Abstract
We expressed rat Nav1.6 sodium channels with or without the rat β1 subunit in human embryonic kidney (HEK293) cells and evaluated the effects of the pyrethroid insecticides tefluthrin and deltamethrin on whole-cell sodium currents. In assays with the Nav1.6 α subunit alone, both pyrethroids prolonged channel inactivation and deactivation and shifted the voltage dependence of channel activation and steady-state inactivation toward hyperpolarization. Maximal shifts in activation were ~18 mV for tefluthrin and ~24 mV for deltamethrin. These compounds also caused hyperpolarizing shifts of ~10–14 mV in the voltage dependence of steady-state inactivation and increased in the fraction of sodium current that was resistant to inactivation. The effects of pyrethroids on the voltage-dependent gating greatly increased the size of sodium window currents compared to unmodified channels; modified channels exhibited increased probability of spontaneous opening at membrane potentials more negative than the normal threshold for channel activation and incomplete channel inactivation. Coexpression of Nav1.6 with the β1 subunit had no effect on the kinetic behavior of pyrethroid-modified channels but had divergent effects on the voltage-dependent gating of tefluthrin- or deltamethrin-modified channels, increasing the size of tefluthrin-induced window currents but decreasing the size of corresponding deltamethrin-induced currents. Unexpectedly, the β1 subunit did not confer sensitivity to use-dependent channel modification by either tefluthrin or deltamethrin. We conclude from these results that functional reconstitution of channels in vitro requires careful attention to the subunit composition of channel complexes to ensure that channels in vitro are faithful functional and pharmacological models of channels in neurons.
Keywords: voltage-gated sodium channel, Nav1.6 isoform, β subunits, functional reconstitution, pyrethroid insecticide, HEK293 cells
Introduction
Pyrethroids are synthetic analogs of the insecticidal constituents of natural insecticide pyrethrum (Elliott, 1989). Introduced more than three decades ago, pyrethroids remain in widespread use in a variety of agricultural contexts, in human health to control insect vectors of malaria and other diseases, and in household pest control. In 2013, pyrethroids held 17% of the world insecticide market (Sparks, 2013).
Pyrethroids prolong the opening of voltage-gated sodium channels, thereby disrupting normal nerve function (Soderlund, 1995). The large, pore-forming (~260 kDa) α subunits of voltage-gated sodium channels contain structural domains that confer voltage-dependent gating and the pharmacological properties of the channel (Catterall, 2000). Mammalian genomes contain nine α subunit isoforms (designated Nav1.1 – Nav1.9), four of which (Nav1.1, Nav1.2, Nav1.3, and Nav1.6) are expressed in the brain (Goldin, 2001) and represent putative targets for the central neurotoxic effects of pyrethroids (Soderlund et al., 2002).
Native sodium channels in the mammalian brain are heteromultimers comprising one α subunit and two smaller (33–36 kDa) auxiliary β subunits that modulate channel gating and regulate channel trafficking and expression in the cell membrane (Goldin, 2001; Meadows and Isom, 2005). Mammalian genomes contain four genes for sodium channel β subunits, designated β1 – β4 (Patino and Isom, 2010). Individual neurons express multiple sodium channel α and β subunit isoforms and contain multiple functionally and pharmacologically distinct sodium channel subunit complexes (Felts et al., 1997; Whitaker et al., 2000; Whitaker et al., 2001). However, the subunit compositions of native sodium channel complexes remain to be established.
The expression of multiple sodium channel complexes in individual neurons complicates the use of native neuronal tissue to identify the most sensitive molecular targets for pyrethroids and characterize pyrethroid action at those targets. However, functional reconstitution of sodium channel complexes in vitro by expression either in unfertilized oocytes of the frog Xenopus laevis or in mammalian cells in culture allows direct investigation of the functional and pharmacological properties of different sodium channel isoforms and complexes of different subunit composition. Studies of the pyrethroid sensitivity of five different rat sodium channel isoforms in the Xenopus oocyte system identified three isoforms (Nav1.3, Nav1.6 and Nav1.8) that are relatively sensitive to pyrethroid modification and two isoforms (Nav1.2 and Nav1.7) that are much less sensitive (Soderlund, 2012b). The identification of the Nav1.6 isoform as pyrethroid-sensitive is significant because it is the most abundant isoform in the adult brain (Auld et al., 1988) and is expressed preferentially at nodes of Ranvier and synapses (Caldwell et al., 2000), where it is likely to play key roles in both electrical and chemical signaling. The abundance, functional importance and pyrethroid sensitivity of the Nav1.6 isoform suggest that it is likely to be the primary molecular target for pyrethroid intoxication in the brain.
The Xenopus oocyte expression system has also been employed to identify functional roles for sodium channel β subunits as modulators of pyrethroid action on sodium channels. Coexpression of the rat Nav1.2 or Nav1.3 sodium channel α subunit with either the rat β1 or β3 subunit enhances the sensitivity of these channels to modification by pyrethroids in the resting state (Smith and Soderlund, 1998; Meacham et al., 2008). Similarly, coexpression of the rat Nav1.6 α subunit with the rat β1 and β2 subunit, both individually and in combination, revealed that use-dependent enhancement of channel modification by tefluthrin, which reflects preferential binding to the channel in the open state (McCavera and Soderlund, 2012), requires coexpression with the β1 subunit (Tan and Soderlund, 2011b).
Despite the widespread use of Xenopus oocytes for the functional reconstitution of ion channels and neurotransmitter receptors, the properties of channels and receptors reconstituted in oocytes may not fully reproduce those of native channels and receptors due to species differences in the composition and structure of the cell membrane environment and post-translational modification of membrane proteins (Goldin, 2006). The HEK293 cell line, derived from human embryonic kidney tissue (Graham et al., 1977), has been widely employed as an alternative to Xenopus oocytes for the expresion of a variety of ion channels and receptors, including mammalian sodium channels (Thomas and Smart, 2005). HEK293 cells exhibit many neuron-like qualities in culture and express more than 60 neuronal genes, including neurofilament proteins and neuroreceptor and ion channel subunits (Shaw et al., 2002; Thomas and Smart, 2005).
Previously we employed the HEK293 cell system to express heterotrimeric sodium channel complexes containing the rat Nav1.6, β1, and β2 subunits and described the action of the pyrethroid insecticides tefluthrin and deltamethrin on the expressed channels (He and Soderlund, 2011). This study identified significant differences between these heterotrimeric channels expressed either in HEK293 cells or Xenopus oocytes (Tan and Soderlund, 2010) both in their functional properties and their modification by pyrethroids. However, this study did not address whether the modulatory effects β1 subunit observed previously in the Xenopus oocyte system also occurred in HEK293 cells. Recently, we created HEK293 cell lines expressing the rat Nav1.6 sodium channel α subunit either alone or in combination with the rat β1 subunit and employed these cell lines to examine the impact of the β1 subunit on the expression and functional properties of Nav1.6 channels (He and Soderlund, 2014). Here we report the extension of these studies in which we have used the same cell lines to assess the impact of the β1 subunit on the modification of Nav1.6 sodium channels in the HEK293 cell expression system. Our results provide evidence that the modulatory effects of the β1 subunit in HEK293 cells differ from those observed previously in Xenopus oocytes. Moreover, coexpression with the β1 subunit differentially affects channel modification by the pyrethroids tefluthrin and deltamethrin.
Materials and methods
Cell lines
The construction, characterization and maintenance of stably-transformed HEK293 cell lines expressing the rat Nav1.6 sodium channel α subunit alone (designated HEK-Nav1.6 cells) or in combination with the rat β1 auxiliary subunit (designated HEK-Nav1.6β1 cells) are described in a previous publication (He and Soderlund, 2014).
