Abstract
The strongly immunogenic environment in autoimmune diseases such as lupus may pose a stringent barrier to transplantation. Despite available murine models of lupus, transplant tolerance in this setting has yet to be fully investigated in highly penetrant genetic models of disease. Such studies are of clear clinical importance because lupus is a transplant indication in which transplanted kidneys have a substantially increased risk of rejection including a role for recurrent nephritis. In the fully penetrant B6.SLE123 mouse, we determined that CD4 T follicular helper and germinal center B cells were significantly expanded compared with healthy controls. We traced this expansion to resistance of effector CD4 T and B cells in B6.SLE123 mice to regulation by either CD4 T regulatory cells (CD4Tregs) or CD8 T regulatory cells (CD8Tregs), despite demonstrating normal function by Tregs in this strain. Finally, we determined that B6.SLE123 mice resist anti-CD45RB–mediated tolerance induction to foreign islet allografts, even in the absence of islet autoimmunity. Overall, B6.SLE123 lupus-prone mice are highly resistant to transplant tolerance induction, which provides a new model of failed tolerance in autoimmunity that may elucidate barriers to clinical transplantation in lupus through further cellular and genetic dissection.
Introduction
Systemic lupus erythematous (SLE) is driven by unchecked collaboration between autoreactive T and B lymphocytes. These deleterious immune interactions manifest in the production of tissue-destructive autoantibodies and generalized inflammation, which can result in damage to multiple organs. Immune complex deposition and T cell activation drive glomerular injury to cause lupus nephritis, which, in 2004, accounted for approximately 1.1% of all cases of end-stage renal disease (ESRD) in the United States. Accordingly, of the 17 106 kidney transplants performed in 2014 in the United States, 446 cases were due to lupus-related ESRD, based on Organ Procurement and Transplantation Network data as of April 1, 2015. Yet, despite intense immunosuppression, patients with SLE remain at risk for recurrent lupus nephritis in their kidney grafts, a scenario that places patients at a four-fold higher risk of organ rejection (1). Thus, achieving graft tolerance in the highly immunogenic lupus environment requires overcoming both recurrent autoimmunity against the grafted tissue and alloimmunity propagated by foreign tissue antigens.
Despite a significant body of work defining tolerance induction in mice and additional evidence that autoimmunity poses a substantial barrier to allograft tolerance (2), tolerance induction in the setting of lupus is poorly understood. In the setting of diabetic autoimmunity, treatment with donor-specific transfusion (DST) plus anti-CD40L, anti-CD45RB, or anti–LFA-1 fails to promote long-term graft acceptance in autoimmune nonobese diabetic (NOD) mice (3–6). These mice rapidly reject both islet allografts (which are subject to recurrent autoimmunity and alloimmunity) and cardiac allografts (which are subject only to alloimmunity). It remains unanswered how underlying autoimmunity contributes to graft rejection and to what degree these barriers can be generalized.
In the present study, we examine immune regulation and tolerance induction in a fully penetrant model of lupus, the B6.SLE123 mouse, which harbors the lupus-promoting NZM2410 congenic regions SLE1, SLE2, and SLE3 (7). We determine that these mice, unlike wild-type B6 mice, are resistant to anti-CD45RB-mediated tolerance induction to foreign islet allografts, which we have studied to limit the contribution of recurrent autoimmunity. Mechanistically, effector CD4 T and B cells from this strain resist suppression mediated by both CD4 T regulatory cells (CD4Tregs) and CD8 T regulatory cells (CD8Tregs). We demonstrate that resistance to immune regulation in the autoimmune lupus setting may pose a stringent barrier to transplant tolerance induction and provide a new model for investigation of this clinical challenge.
Materials and Methods
Animals
C3H/HeJ (C3H), C57BL6/J (B6), C57BL6/J.RAG1−/− (B6.RAG), C57BL6/J. SLE1.SLE2.SLE3 (B6.SLE123), and NOD/ShiLtJ (NOD) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). B6.SLE123 mice were developed by Edward K. Wakeland at the University of Texas Southwestern Medical Center in Dallas, TX (7). Mice were housed in a specific-pathogen–free facility at Vanderbilt University. The Institutional Animal Care and Use Committee at Vanderbilt University approved all procedures carried out during this study.
Flow cytometry
Splenocytes were stained with fluorophore-conjugated antibodies purchased from either BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), or Cell Signaling Technologies (Danvers, MA): B220 (RA3-6B2), Bcl-6 (K112-91), CD4 (RM4-5), CD8a (53-6.7), CD25 (7D4), CD95 (Jo2), CD122 (TM-B1), Foxp3 (FJK-16s), IgM (II/41), Ly49C/F/I/H (14B11), PD-1 (J43), pSMAD2/3 (D27F4), and pSMAD1/5/8 (D5B10). Samples were acquired on a BD LSR Fortessa Flow Cytometer and analyzed by using FlowJo software (TreeStar, Ashland, OR).
