Abstract
Recently approved, interferon-free medication regimens for treating hepatitis C are highly effective but extremely costly. We aimed to identify cost-effective strategies for managing treatment-naïve US Veterans with new hepatitis C medication regimens. We developed a Markov model with 1-year cycle length for a cohort of 60-year old Veterans with untreated genotype 1 hepatitis C seeking treatment in a typical year. We compared using sofosbuvir/ledipasvir or ombitasvir/ritonavir/paritaprevir/dasabuvir to treat: (1) any patient seeking treatment, (2) only patients with advanced fibrosis or cirrhosis, or (3) patients with advanced disease first and healthier patients one year later. The previous standard of care, sofosbuvir/simeprevir or sofosbuvir/pegylated interferon/ribavirin, was included for comparison. Patients could develop progressive fibrosis, cirrhosis, or hepatocellular carcinoma, undergo transplantation, or die. Complications were less likely after sustained virologic response. We calculated the incremental cost per quality-adjusted life year (QALY) and varied model inputs in one-way and probabilistic sensitivity analyses. We used the Veterans Health Administration perspective with a lifetime time horizon and 3% annual discounting. Treating any patient with ombitasvir-based therapy was the preferred strategy ($35,560; 14.0 QALYs). All other strategies were dominated (greater costs/QALY gained than more effective strategies). Varying treatment efficacy, price and/or duration changed the preferred strategy. In probabilistic sensitivity analysis, treating any patient with ombitasvir-based therapy was cost-effective in 70% of iterations at a $50,000/QALY threshold and 65% of iterations at a $100,000/QALY threshold.
Conclusion
Managing any treatment-naïve genotype 1 hepatitis C patient with ombitasvir-based therapy is the most economically efficient strategy, although price and efficacy can impact cost-effectiveness. It is economically unfavorable to restrict treatment to patients with advanced disease or use a staged treatment strategy.
Keywords: Markov model, interferon-free, ombitasvir, sofosbuvir, ledipasvir
Hepatitis C (HCV) affects over 174 million people worldwide and over 3 million people in the US (1, 2). Although patients often remain asymptomatic for years, chronic HCV infection is a leading cause of liver cirrhosis and hepatocellular carcinoma and the most common indication for liver transplantation in the US (3, 4). Patients with HCV experience substantially higher mortality than the general population (5). Though there are 7 HCV genotypes, approximately 75% of US patients are infected with genotype 1. Successful HCV treatment leads to sustained virologic response, improving quality of life and reducing morbidity and mortality (5–7). However, due in part to the poor efficacy, low rates of HCV testing, and eligibility restrictions for prior therapeutic options, many patients with HCV remain untreated (8).
Recently approved HCV drug regimens have dramatically improved treatment efficacy, but high drug prices have necessitated novel strategies for determining which patients would benefit most from treatment. Historically, HCV treatment regimens have included pegylated interferon, ribavirin and direct acting antiviral drugs (telaprevir or boceprevir). These regimens required up to 48 weeks of therapy, were only modestly efficacious, and caused significant dose-limiting morbidity (9, 10). In 2013, the Food and Drug Administration approved two new drugs, sofosbuvir and simeprevir, which improved treatment efficacy to over 90% in many patient subgroups (11, 12). These regimens still included poorly tolerated interferon for most patients and cost up to $1800 per dose. With these high treatment costs, two studies evaluating restricting treatment to patients with advanced liver disease concluded that treating all patients was more cost-effective (13, 14). One of these studies found that it was cost-effective to prioritize those with advanced disease in select patient subgroups (14). Since these analyses, a new wave of interferon-free regimens received approval from the Food and Drug Administration, including sofosbuvir/ledipasvir (SOF/LDV) and a multidrug regimen of ombitasvir, ritonavir, paritaprevir and dasabuvir (3D), with or without ribavirin. Both of these regimens result in nearly universal cure rates with lower costs than sofosbuvir/simeprevir and without the adverse effects or eligibility restrictions of interferon-based regimens. 3D is similarly effective and less expensive per dose than SOF/LDV, but requires multiple daily pills for 12–24 weeks, compared to 8–12 weeks of a single daily dose of SOF/LDV (15–19). In addition, 3D includes ritonavir, which has drug interactions precluding its use in some patients, and may require ribavirin, which may cause dose-limiting anemia (16, 17). Both regimens are more costly than sofosbuvir/ribavirin/interferon, with wholesale prices of up to $1125 per dose. High drug prices have led many healthcare systems to restrict access to novel HCV drug regimens, but there is no evidence that this is based on cost-effectiveness (20).
The Veterans Health Administration (VA) is a leading provider of HCV care in the US and a useful model for evaluating changes in treatment policy. HCV prevalence is two-fold greater in Veterans than the general US population with more than 170,000 HCV positive Veterans currently receiving VA healthcare; over 75% have never received antiviral therapy (21). VA’s unified national electronic medical record system and its national Hepatitis C Clinical Case Registry provide extensive data about the natural history and treatment costs and distinguish VA as an excellent system in which to model changes in treatment policy.
With the advent of interferon-free therapy, optimal treatment for genotype 1 HCV remains unclear. Because of differences in drug price, treatment duration, efficacy, and quality of life associated with SOF/LDV and 3D, it remains unclear which drug regimen is most cost-effective. Because newer regimens are so costly, it is important to determine how they compare to previously used sofosbuvir regimens and to assess whether alternative strategies, such as prioritizing patients with advanced disease, may now be cost-effective. Thus, we compared the cost-effectiveness of managing a cohort of patients with treatment-naïve genotype 1 HCV using SOF/LDV versus 3D, and sought to determine whether certain patients should be prioritized for treatment.