Electrophysiology
On the day prior to assay, cells were plated at low density in 35-mm Petri dishes. For electrophysiological assays, cells (24 – 48 h after plating) were rinsed three times with extracellular perfusion medium that contained (mM): NaCl (140), KCl (5), CaCl2 (2), MgCl2 (1), and HEPES (10) at pH 7.40 (adjusted with 2M NaOH). Whole-cell patch clamp recordings were conducted at room temperature (23–27 °C) using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA). Cells were perfused at ~350 μl/min with extracellular medium using a custom-fabricated passive perfusion manifold and a disposable plastic recording chamber insert (~240 μl volume; Warner Instruments, Hamden, CT). The intracellular solution contained (in mM): NaCl (35), CsF (105), MgCl2 (2), EGTA (10), and HEPES (10) at pH 7.20 (adjusted with 2M CsOH). The final osmolarity of both solutions was 295 – 305 mOsm. Fire-polished patch electrodes were fabricated from borosilicate glass capillaries (1.5 mm O.D.; 1.0 mm I.D.; World Precision Instruments Inc., Sarasota, FL) using a P-87 puller (Sutter Instruments, Novato, CA) to give a resistance of 1–2 MΩ when filled with intracellular solution. The ground electrode was a bridge of 1% agar in extracellular medium in a glass pipet. Output signals were filtered at 2 kHz and sampled at 50 kHz (DigiData 1322A; Molecular Devices). Voltage errors were minimized using 70–80% series resistance compensation. Leak currents were corrected using the P/4 method (Bezanilla and Armstrong, 1977). Membrane potentials were not corrected for junction potential (~2.3mV at 23.5°C). Data were acquired using pClamp 10.2 (Molecular Devices) software. Following the establishment of a stable holding potential (−120 mV) under voltage clamp, sodium currents were sampled using 40-ms step depolarizations to −15 mV at a frequency of 0.05 Hz for ~20 min to achieve stable sodium current amplitudes prior to initiating other protocols. To determine the voltage dependence of activation, cells were clamped at a membrane potential of −120 mV and currents were measured during a 40-ms depolarizing test pulse to potentials from −80 mV to 0 mV in 5-mV increments. To determine the voltage dependence of steady-state inactivation, cells were clamped at a membrane potential of −120 mV followed by a 100-ms conditioning prepulse to potentials from −120 mV to 0 mV in 5-mV increments and then a 40-ms test pulse to −15 mV. For determinations of use dependence, cells were given trains of up to 100 5-ms conditioning prepulses to 10 mV at a frequency of 20 Hz followed by a 40-ms test pulse to −15 mV. In some experiments, tetrodotoxin (TTX, Sigma-Aldrich, St. Louis, MO; 500 nM final concentration) was used to visualize currents and voltage-clamp artifacts unrelated to sodium channel expression.
Assays with pyrethroids
Stock solutions of deltamethrin (Fig. 1; 99.5%; Bayer CropScience, Research Triangle Park, NC) and tefluthrin (Fig. 1; 98.8%; Syngenta, Bracknell, Berks., UK) in DMSO were diluted in extracellular medium just prior to use to achieve final concentrations of 0.01 – 10 μM (deltamethrin) or 0.01 – 100 μM (tefluthrin) and applied through the perfusion system. The final concentration of DMSO in extracellular medium did not exceed 0.1%, a concentration that had no effect on sodium currents. Recording chamber inserts employed in experiments with insecticides were used only once to prevent cross-contamination of cells. Following the characterization of control currents, each cell was clamped at −120 mV and sodium currents elicited by 40-ms pulses to −15 mV were sampled for 3 – 5 min at a frequency of 0.05 Hz to confirm the stability of sodium current amplitudes prior to experiments with insecticides. The last sampled control current from this series was used to normalize the amplitudes or conductances of pyrethroid-modified currents in each cell. Pyrethroids were applied by perfusion in extracellular medium and the development of pyrethroid modification was monitored until stable (~20–22 min) by assessing the increase in the sodium tail current observed following 40-ms test pulses from −120 mV to −15 mV at a frequency of 0.05 Hz. The voltage dependence of activation and steady-state inactivation and the effects of repeated stimulation on channel modification were measured as described above. All experiments with pyrethroids employed 10-s intervals between pulses or pulse trains to permit the complete decay of pyrethroid-modified currents.
Fig. 1.
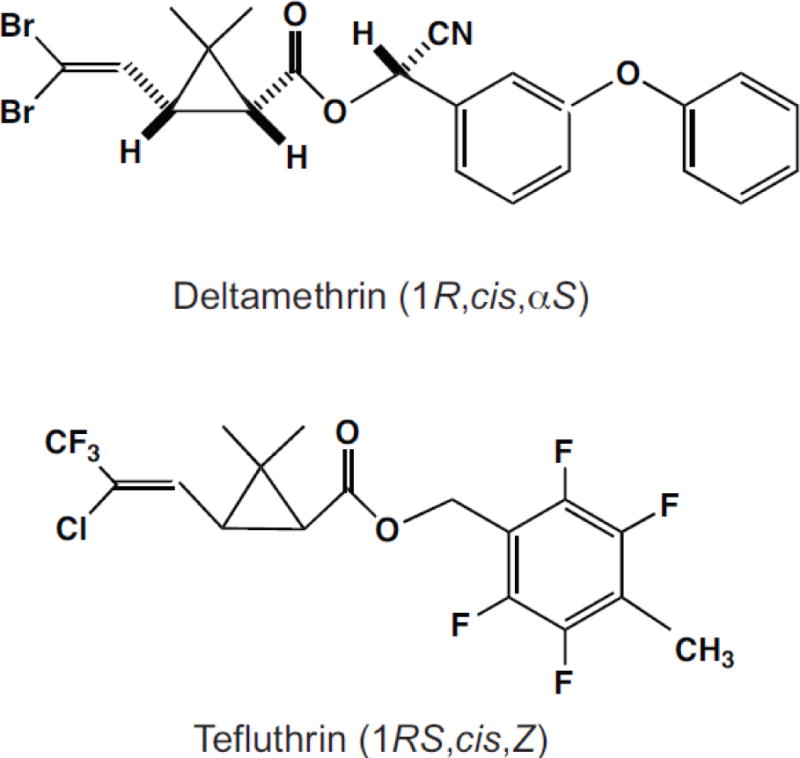
Structures and isomeric compositions of deltamethrin and tefluthrin.
Data analysis
Data were acquired and analyzed using pClamp 10.2 (Molecular Devices) and Origin 8.1 (OriginLab Corp., Northampton, MA). For each cell, currents from activation experiments were converted to sodium conductances and plotted as a function of test potential using the Boltzmann equation [y = (A1 – A2)/(1+e(x−x0)/dx) + A2] to give values for V0.5 (potential causing half-maximal activation) and K (slope factor). Similarly, currents from steady-state inactivation experiments with each cell were plotted as a function of prepulse potential and fitted to the Boltzmann equation. The initial conductance of the pyrethroid-induced sodium tail current, normalized to the conductance of the peak current measured in the same cell prior to pyrethroid exposure, was employed to calculate the fraction of pyrethroid-modified sodium channels (Tatebayashi and Narahashi, 1994). Statistical analyses were performed in Prism 5.0 (GraphPad Software, La Jolla, CA). Comparisions between two means employed Student’s unpaired t-test. Comparisons among three or more mean values employed one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for statistical significance. Comparisons with values of P < 0.05 were considered statistically significant.
Results
Pyrethroid-modified currents
In the absence of insecticide, sodium currents in HEK-Nav1.6 and HEK-Nav1.6β1 cells activate and inactivate rapidly within ~10 msec of depolarization, and late currents (measured at the end of a 40-ms depolarization) are <1.5% of the peak transient current (He and Soderlund, 2014). Resting modification of sodium channels by tefluthrin or deltamethrin, evident in the development of prominent, persistent late currents during depolarizing pulses and slowly-decaying tail currents following repolarization, increased gradually during perfusion and reached apparent equilibrium by ~22 min (Fig. 2). Accordingly, we assessed the effects of tefluthrin and deltamethrin on sodium channels in the resting state by equilibrating HEK-Nav1.6 or HEK-Nav1.6β1 cells with pyrethroid for 20–22 min at −120 mV and sampling peak transient currents and tail currents using low-frequency depolarizations.
Fig. 2.
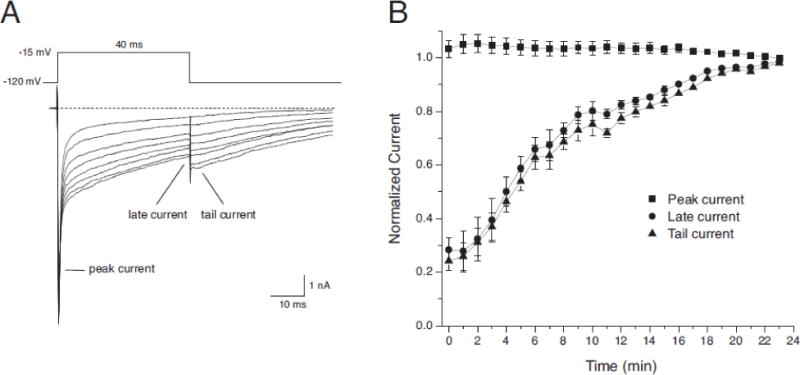
Time course of modification of Naa1.6 channels during perfusion with 1 μM tefluthrin. (A) Currents recorded from a representative cell at 3-min intervals (0–21 min) during perfusion with 1 μM tefluthrin showing the time-dependent increase in the tefluthrin-induced late and tail currents. (B) Time course of modification of Nav1.6 channels by 10 μM tefluthrin from multiple experiments such as that shown in panel A. Peak, late and tail current amplitudes for each cell were normalized to the amplitude of the corresponding current recorded after 23 minutes. Each data point is the mean of 9 experiments with different cells; bars show SE values larger than the data point symbols.
