Abstract
Both type 1 and type 2 diabetes are associated with cardiac fibrosis that may reduce myocardial compliance, contribute to the pathogenesis of heart failure, and trigger arrhythmic events. Diabetes-associated fibrosis is mediated by activated cardiac fibroblasts, but may also involve fibrogenic actions of macrophages, cardiomyocytes and vascular cells. The molecular basis responsible for cardiac fibrosis in diabetes remains poorly understood. Hyperglycemia directly activates a fibrogenic program, leading to accumulation of advanced glycation end-products (AGEs) that crosslink extracellular matrix proteins, and transduce fibrogenic signals through reactive oxygen species generation, or through activation of Receptor for AGEs (RAGE)-mediated pathways. Pro-inflammatory cytokines and chemokines may recruit fibrogenic leukocyte subsets in the cardiac interstitium. Activation of transforming growth factor-β/Smad signaling may activate fibroblasts inducing deposition of structural extracellular matrix proteins and matricellular macromolecules. Adipokines, endothelin-1 and the renin-angiotensin-aldosterone system have also been implicated in the diabetic myocardium. This manuscript reviews our current understanding of the cellular effectors and molecular pathways that mediate fibrosis in diabetes. Based on the pathophysiologic mechanism, we propose therapeutic interventions that may attenuate the diabetes-associated fibrotic response and discuss the challenges that may hamper clinical translation.
1. INTRODUCTION
Epidemiologic studies have documented a strong association between diabetes and heart failure [1]. Data from the Framingham study demonstrated a 2-fold higher risk of heart failure in male diabetics and a 5-fold increase in risk in female patients with diabetes [2]. In the Reduction of Atherothrombosis for Continued Health (REACH) registry, an international study of patients with established atherothrombotic disease, or at high risk for of atherothrombosis, diabetics exhibited a 33% higher risk of hospitalization due to heart failure [3]. Moreover, diabetes adversely affects prognosis in patients with heart failure. In the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program, diabetes was an independent predictor of morbidity and mortality in patients with heart failure, in both groups with systolic dysfunction and with preserved ejection fraction [4].
Despite its high clinical significance and important contribution to morbidity and mortality, diabetes-associated heart failure remains understudied [5]. From a pathophysiologic perspective, the increased incidence of heart failure in diabetics can be attributed to several factors. First, patients with diabetes have an increased incidence of coronary artery disease and develop atherosclerotic lesions at a younger age, often exhibiting multivessel disease and involvement of distal coronary segments. Second, hypertension is commonly found in both type 1 and type 2 diabetics [6] and may also be involved in the pathogenesis of diabetes-associated heart failure. Third, diabetes can cause distinct pathologic alterations in the myocardium, independent of its effects on blood pressure and coronary atherosclerosis. Histopathologic studies in myocardial samples from patients with diabetes [7] suggested a distinct entity, termed “diabetic cardiomyopathy” that may occur in the absence of coronary disease or other concomitant conditions and may contribute to the development of heart failure. In both human patients and in animal models of diabetes, interstitial and perivascular fibrosis are prominent characteristics of diabetic cardiomyopathy. Deposition of extracellular matrix proteins in the cardiac interstitium, and cross-linking of the matrix increase myocardial stiffness and may mediate diastolic dysfunction in the diabetic myocardium. Considering the high prevalence of heart failure with preserved ejection fraction (HFpEF) in diabetics, fibrotic myocardial remodeling may be critically implicated in the pathogenesis of diabetes-associated heart failure. The current manuscript reviews our understanding of the pathophysiology of diabetic cardiac fibrosis. We identify the cellular effectors mediating fibrosis in the diabetic heart, and discuss the molecular signals that may activate the fibrogenic response. Finally, we propose therapeutic interventions that may attenuate myocardial fibrosis, targeting heart failure in diabetic patients.
2. CARDIAC FIBROSIS IN DIABETICS
Extensive evidence has documented the presence of myocardial fibrosis in patients with diabetes. Cardiac magnetic resonance imaging often identifies myocardial scars (replacement fibrosis) in diabetics without a history of myocardial infarction; these findings likely reflect silent coronary events [8],[9]. Numerous histopathologic studies have demonstrated that cardiac fibrosis in diabetic patients occurs independently of coronary atherosclerosis or hypertension. Regan and co-workers showed that patients with adult-onset diabetes may exhibit extensive perivascular, interstitial, and even replacement fibrosis, in the absence of hypertension or coronary artery disease [10]. Myocardial fibrosis in diabetics is often accompanied by cardiomyocyte hypertrophy and by evidence of microvascular abnormalities, such as thickening of the capillary basement membrane [11]. Diabetes-associated interstitial fibrosis is associated with accumulation of type I and III collagen, involves both left [12],[13] and right ventricle [14], and has been described in both type 1 and type 2 diabetes [15],[16]. Relations between diabetes-associated fibrosis and cardiac function have not been systematically investigated. However, in a study examining biopsies from patients with heart failure in the absence of coronary disease, diabetes was associated with increased collagen levels only in patients with reduced ejection fraction [17]. Diabetes is also associated with accentuation of fibrotic changes in patients with other cardiac conditions. In patients with aortic stenosis, diabetes was associated with worse myocardial stiffness and increased myocardial collagen content [18].
3. FIBROSIS IN ANIMAL MODELS OF DIABETES
Animal models of diabetes provide strong support to the association between diabetes and myocardial fibrosis. The severity of cardiac fibrosis and left ventricular dysfunction in experimental models of diabetes is dependent on the species, genetic background, gender and age of the animals studied, the etiology of diabetes and the presence of concomitant pathophysiologic conditions (such as hypertension, dyslipidemias, etc.) [19].
Streptozotocin-induced diabetes models have been extensively used to investigate the complications of type 1 diabetes. In rodent models, steptozotocin induces β cell toxicity and death, and triggers a T cell-mediated immune response, simulating human insulin-dependent diabetes [20]. In both mice and rats, streptozotocin-induced diabetes is associated with interstitial myocardial fibrosis, accompanied by cardiomyocyte hypertrophy, induction of pro-fibrotic and hypertrophy-associated genes, and microvascular rarefaction [21],[22],[23],[24],[25]. In contrast, in a genetic model of insulin-dependent type 1 diabetes, the Ins2WT/C96Y Akita mouse, diastolic dysfunction was associated with a lipotoxic cardiomyopathy, in the absence of significant cardiac fibrosis and cardiomyocyte hypertrophy [26].
Pro-fibrotic effects of type 1 diabetes on the myocardium have also been demonstrated in large animal models. Mongrel dogs rendered diabetic through administration of alloxan developed significant myocardial fibrosis [27]. Alloxan-induced insulin-dependent diabetes had similar effects on rhesus monkeys, causing a 2-fold increase in left ventricular collagen content, in the absence of hypertrophy [28].