Alloimmunization and alloantibody titer analysis
Twenty million splenocytes from major histocompatibility (MHC)-mismatched C3H mice (H-2k) were intravenously injected into recipient B6 and B6.SLE123 mice (H-2b). Sera was isolated on day 14 and incubated with target C3H splenocytes at a 1:25 dilution. Splenocytes were stained with antibodies directed at CD3e, immunoglobulin IgM, IgG1 (A85-1), IgG2a (m2a-15F8), IgG2b (m2b-25G4), and IgG3 (SB76b) (Southern Biotech, Birmingham, AL). The median fluorescence intensity (MFI) of bound mouse Ig on target C3H CD3 T cells was used to assess relative alloantibody titer.
Ex vivo CD4Treg suppression assay
CD4+ CD25−effector T cells and CD4+ CD25+ Tregs were isolated from B6 or B6.SLE123 spleens by using a CD4+ CD25+ Treg isolation kit (Miltenyi, San Diego, CA). Effector CD4 T cells and CD4Tregs were resuspended in complete RPMI media (10% fetal calf serum plus 1% penicillin/streptomycin) and plated in 96-well round-bottomed plates at Treg:T effector ratios ranging from 1:2 to 1:8 (8). Cells were stimulated with anti-CD3 (1 µg/mL, 145-2C11; BD) and anti-CD28 (1 µg/mL, 37.51; BD) and incubated for 72 h. For transforming growth factor-β (TGF-β) suppression assays, splenocytes were incubated with anti-CD3/CD28 (1 µg/mL) with or without 10 µg/mL TGF-β (R&D Systems, Minneapolis, MN) for 72 h. Supernatants were collected and analyzed for interferon-γ (IFNγ) by enzyme-linked immunosorbent assay (ELISA) (BD). The percent suppression was calculated as follows: [(IFNγ production in each condition/IFNγ T effector cells only) * 100].
In vivo CD8Treg suppression assay
B6 and B6.SLE123 mice were injected intraperitoneally with 100 µg of nitrophenyl (NP)33–keyhole limpet hemocyanin (KLH) (BioSearch Technologies, Petaluma, CA) emulsified in complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO). Seven days later, splenic CD8+ T cells were isolated by using magnetic activated cell sorting (MACS) (Ly-2, Miltenyi) and then sorted by using fluorescent activated cell sorting (FACS) to select for CD8Treg (CD8+ CD122+ Ly49+) and non-CD8Treg (CD8+ CD122+ Ly49−) populations (BD FACsAria III). FACS-sorted CD8Tregs or non-CD8Tregs from B6 or B6.SLE123 mice were intravenously injected into recipient B6.RAG mice. Reconstituted mice received MACS-purified splenic B cells (2 million) and CD4+ CD25−T cells (1 million) from naïve B6 or B6.SLE123 donors. Mice were injected intraperitoneally with 100 µg of NP33-KLH/CFA. Ten days after initial immunization, mice were boosted with 50 µg of NP33-KLH emulsified in incomplete Freund’s adjuvant (Sigma-Aldrich). Seven days after boosting, the anti-NP8 IgG response was measured via ELISA (NP8-BSA; Biosearch Technologies) (9).
Anti-insulin IgG ELISA
To detect circulating anti-insulin IgG, 96-well MaxiSORP plates (eBio) were coated overnight at 4°C with 1 µg/mL human insulin (Sigma) diluted in borate-buffered saline. Serum was diluted 1:100 in wash buffer. Supernatant from the hybridoma mAb125, which produces anti-insulin IgG (kindly provided by Tom Thomas, Vanderbilt, Nashville, TN), was used as a positive control. Specific binding was verified by competition with 100 µg/mL of free, unbound human insulin (10).
Islet transplantation and anti-CD45RB–mediated tolerance induction
Subcapsular renal islet transplantation was carried out as previously described (11). Briefly, chemically diabetic B6 and B6.SLE123 mice (H-2b) were transplanted with 400 allogeneic C3H islets (H-2k). Treated mice were injected with 100 µg of anti-CD45RB antibody (BioXCell, West Lebanon, NH) on days 0, 1, 3, 5, and 7 after transplantation. Graft rejection was recorded when recipient mice demonstrated glucose readings above 250 mg/dL on 2 consecutive days.
Statistics
Statistical analysis was performed with GraphPad Prism V5 (La Jolla, CA), using the Student’s t-test for comparison of two normally distributed conditions. One- or two-way analysis of variance followed by Bonferroni post-test was used to compare multiple groups. Analysis of the anti-NP response was analyzed via performing a semilogarithmic linear regression analysis followed by y-intercept and slope curve comparison. Graft rejection was graphed as Kaplan–Meier curve and compared by log-rank statistical analysis. Statistical comparisons with p ≤ 0.05 values were deemed significant.