Figure 3 shows representative control and pyrethroid-modified currents from HEK-Nav1.6 (Fig. 3A) and HEK-Nav1.6β1 (Fig. 3B) cells. Both pyrethroids induced hybrid currents comprising a rapidly-decaying unmodified component and a more persistent pyrethroid-modified component. Tefluthrin slowed the time course of transient current decay during a depolarizing pulse, producing a late current that persisted throughout a 40-ms depolarization. Tefluthrin also slowed the rate of channel deactivation following repolarization, producing a slowly-decaying sodium tail current. First-order time constants for both the tefluthrin late and tail currents were 25–35 ms.
Fig. 3.
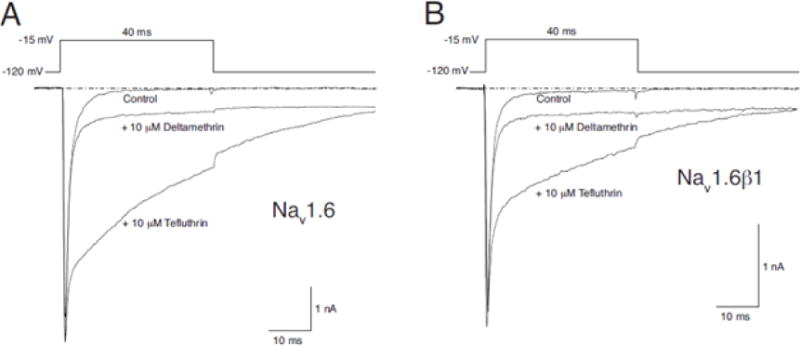
Representative control and pyrethroid-modified current traces recorded during a 40-ms step depolarization from −120 mV to −15 mV from HEK-Nav1.6 (A) and HEK-Nav1.6β1 (B) cells.
Deltamethrin slowed the rates of channel inactivation and deactivation to a greater extent than tefluthrin, inducing late and tail currents with no detectable decay during or after a 40-ms depolarization. The overall the extent of channel modification by deltamethrin from the resting state at all concentrations examined (reflected in the relative amplitudes of the late current and peak transient current) was lower for deltamethrin than tefluthrin.
We previously showed that the β1 subunit causes a small but significant acceleration in the kinetics of fast inactivation of Nav1.6 sodium channels in HEK293 cells (He and Soderlund, 2014). However we found no significant effect of the β1 subunit on the rate of first-order decay of tefluthrin-induced late and tail currents (data not shown). The extreme persistence of deltamethrin-induced late and tail currents precluded an assessment of the effects of the β1 subunit on the decay of these currents.
Voltage-dependent activation
Figure 4 illustrates the concentration-dependent effects of tefluthrin and deltamethrin on the voltage dependence of sodium channel activation in HEK-Nav1.6 cells and HEK-Nav1.6β1 cells, and Table 1 summarizes the statistical analyses of these data. Both tefluthrin (Figs. 4A and 4C) and deltamethrin (Figs. 4B and 4D) produced concentration-dependent hyperpolarizing shifts in the V0.5 values for channel activation. Although the trend of this effect was consistent across pyrethroid concentrations in both cell lines, differences in V0.5 values at different insecticide concentrations were statistically significant only for some of the binary comparisons with tefluthrin. Neither insecticide affected the slope of the voltage response curve in HEK-Nav1.6 cells. Tefluthrin at concentrations above 0.1 μM increased the numerical slope factor of the voltage response curve, whereas deltamethrin had no significant effect on this parameter.
Fig. 4.
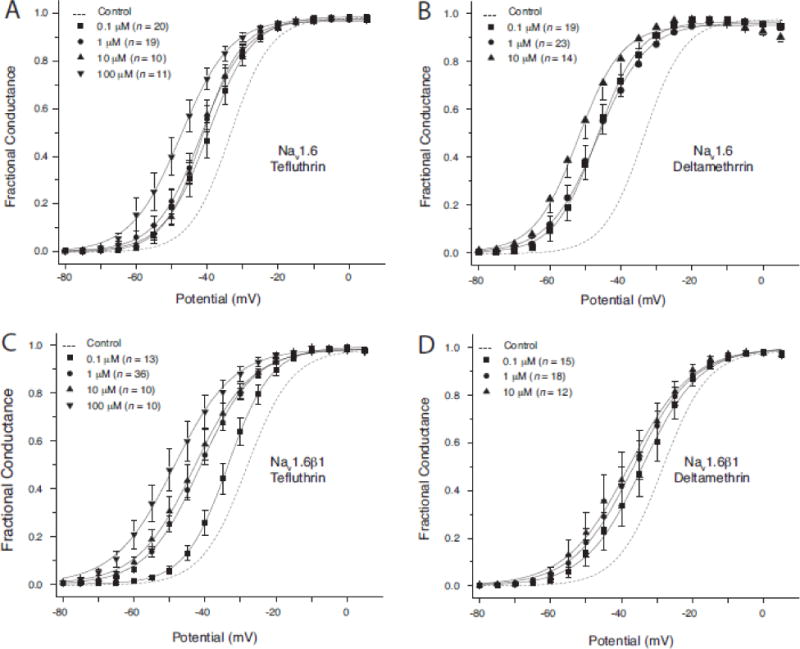
Concentration-dependent modification of the voltage dependence of activation of sodium channels in HEK-Nav1.6 (A, B) and HEK-Nav1.6β1 (C, D) cells by tefluthrin (A, C) and deltamethrin (B, D). Values for the conductance of peak sodium current were plotted as a function of test potential and curves were drawn by fitting mean values to the Boltzmann equation. Values are the means of the indicated number of determinations with different cells; bars show SE values larger than the data point symbols. Dashed lines show the curve obtained by fitting mean control values to the Boltzmann equation (He and Soderlund, 2014).
Table 1.
Effects of tefluthrin and deltamethrin on the voltage dependence of activation of sodium channels expressed in HEK-Nav1.6 and HEK-Nav1.6β1 cells.a
| HEK-Nav1.6 cells | HEK-Nav1.6β1 cells | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Condition | V0.5 | K | n | V0.5 | K | n |
| Control | −35.2 ± 0.8*b | 4.19 ± 0.17* | 64 | −28.6 ± 0.8*c | 4.90 ± 0.12* | 65 |
| + Tefluthrin | ||||||
| 0.1 μM | −39.9 ± 1.1† | 3.50 ± 0.34* | 20 | −33.6 ± 1.5* | 5.02 ± 0.19* | 13 |
| 1 μM | −41.5 ± 1.4†‡ | 4.44 ± 0.34* | 19 | −41.6 ± 1.2† | 6.17 ± 0.32† | 36 |
| 10 μM | −42.0 ± 1.2†‡ | 4.79 ± 0.49* | 10 | −43.6 ± 2.2†‡ | 6.85 ± 0.33† | 10 |
| 100 μM | −47.7 ± 2.2‡ | 5.20 ± 0.60* | 11 | −48.9 ± 2.8‡ | 6.57 ± 0.32† | 10 |
| + Deltamethrin | ||||||
| 0.1 μM | −45.3 ± 1.6† | 3.87 ± 0.29* | 19 | −34.9 ± 2.6† | 4.39 ± 0.25* | 15 |
| 1 μM | −45.6 ± 1.9† | 4.62 ± 0.37* | 23 | −37.2 ± 2.3† | 5.17 ± 0.32* | 18 |
| 10 μM | −50.3 ± 2.0† | 4.41 ± 0.25* | 14 | −38.3 ± 3.2† | 4.84 ± 0.31* | 12 |
Values calculated from fits of the data from the indicated number of individual experiments to the Boltzmann equation; V0.5, midpoint potential (mV) for voltage-dependent activation; K, slope factor; data in the absence of insecticides are pooled values for all HEK-Nav1.6 and HEK-Nav1.6β1 cells tested (He and Soderlund, 2014).