METHODS
Model Structure and Perspective
We created a Markov state-transition model with one-year cycle length to evaluate treatment strategies in a cohort of previously untreated, 60-year-old US Veterans with genotype 1 HCV mono-infection. The cohort did not include patients with decompensated cirrhosis or HIV co-infection at baseline. We used a lifetime time horizon and took a VA perspective, including drug and medical costs. We conducted sensitivity analyses including relative prices (i.e., differences in cost between regimens) for each treatment regimen to make our results generalizable to systems with alternative price structures. Future costs and utilities were discounted 3% per year. Costs were adjusted to 2014 US dollars using the Consumer Price Index.
Model Cohort
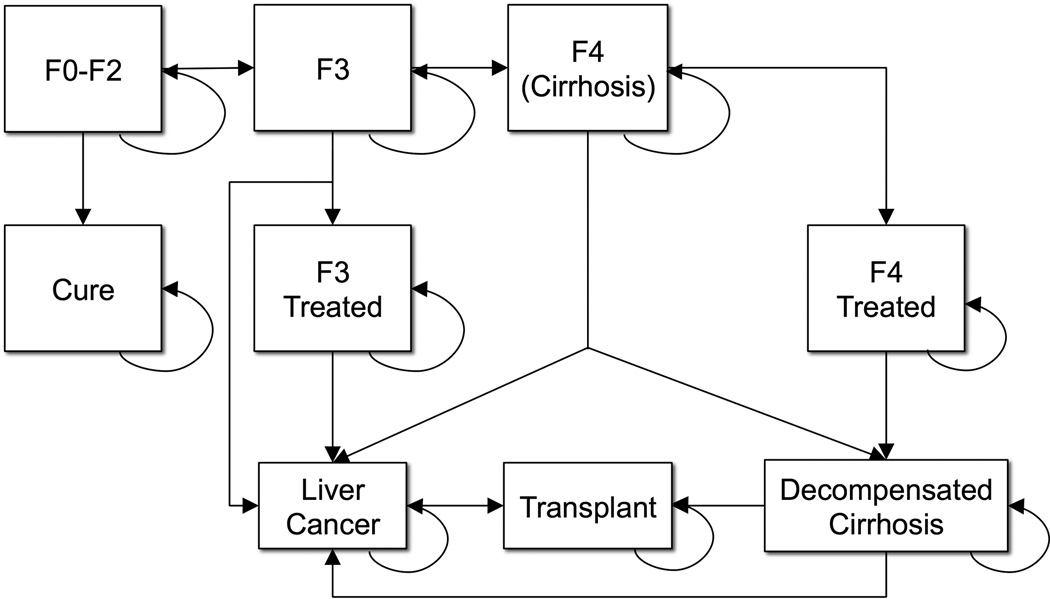
We examined a hypothetical cohort of untreated patients with HCV seeking treatment in VA in a given year, with an average age and distribution of fibrosis similar to this VA patient population in 2013 (Table 1). We defined chronic HCV severity using the Meta-analysis of Histologic Data in Viral Hepatitis (METAVIR) histologic scoring system: F0, no hepatic fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, many septa without cirrhosis; F4, cirrhosis. After treatment, patients could experience sustained virologic response, remain infected and progress through stages of fibrosis, develop cirrhosis or hepatocellular carcinoma, undergo liver transplantation, or die (Figure 1). Age-specific, annual all-cause mortality was estimated using CDC 2009 US life tables. Excess mortality associated with HCV infection was estimated using METAVIR stage and treatment status. For Veterans with F0–F2 fibrosis, we assumed that after sustained virologic response, annual treatment costs, quality-adjusted life years (QALYs), morbidity and mortality would be similar to uninfected Veterans. For those with F3 or F4 disease, we assumed that morbidity and mortality were significantly reduced after sustained virologic response (Table 1). Each year, patients accrued the costs and QALYs associated with their current Markov state. Only one state transition was possible during each model cycle, and progression occurred according to previously established transition probabilities (Table 1).
Table 1.