Results
Lupus-prone B6.SLE123 mice possess expanded splenic CD4 T follicular helper cell and germinal center B cells, fluctuating CD4Treg and CD8Treg populations, and generate an exaggerated alloantibody response
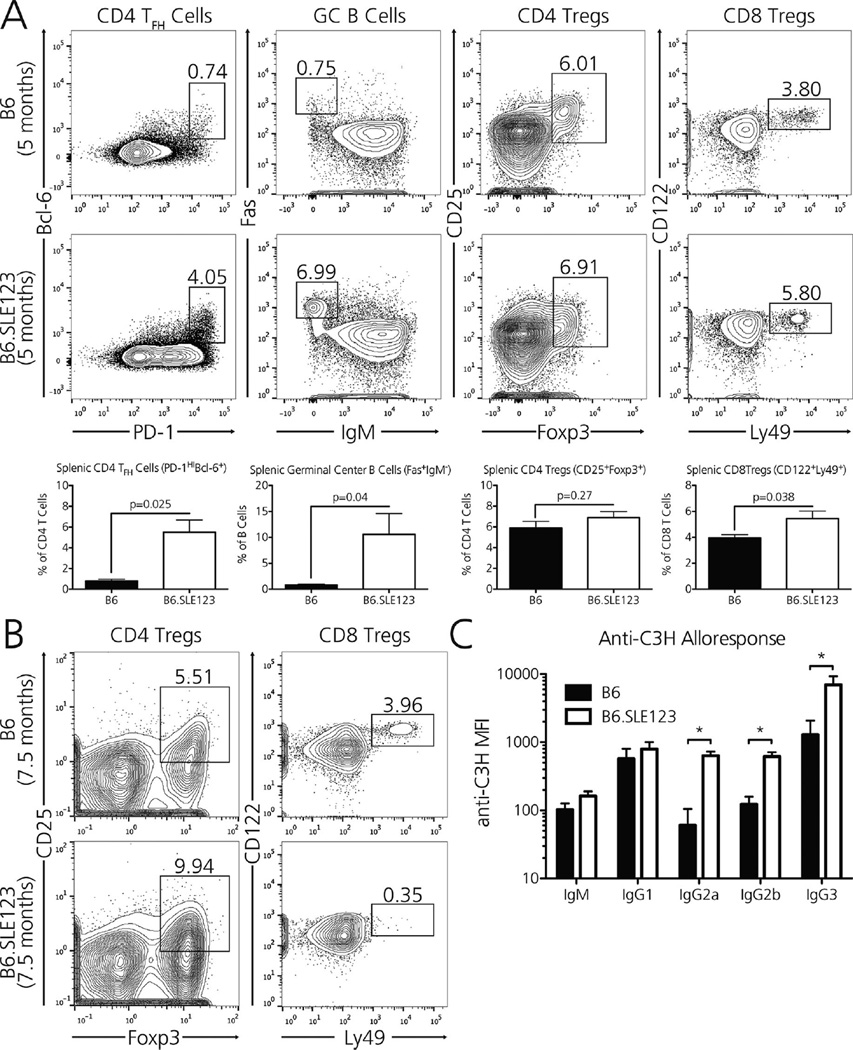
Lupus-prone mouse strains harbor expanded and activated populations of effector CD4 T and B cells, which promote the generation of autoantibodies and the generalized inflammation characteristic of human SLE. T follicular helper (TFH) cells, which promote the activation and expansion of germinal center (GC) B cells, may be prominent in active autoimmunity (12) and are required for SLE progression in Roquinsan/san (sanroque) mice (13). Thus, as expected, 5-month-old B6.SLE123 mice harbored seven times as many splenic PD-1HIBcl-6+ TFH cells and 13 times as many Fas+ IgM− GC B cells as age-matched B6 controls (Figure 1A).
Figure 1. Lupus-prone B6.SLE123 mice possess expanded splenic CD4 TFH and GC B cells, fluctuating CD4Treg and CD8Treg populations, and generate an exaggerated alloantibody response.
(A) Five-month-old B6.SLE123 mice have expanded splenic populations of CD4 TFH cells (CD4+ PD-1HIBcl-6+), GC B cells (B220+ Fas+IgM−), CD4Tregs (CD4+ CD25+ Foxp3+), and CD8Tregs (CD8+ CD122+ Ly49+), as observed in the contour plots. (B) Compared with 7.5-month-old B6 mice (top), similarly aged B6.SLE123 mice (bottom) possess an expanded population of splenic CD4Tregs yet completely lack CD8 Tregs, the latter of which may permit unchecked CD4/B cell immunity characteristic of proliferative glomerulonephritis seen in lupus. (C) B6.SLE123 mice (H-2b) generate an exaggerated IgG2a, IgG2b, and IgG3 alloantibody response 14 days after immunization with MHC-mismatched splenocytes from C3H mice (H-2k). N = 5 mice per strain. Significance was determined by Student t-test, *p < 0.05.