Values in each column for control and concentrations of either tefluthrin or deltamethrin that are marked with the different symbols were significantly different.
Taken together, the results shown in Fig. 4 suggest that coexpression with the β1 subunit exerts opposite effects on the hyperpolarizing shifts in the activation curves obtained with tefluthrin and deltamethrin. Figure 5 shows the magnitude of the shift in V0.5 values for Nav1.6 channels and Nav1.6β1 channels. This figure also includes previously-published data for Nav1.6β1β2 channels (He and Soderlund, 2011) for comparison. Coexpression of the Nav1.6 α subunit with the β1 subunit increased the magnitude of the activation potential shift for tefluthrin but had no effect on that for deltamethrin. Coexpressing with both β subunits did not alter the tefluthrin-dependent shift in activation compared to that observed with Nav1.6β1 but decreased the magnitude of the deltamethrin-dependent activation potential shift compared to that observed for Nav1.6β1.
Fig. 5.
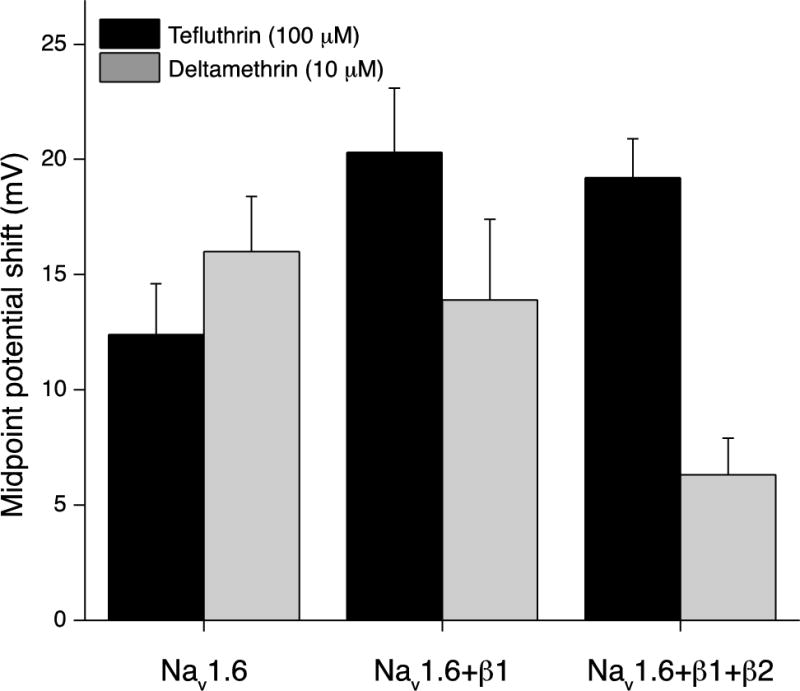
Effect of coexpression with β subunits on the magnitude of the shift in V0.5 values for Nav1.6 sodium channel activation caused by tefluthrin (100 μM) or deltamethrin (10 μM). Values for HEK-Nav1.6 and HEK-Nav1.6β1 cells were calculated by subtracting the mean control V0.5 values from the mean V0.5 values measured in the presence of insecticide; bars show SE values as in Tables 1 and 2. Comparable values for HEK-Nav1.6β1β2 cells were calculated from previously-published data (He and Soderlund, 2011) and are provided here for comparison.
The concentration dependence of the activation parameters illustrated in Fig. 4 and Table 1 reflects the heterogeneous responses of mixed populations of modified and unmodified channels because the solubilities of tefluthrin and deltamethrin attainable in aqueous medium were insufficient to give concentrations that saturated the response. To assess gating properties of only the pyrethroid-modified population of sodium channels, we measured the voltage dependence of the pyrethroid-induced late currents and compared them to the control (unmodified) current and the pyrethroid-modified peak transient current. Figure 6 illustrates this approach with HEK-Nav1.6 cells and 100 μM tefluthrin. Figure 6A shows normalized current – voltage plots for the control peak transient current, the peak transient current measured in the presence of 100 μM tefluthrin, and the late current induced by 100 μM tefluthrin. In the absence of insecticide, depolarization to −20 mV gave the largest-amplitude peak transient current. Exposure to 100 μM tefluthrin shifted the peak transient current maximum to −30 mV but shifted the maximum for the late current to −40 mV. Plots of the conductance transformations of these data (Fig. 6B) show that the peak transient current measured in the presence of 100 μM tefluthrin underestimated the effect of tefluthrin on the voltage dependence of tefluthrin modified channels, which is reflected in the voltage responses of the late current.
Fig. 6.
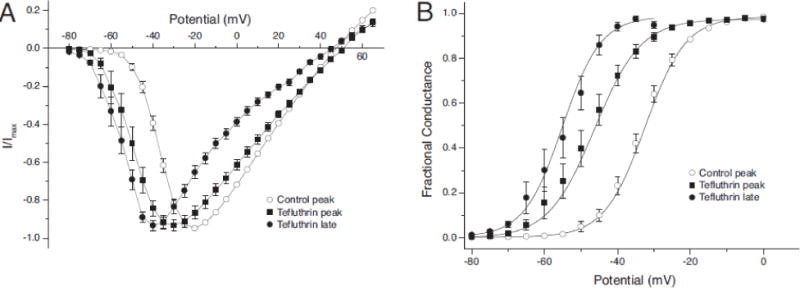
Voltage dependence of the control peak current and the peak and late currents induced by 100 μM tefluthrin in assays with HEK-Nav1.6 cells. (A) Normalized current – voltage plots for control peak sodium currents (He and Soderlund, 2014) and for the peak currents (as in Fig. 3A), and late currents (measured at the end of a 40-ms depolarizing pulse) following exposure to tefluthrin. Values are the means of 64 (control) or 11 (+tefluthrin) determinations with different cells; bars show SE values larger than the data point symbols. (B) Plots of the conductance transformations of data in Fig. 6A; curves were drawn by fitting mean values to the Boltzmann equation.
Table 2 gives V0.5 and K values for late currents induced by 100 μM tefluthrin and 10 μM deltamethrin in assays with HEK-Nav1.6 and HEK-Nav1.6β1 cells based on analyses such as that shown in Fig. 6. Coexpression with the β1 subunit had no effect on either the voltage dependence of the tefluthrin-induced late current or the slope of the voltage-response curve. By contrast, coexpression with the β1 subunit significantly shifted the voltage dependence of the deltamethrin induced late current by ~ 10 mV in the direction of depolarization without significantly affecting the slope of the voltage response.
Table 2.
The voltage dependence of late currents in HEK-Nav1.6 and HEK-Nav1.6β1 cells induced by tefluthrin and deltamethrin.a
| Tefluthrin (100 μM) | Deltamethrin (10 μM) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Cell line | V0.5 | K | n | V0.5 | K | n |
| HEK-Nav1.6 | −52.8 ± 1.8*b | 4.85 ± 0.37* | 11 | −59.1 ± 1.8* | 4.35 ± 0.60* | 12 |
| HEK-Nav1.6β1 | −54.2 ± 2.1* | 5.62 ± 0.36* | 10 | −48.5 ± 3.7† | 5.51 ± 0.53* | 9 |
Values are means ± SE calculated from fits of individual data sets obtained in the presence of tefluthrin or deltamethrin to the Boltzmann equation (see Fig. 6B) ; V0.5, midpoint potential (mV) for voltage-dependent activation or inactivation; K, slope factor; n, number of replicate experiments.
Values in each column that are marked with the different symbols were significantly different.
Steady-state fast inactivation
Figure 7 illustrates the effects of tefluthrin and deltamethrin on the voltage dependence of steady-state fast inactivation of sodium channels in HEK-Nav1.6 cells and HEK-Nav1.6β1 cells, and Table 3 summarizes the statistical analyses of these data. Both tefluthrin (Figs. 7A and 7C) and deltamethrin (Figs. 7B and 7D) caused concentration-dependent hyperpolarizing shifts in the V0.5 for steady-state inactivation. However, the magnitude of these shifts was smaller than those found for activation and only some of the binary comparisons were statistically significant. In both cell lines, neither insecticide significantly altered the slopes of the voltage response curves for steady-state inactivation (Table 3).
Fig. 7.