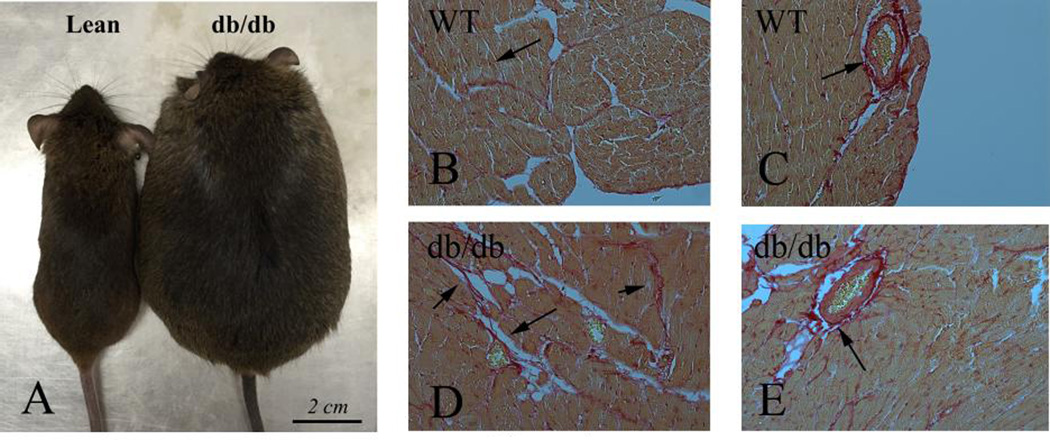
Cardiac fibrosis has also been documented in experimental models of type 2 diabetes (Figure 1). db/db mice express a truncated leptin receptor and are resistant to the central effects of leptin. These animals develop severe obesity at 1–2 months of age, associated with overt diabetes. Both histochemical staining techniques and biochemical assays have consistently documented cardiac fibrosis in db/db mice at 4–6 months of age [29],[30],[31], accompanied by cardiomyocyte hypertrophy and diastolic dysfunction [32],[31]. Leptin-deficient ob/ob animals also develop severe obesity, insulin resistance and cardiac hypertrophy at a young age [33]. However, evidence demonstrating fibrotic remodeling in ob/ob hearts has been inconsistent. 20-week old ob/ob in a C57BL/6J background exhibited significant perivascular cardiac fibrosis associated with elevated expression of Transforming Growth Factor (TGF)-β1 and Plasminogen activator inhibitor (PAI)-1, indicating activation of matrix-preserving pathways [34]. In contrast, another study demonstrated that 36-week-old ob/ob mice exhibited cardiac hypertrophy and diastolic dysfunction, but had comparable collagen content with lean WT animals [35]. Gender-specific effects, the relative sensitivity of various techniques used to assess fibrosis, and differences in the diets fed to the animals may explain the conflicting findings. Genetic models of type diabetes in the rat also exhibit cardiac fibrosis. Zucker rats have a leptin receptor missense mutation and develop severe obesity and insulin resistance. These animals have perivascular myocardial fibrosis, associated with cardiomyocyte hypertrophy [36] and diastolic dysfunction [37].
Figure 1.
Cardiac fibrosis in experimental models of diabetes. A. db/db mice develop severe obesity and diabetes, associated with myocardial fibrosis. B–E. Sirius red staining labels collagen (red – arrows) in the cardiac interstitium (B) and in perivascular areas (C) in lean and db/db mice (D–E). db/db animals exhibit expansion of the interstitial space (D) and perivascular accumulation of collagen (E).
The effects of diet-induced type 2 diabetes and metabolic syndrome on the myocardial interstitium are more subtle. Male C57/BL6J mice fed a high-fat/high-carbohydrate diet developed diabetes associated with ventricular hypertrophy, interstitial fibrosis and diastolic dysfunction after 6–8 months of feeding [38],[39]. Feeding of male C57/BL6J mice with a high-fat diet required 16 months for development of significant cardiac hypertrophy and myocardial fibrosis [40].
Animal model experiments support the clinical evidence suggesting that diabetes accentuates fibrosis induced by other pathophysiologic conditions. Streptozotocin-induced insulin-dependent diabetes increased hypertensive myocardial fibrosis in rats [41]. Moreover, non-insulin dependent diabetes in mice increased myocardial susceptibility to hypertensive hypertrophy and fibrosis [42].
4. THE CELL BIOLOGY OF DIABETES-ASSOCIATED CARDIAC FIBROSIS
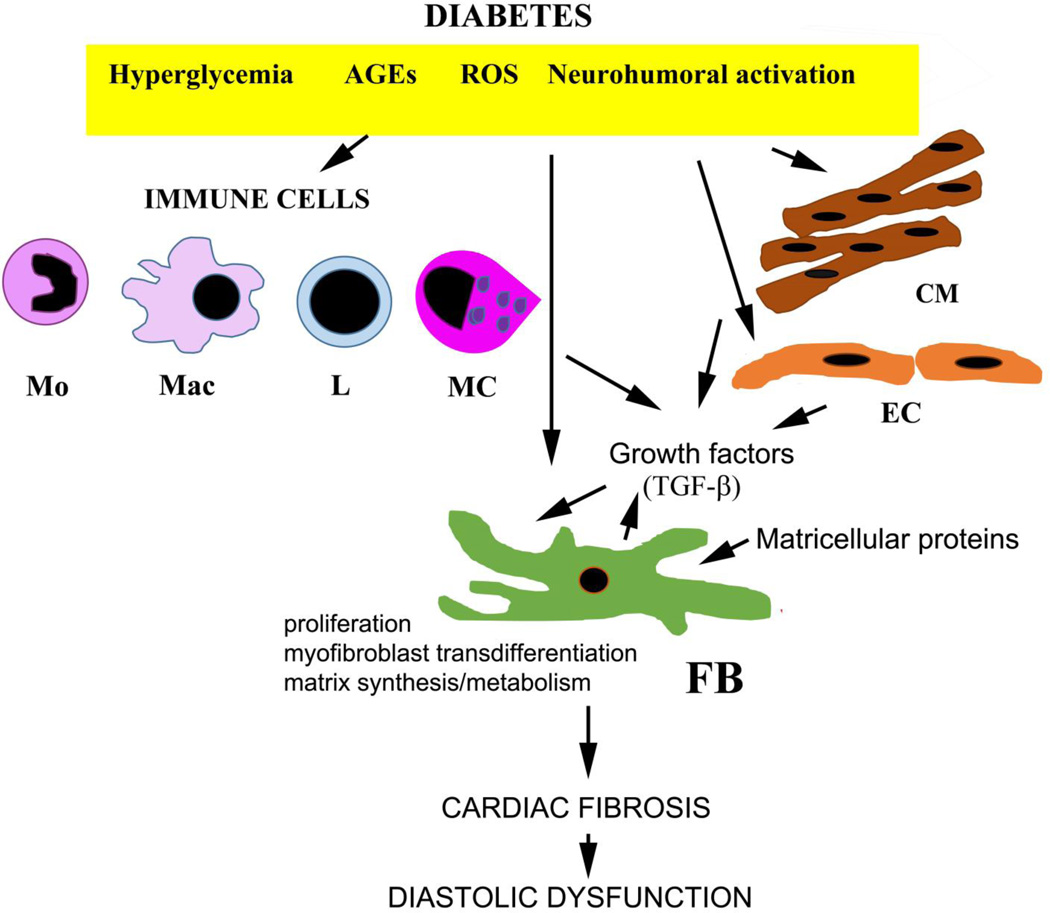
The adult mammalian myocardium contains large populations of non-cardiomyocytes, enmeshed into the interstitial matrix network, including fibroblasts, pericytes vascular smooth muscle cells, endothelial cells, mast cells, macrophages and dendritic cells. Although fibroblasts, as the main matrix-producing cells, are considered critical cellular effectors of fibrosis, other populations of myocardial cells may also contribute to the fibrotic process by modulating fibroblast phenotype and function (Figure 2). Although the cell biological basis of cardiac fibrosis has been extensively investigated in models of myocardial infarction and cardiac pressure overload [43], much less is known regarding the cells responsible for diabetes-associated fibrotic cardiac remodeling.
Figure 2.
The cell biology of diabetes-associated cardiac fibrosis. Diabetes-associated hyperglycemia, generation of advanced glycation end-products (AGEs) and reactive oxygen species (ROS) and neurohumoral activation directly activate resident cardiac fibroblasts and may induce a proliferative response and a matrix-synthetic phenotype. Induction and activation of fibrogenic growth factors (such as TGF-β) may play an important role in fibroblast stimulation. Immune cells (monocytes/Mo, macrophages/Mac, lymphocytes/L and mast cells/MC) may contribute to the fibrotic response by secreting pro-fibrotic mediators. Cardiomyocytes (CM) and endothelial cells (EC) may also secrete growth factors and modulate fibroblast phenotype. Endothelial cells and pericytes may transdifferentiate into fibroblasts contributing to the expansion of the fibroblast population in diabetic hearts. Deposition of matricellular proteins (such as thrombospondin-1) in the diabetic myocardium may promote a pro-fibrotic phenotype in interstitial cells.