Both CD4Tregs and CD8Tregs attenuate the germinal center reaction before it results in tissue-destructive immunity (14,15). We investigated whether lupus-prone B6.SLE123 possessed altered populations of these regulatory cells. Although B6.SLE123 mice possessed slightly increased percentages of CD4Tregs and CD8Tregs at 5 months (Figure 1A), immune regulation may be severely compromised in these lupus-prone mice as TFH cells outnumber CD4Treg and CD8Treg populations by 0.7:1 and 3.3:1, respectively. In comparison, target TFH cells are in approximately 0.09:1 and 0.3:1 ratios with CD4Tregs and CD8Tregs in the background nonautoimmune B6 strain (p = 0.04 by Mann-Whitney test). We also note that CD4Tregs in B6.SLE123 mice demonstrate decreased levels of CD25 expression (B6.SLE123 vs. B6: 249 ± 5 vs. 401 ± 9, p < 0.001 by Student t-test), a phenotype that has been observed in patients with SLE (16). Finally, although aged B6.SLE123 mice possess significantly increased numbers of CD25+ Foxp3+ CD4Tregs compared with their 7.5-month-old B6 counterparts (B6.SLE123 vs. B6: 9.9 ± 0.73% vs. 5.6 ± 0.29% of CD4 T cells, p < 0.001 by Student t-test), the number of CD122+ Ly49+ CD8 Tregs is decreased to nearly undetectable levels by 7.5 months of age (B6.SLE123 vs. B6: 0.56 ± 0.24% vs. 4.0 ± 0.04% of CD8 T cells, p < 0.005 by Student t-test), a time at which these mice develop progressive glomerulonephritis (Figure 1B).
To determine whether the expanded and potentially unregulated TFH and GC B cells in lupus-prone B6.SLE123 mice resulted in enhanced alloimmunity, we immunized both B6 and B6.SLE123 strains with MHC-mismatched C3H splenocytes (H-2k). Fourteen days after immunization, lupus-prone mice generated significantly higher anti-C3H IgG2a, IgG2b, and IgG3 alloantibody titers than non-autoimmune mice (p < 0.05 by Student t-test within each Ig isotype; Figure 1C).
Effector CD4 T cells from lupus-prone mice resist CD4Treg-mediated suppression of the IFNγ response
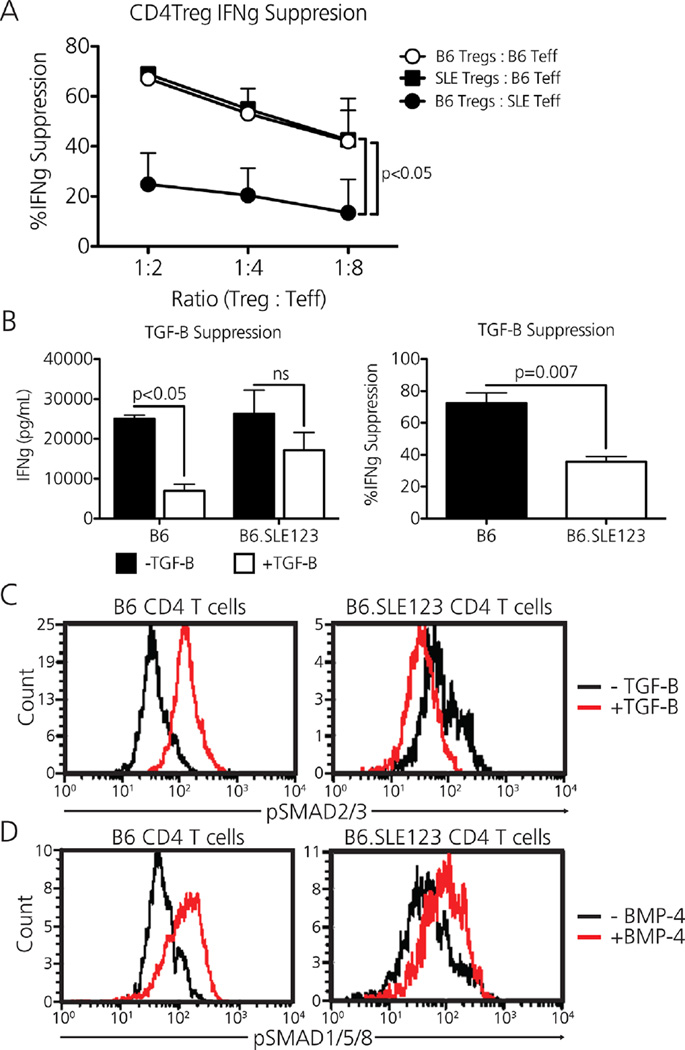
CD4 T effector cells from lupus-prone B6.SLE123 mice resist CD4Treg-mediated suppression of the proliferative response (8). Because IFNγ produced by activated CD4 T helper cells directly augments IgG2b alloantibody production (17) (Figure 1C), we questioned whether CD4 T effector cells from this lupus-prone strain would similarly resist CD4Treg-based suppression of the IFNγ response. Naïve CD4Tregs (CD4+ CD25+) were sorted from B6 and B6.SLE123 mice by MACS and plated with MACS-purified CD4 T effector cells (CD4+ CD25−) from either B6 or B6.SLE123 mice, along with the stimulus anti-CD3 and anti-CD28 in a crossover platform. Although CD4Tregs from B6.SLE123 mice suppressed IFNγ production by CD4 T effector cells from B6 mice to similar levels as the B6 syngeneic system, CD4Tregs from B6 mice failed to suppress IFNγ production by CD4 T effector cells from B6.SLE123 mice (Figure 2A), indicating that B6.SLE123 CD4Tregs were similarly functional despite the slight decrease in CD25 MFI reported earlier.
Figure 2. Effector CD4 T cells from lupus-prone mice resist CD4Treg-mediated suppression of the IFNγ response.