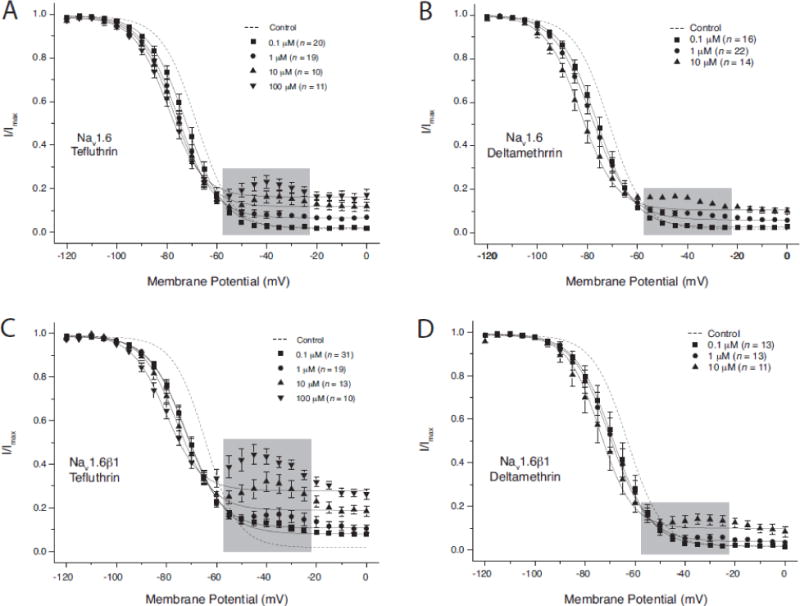
Concentration-dependent modification of the voltage dependence of steady-state inactivation of sodium channels in HEK-Nav1.6 (A, B) and HEK-Nav1.6β1 (C, D) cells by tefluthrin (A, C) and deltamethrin (B, D). Normalized amplitudes of peak sodium currents were plotted as a function of test potential and curves were drawn by fitting mean values to the Boltzmann equation. Data points in the shaded regions were omitted from the fits of data obtained in the presence of pyrethroids (see text for details). Values are the means of the indicated number of determinations with different cells; bars show SE values larger than the data point symbols. Dashed lines show the curve obtained by fitting mean control values to the Boltzmann equation (He and Soderlund, 2014).
Table 3.
Effects of tefluthrin and deltamethrin on the voltage dependence of steady-state inactivation of sodium channels expressed in HEK-Nav1.6 and HEK-Nav1.6β1 cells.a
| HEK-Nav1.6 cells | HEK-Nav1.6β1 cells | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Condition | V0.5 | K | n | V0.5 | K | n |
| Control | −68.7 ± 0.7* | 6.50 ± 0.10* | 63 | −63.5 ± 0.8* | 6.10 ± 0.08* | 66 |
| + Tefluthrin | ||||||
| 0.1 μM | −72.4 ± 1.2*†b | 6.91 ± 0.22* | 18 | −71.7 ± 1.1† | 6.40 ± 0.13* | 31 |
| 1 μM | −75.7 ± 1.1† | 6.74 ± 0.19* | 18 | −72.2 ± 1.2†‡ | 6.25 ± 0.15* | 19 |
| 10 μM | −77.1 ± 1.2† | 6.47 ± 0.44* | 11 | −76.0 ± 1.0†‡ | 6.04 ± 0.15* | 13 |
| 100 μM | −79.0 ± 1.2‡ | 6.35 ± 0.27* | 10 | −80.4 ± 1.7‡ | 5.94 ± 0.30* | 10 |
| + Deltamethrin | ||||||
| 0.1 μM | −75.9 ± 1.4† | 6.63 ± 0.21* | 16 | −68.2 ± 2.4* | 5.88 ± 0.16* | 13 |
| 1 μM | −78.1 ± 1.3†‡ | 6.80 ± 0.19* | 22 | −69.5 ± 2.4* | 6.08 ± 0.19* | 11 |
| 10 μM | −82.7 ± 1.7‡ | 6.63 ± 0.34* | 14 | −73.4 ± 2.6† | 5.60 ± 0.30* | 11 |
Values calculated from fits of the data from the indicated number of individual experiments to the Boltzmann equation; V0.5, midpoint potential (mV) for voltage-dependent inactivation; K, slope factor; data in the absence of insecticides are pooled values for all cells tested from He and Soderlund (He and Soderlund, 2014). Data for prepulse potentials from −55 mV to −25 mV in the presence of insecticides were omitted from fits of inactivation data to the Boltzmann equation; see text for explanation.
Values in each column for control and concentrations of either tefluthrin or deltamethrin that are marked with the different symbols were significantly different.
Taken together, the results shown in Fig. 7 and Table 3 suggest that coexpression with the β1 subunit differentially affects the hyperpolarizing shifts in the inactivation curves for tefluthrin and deltamethrin. Coexpression with the β1 subunit had no significant effect on the measured midpoint potentials for inactivation at all tefluthrin concentrations. By contrast, coexpression with the β1 subunit caused statistically-significant (P < 0.05) depolarizing shifts of 7.7–9.2 mV at the three deltamethrin concentrations examined.
Figure 8 illustrates the differential impact of coexpression with the β1 subunit on Nav1.6 sodium channel inactivation in the presence of either tefluthrin or deltamethrin by comparing the magnitude of the hyperpolarizing shift in V0.5 values for Nav1.6 channels and Nav1.6β1 channels at high insecticide concentrations. This figure also includes previously-published data for Nav1.6β1β2 channels (He and Soderlund, 2011). Coexpression of the Nav1.6 α subunit with the β1 subunit increased the magnitude of the inactivation potential shift for tefluthrin by 6.6 mV. By contrast, coexpression of the Nav1.6 α subunit with the β1 subunit decreased the magnitude of the activation potential shift for deltamethrin by 4.1 mV. Coexpressing Nav1.6 with both β subunits did not significantly alter the insecticide-dependent shifts in midpoint potentials for inactivation compared to those observed with Nav1.6β1 channels.
Fig. 8.
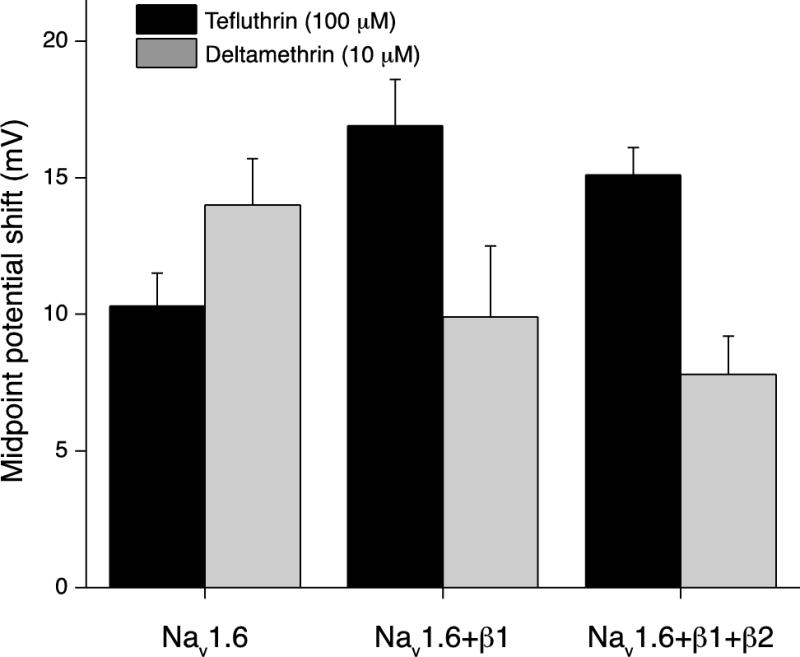
Effect of coexpression with β subunits on the magnitude of the shift in V0.5 values for Nav1.6 sodium channel inactivation caused by tefluthrin (100 μM) or deltamethrin (10 μM). Values for HEK-Nav1.6 and HEK-Nav1.6β1 cells were calculated by subtracting the mean control V0.5 values from the mean V0.5 values measured in the presence of insecticide; bars show SE values as in Tables 1 and 2. Comparable values for HEK-Nav1.6β1β2 cells were calculated from previously-published data (He and Soderlund, 2011) and are provided here for comparison.