Hepatitis C Cohort Characteristics, Natural History, Costs, and Utilities
| Description | Base Case | Low | High | Distribution | Source |
|---|---|---|---|---|---|
| Cohort Characteristics | |||||
| Age (years) | 60 | 50 | 70 | Gamma | VA CCR |
| F0–2 (%) | 0.76 | 0.56 | 0.85 | Dirichlet | (34, 40) |
| F3 (%) | 0.12 | 0.11 | 0.44 | Dirichlet | (34, 40) |
| Interferon-Ineligible (%) | 0.37 | 0.20 | 0.57 | Beta | (41) |
| Genotype 1a (%) | 0.65 | 0.50 | 0.75 | Beta | (42, 43) |
| <6 million HCV RNA | 0.59 | 0 | 0.99 | Beta | (19) |
| Risk of Disease Progression (%) | |||||
| F0–2 to F3 | 0.12 | 0.11 | 0.13 | Beta | (44) |
| F3 to F4 | 0.12 | 0.09 | 0.14 | Beta | (44) |
| F3 to HCC | 0.01 | 0 | 0.03 | Beta | (45) |
| F4 to DC | 0.04 | 0.01 | 0.04 | Beta | (45, 46) |
| F4 to HCC | 0.03 | 0.01 | 0.08 | Beta | (45, 47) |
| DC to HCC | 0.07 | 0.03 | 0.08 | Beta | (48) |
| DC to Transplant | 0.03 | 0.02 | 0.06 | Beta | (40, 49) |
| HCC to Transplant | 0.04 | 0 | 0.14 | Beta | (50, 51) |
| Progression After SVR (%) | |||||
| F3 to HCC | 0.007 | 0.006 | 0.008 | Beta | (5) |
| F4 to DC | 0.005 | 0.002 | 0.096 | Beta | (25) |
| F4 to HCC | 0.005 | 0 | 0.019 | Beta | (13, 33, 53) |
| Mortality Rates | |||||
| Hepatitis C (RR)* | 2.37 | 1.28 | 4.38 | Lognormal | (52) |
| Cirrhosis (RR)† | 2.50 | 1.23 | 5.08 | Lognormal | (6) |
| SVR after F0–3 (RR)* | 1.00 | ||||
| SVR after F4 (RR)‡ | 0.39 | 0.14 | 0.65 | Lognormal | (5–7) |
| DC (%) | 0.10 | 0.04 | 0.21 | Beta | (48) |
| HCC (%) | 0.43 | 0.34 | 0.51 | Beta | (46) |
| Transplant Year 1 (%) | 0.14 | 0.06 | 0.42 | Beta | (53, 54) |
| Transplant Year 2+ (%) | 0.03 | 0.02 | 0.11 | Beta | (54) |
| Annual Follow-Up Costs (2014 $US) | |||||
| F0–3 | $190 | $90 | $555 | Gamma | (25) |
| F4 | $1,264 | $740 | $1,789 | Gamma | (25) |
| DC | $16,214 | $12,971 | $40,076 | Gamma | (25) |
| HCC Treatment | $50,754 | $26,124 | $75,384 | Gamma | (25) |
| Transplant Year 1 | $310,023 | $248,019 | $372,028 | Gamma | (25) |
| Transplant Year 2+ | $46,985 | $37,588 | $56,382 | Gamma | (25) |
| SVR (F0–2) | $0 | ||||
| Utilities before SVR | |||||
| F0–2 | 0.85 | 0.83 | 0.87 | Beta | (53, 55) |
| F3 | 0.79 | 0.77 | 0.81 | Beta | (53, 55) |
| F4 | 0.76 | 0.67 | 0.79 | Beta | (53, 55) |
| DC | 0.69 | 0.44 | 0.69 | Beta | (25) |
| HCC | 0.67 | 0.6 | 0.72 | Beta | (25) |
| Transplant Year 1 | 0.5 | 0.3 | 0.8 | Beta | (25) |
| Transplant Year 2+ | 0.77 | 0.57 | 0.77 | Beta | (25) |
| Utilities After SVR | |||||
| F0–2 | 0.92 | 0.9 | 0.94 | Beta | (25) |
| F3 | 0.86 | 0.84 | 0.88 | Beta | (25) |
| F4 | 0.83 | 0.81 | 0.85 | Beta | (25) |
Note: DC - decompensated cirrhosis, F0–2, F3, F4 - METAVIR stages of hepatic fibrosis, G1a - genotype 1a, HCC - hepatocellular carcinoma, RR - relative risk, SVR - sustained virologic response, VA CCR – VA Clinical Case Registry 2013
- compared to all-cause mortality,
- compared to F0–2,
- compared to pre-treatment state.
Figure 1. Markov State-Transition Model Simulating the Natural History of Hepatitis C.
Note: Transition probabilities derived from recent population-based studies. F0–2, F3 and F4 represent METAVIR stages of hepatic fibrosis. F3 and F4 treated states involve reduced risks of liver-related morbidity and mortality compared to untreated states.
Model Assumptions
To model the natural history of HCV, we made a number of assumptions. The METAVIR score has been used more widely in the literature than the FIB-4 scoring system used in VA. Because FIB-4 scores of 3.25 or above correlate with biopsy results demonstrating advanced liver disease, we estimated that 50% of patients with FIB-4 scores above 3.25 had METAVIR F3 disease, while the others had METAVIR F4 disease (22). We also assumed that liver transplantation would not occur after age 75 (23). Finally, we assumed no additional costs for HCV sub-genotyping because this is routinely performed for patients with HCV in VA.
Costs and Effectiveness
We included SOF/LDV and 3D drug regimens recommended as first-line therapy by the American Association for the Study of Liver Diseases/Infectious Disease Society of America in August 2015 (Supplementary Table 1). We obtained VA drug costs from VA Pharmacy Benefits Management and varied them by ±25% in sensitivity analyses. Because VA prices for SOF/LDV and 3D are not publicly available, we used estimated VA prices that have been reported in the public domain (24). We varied the absolute and relative prices of these regimens over the full range of possibilities in sensitivity analyses. We also estimated medical monitoring costs based on estimates from recent literature reviews based on data from Medicare and managed care systems (13, 25). Costs included a pre-treatment office visit, complete blood count, complete metabolic panel, and viral load measurement; monthly office visits and metabolic panels during treatment; quarterly on-treatment viral load measurements; and a post-treatment office visit, viral load measurement, and metabolic panel.
Treatment regimen efficacy was obtained from recent clinical trials (Table 2). To account for potential differences between trial efficacy and real-world effectiveness, we varied each regimen’s efficacy by ±25% in sensitivity analyses. Because there is some evidence that patients with low pre-treatment viral loads may only require 8 weeks of treatment with SOF/LDV, we included an 8-week regimen in sensitivity analysis using the proportion of eligible patients found in the ION-3 study (19). In addition, because some clinicians are using 12 weeks of 3D/RBV for genotype 1a cirrhotic patients, we included this regimen in sensitivity analyses. The utility of each treatment regimen was estimated based on patient reports of treatment-related quality-of-life from sofosbuvir clinical trials (26–28). In the base case, we assumed that the utility of using SOF/LDV or 3D was similar to sofosbuvir/simeprevir, and the utility of using 3D/ribavirin was similar to sofosbuvir/ribavirin.
Table 2.