The fibroblasts
As the main matrix-producing cells in the cardiac interstitium, fibroblasts are critically involved in all cardiac fibrotic conditions [44],[45]. In the infarcted and remodeling myocardium, fibroblasts undergo myofibroblast transdifferentiation, expressing contractile proteins, such as α-smooth muscle actin (α-SMA), and synthesizing large amounts of extracellular matrix proteins [46],[47],[48],[49]. Although diabetes-associated cardiac fibrosis likely involves expansion and activation of the resident fibroblast population, whether fibroblasts in diabetic hearts undergo a similar process of myofibroblast conversion remains unknown. In vitro studies characterizing fibroblasts isolated from diabetic hearts provide robust evidence of activation. Cardiac fibroblasts harvested from obese diabetic Zucker rat hearts exhibited greater ability to contract gels, increased proliferative activity, and elevated α-SMA expression, consistent with a myofibroblast phenotype [50]. Cardiac fibroblasts isolated from db/db mice exhibited a matrix-preserving phenotype, associated with increased expression of collagen and protease inhibitors [51]. Atrial fibroblasts derived from patients with type 2 diabetes also showed evidence of activation, exhibiting high collagen synthesis [52]. Hyperglycemia, activation of the Renin-angiotensin-aldosterone (RAAS) system and fibrogenic growth factors induced by metabolic dysregulation may be involved in activation of cardiac fibroblasts in diabetic hearts.
Monocytes and macrophages
The myocardium contains a resident macrophage population [53],[54] that is enriched following cardiac injury through recruitment of monocytes [55],[56]. Macrophages are highly plastic cells, capable of acquiring a fibrogenic phenotype that may activate fibroblasts following myocardial injury, or pressure overload [57],[58],[59]. Infiltration of the diabetic myocardium with monocytes and macrophages has been demonstrated in models of type 1 and type 2 diabetes [60],[61],[62]; these cells may contribute to fibrotic remodeling of the ventricle by secreting a wide range of fibrogenic mediators. Whether recruitment or activation of fibrogenic subsets of monocytes/macrophages mediates fibrosis in diabetic hearts has not been directly tested.
Lymphocytes
A growing body of evidence suggests that lymphocyte subpopulations modulate fibroblast phenotype [63], and may mediate fibrotic responses in the remodeling myocardium [64],[65]. Whether alterations in lymphocyte phenotype are implicated in the pathogenesis of diabetes-associated cardiac fibrosis remains unknown.
Endothelial cells and pericytes
In the infarcted and in the pressure overloaded heart, endothelial to mesenchymal transition (EndMT) contributes to cardiac fibrosis, by providing an additional pool of activated fibroblasts [66],[67]. In models of type 1 diabetes, associative evidence supports the involvement of EndMT in the expansion of fibroblasts in the cardiac interstitium [68]. Pericytes are also capable of myofibroblast conversion and may acquire a fibroblast-like phenotype in diabetic states [69]. Moreover, vascular cells may participate in cardiac fibrosis by secreting mediators that activate fibroblasts. Hard documenting the involvement of vascular cells in diabetes-associated fibrosis is lacking.
Mast cells
Mast cells are capable of producing fibrogenic growth factors and proteases and have been implicated in the pathogenesis of cardiac fibrosis in models of myocardial infarction, cardiac pressure overload and cytokine overexpression [70],[71],[72]. In a mouse model of type 1 diabetes, delayed accumulation of mast cells has been implicated in defective healing [73]. Whether mast cells are involved in diabetes-associated cardiac fibrosis remains unknown.
Cardiomyocytes
Cardiomyocytes may play a critical role in diabetes-associated cardiac fibrosis through several distinct mechanisms. First, diabetes and metabolic dysfunction may exert toxic effects on the cardiomyocytes, eventually leading to irreversible injury and cell death [74],[75]. Fibrosis in diabetics may reflect replacement of dead cardiomyocytes with fibrous tissue, rather than direct activation of fibroblasts or immune cells. Second, hyperglycemia may promote a fibrogenic phenotype in cardiomyocytes, inducing synthesis and release of growth factors and cytokines that induce fibroblast proliferation and activation. Third, cardiomyocytes in diabetic hearts may express pro-inflammatory mediators that trigger fibrosis through activation of immune cells. Robust experimental data supporting these cellular mechanisms are lacking.
5. MOLECULAR SIGNALS IMPLICATED IN DIABETES-ASSOCIATED CARDIAC FIBROSIS
Experimental evidence suggests that diabetes-associated cardiac fibrosis may involve activation of several distinct, but overlapping, fibrogenic pathways, including neurohumoral signals, inflammatory cytokines and growth factors, endothelin-1, adipokines, reactive oxygen species (ROS) and deposition of matricellular proteins in the cardiac interstitium. It is likely that the significance of each one of these pathways may be dependent on the severity and pathophysiologic basis of diabetes and on the presence of concommittant conditions, such as dyslipidemia and hypertension.
Hyperglycemia activates a pro-fibrotic program
In vitro, high glucose stimulates fibroblast proliferation, promotes myofibroblast transdifferentiation, and activates transcription and secretion of extracellular matrix proteins [76],[77],[78]. The stimulatory effects of glucose have been attributed to activation of angiotensin II and TGF-β signaling [79],[76], ROS generation [80] and subsequent stimulation of Erk [81] pathways. However, the significance of these findings in vivo is unclear, as robust evidence demonstrating that diabetes-associated myocardial fibrosis is due to hyperglycemia is lacking. Although clinical data examining relations between serum glucose and cardiac fibrosis are lacking, randomized controlled trials do not support the notion that intensive glycemic control reduces the incidence of heart failure in diabetics [1]. Moreover, correction of hyperglycemia does not consistently attenuate diabetes-associated fibrosis in extracardiac tissues. In rats with streptozotocin-induced type 1 diabetes, tight glycemic control did not affect the development of renal fibrosis [82]. Thus, the role of hyperglycemia in mediating fibrotic remodeling of the diabetic heart remains unclear.
Neurohumoral activation
Diabetes activates the myocardial RAAS; increased activity of local RAAS in diabetic hearts may induce a pro-fibrotic program in cardiac fibroblasts, while promoting functional abnormalities in cardiomyocytes [83]. In vivo studies have documented increased myocardial levels of angiotensin II and augmented density of angiotensin II type 1 (AT1) receptors in experimental models of type 1 and type 2 diabetes [84],[85]. Most of the evidence on the role of angiotensin signaling in diabetes-associated cardiac fibrosis is derived from pharmacologic inhibition studies. In three different models of type 2 diabetes (Zucker rats, db/db and ob/ob mice) ACE inhibition decreased collagen deposition, and reduced perivascular coronary fibrosis, attenuating TGF-β levels [86],[32]. Experiments using AT1 blockers suggested that the profibrotic effects of angiotensin II in diabetes are mediated through AT1 signaling [34], [87]. The protective effects of ACE inhibition and AT1 blockade in diabetes-associated cardiac fibrosis are also observed in models of type 1 diabetes [88].
Several molecular cascades may transduce pro-fibrotic angiotensin II signaling in the diabetic heart. First, angiotensin-mediated AT1 signaling may increase TGF-β expression and activation [89], stimulating Smad-dependent and Smad-independent signaling. Second, angiotensin II may accentuate TGF-β responses by inducing expression of the pro-fibrotic TGF-β co-receptor endoglin [90]. Third, AT1 activation may generate ROS in the diabetic myocardium promoting fibroblast activation [32],[91].