The results shown in Fig. 7 also illustrate two additional effects of pyrethroids on steady-state inactivation. First, the extent of voltage-dependent inactivation was reversed at conditioning potentials between −55 mV and −25 mV. The magnitude of this effect varied with pyrethroid concentration, thus implying an effect directly related to channel modification. With tefluthrin, the maximum reversal of inactivation occurred at potentials causing maximal activation of pyrethroid-modified channels (Fig. 6A). We infer that the sodium currents measured during these steady-state inactivation experiments included components of sodium current resulting from the activation of pyrethroid-modified but non-inactivated channels during conditioning prepulses to potentials from −55 mV to −25 mV. To correct for this effect, we omitted the data from −55 mV to −25 mV from the fits of these results to the Boltzmann equation that resulted in the V0.5 and K values reported in Table 3. Coexpression with the β1 subunit significantly increased the magnitude of the current measured at 45 mV (peak reversal) in the presence of all tefluthrin concentrations but did not significantly alter the magnitude of peak reversal at all deltamethrin concentrations.
Second, both tefluthrin or deltamethrin increased the fraction of sodium current that was resistant to inactivation during strong depolarizing prepulses (e.g., to 0 mV) in a concentration-dependent manner. Coexpression with the β1 subunit significantly increased the magnitude of the inactivation-resistant current at all tefluthrin concentrations (Fig. 9). The inactivation-resistant currents caused by deltamethrin (0.1, 1, and 10 μM) in HEK-Nav1.6 cells were similar in magnitude to those shown in Fig. 9 for tefluthrin at the same concentrations but were not increased by coexpression with the β1 subunit (data not shown).
Fig. 9.
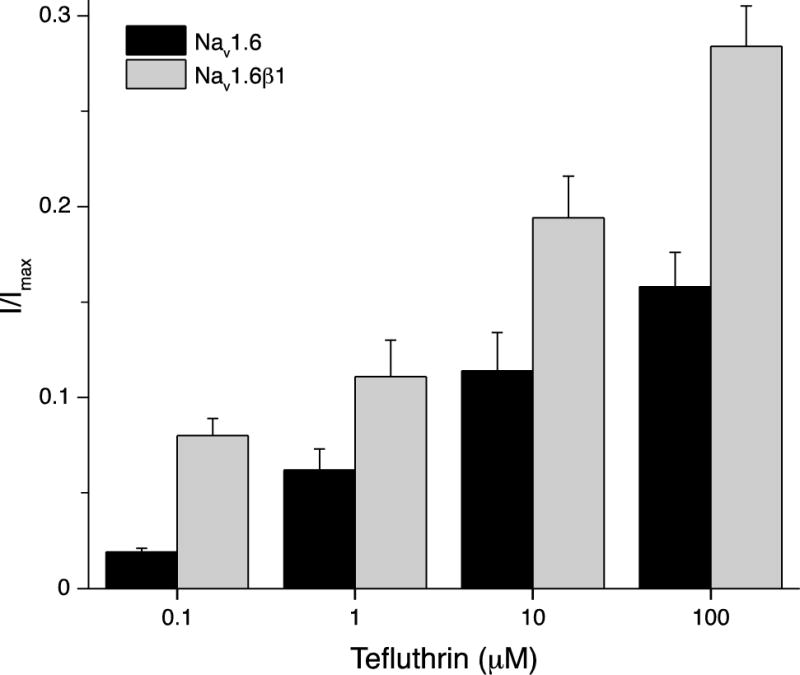
Effect of coexpression with the β1 subunit on tefluthrin-induced, inactivation-resistant currents carried by Nav1.6 sodium channels. Values are means ±SE of normalized fractional current (I/Imax) measured following conditioning depolarizations to 0 mV in either HEK-Nav1.6 or HEK-Nav1.6β1 cells following exposure to four concentrations of tefluthrin (see also Figs. 7 and 8).
Sodium window currents
The area under the intersection of the curves for the voltage dependence of activation and steady-state fast inactivation is the sodium window current, which comprises a range of membrane potentials at which sodium channels are predicted to spontaneously activate but not inactivate. The voltage-dependent gating of Nav1.6 channels is tightly regulated, yielding small window currents (see Fig. 10A). However, the combined impact of pyrethroids on activation and steady-state inactivation significantly increased sodium window currents compared to control channels (Fig. 10). Tefluthrin at 10 μM markedly increased the probability of channel opening across a wide range of membrane potentials at which unmodified channels were either unresponsive or inactivated. Comparison of results obtained with HEK-Nav1.6 cells (Fig. 10A) and HEK-Nav1.6β1 cells (Fig. 10B) showed that coexpression with the β1 subunit further increased sodium window currents in tefluthrin-modified channels through effects on both voltage-dependent activation and inactivation. Specifically, the β1 subunit shifted the threshold for activation of modified channels further in the direction of hyperpolarization relative to control channels (as in Fig. 5), increased the amplitude of the reversal of inactivation at potentials between −55 mV and −25 mV, and increased the relative amplitude of the inactivation-resistant current (as in Fig. 9). Deltamethrin at 10 μM also markedly increased the probability of channel opening across a wide range of membrane potentials, but these effects were smaller than those found with tefluthrin. By contrast to results obtained with tefluthrin, coexpression with the β1 appeared to slightly diminish the impact of deltamethrin on window currents (compare Figs. 10C and 10D).
Fig. 10.
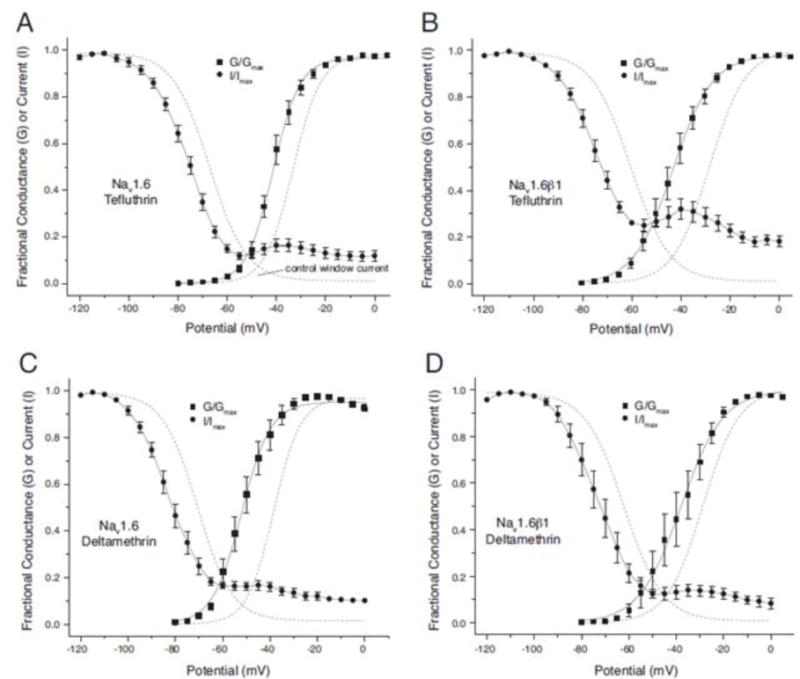
Effects of tefluthrin (A, B) or deltamethrin (C, D) on sodium window currents in HEK-Nav1.6 (A, C) and HEK-Nav1.6β1 (B, D) cells. Each panel shows voltage dependence plots for activation and steady-state inactivation in the presence of 10 μM tefluthrin or 10 μM deltamethrin in taken from data in Figs. 4 and 7. Dashed lines show the curve obtained by fitting mean control values for Nav1.6 and HEK-Nav1.6β1 cells to the Boltzmann equation (He and Soderlund, 2014).
Resting and use-dependent modification
Evidence for a requirement for the β1 subunit to observe use-dependent modification of Nav1.6 sodium channels expressed in Xenopus oocytes by pyrethroids (Tan and Soderlund, 2010), together with our previous finding of use-dependent enhancement of modification of Nav1.6β1β2 channels expressed in HEK293 cells by deltamethrin (He and Soderlund, 2011), led us to explore the effect of coexpression with the β1 subunit on use-dependent modification by pyrethroids of Nav1.6 sodium channels expressed in HEK293 cells. To assess use-dependent modification, we examined the extent of channel modification during a 40-ms test depolarization following either 0 or 100 brief (5 ms, 20 Hz) depolarizing prepulses. The β1 subunit had no statistically significant effect on either resting (0 prepulses) or use-dependent (100 prepulses) modification of Nav1.6 channels by 10 μM deltamethrin (Fig. 11). Figure 11 also includes results from our previous study (He and Soderlund, 2011) that illustrate the extent of use-dependent enhancement of modification with Nav1.6β1β2 channels. Similar experiments with 10 μM tefluthrin found no evidence of statistically-significant use-dependent enhancement of the modification in either the absence or presence of the β1 subunit (data not shown).
Fig. 11.