Hepatitis C Treatment Parameters
| Parameters | Base Case | Low | High | Distribution | Source |
|---|---|---|---|---|---|
| Treatment Efficacy | |||||
| Sofosbuvir/Interferon/RBV | 0.92 | 0.69 | 1.00 | Beta | (11, 56) |
| Sofosbuvir/Interferon/RBV (F4) | 0.80 | 0.60 | 1.00 | Beta | (11) |
| Sofosbuvir/Simeprevir | 0.95 | 0.71 | 1.00 | Beta | (12) |
| Sofosbuvir/Simeprevir (F4) | 0.94 | 0.71 | 1.00 | Beta | (12) |
| SOF/LDV (8 weeks) | 0.94 | 0.71 | 1.00 | Beta | (19) |
| SOF/LDV (12 weeks) | 0.96 | 0.72 | 1.00 | Beta | (18, 19) |
| SOF/LDV (F4) | 0.97 | 0.73 | 1.00 | Beta | (18) |
| 3D/RBV Genotype 1a | 0.96 | 0.72 | 1.00 | Beta | (15, 16) |
| 3D/RBV Genotype 1a (F4) | 0.94 | 0.71 | 1.00 | Beta | (17) |
| 3D Genotype 1b | 0.99 | 0.74 | 1.00 | Beta | (16) |
| 3D Genotype 1b (F4) | 0.99 | 0.74 | 1.00 | Beta | (16, 57) |
| Treatment Disutilities | |||||
| Sofosbuvir/RBV | −0.04 | −0.05 | −0.03 | Beta | (26, 27) |
| Sofosbuvir/Interferon/RBV | −0.11 | −0.12 | −0.09 | Beta | (26, 27) |
| Sofosbuvir/Simeprevir | 0 | −0.04 | 0 | Beta | (53) |
| Drug Costs (weekly, ±25%) | |||||
| Interferon | $178 | $134 | $223 | Gamma | VA PBM |
| Ribavirin | $42 | $32 | $53 | Gamma | VA PBM |
| Simeprevir | $2,641 | $1,981 | $3,301 | Gamma | VA PBM |
| Sofosbuvir | $3,796 | $2,847 | $4,745 | Gamma | VA PBM |
| SOF/LDV | $3,440 | $500 | $7,875 | Gamma | (24) |
| 3D | $2,094 | $500 | $6,933 | Gamma | (24) |
| Medical Monitoring Costs (each, ±25%) | |||||
| Office visits | $76.19 | $57.14 | $95.24 | Gamma | (13, 25) |
| Complete blood count | $10.32 | $7.74 | $12.90 | Gamma | (13, 25) |
| Complete metabolic panel | $15.27 | $11.45 | $19.09 | Gamma | (13, 25) |
| Quantitative HCV PCR | $61.89 | $46.42 | $77.36 | Gamma | (13, 25) |
Note: HCV – hepatitis C, 3D – ombitasvir, ritonavir, paritaprevir, dasabuvir ± ribavirin, PCR – polymerase chain reaction test, SOF/LDV – sofosbuvir/ledipasvir, VA PBM - VA Pharmacy Benefits Management, RBV – ribavirin
Treatment Strategies
We compared seven HCV treatment strategies for both SOF/LDV and 3D. Five compared using SOF/LDV or 3D to treat: (1) any patient seeking treatment, (2) only patients with cirrhosis, (3) only patients with F3–F4 disease, (4) patients with cirrhosis first and then patients with F0–3 disease the following year, or (5) patients with F3–4 disease in the first year, and those with F0–2 disease one year later. In addition to a no treatment strategy, we also included the previous recommendation of the American Association for the Study of Liver Diseases to use sofosbuvir/interferon/ribavirin for all eligible patients and sofosbuvir/simeprevir for interferon-ineligible patients. Treating only F0–2 patients was considered ethically unjustifiable and was not included in our analyses.
Analyses
In our base case analysis, we calculated the incremental cost-effectiveness ratio (ICER), the additional cost required to derive additional QALYs for a given treatment strategy compared to a less costly and less effective strategy. Strategies that were more costly and less effective or had higher ICERs than more effective strategies were considered dominated (29). Though the VA does not use cost-effectiveness thresholds to make treatment decisions, thresholds of $50,000 or $100,000 per QALY are often considered reasonable in contemporary cost-effectiveness studies (30). After determining the preferred strategy, we compared the overall budget and public health impact of treating any patient vs. staged treatment for a hypothetical cohort of 100,000 Veterans with genotype 1 hepatitis C. We conducted one-way sensitivity analyses to determine whether varying any single model input changed the preferred strategy and included estimates for the general population in all ranges. Finally, we conducted Monte Carlo probabilistic sensitivity analyses in which all model inputs were simultaneously varied. Values were sampled from each variable’s probability distribution over 5,000 iterations to determine the likelihood that a given strategy would be cost-effective (31). Distributions were chosen based on parameter characteristics: beta distributions were used for transition probabilities, treatment efficacy, annual mortality rates, utilities, and cohort characteristics; gamma distributions were used for model costs; Dirichlet distributions were used for fibrosis staging; and log-normal distributions were used for relative risks of mortality (Tables 1 & 2). Analyses were performed using TreeAge Pro 2014 (TreeAge Software, Inc., Williamstown, MA).
RESULTS
Validation
We validated the model using the no treatment strategy ($38,246, 9.0 QALYs). Our results are similar to those in recent cost-effectiveness analyses (32, 33). To further validate the model, we created survival and state probability curves for the no treatment strategy, which were compared to recent estimates of the changing natural history of HCV (34). Our estimates of the magnitude and timing of the peak annual prevalence for decompensated cirrhosis, hepatocellular carcinoma, and overall survival were similar to reported values (±15% relative to previous estimates).