Aldosterone may also be an important downstream effector of angiotensin-mediated fibrogenic actions on the myocardium [92]. Experimental evidence suggests that aldosterone antagonism attenuates fibrosis in a rat model of type 1 diabetes [93] and in a mouse model of diet-induced obesity [94]. Clinical studies support an important role for aldosterone signaling in myocardial fibrosis associated with diabetes and obesity. In a prospective randomized controlled clinical study, a 6-month course of the aldosterone antagonist spironolactone in patients with obesity, but without other comorbidities, reduced levels of serological markers of collagen synthesis and improved myocardial compliance and diastolic function [95].
Pro-inflammatory cytokines and chemokines
Activation of immune pathways and induction of pro-inflammatory signaling are associated with fibrosis [96]. Pro-inflammatory cytokines (such as Tumor Necrosis Factor (TNF)-α, and Interleukin (IL)-1β) modulate fibroblast phenotype and have been implicated in the pathogenesis of heart failure. In cardiac fibroblasts, TNF-α stimulation induces proliferation and enhances collagen synthesis [97]. IL-1β on the other hand, delays myofibroblast conversion, promoting α matrix-degrading pro-inflammatory fibroblast phenotype [98]. In a model of streptozotocin-induced type 1 diabetes TNF-α and IL-1β were upregulated in the myocardium [99],[100]. However, experiments examining the effects of cytokine inhibition in rodent models of diabetes have produced conflicting results [101],[102] that may be explained by differences in experimental models, the specific anti-cytokine strategy used, and different methodologies for assessment of cardiac remodeling. The cellular targets of TNF-α and the molecular signals responsible for activation of a pro-fibrotic program in the diabetic heart remain unknown.
Chemokines may also be implicated in cardiac fibrosis through recruitment of fibrogenic monocyte subsets, or through direct actions on fibroblasts [103]. The CC chemokine Monocyte Chemoattractant Protein (MCP)-1/CCL2 mediates ischemic cardiac fibrosis, predominantly through effects on macrophages [104],[55]. Moreover, the CXC chemokine Stromal cell-Derived Factor (SDF)-1/CXCL12 may promote fibrosis by mediating recruitment of bone marrow-derived cells [105]. Evidence suggesting a role for chemokine-mediated pathways in diabetes-associated cardiac fibrosis is limited. Although myocardial MCP-1 expression was increased in a rat model of type 2 diabetes [106], whether induction of the chemokine plays a critical role in fibrosis of the diabetic heart has not been investigated. SDF-1 blockade through inhibition of its receptor CXCR4 attenuated cardiac fibrosis in rodent models of type 1 and type 2 diabetes [107].
TGF-β
TGF-βs are highly pleiotropic mediators that have been extensively implicated in the pathogenesis of tissue fibrosis [108],[109]. TGF-β1 mediates myofibroblast transdifferentiation, stimulates matrix transcription, and promotes a matrix-preserving phenotype in fibroblasts by inducing synthesis of protease inhibitors [108]. Increased myocardial expression of TGF-β has been consistently reported in models of type 1 and type 2 diabetes and is associated with cardiac fibrosis [100],[110],[111],[86]. TGF-β induction in diabetic hearts may be mediated through angiotensin II activation [86], or may involve direct actions of high glucose and leptin on TGF-β transcription, secretion, and activation [112],[113].
Because TGF-β exerts a wide range of actions on all cell types involved in cardiac remodeling, dissecting its biological actions on the diabetic heart is challenging. Pro-fibrotic actions of TGF-β may involve both Smad-dependent and Smad-independent pathways [114],[115]. Experiments using a mouse model of type 2 diabetes demonstrated that global loss of Smad3 reduces cardiac fibrosis and improves myocardial compliance, attenuating myocardial oxidative stress [31]. Whether the pro-fibrotic actions of Smad3 in the diabetic heart reflect direct actions on cardiac fibroblasts, or effects on cardiomyocytes, immune and vascular cells remains unknown.
Endothelin (ET)-1
ET-1, is a potent vasoconstrictor and pro-fibrotic peptide, produced by vascular endothelial cells in response to stimulation with cytokines, angiotensin II, or hypoxia. Experimental evidence suggests that ET-1 expression is induced in experimental models of type 1 and type 2 diabetes [68],[116]. In streptozotocin-induced diabetic mice, endothelial cell-specific loss of ET-1 attenuated myocardial fibrosis, reducing endothelial to mesenchymal transdifferentiation [68]
Oxidative stress
ROS generation is has been extensively implicated in the pathogenesis of cardiac fibrosis Fibrogenic actions of angiotensin II, cytokines and growth factors are, at least in part, dependent on ROS. Extensive evidence demonstrates accentuated oxidative stress in experimental models of type 1 and type 2 diabetes [117]. Streptozotocin-induced diabetic rats exhibited increased glutathione oxidation and augmented lipid hydroperoxide levels in the myocardium [118]. In db/db hearts, mitochondrial generation of ROS is markedly increased and is associated with peroxidation of lipids and proteins [119]. Hyperglycemia and insulin resistance may increase myocardial ROS generation in diabetic animals. Moreover, diabetes is associated with attenuated myocardial activation of antioxidant enzymes, such as manganese superoxide dismutase and glutathione peroxidase 1 [120],[121], suggesting that defective free radical scavenging may also contribute to the accentuated oxidative stress.
The role of oxidative stress in diabetes-associated cardiac fibrosis is supported predominantly by pharmacologic interventions. In models of type 1 or type 2 diabetes, various pharmacological approaches that inhibit oxidative stress, or reduce the level of oxidative modification attenuated cardiac interstitial fibrosis [122], reduced cardiomyocyte hypertrophy, and decreased diastolic dysfunction [39],[32].
The advanced glycation end-products (AGE)/Receptor for AGE (RAGE) axis
After prolonged exposure to aldose sugars, proteins and lipids undergo non-enzymatic glycation and oxidation, leading to formation of AGEs [123]. In diabetic tissues, accelerated accumulation of AGEs may mediate inflammation and fibrosis. AGEs accumulate in both intracellular and extracellular space and may play a key role in diabetes-associated cardiac fibrosis through several distinct mechanisms. First, AGEs crosslink collagens and laminins in the extracellular matrix and may reduce cardiac compliance, causing diastolic dysfunction. Second, AGEs may bind to RAGEs, cell surface receptors that when activated modulate cellular phenotype. In fibroblasts, AGE/RAGE signaling stimulates inflammatory gene synthesis, accentuates expression of matrix proteins, and stimulates proliferation [124]. The pro-fibrotic effects of RAGE may be mediated, at least in part, through TGF-β [125] and AT-1 cascades [126]. Third, AGEs generate ROS and may promote fibrosis by increasing oxidative stress. Fourth, AGEs may modulate macrophage phenotype inducing a pro-fibrotic program. The in vivo role of these potential mechanisms in mediating diabetes-associated cardiac fibrosis has not been systematically studied. In db/db mice, RAGE blockade protected from the development of diastolic dysfunction, attenuating myocardial collagen expression [127]. However, the cellular basis and molecular mechanisms for these effects remain unknown.
Adipokines
Adipose tissue does not serve only as a depot for stored fat, but also secretes large amounts of bioactive mediators, termed adipokines. Of these pleiotropic molecules, leptin and adiponectin have been implicated in the pathogenesis of tissue fibrosis.
Leptin is involved in the pathogenesis of cardiac remodeling in type 2 diabetes, obesity and metabolic dysfunction through effects on both cardiomyocytes and cardiac fibroblasts. In patients with uncomplicated obesity, elevated circulating leptin is associated with increased left ventricular mass [128]. Conflicting data are available on the effects of leptin on cardiomyocytes, suggesting both hypertrophic and anti-hypertrophic actions [129],[130]. Moreover, leptin is capable of activating fibroblasts, inducing activation of MMPs [131],[132]. In vivo, exogenous leptin administration in ob/ob mice significantly increased interstitial fibrosis [133]. The relative significance of the cellular actions of leptin on cardiomyocytes and fibroblasts in vivo remains unknown.