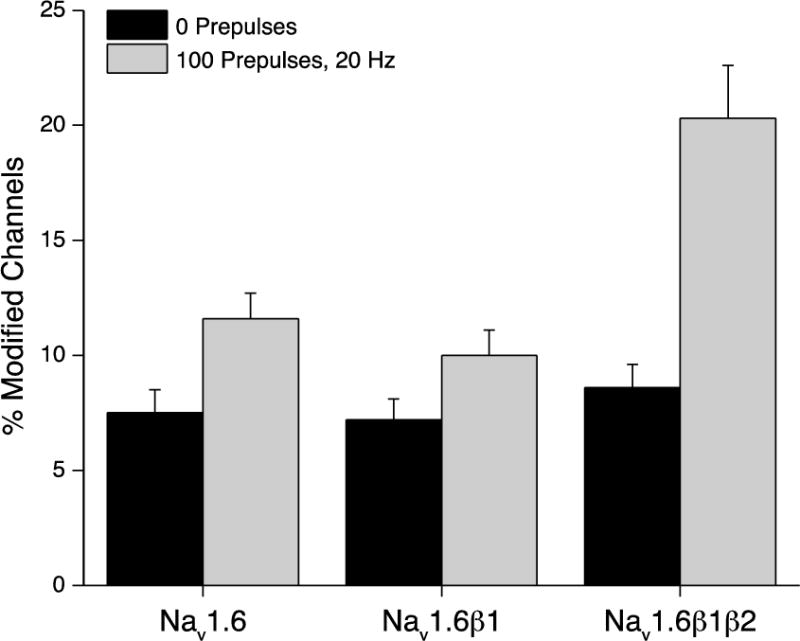
Effect of coexpression with the β1 subunit on the resting (0 prepulses) and use-dependent (100 prepulses) modification of Nav1.6 sodium channels by 10 μM deltamethrin. Values are means ± SE of 7 determinations. Comparable values for HEK-Nav1.6β1β2 cells from previously-published data (He and Soderlund, 2011) are provided here for comparison.
Discussion
Actions of tefluthrin and deltamethrin on Nav1.6 sodium channels
Most functional and pharmacological properties of voltage-gated sodium channels are intrinsic to the large, pore-forming α subunit (Catterall, 2000). We therefore overexpressed rat Nav1.6 sodium channel α subunits by stable transformation of HEK293 cells and characterized the actions of tefluthrin and deltamethrin on the expressed channels to provide a baseline for assessing the modulatory effects of the auxiliary β1 subunit. We selected tefluthrin and deltamethrin as examples of the two structural classes of pyrethroids (Type I and and Type II) that produce different syndromes of acute intoxication (T and CS, respectively) in mammals (Soderlund, 2012a). Our continued use of tefluthrin and deltamethrin as example compounds also facilitates direct comparison of the results of this study with our previous studies of the action of pyrethroids on sodium channels expressed either in Xenopus oocytes (Tan and Soderlund, 2009; Tan and Soderlund, 2010; Tan and Soderlund, 2011a; Tan and Soderlund, 2011b) or HEK293 cells (He and Soderlund, 2011).
Exposure of voltage-clamped HEK-Nav1.6 cells to pyrethroids at a hyperpolarized membrane potential, which placed channels predominantly in the resting state, resulted in profound modification of channel kinetics and voltage-dependent gating upon subsequent membrane depolarization. Both tefluthrin and deltamethrin produced a persistent “late” current during membrane depolarization and a sodium tail current following membrane repolarization. The induction of late and tail currents is a hallmark of pyrethroid action on sodium channels in both native neurons and heterologous expression systems (Soderlund et al., 2002; Soderlund, 2010b). The greater persistence of deltamethrin-induced late and tail currents is also typical of results obtained in other experimental systems with deltamethrin and other Type II pyrethroid structures (Soderlund et al., 2002).
In addition to their effects on gating kinetics, tefluthrin and deltamethrin also altered the voltage-dependent gating of Nav1.6 channels in HEK293 cells. Both insecticides caused concentration-dependent hyperpolarizing shifts in the voltage dependence of channel activation. However, the effects of both insecticides on the voltage dependence of activation measured using the peak transient sodium current underestimated the extent of these hyperpolarizing shifts because the pyrethroid-modified currents were composites of currents carried by pyrethroid-modified and unmodified channels at the highest pyrethroid concentrations tested.
Tefluthrin and deltamethrin also produced complex effects on the voltage dependence of steady-state fast inactivation of Nav1.6 channels. These compounds also shifted the voltage dependence of fast inactivation in the direction of hyperpolarization, but these shifts were smaller in magnitude than the corresponding hyperpolarizing shifts in the voltage dependence of activation found for each compound at the same concentration. Both tefluthrin and deltamethrin significantly also increased the population of Nav1.6 channels that were resistant to inactivation even after strong depolarizations.
The overall impact of tefluthrin and deltamethrin on the voltage dependence of channel gating is best gauged by considering the effects of these compounds on sodium window currents. The window current describes the range of membrane potentials at which channels are predicted to be persistently open and conducting a current (Attwell et al., 1979). The size of the window currents varies among sodium channel isoforms; larger window currents are correlated with significant persistent currents in voltage-clamp assays (Dib-Hajj et al., 2009). The rat Nav1.6 isoform produces small window currents (see Fig. 10) and correspondingly small persistent currents (see Fig. 3). The combined effects of either tefluthrin or deltamethrin on the voltage dependence of activation and steady-state fast inactivation greatly increased the size of sodium window currents compared to unmodified channels. Thus, these compounds function as persistent sodium channel activators by increasing the probability of channel opening at membrane potentials more negative than the normal threshold for channel activation and preventing complete channel inactivation following strong depolarizations.
The effects of tefluthrin and deltamethrin on the kinetics and voltage-dependent gating of Nav1.6 channels expressed in HEK293 cells were fully consistent with the effects of these compounds on Nav1.6 channels expressed as heterotrimeric complexes with the rat β1 and β2 (i.e., Nav1.6β1β2 channels) in HEK293 cells that we described previously (He and Soderlund, 2011). Thus, these effects are intrinsic to the interaction between pyrethroids and the Nav1.6 α subunit and are not mediated by the auxiliary β subunits. However, the effects of these compounds on Nav1.6 channels in the HEK293 cell expression system described here differ markedly from their effects on the same channels expressed in the absence of β subunits in the Xenopus oocyte expression system (Tan and Soderlund, 2011b). In Xenopus oocytes tefluthrin and deltamethrin also cause characteristic pyrethroid-like effects on the kinetics of channel inactivation and deactivation, producing slowly-decaying late and tail currents under voltage clamp conditions. However, these pyrethroids have no effect on the voltage-dependent gating of Nav1.6 channels in Xenopus oocytes. The significant hyperpolarizing shifts in the voltage dependence of Nav1.6 channel gating found in the present study nevertheless are consistent with previous results obtained in assays with tefluthrin on sodium channels in rat GH3 pituitary tumor cells (Wu et al., 2009) and with deltamethrin and tetramethrin on the TTX-sensitive and TTX-resistant components of the sodium current in rat dorsal root ganglion neurons (Tatebayashi and Narahashi, 1994; Tabarean and Narahashi, 1998).
The majority of experiments described here involved equilibration of Nav1.6 channels at membrane potentials well below the activation threshold and therefore represent the effects of channel modification in the resting state. However, evidence for preferential modification of some sodium channel isoforms in the open state by at least some pyrethroids (Soderlund, 2010a) led us to examine the effect of repeated short depolarizing prepulses on the modification of Nav1.6 channels by tefluthrin and deltamethrin. Consistent with results of similar assays of Nav1.6 channels in the absence of β subunits in the Xenopus oocyte expression system (Tan and Soderlund, 2011b), we found no evidence for preferential modification of open Nav1.6 channels upon expression in HEK293 cells.
Effects of the β1 auxiliary subunit on the modification of Nav1.6 sodium channels by tefluthrin and deltamethrin
Previously, we showed that coexpression of the rat Nav1.6 sodium channel α subunit with the rat β1 subunit in HEK293 cells produced depolarizing shifts in the voltage dependence of both activation and steady-state fast inactivation (He and Soderlund, 2014). Here, we report that the β1 subunit also modified the voltage-dependent gating of pyrethroid-modified Nav1.6 channels. Moreover, the effects of the β1 subunit were markedly different on channels modified by tefluthrin or deltamethrin.