Base Case Analysis
In the base case, we found that treating any patient with 3D was the least costly and most effective strategy ($35,560, 14.0 QALYs) and was cost-saving compared to no treatment ($38,246, 9.0 QALYs). Four additional strategies were less costly and more effective than no treatment: staged treatment of F3/F4 disease first with 3D, staged treatment of F4 disease first with 3D, treatment of only F3/F4 disease with 3D, and treatment of only F4 disease with SOF/LDV (Table 3). Treating any patient with SOF/LDV cost an additional $13,759 and yielded 0.01 fewer QALYs ($49,319, 13.9) compared to treating any patient with 3D. All other strategies, including the previous standard of care, sofosbuvir with interferon and ribavirin or sofosbuvir with simeprevir, were dominated (Table 3). Based on these results, treating any patient with 3D would lead to $67.6 million in lifetime cost-savings and 29,000 additional QALYs compared to treating patients with F3/F4 disease first, for a cohort of 100,000 Veterans with genotype 1 hepatitis C.
Table 3.
Cost-Effectiveness of Treating Hepatitis C Among US Veterans: Base Case Results
| Strategy | Costs | QALYs | ICER ($/QALY) |
|---|---|---|---|
| Treat Any: 3D | $35,560 | 14.0 | -- |
| Staged F3/F4 First: 3D | $36,235 | 13.7 | Dominated |
| Staged F4 First: 3D | $36,458 | 13.6 | Dominated |
| Treat when F3/4: 3D | $37,345 | 12.3 | Dominated |
| Treat when F4: SOF/LDV | $37,845 | 10.5 | Dominated |
| Treat None | $38,246 | 9.0 | Dominated |
| Treat when F4: 3D | $38,596 | 10.5 | Dominated |
| Treat when F3/4: SOF/LDV | $47,092 | 12.3 | Dominated |
| Treat Any: SOF/LDV | $49,319 | 13.9 | Dominated |
| Staged F4 First: SOF/LDV | $49,327 | 13.6 | Dominated |
| Staged F3/F4 First: SOF/LDV | $49,430 | 13.7 | Dominated |
| Treat Any: Previous SOC | $68,433 | 13.7 | Dominated |
Note: F3, F4 – METAVIR stages of fibrosis, ICER – incremental cost-effectiveness ratio, 3D – ombitasvir/ritonavir/paritaprevir/dasabuvir, Previous SOC – sofosbuvir with pegylated interferon/ribavirin or simeprevir, QALY – quality adjusted life-year, SOF/LDV – sofosbuvir/ledipasvir
One-Way Sensitivity Analyses
In one-way sensitivity analysis, cost-effectiveness ratios were impacted by changes in several key variables, including the proportion of patients with early-stage disease and the efficacy and relative costs of each drug regimen (Table 4). Treating any with SOF/LDV was cost-effective at a $50,000 per QALY threshold if SOF/LDV cost <$2,250 per week, 3D cost >$3,195 per week, or if 3D was <86% effective for patients with genotype 1a disease or <80% effective for patients with genotype 1b disease. Treating any with SOF/LDV was cost-effective at a $100,000 per QALY threshold if SOF/LDV cost <$2,220 per week, 3D cost >$3,220 per week, 3D was <91% effective for genotype 1a or <89% effective for genotype 1b, or if SOF/LDV was 100% effective for non-cirrhotic patients. The ICER was robust to variations in all other model parameters, including cohort age, as well as alternative regimens such as 8 weeks of SOF/LDV for early-stage patients or 12 weeks of 3D for genotype 1a cirrhotic patients.
Table 4.
Scenarios in Which SOF/LDV is Cost-Effective Compared to 3D for Treating Veterans with Hepatitis C*
| $50,000 per QALY | $100,000 per QALY |
|---|---|
| Cost of SOF/LDV <$2,250/week | Cost of SOF/LDV <$2,220/week |
| Cost of 3D >$3,195/week | Cost of 3D >$3,220/week |
| 3D <86% effective for genotype 1a (F0–F3) | 3D <91% effective for genotype 1a (F0–F3) |
| 3D <80% effective for genotype 1b (F0–F3) | 3D <89% effective for genotype 1b |
| SOF/LDV 100% effective (F0–F3) |
Note: Calculated using one-way sensitivity analyses comparing drug regimens for the "Treat Any" strategy. 3D: ombitasvir, ritonavir, paritaprevir, dasabuvir ± ribavirin, F0–F3: METAVIR Stages of Hepatic Fibrosis, SOF/LDV: sofosbuvir/ledipasvir
Probabilistic Sensitivity Analysis
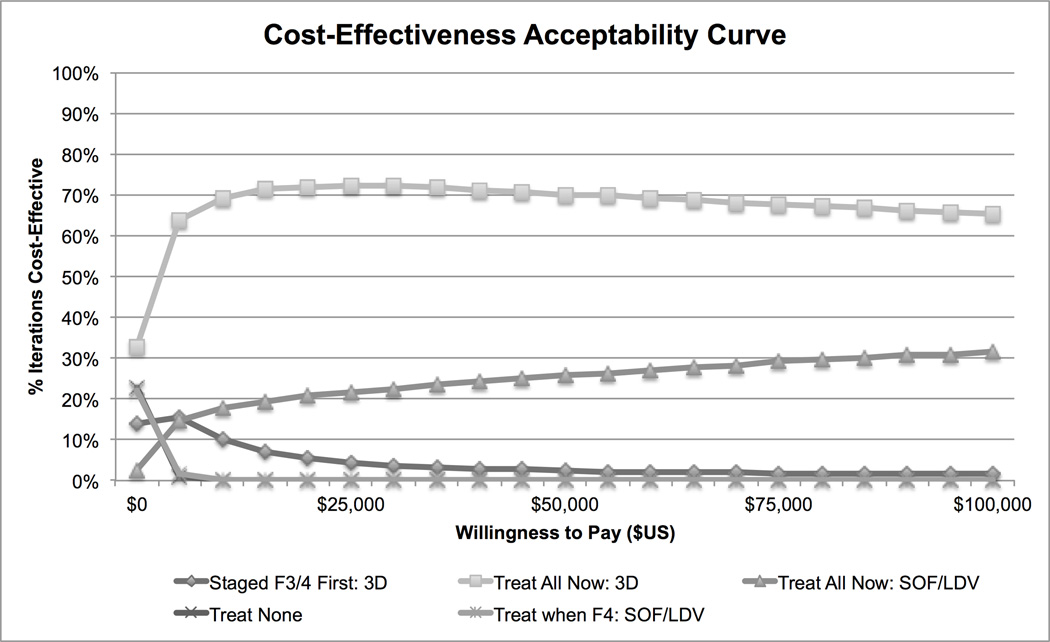
In probabilistic sensitivity analysis, treating any patient was preferred in the majority of iterations. At a willingness-to-pay threshold of $50,000/QALY, treating any with 3D was preferred in 70% of iterations, while treating any with SOF/LDV was preferred in 20% of iterations (Figure 2). At a $100,000/QALY threshold, treating any was cost-effective in 65% of iterations with 3D and 28% of iterations with SOF/LDV.