Adiponectin, an adipokine with anti-inflammatory, cardioprotective and anti-atherogenic properties [134],[135] may also regulate cardiac fibrosis. In vitro studies have suggested conflicting effects of adiponectin on fibroblast migration [136],[137]. In vivo, adiponectin exerted anti-fibrotic effects in a model of angiotensin-induced cardiac remodeling [138]. In db/db mice, exogenous adiponectin reduced cardiac hypertrophy activating AMPK signaling [139]. The role of adiponectin in diabetic cardiac fibrosis remains unknown.
The role of the matricellular proteins
Following injury, the extracellular matrix is enriched through deposition of matricellular proteins, extracellular macromolecules that do not play a structural role, but transduce signaling cascades, modulating cell:cell and cell:matrix interactions. Several members of the matricellular family, including the thrombospondins (TSPs), tenascins-C and X, periostin, osteonectin, osteopontin, periostin, etc. are induced in the remodeling heart and regulate inflammatory, fibrotic and angiogenic responses [140]. TSP-1 is induced by high glucose [141] and is consistently upregulated in diabetes, obesity and metabolic dysfunction in both animal models and human patients [142],[143]. TSP-1 induction in the diabetic heart promotes matrix-preserving actions, while causing capillary rarefaction in db/db mice through effects on angiopoietin-2 synthesis [30]. Thus, induction of TSP-1 may mediate both fibrosis and vascular loss in the diabetic myocardium.
MicroRNAs
MicroRNAs (miRNAs) are short noncoding RNAs that function as regulators of gene expression and are involved in virtually all cellular responses. A growing body of evidence suggests a critical role for miRNAs in cardiac fibrosis [144]. miRNAs have multiple targets, including cytokines and growth factors, extracellular matrix proteins, proteases and matricellular macromolecules. Although published investigations suggest critical roles for several miRNAs in cardiac fibrosis induced by pressure overload or myocardial infarction, evidence indicating involvement of specific miRNAs in diabetes-associated cardiac fibrosis is limited. Recent studies investigated the myocardial miRNA landscape in a mouse model of type 1 diabetes [145],[146]. Diabetic hearts had alterations in levels of several miRNAs implicated in the pathogenesis of fibrosis, exhibiting upregulation of miR-125b and miR-199a, and downregulation of miR-150, miR-29b and miR-30a. Importantly, dysregulated expression of fibrosis-associated miRNAs remained altered in animals receiving insulin therapy to achieve intensive glycemic control [146]. Experimental studies examining whether these alterations play a causative role in the pathogenesis of diabetic cardiac fibrosis have not yet been performed. A recent investigation suggested that miR-133 downregulation may be involved in fibrotic remodeling of the diabetic heart. In a model of type 1 diabetes, cardiac fibrosis was associated with suppressed myocardial miR-133 expression; miR-133 overexpression attenuated the fibrotic response attenuating Erk and Smad activation [147].
6. TARGETING DIABETIC CARDIAC FIBROSIS: CHALLENGES AND OPPORTUNITIES
Clinical evidence suggests that diabetics with evidence of cardiac fibrosis, assessed through cardiac magnetic resonance imaging, have increased mortality and higher incidence of hospitalizations due to heart failure [148]. Several mechanisms may contribute to the increased risk. First, myocardial fibrosis may reduce ventricular compliance causing HFpEF. Second, diabetes-associated atrial fibrosis may induce atrial fibrillation, precipitating heart failure, and increasing the incidence of stroke. Third, ventricular fibrosis may be responsible for the increased risk of ventricular arrhythmias and sudden death observed in diabetic individuals [149]. Fourth, diabetes-related perturbation of the reparative response following infarction may result in faulty healing, adverse remodeling and development of post-infarction heart failure. Thus, on a theoretical basis, attenuation of cardiac fibrosis may reduce morbidity and mortality in diabetic patients.
Which strategies could be used to reduce cardiac fibrosis in diabetics? Considering the potentially critical involvement of high glucose in the pathogenesis of fibrosis, it would be reasonable to hypothesize that tight glycemic control may be effective in attenuation of cardiac fibrosis. Although poor glycemic control is associated with an increased incidence of heart failure [150], intensive glucose lowering failed to reduce cardiovascular events [151], the risk of heart failure [152] and the incidence of new-onset atrial fibrillation [153]. Unfortunately, relations between outcome and effects on cardiac fibrosis have not been studied.
Clearly, additional pharmacologic strategies are needed to inhibit and reverse fibrosis and to prevent the development of heart failure in diabetics. Established approaches inhibiting the RAAS through the use of ACE inhibitors, angiotensin receptor blockers and aldosterone antagonists may be valuable and have an excellent record of safety. Whether these approaches exert beneficial actions through attenuation of fibrosis is unclear. Novel pharmacologic strategies targeting the ROS system, AGE-mediated crosslinking, ET-1 or the TGF-β system hold significant promise. However, such approaches may also carry significant risks, due to the need for prolonged therapy and the importance of these molecular signals in homeostasis and tissue repair [154].
Attenuation of fibrotic remodeling following myocardial infarction may represent a more attractive therapeutic opportunity to reduce the risk of heart failure in diabetics. A brief therapeutic intervention may be effective in protecting the infarcted heart from excessive fibrosis in diabetic patients surviving an acute myocardial infarction. Clinical evidence suggests that diabetics have an increased incidence of post-infarction heart failure predominantly due to diastolic dysfunction [155]. Overactive angiotensin or TGF-β/Smad signaling may drive the reparative process towards an excessive fibrotic response. Dissection of the mechanisms responsible for defective cardiac repair in diabetics, and use of biomarkers or imaging studies to identify patients with specific pathophysiologic defects are needed to design personalized therapeutic approaches in order to reduce fibrosis and prevent the development of post-infarction heart failure [156].
7. CONCLUSIONS
Diabetes-associated cardiac fibrosis may be a major contributor to morbidity and mortality by causing heart failure and by increasing the incidence of arrhythmic events. Our understanding of the cellular events and molecular pathways involved in the pathogenesis of cardiac fibrosis remains limited. As a result, effective therapies targeting the fibrotic response in diabetic are lacking. Studies are needed to dissect the diabetes-associated molecular signals that activate fibroblasts in the cardiac interstitium, and to understand the fundamental basis for the link between metabolic dysfunction and fibrosis.
Highlights.
Diabetes is associated with cardiac fibrosis.
Diabetic cardiac fibrosis contributes to diastolic dysfunction and arrhythmogenesis.
Activated fibroblasts are the main effector cells in diabetic fibrosis.
Neurohumoral and inflammatory pathways may activate diabetic fibroblasts.
Induction of matricellular proteins in the diabetic myocardium promotes fibrosis.