(A) B6.SLE123 effector CD4 T cells resist B6 CD4Treg-mediated suppression of the IFNγ response. (B) Plated B6. SLE123 splenocytes are resistant to TGF-β–mediated suppression of the IFNγ response. (C) B6.SLE123 CD4+ T cells inappropriately decrease SMAD2/3 phosphorylation when exposed to TGF-β; however, these cells appropriately phosphorylate SMAD1/5/8 in response to BMP-4 (D). N = 3–5 mice per strain/condition. Significance was determined by two-way analysis of variance followed by Bonferroni post-test or by Student t-test, where appropriate.
To elucidate why CD4 T effector cells from these lupus-prone mice resisted CD4Treg-based suppression, we explored the response to TGF-β because this cytokine directly dampens the IFNγ response via a CD4Treg-dependent mechanism (18). Whole splenocytes from B6.SLE123 mice activated in the presence of exogenous TGF-β showed resistance to suppression of IFNγ production compared with control (Figure 2B). This finding was supported by reduced pSMAD-2/3 expression (Figure 2C) in B6.SLE123 CD4T cells when exposed to TGF-β. pSMAD-1/5/8 expression in response to BMP-4 (Figure 2D) stimulation was intact in B6.SLE123 CD4 T cells, indicating that the pSMAD defect was specific to the canonical TGF-β signaling pathway (19).
Effector CD4 T and B cells from lupus-prone mice resist CD8Treg-mediated suppression
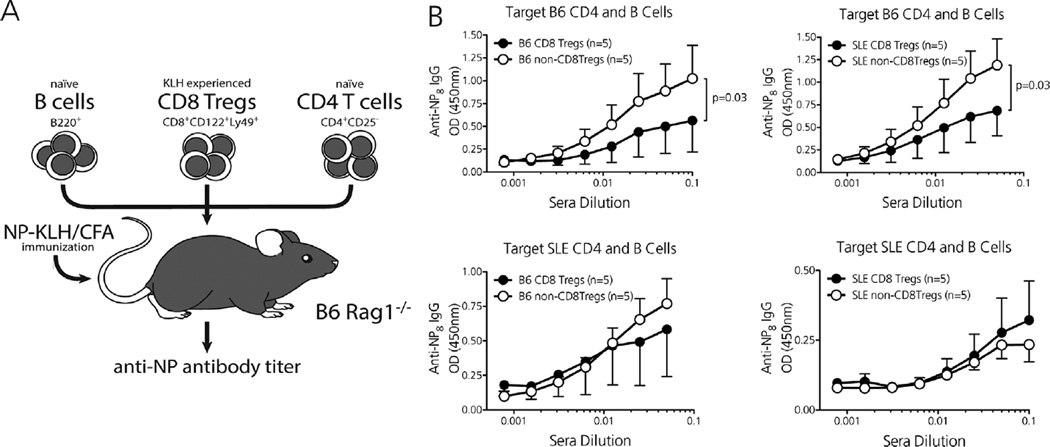
Extending our experimental crossover platform, we determined whether effector CD4 T and B cells from B6.SLE123 mice also resisted CD8Treg-mediated suppression (9). Donor B6 and B6.SLE123 mice were immunized with KLH and CD8Treg (CD8+ CD122+ Ly49+), and non-CD8Treg (CD8+ CD122+ Ly49−) populations were purified via FACS. Immunodeficient B6.RAG mice were then injected with either of these CD8Treg or non-CD8Treg populations, along with MACS-purified CD4+ CD25−T and B220+ B cells from either antigen-naive B6 or B6.SLE123 mice (Figure 3A). Recipient mice were immunized and boosted with the original test stimulus NP-KLH, and the high-affinity anti-NP antibody response was compared between groups by use of ELISA. Whereas CD8Tregs from both B6 and B6.SLE123 mice suppressed the high-affinity antibody response when targeting B6 CD4 T and B cells, target B6.SLE123 CD4 T and B cells resisted suppression when transferred with CD8Tregs from either donor (Figure 3B). Thus, although B6.SLE123 CD8Tregs are able to target effector cells from nonautoimmune mice, target effector cells from autoimmune lupus-prone mice resist CD8Treg-mediated suppression, which could promote an unchecked antibody response.
Figure 3. Effector CD4 T and B cells from lupus-prone mice resist CD8Treg-mediated suppression.
(A) In vivo CD8Treg suppression assay in which KLH-activated CD8Tregs and naïve CD4+ CD25−T cells/whole B220+ B cells are transferred to immunodeficient B6.RAG recipients that are immunized and boosted with the test stimulus NP-KLH. A similar crossover platform was used as described in Figure 2A. (B) CD8Tregs from B6 and B6.SLE123 mice potently suppress the high-affinity anti-NP8 IgG response when targeting nonautoimmune CD4/B cells (top). Effector CD4/B cells from lupus-prone B6.SLE123 mice resist suppression when targeted byCD8Tregs from either B6 or B6. SLE123 mice (bottom). N = 5 mice per experimental group. Significance determined by performing a semilogarithmic linear regressional analysis followed by y-intercept and slope curve comparison.