Figure 2. Probabilistic Sensitivity Analysis of Treatment Strategies for Treatment-Naïve Genotype 1 Hepatitis C Cost-Effective in >5% of Iterations.
Note: F3 and F4 – METAVIR stages of hepatic fibrosis, 3D – ombitasvir, ritonavir, paritaprevir, dasabuvir ribavirin, SOF/LDV – sofosbuvir/ledipasvir. Strategies that were cost-effective in <5% of iterations are not depicted. These strategies included: treating all with the previous standard of care, treating when F4 with 3D, treating when F3/F4 with 3D or SOF/LDV, staged treatment of F3/F4 first with SOF/LDV, and staged treatment of F4 first with 3D or SOF/LDV.
DISCUSSION
In this cost-effectiveness analysis, we found that, for a cohort of treatment-naïve genotype 1 HCV-infected Veterans, managing patients with 3D regardless of disease status was the most economically efficient strategy. We found it economically unfavorable to treat patients with SOF/LDV, restrict treatment to patients with METAVIR F3–F4 disease or use a staged treatment strategy. The cost-effectiveness of 3D depended on the efficacy and price of each drug regimen and the proportion of patients with early-stage disease.
We demonstrated that regimens using 3D were less costly and more effective than those based on SOF/LDV. Varying the price and efficacy of each drug regimen could change the preferred strategy. While the scenarios that change the preferred strategy are unlikely based on estimated VA prices and data from recent clinical trials, large variations between trial efficacy and real-world effectiveness could impact the cost-effectiveness of each drug regimen. However, when we took this uncertainty into account in probabilistic sensitivity analyses, we found that treating any patient with 3D was still preferred up to 70% of the time. Ultimately, real-world effectiveness data, and future price negotiations will determine the most cost-effective strategy.
In addition, we found that restricting treatment to patients with advanced disease or treating patients with advanced disease first was not cost-effective at $50,000 or $100,000/QALY. This was because these strategies had higher ICERs than treating any eligible patient and were eliminated from further consideration based on current guidelines (29). Under these strategies, VA would incur lower initial and annual treatment costs by restricting treatment eligibility. However, because patients with advanced disease have a higher risk of morbidity and mortality even after successful treatment, patients would still incur years of post-treatment follow-up costs that ultimately exceed savings in treatment costs. Furthermore, requiring patients with early-stage disease to progress before treatment increases morbidity and mortality, reducing the number of QALYs associated with these strategies. These findings are similar to previous studies, in which staging-guided therapy was not favorable compared to treating all patients (13). In one study, staging-guided therapy was cost-effective for patients with cirrhosis, but only when compared to waiting one year for treatment with future regimens (14). These results suggest that treating healthier patients is more cost-effective than treating sicker ones. However, strategies favoring treatment of healthier patients are clinically and ethically unfavorable; treating sicker patients first is ethically ideal. In practice, it is unlikely that all cirrhotic patients can be quickly identified and prepared for treatment, so there may be opportunities for healthy patients to be treated as well. Thus it may be preferable to implement a triage policy similar to that employed in emergency rooms, in which efforts are made to identify and treat the sickest patients, but healthier patients are also treated whenever possible.
We also found that interferon-free treatment regimens were preferred to the previous standard of care. This is likely because new interferon-free regimens are more efficacious, are associated with improved quality of life compared to interferon-containing regimens, and are less costly than sofosbuvir/simeprevir. Our findings are consistent with prior studies, in which interferon-free regimens were less costly and more effective than previous therapeutic options. For example, in one study, the authors identified price thresholds under which SOF/LDV could be the least costly, most effective strategy, however they did not include the 3D regimen in their analysis (35). Another study found that interferon-free regimens dominated earlier sofosbuvir-based treatment options for the general population, even at wholesale prices (36).