ACKNOWLEDGMENTS
Dr Frangogiannis’ laboratory is supported by NIH grants R01 HL76246 and R01 HL85440. Ilaria Russo is supported by training grants from the Fondazione Cassa di Risparmio di Lucca and the Fondazione Banca del Monte di Lucca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385:2107–2117. doi: 10.1016/S0140-6736(14)61402-1. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 3.Cavender MA, Steg PG, Smith SC, Jr, Eagle K, Ohman EM, Goto S, et al. Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years From the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132:923–931. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 5.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 6.Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380:601–610. doi: 10.1016/S0140-6736(12)60987-8. [DOI] [PubMed] [Google Scholar]
- 7.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 8.Kwong RY, Sattar H, Wu H, Vorobiof G, Gandla V, Steel K, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118:1011–1020. doi: 10.1161/CIRCULATIONAHA.107.727826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkbey EB, Backlund JY, Genuth S, Jain A, Miao C, Cleary PA, et al. Myocardial structure, function, and scar in patients with type 1 diabetes mellitus. Circulation. 2011;124:1737–1746. doi: 10.1161/CIRCULATIONAHA.111.022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, et al. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest. 1977;60:884–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawaguchi M, Techigawara M, Ishihata T, Asakura T, Saito F, Maehara K, et al. A comparison of ultrastructural changes on endomyocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessels. 1997;12:267–274. doi: 10.1007/BF02766802. [DOI] [PubMed] [Google Scholar]
- 12.Fischer VW, Barner HB, Larose LS. Pathomorphologic aspects of muscular tissue in diabetes mellitus. Hum Pathol. 1984;15:1127–1136. doi: 10.1016/s0046-8177(84)80307-x. [DOI] [PubMed] [Google Scholar]
- 13.van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990;82:848–855. doi: 10.1161/01.cir.82.3.848. [DOI] [PubMed] [Google Scholar]
- 14.Nunoda S, Genda A, Sugihara N, Nakayama A, Mizuno S, Takeda R. Quantitative approach to the histopathology of the biopsied right ventricular myocardium in patients with diabetes mellitus. Heart Vessels. 1985;1:43–47. doi: 10.1007/BF02066486. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland CG, Fisher BM, Frier BM, Dargie HJ, More IA, Lindop GB. Endomyocardial biopsy pathology in insulin-dependent diabetic patients with abnormal ventricular function. Histopathology. 1989;14:593–602. doi: 10.1111/j.1365-2559.1989.tb02200.x. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–36. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 18.Falcao-Pires I, Hamdani N, Borbely A, Gavina C, Schalkwijk CG, van der Velden J, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–1159. doi: 10.1161/CIRCULATIONAHA.111.025270. [DOI] [PubMed] [Google Scholar]
- 19.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res. 2014;164:323–335. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen X, Bornfeldt KE. Mouse models for studies of cardiovascular complications of type 1 diabetes. Ann N Y Acad Sci. 2007;1103:202–217. doi: 10.1196/annals.1394.004. [DOI] [PubMed] [Google Scholar]
- 21.Huynh K, McMullen JR, Julius TL, Tan JW, Love JE, Cemerlang N, et al. Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy. Diabetes. 2010;59:1512–1520. doi: 10.2337/db09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ares-Carrasco S, Picatoste B, Benito-Martin A, Zubiri I, Sanz AB, Sanchez-Nino MD, et al. Myocardial fibrosis and apoptosis, but not inflammation, are present in long-term experimental diabetes. Am J Physiol Heart Circ Physiol. 2009;297:H2109–H2119. doi: 10.1152/ajpheart.00157.2009. [DOI] [PubMed] [Google Scholar]
- 23.Hao PP, Yang JM, Zhang MX, Zhang K, Chen YG, Zhang C, et al. Angiotensin-(1–7) treatment mitigates right ventricular fibrosis as a distinctive feature of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;308:H1007–H1019. doi: 10.1152/ajpheart.00563.2014. [DOI] [PubMed] [Google Scholar]
- 24.Meloni M, Descamps B, Caporali A, Zentilin L, Floris I, Giacca M, et al. Nerve growth factor gene therapy using adeno-associated viral vectors prevents cardiomyopathy in type 1 diabetic mice. Diabetes. 2012;61:229–240. doi: 10.2337/db11-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu R, Oudit GY, Wang X, Zhang L, Ussher JR, Lopaschuk GD, et al. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol. 2009;297:H2096–H2108. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 27.Regan TJ, Wu CF, Yeh CK, Oldewurtel HA, Haider B. Myocardial composition and function in diabetes. The effects of chronic insulin use. Circ Res. 1981;49:1268–1277. doi: 10.1161/01.res.49.6.1268. [DOI] [PubMed] [Google Scholar]
- 28.Haider B, Yeh CK, Thomas G, Oldewurtel HA, Lyons MM, Regan TJ. Influence of diabetes on the myocardium and coronary arteries of rhesus monkey fed an atherogenic diet. Circ Res. 1981;49:1278–1288. doi: 10.1161/01.res.49.6.1278. [DOI] [PubMed] [Google Scholar]
- 29.Khaidar A, Marx M, Lubec B, Lubec G. L-arginine reduces heart collagen accumulation in the diabetic db/db mouse. Circulation. 1994;90:479–483. doi: 10.1161/01.cir.90.1.479. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee DW, Saxena A, et al. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res. 2013;113:1331–1344. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, et al. Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice. Circ Heart Fail. 2015;8:788–798. doi: 10.1161/CIRCHEARTFAILURE.114.001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huynh K, Kiriazis H, Du XJ, Love JE, Jandeleit-Dahm KA, Forbes JM, et al. Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia. 2012;55:1544–1553. doi: 10.1007/s00125-012-2495-3. [DOI] [PubMed] [Google Scholar]
- 33.Sloan C, Tuinei J, Nemetz K, Frandsen J, Soto J, Wride N, et al. Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes. 2011;60:1424–1434. doi: 10.2337/db10-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaman AK, Fujii S, Goto D, Furumoto T, Mishima T, Nakai Y, et al. Salutary effects of attenuation of angiotensin II on coronary perivascular fibrosis associated with insulin resistance and obesity. J Mol Cell Cardiol. 2004;37:525–535. doi: 10.1016/j.yjmcc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Van den Bergh A, Vanderper A, Vangheluwe P, Desjardins F, Nevelsteen I, Verreth W, et al. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res. 2008;77:371–379. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 36.Fredersdorf S, Thumann C, Ulucan C, Griese DP, Luchner A, Riegger GA, et al. Myocardial hypertrophy and enhanced left ventricular contractility in Zucker diabetic fatty rats. Cardiovasc Pathol. 2004;13:11–19. doi: 10.1016/S1054-8807(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 37.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, et al. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology. 2013;154:2501–2513. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zibadi S, Vazquez R, Moore D, Larson DF, Watson RR. Myocardial lysyl oxidase regulation of cardiac remodeling in a murine model of diet-induced metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H976–H982. doi: 10.1152/ajpheart.00398.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin F, Siwik DA, Luptak I, Hou X, Wang L, Higuchi A, et al. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. S1–S6. doi: 10.1161/CIRCULATIONAHA.111.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calligaris SD, Lecanda M, Solis F, Ezquer M, Gutierrez J, Brandan E, et al. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One. 2013;8:e60931. doi: 10.1371/journal.pone.0060931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Factor SM, Bhan R, Minase T, Wolinsky H, Sonnenblick EH. Hypertensive-diabetic cardiomyopathy in the rat: an experimental model of human disease. Am J Pathol. 1981;102:219–228. [PMC free article] [PubMed] [Google Scholar]