Lupus-prone mice lack ongoing islet immunity yet resist anti-CD45RB-mediated tolerance induction to islet allografts
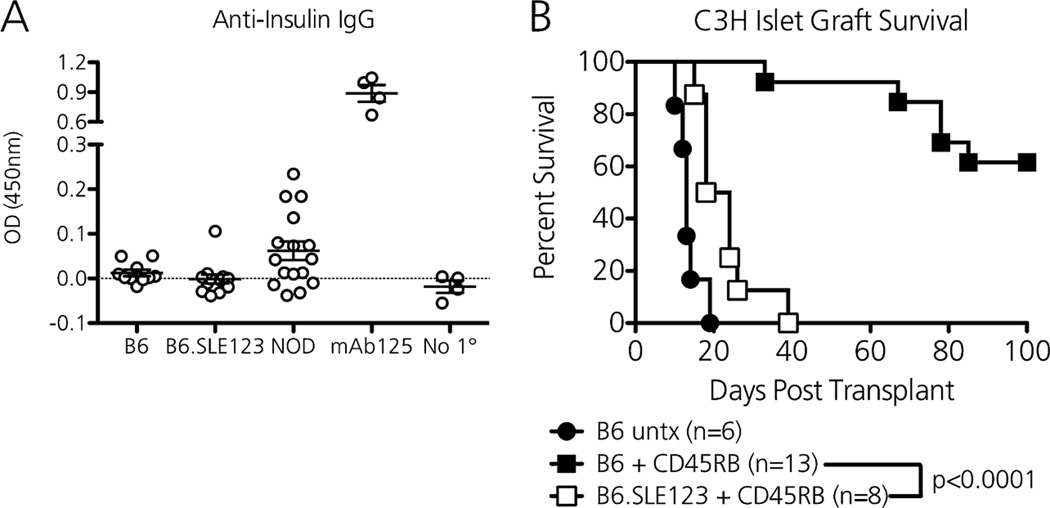
Anti-CD45RB induces permanent tolerance to foreign allografts via activation of antigen-specific, graft-protective regulatory cells (20–22). After a 7-day course of anti-CD45RB, the absolute number of splenic Foxp3+ CD4Tregs expanded 1.74-fold in B6 mice and 1.76-fold in B6.SLE123 mice, and the percentage of proliferating Ki67+ CD8Tregs increased 2.28-fold in B6 mice and 1.48-fold in B6.SLE123 mice, although the absolute numbers of CD8Tregs were unchanged in both. Statistically, there were no significant differences in anti-CD45RB-mediated Treg expansion between treated B6 and B6.SLE123 mice. Despite similar Treg expansion in B6.SLE123 mice, we hypothesized that these lupus-prone mice would resist anti-CD45RB–mediated tolerance induction to foreign allografts because their regulatory cells would not control anti-graft effector CD4 T and B cells. We performed islet allograft transplantation after determining that B6.SLE123 mice lacked preformed anti-insulin IgG that would expose the graft to ongoing, islet-specific immunity (Figure 4A). As a positive control, NOD mice, which resist anti-CD45RB–mediated tolerance induction to islet allografts, possessed circulating anti-insulin IgG. A 7-day course of anti-CD45RB induced long-term tolerance to MHC-mismatched C3H islet allografts (H-2k) when transplanted into chemically diabetic non-autoimmune B6 mice (Figure 4B, median survival time [MST] >100 days vs. MST 13 days in untreated controls). However, when lupus-prone B6.SLE123 mice were transplanted with C3H islets and administered anti-CD45RB, all recipients rejected their islet allografts by 40 days (MST 21 days) (Figure 4B). Thus, even in the absence of underlying organ-specific immunity, the autoimmune setting of lupus is highly resistant to transplant tolerance induction.
Figure 4. Lupus-prone mice lack ongoing islet immunity yet resist anti-CD45RB–mediated tolerance induction to islet allografts.
(A) Unlike type 1 diabetes (T1D)-prone NOD mice (n = 14) that develop islet-specific immunity, B6 (n = 10) and B6.SLE123 (n = 12) mice lack circulating anti-insulin IgG (serum prepared from 8-week-old mice). Supernatant from the hybridoma mAb125 (anti-insulin IgG producing) was used a positive control. No 1° represents plates coated without serum (negative control). (B) Despite lacking islet-specific autoimmunity, lupus-prone B6.SLE123 mice are completely resistant to anti-CD45RB–mediated tolerance induction to C3H islet allografts (open squares, n = 8, MST 21 days). Anti-CD45RB therapy induces long-term tolerance in nonautoimmune B6 mice (closed squares, n = 13, MST >100 days), whereas untreated B6 mice rapidly reject C3H islet allografts (closed circles, n = 6, MST 13 days). Significance was determined by the log-rank test.
Discussion
SLE is a complex immunologic disease that results in permanent, multiorgan dysfunction. Lupus nephritis represents a significant cause of ESRD in the United States, culminating in approximately 500 renal transplants and 50 retransplants each year. Thus, attenuating autoantibody production and lymphocyte activation before kidney damage results remains a critical barrier to preventing lupus-related ESRD. In the current study, we demonstrate that lupus-prone B6.SLE123 mice, despite having no islet-specific immunity, are completely resistant to anti-CD45RB–mediated tolerance induction to islet allografts. Because this therapy activates graft-protective regulatory cells, we relate this strain’s resistance to our observation that effector cells from these mice are highly resistant to both CD4Treg- and CD8Treg-mediated suppression. As future studies move toward linking this resistance to graft rejection directly, it will be vital to understand how Tregs of both the CD4 and CD8 class interact with each other to regulate the immune response to transplant antigens.