While we demonstrate that treating any patient is cost-effective compared to more restrictive treatment strategies, practical limitations influence the clinical application of these findings. Whereas VA policy supports HCV treatment in all patient populations, clinical capacity and financial limitations dictate that it will take several years to treat the hundreds of thousands of patients with HCV managed in VA. Even without clinical capacity constraints, treating only 70,000 untreated VA HCV patients at a discounted price of $50,000 per treatment course would require $3.5 billion in pharmacy costs for HCV alone. By comparison, in 2014, HCV treatment accounted for $520 million of the $4.8 billion in total pharmacy purchasing through the VA Pharmaceutical Prime Vendor (Vincent Calabrese, VA Pharmacy Benefits Management, Hines, IL, written communication, 2/10/15). Due to limited resources, clinicians will ultimately determine when to treat individual patients.
Our results have important policy implications for the VA and may be more broadly applicable to state Medicaid and national Medicare systems, which assume both the costs and benefits of treatment. In practice, resource constraints limit treatment capabilities. In fact, in 2015, VA allocated $697 million for hepatitis C treatment in 2015, but projects that the true cost will actually exceed $1.1 billion, resulting in a $400 million budgetary shortfall (37). To address this issue, Congress recently passed legislation granting VA access to an additional $500 million in funds from the Veterans Choice program, which allows eligible Veterans to receive treatment from non-VA providers (38). Our analyses suggest that such efforts to improve treatment capacity in the short-term could ultimately lead to substantial long-term improvements in health outcomes and reduced costs for patients with HCV. To improve throughput, VA is also considering a number of potential strategies, including using primary care and telehealth providers to manage uncomplicated cases. Similar strategies could be employed by other healthcare systems to improve the public health impact of HCV treatment.
Our study has some limitations. First, instead of modeling fibrosis regression, we used stage-specific progression rates to account for slower disease progression after sustained virologic response. Second, we did not stratify our analyses by gender or race/ethnicity because neither parameter has been demonstrated to impact sustained virologic response in recent trials. Third, our analyses do not consider aggregate cost, clinic availability or differing models of care. Fourth, our analyses are conducted from the VA perspective, including VA-specific drug pricing. To improve the generalizability of our results, we included general population data in ranges used for sensitivity analyses. We also demonstrated the drug prices & efficacy necessary for cost-effectiveness at each cost-effectiveness threshold, making our results relevant to systems with other price structures. Fifth, we used non-VA data to estimate the costs of on-treatment medical monitoring. While this is a limitation for our base case analyses, it may improve the generalizability of our results. Finally, prescribing patterns may be influenced by emerging post-marketing surveillance data, such as the FDA’s recently expanded warning about the use of 3D in Child-Pugh B & C cirrhosis (39).
In conclusion, we determined that it is economically efficient to manage treatment naïve US Veterans with genotype 1 HCV using novel interferon-free regimens. Still, we demonstrate that treatment efficacy is an important aspect of cost-effectiveness. In addition to monitoring the real world effectiveness of both drugs, it will become important to identify predictors of adherence, sustained virologic response, and reinfection after successful treatment. Although indications and options for interferon-free medications will continue to evolve, we anticipate that these changes will only increase the cost-effectiveness of treatment over time. Interferon-free regimens for genotype 1 HCV can confer long-term health benefits for US Veterans and are cost-effective regardless of fibrosis status.
Supplementary Material
ACKNOWLEDGMENTS
AC was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR000145. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the VA Healthcare Systems.
SR and VR are conducting unrelated research projects funded by Gilead Sciences.
List of Abbreviations
- HCV
Hepatitis C
- SOF/LDV
sofosbuvir/ledipasvir
- 3D
ombitasvir, ritonavir, paritaprevir, dasabuvir
- VA
Veterans Health Administration
- METAVIR
Meta-analysis of Histologic Data in Viral Hepatitis
- QALY
Quality-adjusted life years
- ICER
Incremental Cost-Effectiveness Ratio
Footnotes
Potential conflict(s) of interest: All additional authors report no conflicts of interest.
Contributor Information
Alexis P. Chidi, Email: apc10@pitt.edu.
Shari Rogal, Email: rogalss@upmc.edu.
Cindy L. Bryce, Email: bryce99@pitt.edu.
Michael J. Fine, Email: Michael.fine@va.gov.
Chester B. Good, Email: chester.good@va.gov.
Larissa Myaskovsky, Email: larissa.myaskosvksy@va.gov.
Vinod K. Rustgi, Email: rustgivk@upmc.edu.
Allan Tsung, Email: tsunga@upmc.edu.