- 42.van Bilsen M, Daniels A, Brouwers O, Janssen BJ, Derks WJ, Brouns AE, et al. Hypertension is a conditional factor for the development of cardiac hypertrophy in type 2 diabetic mice. PLoS One. 2014;9:e85078. doi: 10.1371/journal.pone.0085078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 47.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 48.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000;48:89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 49.van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 50.Fowlkes V, Clark J, Fix C, Law BA, Morales MO, Qiao X, et al. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci. 2013;92:669–676. doi: 10.1016/j.lfs.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutchinson KR, Lord CK, West TA, Stewart JA., Jr Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS One. 2013;8:e72080. doi: 10.1371/journal.pone.0072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sedgwick B, Riches K, Bageghni SA, O’Regan DJ, Porter KE, Turner NA. Investigating inherent functional differences between human cardiac fibroblasts cultured from nondiabetic and Type 2 diabetic donors. Cardiovasc Pathol. 2014;23:204–210. doi: 10.1016/j.carpath.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 56.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartupee J, Mann DL. Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.016. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.015. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urbina P, Singla DK. BMP-7 attenuates adverse cardiac remodeling mediated through M2 macrophages in prediabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2014;307:H762–H772. doi: 10.1152/ajpheart.00367.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fukuda M, Nakamura T, Kataoka K, Nako H, Tokutomi Y, Dong YF, et al. Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. J Hypertens. 2010;28:340–352. doi: 10.1097/HJH.0b013e32833366cd. [DOI] [PubMed] [Google Scholar]
- 62.Fukuda M, Nakamura T, Kataoka K, Nako H, Tokutomi Y, Dong YF, et al. Ezetimibe ameliorates cardiovascular complications and hepatic steatosis in obese and type 2 diabetic db/db mice. J Pharmacol Exp Ther. 2010;335:70–75. doi: 10.1124/jpet.110.170373. [DOI] [PubMed] [Google Scholar]
- 63.Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol. 2014;307:H1233–H1242. doi: 10.1152/ajpheart.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, et al. Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ Heart Fail. 2015;8:776–787. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131:1019–1030. doi: 10.1161/CIRCULATIONAHA.114.008788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 67.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech. 2011;4:469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–2418. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 69.Humphreys BD. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Semin Nephrol. 2012;32:463–470. doi: 10.1016/j.semnephrol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, et al. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687–698. doi: 10.1161/01.cir.98.7.687. [DOI] [PubMed] [Google Scholar]
- 71.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W, Chancey AL, Tzeng HP, Zhou Z, Lavine KJ, Gao F, et al. The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions. Circulation. 2012;124:2106–2116. doi: 10.1161/CIRCULATIONAHA.111.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishikori Y, Shiota N, Okunishi H. The role of mast cells in cutaneous wound healing in streptozotocin-induced diabetic mice. Arch Dermatol Res. 2014;306:823–835. doi: 10.1007/s00403-014-1496-0. [DOI] [PubMed] [Google Scholar]
- 74.Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56:391–400. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 76.Han DC, Isono M, Hoffman BB, Ziyadeh FN. High glucose stimulates proliferation and collagen type I synthesis in renal cortical fibroblasts: mediation by autocrine activation of TGF-beta. J Am Soc Nephrol. 1999;10:1891–1899. doi: 10.1681/ASN.V1091891. [DOI] [PubMed] [Google Scholar]
- 77.Shamhart PE, Luther DJ, Adapala RK, Bryant JE, Petersen KA, Meszaros JG, et al. Hyperglycemia enhances function and differentiation of adult rat cardiac fibroblasts. Can J Physiol Pharmacol. 2014;92:598–604. doi: 10.1139/cjpp-2013-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aguilar H, Fricovsky E, Ihm S, Schimke M, Maya-Ramos L, Aroonsakool N, et al. Role for high-glucose-induced protein O-GlcNAcylation in stimulating cardiac fibroblast collagen synthesis. Am J Physiol Cell Physiol. 2014;306:C794–C804. doi: 10.1152/ajpcell.00251.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol. 2008;294:H1675–H1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 80.Fiaschi T, Magherini F, Gamberi T, Lucchese G, Faggian G, Modesti A, et al. Hyperglycemia and angiotensin II cooperate to enhance collagen I deposition by cardiac fibroblasts through a ROS-STAT3-dependent mechanism. Biochim Biophys Acta. 2014;1843:2603–2610. doi: 10.1016/j.bbamcr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 81.Tang M, Zhang W, Lin H, Jiang H, Dai H, Zhang Y. High glucose promotes the production of collagen types I and III by cardiac fibroblasts through a pathway dependent on extracellular-signal-regulated kinase 1/2. Mol Cell Biochem. 2007;301:109–114. doi: 10.1007/s11010-006-9401-6. [DOI] [PubMed] [Google Scholar]
- 82.Conway BR, Betz B, Sheldrake TA, Manning JR, Dunbar DR, Dobyns A, et al. Tight blood glycaemic and blood pressure control in experimental diabetic nephropathy reduces extracellular matrix production without regression of fibrosis. Nephrology (Carlton) 2014;19:802–813. doi: 10.1111/nep.12335. [DOI] [PubMed] [Google Scholar]
- 83.Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- 84.Brown L, Wall D, Marchant C, Sernia C. Tissue-specific changes in angiotensin II receptors in streptozotocin-diabetic rats. J Endocrinol. 1997;154:355–362. doi: 10.1677/joe.0.1540355. [DOI] [PubMed] [Google Scholar]
- 85.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toblli JE, Cao G, DeRosa G, Forcada P. Reduced cardiac expression of plasminogen activator inhibitor 1 and transforming growth factor beta1 in obese Zucker rats by perindopril. Heart. 2005;91:80–86. doi: 10.1136/hrt.2003.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zaman AK, Fujii S, Sawa H, Goto D, Ishimori N, Watano K, et al. Angiotensin-converting enzyme inhibition attenuates hypofibrinolysis and reduces cardiac perivascular fibrosis in genetically obese diabetic mice. Circulation. 2001;103:3123–3128. doi: 10.1161/01.cir.103.25.3123. [DOI] [PubMed] [Google Scholar]
- 88.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE. Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun. 2006;339:633–641. doi: 10.1016/j.bbrc.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 90.Chen K, Mehta JL, Li D, Joseph L, Joseph J. Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ Res. 2004;95:1167–1173. doi: 10.1161/01.RES.0000150369.68826.2f. [DOI] [PubMed] [Google Scholar]
- 91.Vazquez-Medina JP, Popovich I, Thorwald MA, Viscarra JA, Rodriguez R, Sonanez-Organis JG, et al. Angiotensin receptor-mediated oxidative stress is associated with impaired cardiac redox signaling and mitochondrial function in insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2013;305:H599–H607. doi: 10.1152/ajpheart.00101.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Essick EE, Sam F. Cardiac hypertrophy and fibrosis in the metabolic syndrome: a role for aldosterone and the mineralocorticoid receptor. Int J Hypertens. 2011;2011:346985. doi: 10.4061/2011/346985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miric G, Dallemagne C, Endre Z, Margolin S, Taylor SM, Brown L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br J Pharmacol. 2001;133:687–694. doi: 10.1038/sj.bjp.0704131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bostick B, Habibi J, DeMarco VG, Jia G, Domeier TL, Lambert MD, et al. Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. Am J Physiol Heart Circ Physiol. 2015;308:H1126–H1135. doi: 10.1152/ajpheart.00898.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kosmala W, Przewlocka-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart. 2013;99:320–326. doi: 10.1136/heartjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 96.Saxena A, Shinde AV, Haque Z, Wu YJ, Chen W, Su Y, et al. The role of Interleukin Receptor Associated Kinase (IRAK)-M in regulation of myofibroblast phenotype in vitro, and in an experimental model of non-reperfused myocardial infarction. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.11.001. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venkatachalam K, Venkatesan B, Valente AJ, Melby PC, Nandish S, Reusch JE, et al. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNF-alpha)-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J Biol Chem. 2009;284:14414–14427. doi: 10.1074/jbc.M809757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, et al. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J Immunol. 2013;191:4838–4848. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suzuki H, Kayama Y, Sakamoto M, Iuchi H, Shimizu I, Yoshino T, et al. Arachidonate 12/15-lipoxygenase-induced inflammation and oxidative stress are involved in the development of diabetic cardiomyopathy. Diabetes. 2015;64:618–630. doi: 10.2337/db13-1896. [DOI] [PubMed] [Google Scholar]
