulgaris (Fabaceae): Nectar robbers as pollinators. Am J Bot 87: 980–985. [PubMed] [Google Scholar]
- 70.Temeles EJ, Pan IL (2002) Effect of nectar robbery on phase duration, nectar volume, and pollination in a protandrous plant. Int J Plant Sci 163: 803–808. [Google Scholar]
- 71.Utelli AB, Roy BA (2001) Causes and consequences of floral damage in Aconitum lycoctonum at high and low elevations in Switzerland. Oecologia 127: 266–273. 10.1007/s004420000580 [DOI] [PubMed] [Google Scholar]
- 72.Zhang YW, Robert GW, Wang Y, Guo YH (2007) Nectar robbing of a carpenter bee and its effects on the reproductive fitness of Glechoma longituba (Lamiaceae). Plant Ecol 193: 1–13. [Google Scholar]
- 73.de Waal C, Barrett SCH, Anderson B (2012) The effect of mammalian herbivory on inflorescence architecture in Ornithophilous babiana (Iridaceae): Implications for the evolution of a bird perch. Am J Bot 99: 1096–1103. 10.3732/ajb.1100295 [DOI] [PubMed] [Google Scholar]
- 74.Navarro L, Guitian J, Guitian P (1993) Reproductive biology of Petrocoptis grandiflora Rothm (Caryophyllaceae), a species endemic to Northwest Iberian Peninsula. Flora 188: 253–261. [Google Scholar]
- 75.Wise MJ, Cummins JJ, De Young C (2008) Compensation for floral herbivory in Solanum carolinense: identifying mechanisms of tolerance. Evol Ecol 22: 19–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each dot corresponds to a report. The horizontal line indicates the mean effect size of the global analysis.
(JPG)
(JPG)
(DOCX)
Data Availability Statement
Data are all contained within the paper and its Supporting information files.