Kenneth J. Smith, Email: smitkj2@upmc.edu.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic Steatohepatitis is the Second Leading Etiology of Liver Disease Among Adults Awaiting Liver Transplantation in the U.S. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 5.Butt AA, Wang X, Moore CG. Effect of hepatitis C virus and its treatment on survival. Hepatology. 2009;50:387–392. doi: 10.1002/hep.23000. [DOI] [PubMed] [Google Scholar]
- 6.Dieperink E, Pocha C, Thuras P, Knott A, Colton S, Ho SB. All-cause mortality and liver-related outcomes following successful antiviral treatment for chronic hepatitis C. Dig Dis Sci. 2014;59:872–880. doi: 10.1007/s10620-014-3050-5. [DOI] [PubMed] [Google Scholar]
- 7.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. Jama. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 8.Cawthorne CH, Rudat KR, Burton MS, Brown KE, Luxon BA, Janney CG, Fimmel CJ. Limited success of HCV antiviral therapy in United States veterans. Am J Gastroenterol. 2002;97:149–155. doi: 10.1111/j.1572-0241.2002.05439.x. [DOI] [PubMed] [Google Scholar]
- 9.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 10.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 12.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014 doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 13.Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60:530–537. doi: 10.1016/j.jhep.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Deuffic-Burban S, Schwarzinger M, Obach D, Mallet V, Pol S, Pageaux GP, Canva V, et al. Should we await IFN-free regimens to treat HCV genotype 1 treatment-naive patients? A cost-effectiveness analysis (ANRS 12188) J Hepatol. 2014 doi: 10.1016/j.jhep.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 16.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 17.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 18.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 19.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 20.Canary LA, Klevens RM, Holmberg SD. Limited Access to New Hepatitis C Virus Treatment Under State Medicaid ProgramsLimited Access to New HCV Treatment. Annals of Internal Medicine. 2015;163:226–228. doi: 10.7326/M15-0320. [DOI] [PubMed] [Google Scholar]
- 21.Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, Maynard C, Sporleder JL, Stenhouse A, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88–96. doi: 10.1002/hep.20502. [DOI] [PubMed] [Google Scholar]
- 22.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 23.Kim WR, Stock PG, Smith JM, Heimbach JK, Skeans MA, Edwards EB, Harper AM, et al. OPTN/SRTR 2011 Annual Data Report: liver. Am J Transplant. 2013;13(Suppl 1):73–102. doi: 10.1111/ajt.12021. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Veterans Affairs. Hepatitis C Treatment: Request for Choice Program Flexibility to Address $500 Million Shortfall in FY 2015. 2015 Jul 31; http://www.natap.org/2015/HCV/hepatitis_c_treatment_summary.pdf. [Google Scholar]
- 25.Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54:1259–1271. doi: 10.1093/cid/cis011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, Nelson D, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in Chronic Hepatitis C (CH-C) J Hepatol. 2014;60:741–747. doi: 10.1016/j.jhep.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Younossi ZM, Stepanova M, Nader F, Jacobson IM, Gane E, Nelson D, Lawitz E, et al. Patient-reported outcomes in chronic hepatitis C patients with cirrhosis treated with sofosbuvir-containing regimens. Hepatology. 2014;59:2161–2169. doi: 10.1002/hep.27161. [DOI] [PubMed] [Google Scholar]
- 28.Hanmer J. Predicting an SF-6D preference-based score using MCS and PCS scores from the SF-12 or SF-36. Value Health. 2009;12:958–966. doi: 10.1111/j.1524-4733.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold MR. Cost-effectiveness in health and medicine. xxiii. New York: Oxford University Press; 1996. p. 425. [Google Scholar]
- 30.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 31.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 32.Chan K, Lai MN, Groessl EJ, Hanchate AD, Wong JB, Clark JA, Asch SM, et al. Cost effectiveness of direct-acting antiviral therapy for treatment-naive patients with chronic HCV genotype 1 infection in the veterans health administration. Clin Gastroenterol Hepatol. 2013;11:1503–1510. doi: 10.1016/j.cgh.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat. 2013;20:847–857. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 34.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161:170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Najafzadeh M, Andersson K, Shrank WH, Krumme AA, Matlin OS, Brennan T, Avorn J, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407–419. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Bastian ND, Griffin PM. Cost-effectiveness of sofosbuvir-based treatments for chronic hepatitis C in the US. BMC Gastroenterol. 2015;15:98. doi: 10.1186/s12876-015-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The State of VA’s Fiscal Year 2015 Budget: Statement of the Honorable Sloan Gibson Deputy Secretary of Veterans Affairs. US House of Representatives Committee on Veterans' Affairs, 114th Congress. 2015 [Google Scholar]
- 38.Surface Transportation and Veterans Health Care Choice Improvement Act of 2015. United States of America; 2015. Public Law 114-41. 114th Congress ed. [Google Scholar]
- 39.Food and Drug Administration. FDA Drug Safety Communication: FDA warns of serious liver injury risk with hepatitis C treatments Viekira Pak and Technivie. 2015 [Google Scholar]
- 40.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. 521, e511–e516. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 41.Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–325. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 42.Hwang EW, Thomas IC, Cheung R, Backus LI. Implications of rapid virological response in hepatitis C therapy in the US veteran population. Aliment Pharmacol Ther. 2012;35:105–115. doi: 10.1111/j.1365-2036.2011.04903.x. [DOI] [PubMed] [Google Scholar]
- 43.Thomas LB, Foulis PR, Mastorides SM, Djilan YA, Skinner O, Borkowski AA. Hepatitis C genotype analysis: results in a large veteran population with review of the implications for clinical practice. Ann Clin Lab Sci. 2012;42:355–362. [PubMed] [Google Scholar]
- 44.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 45.Dienstag JL, Ghany MG, Morgan TR, Di Bisceglie AM, Bonkovsky HL, Kim HY, Seeff LB, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396–405. doi: 10.1002/hep.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 47.Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32:344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 48.Planas R, Balleste B, Alvarez MA, Rivera M, Montoliu S, Galeras JA, Santos J, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823–830. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 50.Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50:89–99. doi: 10.1016/j.jhep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Saab S, Hunt DR, Stone MA, McClune A, Tong MJ. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver Transpl. 2010;16:748–759. doi: 10.1002/lt.22072. [DOI] [PubMed] [Google Scholar]
- 52.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–157. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014 doi: 10.1002/hep.27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:961–972. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 55.Thein HH, Krahn M, Kaldor JM, Dore GJ. Estimation of utilities for chronic hepatitis C from SF-36 scores. Am J Gastroenterol. 2005;100:643–651. doi: 10.1111/j.1572-0241.2005.40976.x. [DOI] [PubMed] [Google Scholar]
- 56.Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, Bernstein DE, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100–2107. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 57.Feld JJ, Moreno C, Trinh R, Tam E, Bourgeois S, Horsmans Y, Elkhashab M, et al. Turquoise-III: safety and efficacy of 12-week ribavirin-free treatment for patients with HCV genotype 1b and cirrhosis. Journal of Viral Hepatitis. 2015;22:134–135. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.