In the nonautoimmune setting, the germinal center reaction is tightly checked by regulatory T lymphocytes (14). However, there exists conflicting evidence on the suppressive capacity of CD4Tregs in the murine setting of lupus. Whereas CD4Tregs from lupus-prone (NZBxNZW)F1 mice demonstrated similar suppressive capacity as CD4Tregs from nonautoimmune Balb/c mice, CD4Tregs from another lupus-prone strain, MRL/MpJ-Fas(lpr/lpr)J mice, demonstrated reduced suppressor function compared with CD4Tregs from nonautoimmune CBA/J mice (23,24). However, these experimental platforms used noncongenic strains as controls. To more accurately define the suppressive capacity of lupus-derived CD4Tregs, we used a B6 congenic system, the B6.SLE123 mouse, in which three lupus-promoting genetic regions from the lupus-prone strain NZW2410 have been introduced: SLE1, which permits the loss of nuclear antigen tolerance; SLE2, which lowers B cell activation threshold; and SLE3, which enhances T cell activation (7). Using a crossover platform, we demonstrate that CD4Tregs from B6.SLE123 mice possess similar suppressive capacity as CD4Tregs from B6 mice when targeting effector CD4 T cells from non-autoimmune B6 mice. We further determined that target CD4 T effector cells from B6.SLE123 mice resist suppression by B6 CD4Tregs. Importantly, not all models of lupus possess effector cell resistance. Lupus-prone BAFF-Tg mice, which overexpress the cytokine BAFF similar to some SLE patients, possess expanded CD4Tregs and regulation-sensitive T effector cells. When transplanted with islets, these mice are partially permissive to long-term allograft tolerance (25), further suggesting that effector cell resistance in the B6.SLE123 model contributes to failed tolerance. The opportunity for genetic dissection in the SLE123 system should permit isolation of the cellular mechanism by which effector cells resist regulation in future studies. In particular, recent work by Wong et al (26) demonstrates that B6 congenic mice harboring only the SLE1b sublocus possess expanded populations of TFH/GC B cells that generate autoantibodies, which may relate to our finding of excess alloantibody production. Because chronic alloantibody represents a growing cause of late-stage organ rejection, further dissection of the contribution of each SLE region may provide new opportunities to control this barrier to long-term transplant success (27).
Whereas CD8Treg control of the germinal center response in lupus is less well defined, CD8Tregs from the lupus-prone B6.SB-Yaa/J mouse strain did not suppress the high-affinity antibody response when targeting nonautoimmune B6 target CD4/B cells (9). Although our evidence demonstrates that CD8Tregs from B6.SLE123 mice maintain suppressive capacity when targeting nonautoimmune B6 target cells, disease progression in these strains are driven by different genetic factors. Notably, duplication of TLR7 in B6.SB-Yaa/J mice may promote the generation of TLR7HI dendritic cells that favor expansion of non-CD8Treg memory cells (CD122+ Ly49−), thereby overriding the generation of protective CD8Tregs (CD122+ Ly49+)(9). In comparison, lupus in the SLE123 system is driven by genetic material from the NZW2410 strain that permits overactivation of CD4/B effector cells that resist CD8Treg-mediated suppression. Despite these conflicting observations, defective CD8Treg suppression due to either CD8Treg intrinsic dysfunction or extrinsic resistance may permit the unchecked germinal center reaction characteristic of lupus.