- 100.Westermann D, Rutschow S, Jager S, Linderer A, Anker S, Riad A, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007;56:641–646. doi: 10.2337/db06-1163. [DOI] [PubMed] [Google Scholar]
- 101.Isic A, Scharin Tang M, Haugen E, Fu M. TNFalpha-antagonist neither improve cardiac remodelling or cardiac function at early stage of heart failure in diabetic rats. Autoimmunity. 2008;41:473–477. doi: 10.1080/08916930802041164. [DOI] [PubMed] [Google Scholar]
- 102.Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102:500–507. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 103.Dobaczewski M, Frangogiannis NG. Chemokines and cardiac fibrosis. Front Biosci (Schol Ed) 2009;1:391–405. doi: 10.2741/s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 105.Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, et al. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol. 2010;176:1735–1742. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu Y, Ohmori K, Chen Y, Sato C, Kiyomoto H, Shinomiya K, et al. Effects of pravastatin on progression of glucose intolerance and cardiovascular remodeling in a type II diabetes model. J Am Coll Cardiol. 2004;44:904–913. doi: 10.1016/j.jacc.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 107.Chu PY, Walder K, Horlock D, Williams D, Nelson E, Byrne M, et al. CXCR4 Antagonism Attenuates the Development of Diabetic Cardiac Fibrosis. PLoS One. 2015;10:e0133616. doi: 10.1371/journal.pone.0133616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carroll JF, Tyagi SC. Extracellular matrix remodeling in the heart of the homocysteinemic obese rabbit. Am J Hypertens. 2005;18:692–698. doi: 10.1016/j.amjhyper.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 111.Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 112.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumpers P, Gueler F, Rong S, Mengel M, Tossidou I, Peters I, et al. Leptin is a coactivator of TGF-beta in unilateral ureteral obstructive kidney disease. Am J Physiol Renal Physiol. 2007;293:F1355–F1362. doi: 10.1152/ajprenal.00003.2007. [DOI] [PubMed] [Google Scholar]
- 114.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest. 2014;124:5225–5238. doi: 10.1172/JCI75331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sen S, Chen S, Feng B, Iglarz M, Chakrabarti S. Renal, retinal and cardiac changes in type 2 diabetes are attenuated by macitentan, a dual endothelin receptor antagonist. Life Sci. 2012;91:658–668. doi: 10.1016/j.lfs.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 117.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 119.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 120.Ballal K, Wilson CR, Harmancey R, Taegtmeyer H. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol Cell Biochem. 2010;344:221–230. doi: 10.1007/s11010-010-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, et al. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–H162. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S, et al. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology. 2008;149:380–388. doi: 10.1210/en.2007-0877. [DOI] [PubMed] [Google Scholar]
- 123.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 124.Zhao LM, Zhang W, Wang LP, Li GR, Deng XL. Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels. Pflugers Arch. 2012;464:613–621. doi: 10.1007/s00424-012-1165-0. [DOI] [PubMed] [Google Scholar]
- 125.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamazaki KG, Gonzalez E, Zambon AC. Crosstalk between the renin-angiotensin system and the advance glycation end product axis in the heart: role of the cardiac fibroblast. J Cardiovasc Transl Res. 2012;5:805–813. doi: 10.1007/s12265-012-9405-4. [DOI] [PubMed] [Google Scholar]
- 127.Nielsen JM, Kristiansen SB, Norregaard R, Andersen CL, Denner L, Nielsen TT, et al. Blockage of receptor for advanced glycation end products prevents development of cardiac dysfunction in db/db type 2 diabetic mice. Eur J Heart Fail. 2009;11:638–647. doi: 10.1093/eurjhf/hfp070. [DOI] [PubMed] [Google Scholar]
- 128.Perego L, Pizzocri P, Corradi D, Maisano F, Paganelli M, Fiorina P, et al. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90:4087–4093. doi: 10.1210/jc.2004-1963. [DOI] [PubMed] [Google Scholar]
- 129.Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 130.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 131.Schram K, Ganguly R, No EK, Fang XP, Thong FSL, Sweeney G. Regulation of MT1-MMP and MMP-2 by Leptin in Cardiac Fibroblasts Involves Rho/ROCK-Dependent Actin Cytoskeletal Reorganization and Leads to Enhanced Cell Migration. Endocrinology. 2011;152:2037–2047. doi: 10.1210/en.2010-1166. [DOI] [PubMed] [Google Scholar]
- 132.Fukui A, Takahashi N, Nakada C, Masaki T, Kume O, Shinohara T, et al. Role of leptin signaling in the pathogenesis of angiotensin II-mediated atrial fibrosis and fibrillation. Circ Arrhythm Electrophysiol. 2013;6:402–409. doi: 10.1161/CIRCEP.111.000104. [DOI] [PubMed] [Google Scholar]
- 133.Zibadi S, Cordova F, Slack EH, Watson RR, Larson DF. Leptin's regulation of obesity-induced cardiac extracellular matrix remodeling. Cardiovasc Toxicol. 2011;11:325–333. doi: 10.1007/s12012-011-9124-0. [DOI] [PubMed] [Google Scholar]
- 134.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 135.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dadson K, Chasiotis H, Wannaiampikul S, Tungtrongchitr R, Xu A, Sweeney G. Adiponectin Mediated APPL1-AMPK Signaling Induces Cell Migration, MMP Activation, and Collagen Remodeling in Cardiac Fibroblasts. J Cell Biochem. 2014;115:785–793. doi: 10.1002/jcb.24722. [DOI] [PubMed] [Google Scholar]
- 137.Cai XJ, Chen L, Li L, Feng M, Li X, Zhang K, et al. Adiponectin inhibits lipopolysaccharide-induced adventitial fibroblast migration and transition to myofibroblasts via AdipoR1-AMPK-iNOS pathway. Mol Endocrinol. 2010;24:218–228. doi: 10.1210/me.2009-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol. 2008;28:863–870. doi: 10.1161/ATVBAHA.107.156687. [DOI] [PubMed] [Google Scholar]
- 139.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282:5704–5714. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 142.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, et al. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57:432–439. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab. 2013;305:E439–E450. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thum T. Noncoding RNAs and myocardial fibrosis. Nat Rev Cardiol. 2014;11:655–663. doi: 10.1038/nrcardio.2014.125. [DOI] [PubMed] [Google Scholar]
- 145.Diao X, Shen E, Wang X, Hu B. Differentially expressed microRNAs and their target genes in the hearts of streptozotocin-induced diabetic mice. Mol Med Rep. 2011;4:633–640. doi: 10.3892/mmr.2011.489. [DOI] [PubMed] [Google Scholar]
- 146.Costantino S, Paneni F, Luscher TF, Cosentino F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv599. epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 147.Chen S, Puthanveetil P, Feng B, Matkovich SJ, Dorn GW, 2nd, Chakrabarti S. Cardiac miR-133a overexpression prevents early cardiac fibrosis in diabetes. J Cell Mol Med. 2014;18:415–421. doi: 10.1111/jcmm.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35:657–664. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bertoia ML, Allison MA, Manson JE, Freiberg MS, Kuller LH, Solomon AJ, et al. Risk factors for sudden cardiac death in post-menopausal women. J Am Coll Cardiol. 2012;60:2674–2682. doi: 10.1016/j.jacc.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 150.Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 151.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 152.Castagno D, Baird-Gunning J, Jhund PS, Biondi-Zoccai G, MacDonald MR, Petrie MC, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J. 2011;162:938–948. e2. doi: 10.1016/j.ahj.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 153.Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study) Am J Cardiol. 2014;114:1217–1222. doi: 10.1016/j.amjcard.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frangogiannis NG. Targeting the transforming growth factor (TGF)-beta cascade in the remodeling heart: benefits and perils. J Mol Cell Cardiol. 2014;76:169–171. doi: 10.1016/j.yjmcc.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Aronson D, Musallam A, Lessick J, Dabbah S, Carasso S, Hammerman H, et al. Impact of diastolic dysfunction on the development of heart failure in diabetic patients after acute myocardial infarction. Circ Heart Fail. 2010;3:125–131. doi: 10.1161/CIRCHEARTFAILURE.109.877340. [DOI] [PubMed] [Google Scholar]
- 156.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]