In conclusion, lupus-prone B6.SLE123 mice resist CD4Treg- and CD8Treg-based immune regulation, which may account for our observation that these mice have expanded germinal center resident TFH and GC B cells that contribute to an exaggerated alloresponse. Although B6.SLE123 mice lack ongoing islet autoimmunity, these mice are completely resistant to tolerance induction to foreign islet allografts. Efforts to promote tolerance to renal allografts in lupus should focus on eliminating or reprogramming anti-graft effector lymphocytes that resist T cell–mediated regulation. Introduction of the B6 congenic B6.SLE123 lupus-prone mouse offers a new model in which resistance to immune regulation and transplant tolerance induction can be mapped to specific cell types and/or genetic loci. In sum, we provide a new platform in which researchers can mechanistically dissect the transplant response in SLE.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants K08-DK090146 (D.J.M.), R03-DK097410 (D.J.M.), a JDRF Career Development Award (D.J.M.), R01-HL088364 (A.S.M.), and T32-GM07347 (Vanderbilt MSTP support for B.T.S.), as well as institutional funds provided by the Vanderbilt Department of Pediatrics (D.J.M.). B.T.S. and D.J.M. wrote the manuscript. B.T.S., A.J.W., A.S.M., and D.J.M. edited the manuscript. All authors designed, performed, and analyzed experiments. Flow cytometry experiments were performed in the VUMC Flow Cytometry Shared Resource, which is supported by NIH grants P30-CA68485 and DK-058404.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- ESRD
end-stage renal disease
- FACS
fluorescent activated cell sorting
- GC
germinal center
- IFNγ
interferon-γ
- Ig
immunoglobulin
- KLH
keyhole limpet hemocyanin
- MACS
magnetic activated cell sorting
- MFI
median fluorescence intensity
- MHC
major histocompatibility
- NOD
nonobese diabetic
- NP
nitrophenyl
- SLE
systemic lupus erythematous
- TFH
CD4 T follicular helper cell
- TGF-β
transforming growth factor-β
- Treg
T regulatory cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Contreras G, Mattiazzi A, Guerra G, et al. Recurrence of lupus nephritis after kidney transplantation. J Am Soc Nephrol. 2010;21:1200–12007. doi: 10.1681/ASN.2009101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10:14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markees TG, Serreze DV, Phillips NE, et al. NOD mice have a generalized defect in their response to transplantation tolerance induction. Diabetes. 1999;48:967–974. doi: 10.2337/diabetes.48.5.967. [DOI] [PubMed] [Google Scholar]
- 4.Molano RD, Pileggi A, Berney T, et al. Prolonged islet allograft survival in diabetic NOD mice by targeting CD45RB and CD154. Diabetes. 2003;52:957–964. doi: 10.2337/diabetes.52.4.957. [DOI] [PubMed] [Google Scholar]
- 5.Berney T, Pileggi A, Molano RD, et al. The effect of simultaneous CD154 and LFA-1 blockade on the survival of allogeneic islet grafts in nonobese diabetic mice. Transplantation. 2003;76:1669–1674. doi: 10.1097/01.TP.0000092525.17025.D0. [DOI] [PubMed] [Google Scholar]
- 6.Moore DJ, Huang X, Lee MK, et al. Resistance to anti-CD45RB-induced tolerance in NOD mice: mechanisms involved. Transpl Int. 2004;17:261–269. doi: 10.1007/s00147-004-0698-3. [DOI] [PubMed] [Google Scholar]
- 7.Morel L, Croker BP, Blenman KR, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilhelm AJ, Rhoads JP, Wade NS, Major AS. Dysregulated CD4+ T cells from SLE-susceptible mice are sufficient to accelerate atherosclerosis in LDLr−/− mice. Ann Rheum Dis. 2015;74:778–785. doi: 10.1136/annrheumdis-2013-203759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H-J, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci USA. 2011;108:2010–2015. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry RA, Kendall PL, Thomas JW. Autoantigen-specific B-cell depletion overcomes failed immune tolerance in type 1 diabetes. Diabetes. 2012;61:2037–2044. doi: 10.2337/db11-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng S, Moore DJ, Huang X, et al. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol. 2007;178:6028–6032. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 12.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linterman MA, Rigby RJ, Wong RK, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linterman MA, Pierson W, Lee SK, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H-J, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Zhang X, Tang F, Zhu L, Liu Y. Reduction of forkhead box P3 levels in CD4+CD25high T cells in patients with new-onset systemic lupus erythematosus. Clin Exp Immunol. 2008;153:182–187. doi: 10.1111/j.1365-2249.2008.03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gracie JA, Bradley JA. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26:1217–1221. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takimoto T, Wakabayashi Y, Sekiya T, et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol. 2010;185:842–855. doi: 10.4049/jimmunol.0904100. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z, Zhong R, Jiang J, et al. Adoptively transferable tolerance induced by CD45RB monoclonal antibody. J Am Soc Nephrol. 1999;10:374–381. doi: 10.1681/ASN.V102374. [DOI] [PubMed] [Google Scholar]
- 21.Deng S, Moore DJ, Huang X, et al. Antibody-induced transplantation tolerance that is dependent on thymus-derived regulatory T cells. J Immunol. 2006;176:2799–2807. doi: 10.4049/jimmunol.176.5.2799. [DOI] [PubMed] [Google Scholar]
- 22.Camirand G, Wang Y, Lu Y, et al. CD45 ligation expands Tregs by promoting interactions with DCs. J Clin Invest. 2014;124:4603–4613. doi: 10.1172/JCI74087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 24.Parietti V, Monneaux F, Décossas M, Muller S. Function of CD4+, CD25+ Treg cells in MRL/lpr mice is compromised by intrinsic defects in antigen-presenting cells and effector T cells. Arthritis Rheum. 2008;58:1751–1761. doi: 10.1002/art.23464. [DOI] [PubMed] [Google Scholar]
- 25.Walters S, Webster KE, Sutherland A, et al. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol. 2009;182:793–801. doi: 10.4049/jimmunol.182.2.793. [DOI] [PubMed] [Google Scholar]
- 26.Wong EB, Khan TN, Mohan C, Rahman ZSM. The lupus-prone NZM2410/NZW strain-derived Sle1b sublocus alters the germinal center checkpoint in female mice in a B cell-intrinsic manner. J Immunol. 2012;189:5667–5681. doi: 10.4049/jimmunol.1201661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RN, Colvin RB. Chronic alloantibody mediated rejection. Semin Immunol. 2012;24:115–121. doi: 10.1016/j.smim.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]