Coexpression with the β1 increased the magnitude of the apparent tefluthrin-induced hyperpolarizing shift in the activation curve for Nav1.6 channels, but this effect was due entirely to a depolarizing shift in the activation curve for unmodified channels (He and Soderlund, 2014); the midpoint potentials for activation of both the composite peak currents and late currents measured in the presence of tefluthrin were not affected by the β1 subunit. By contrast, the β1 subunit had no effect on the magnitude of the midpoint potential shift in peak current activation for deltamethrin-modified channels, because the activation curves for both unmodified and deltamethrin-modified channels were shifted in the direction of depolarization to an equal degree. Thus, the depolarizing shift in the voltage dependence of Nav1.6 channel activation caused by the β1 subunit in the absence of insecticides was abolished by tefluthrin modification but not affected by deltamethrin modification.
The β1 subunit also differentially affected the voltage dependence of steady-state fast inactivation of tefluthrin- and deltamethrin-modified Nav1.6 channels. Coexpression with the β1 subunit increased the magnitude of the hyperpolarizing shift in the inactivation curve measured in the presence of tefluthrin, whereas the β1 subunit had no effect on the inactivation curve measured in the presence of deltamethrin. This result implies that tefluthrin, but not deltamethrin, antagonized the β1 subunit-induced depolarizing shift in the inactivation curve that we observed previously for unmodified channels (He and Soderlund, 2014). The β1 subunit also increased the relative amplitude of the maximum reversal of inactivation caused by tefluthrin following conditioning depolarizations to potentials between −55 mV and −25 mV and the amplitude of the inactivation-resistant component of sodium current measured in the presence of tefluthrin. However, the β1 subunit had no significant effect on either of these inactivation parameters for measured in the presence of deltamethrin.
The divergent effects of the β1 subunit on the voltage-dependent gating of tefluthrin- and deltamethrin-modified Nav1.6 sodium channels are most clearly evident in comparisons of sodium window currents obtained in the absence or presence of the β1 subunit. The combined effects of the β1 subunit on the activation and steady-state fast inactivation of tefluthrin-modified channels substantially increased the size of the tefluthrin-induced window current carried by Nav1.6β1 channels compared to that carried by Nav1.6 channels (compare Figs. 10A and 10B). By contrast the β1 subunit appeared to slightly diminish the size of the deltamethrin-induced window current (compare Figs. 10C and 10D). Thus, the β1 subunit selectively enhanced the ability of tefluthrin to act as a persistent activator of Nav1.6 channels at membrane potentials that would normally place channels in either the resting or inactivated states. The dramatic pyrethroid-induced changes in sodium window currents, and the effects of the β1 subunit on those currents, were not previously observed in assays of pyrethroid action on Nav1.6 and Nav1.6β1 sodium channels expressed in Xenopus oocytes because pyrethroids did not produce significant shifts in the voltage dependence of channel gating in that system (Tan and Soderlund, 2011b).
Assays in Xenopus oocytes of the impact of β subunits on the modification of Nav1.6 channels by pyrethroids revealed that the β1 subunit is required in order to observe use-dependent enhancement of modification by tefluthrin and deltamethrin (Tan and Soderlund, 2011b). To our surprise, we found no evidence for use-dependent effects of either pyrethroid in assays with HEK- Nav1.6β1 cells.
Inferred effects of the β2 auxiliary subunit on the modification of Nav1.6 sodium channels by tefluthrin and deltamethrin
We did not coexpress the auxiliary β2 subunit with the Nav1.6 α subunit because there is no persuasive evidence that such heterodimeric channel complexes exist in any abundance in native nerves. However, we previously described the action of tefluthrin and deltamethrin on heterotrimeric channels formed from the coexpression of the rat Nav1.6 α subunit and the rat β1 and β2 subunit (i.e., Nav1.6β1β2 channels) in HEK293 cells under experimental conditions identical to those employed in the present study (He and Soderlund, 2011). Comparison of results obtained in assays of Nav1.6β1β2 channels with those in the present study allows us to infer the impact of inclusion of β2 subunit on the modification of Nav1.6 channel complexes by pyrethroids.
Comparison of results obtained with HEK-Nav1.6β1 cells (this study) and HEK- Nav1.6β1β2 cells (He and Soderlund, 2011) did not identify any significant effects of the β2 subunit on the voltage-dependent gating of tefluthrin modified channels (see Figs. 5 and 8). However, inclusion of the β2 subunit in heterotrimeric complexes further reduced the magnitude of the hyperpolarizing shift in the midpoint potential for activation of deltamethrin-modified channels (see Fig. 5). Thus, the principal effect of the β2 subunit in Nav1.6β1β2 channels on voltage-dependent gating is a reduction in the size of the deltamethrin-induced window current when compared to Nav1.6β1 channels.
The most significant effect of the β2 subunit in these comparisons is evident in assays of use-dependent channel modification. Whereas the β1 subunit had no effect on use-dependent modification, inclusion of the β2 subunit conferred an approximately two-fold use-dependent enhancement of Nav1.6 channel modification by deltamethrin (see Fig. 11) but not tefluthrin. This result reinforces the importance of auxiliary β subunits as determinants of the preferential binding of certain pyrethroids to receptor sites on the open configuration of mammalian sodium channel α subunits (Soderlund, 2010a). The mechanism by which auxiliary subunits facilitate interactions between some pyrethroids and channels in the open state remains to be determined.
Conclusions
This study, together with our previous report of the action of tefluthrin and deltamethrin on Nav1.6β1β2 channels (He and Soderlund, 2011), represents the first analysis of the impact of sodium channel β subunits on the modification of a mammalian sodium channel by pyrethroid insecticides following functional reconstitution in the HEK293 cell expression system. These studies identified effects of the β subunits on voltage-dependent gating and use-dependent modification that differed markedly from those we found previously in assays of the same sodium channel subunit combinations and insecticides using the Xenopus oocyte expression system (Tan and Soderlund, 2010; Tan and Soderlund, 2011b). Moreover, the actions of these pyrethroids on Nav1.6 channel complexes expressed in HEK293 cells are much more consistent with the actions of the same pyrethroids on channel complexes of unspecified composition in native mammalian neurons (Tatebayashi and Narahashi, 1994; Tabarean and Narahashi, 1998; Wu et al., 2009) than are the actions of these compounds on Nav1.6 complexes expressed in oocytes. We conclude from these results that functional reconstitution of channels in vitro requires careful attention to both the subunit composition of channel complexes and the choice of heterologous expression system to ensure that channels in vitro are faithful functional and pharmacological models of channels in neurons.
Research Highlights.
We expressed Nav1.6 sodium channels with or without β1 subunits in HEK293 cells.
Tefluthrin and deltamethrin shifted channel gating to hyperpolarized potentials.
The β1 subunit had opposite effects on the actions of tefluthrin and deltamethrin.
Auxiliary subunits are required for full reconstitution of channel function.
Channels in HEK293 cells exhibit properties similar to channels in neurons.
Acknowledgments
This work was supported in part by grant number R01-ES013686 from the National Institute of Environmental Health Sciences, National Institutes of Health. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences. We thank P. Adams and S. Kopatz for technical assistance.
Role of Funding Sources
The National Institute of Environmental Health Sciences provided financial support but no other input into this project or the manuscript derived from it.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements for Authors
Neither B. He nor D. M. Soderlund has conflicts of interest regarding the research described in this manuscript.
References
- Attwell D, Cohen I, Eisner D, Ohba M, Ojeda C. The steady state TTX-sensitive (“window”) sodium current in cardiac Purkinje fibres. Pflugers Archiv. 1979;379:137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel a subunit with novel gating properties. Neuron. 1988;1:449–461. doi: 10.1016/0896-6273(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel. Journal of General Physiology. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized nodes of Ranvier, dendrites, and synapses. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Waxman SG. Voltage-gated sodium channels: therapeutic targets for pain. Pain Medicine. 2009;10:1260–1269. doi: 10.1111/j.1526-4637.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- Elliott M. The pyrethroids: early discovery, recent advances and the future. Pestic Sci. 1989;27:337–351. [Google Scholar]
- Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG. Sodium channel a-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): different expression patters in developing rat nervous system. Molecular Brain Research. 1997;45:71–82. doi: 10.1016/s0169-328x(96)00241-0. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annual Review of Physiology. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Expression of ion channels in Xenopus oocytes. In: Clare JJ, Trezise DJ, editors. Expression and analysis of recombinant ion channels. Wiley VCH Verlag GmbH & Co KGaA; Weinheim: 2006. pp. 1–25. [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. Journal of General Virology. 1977;36:59–77. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- He B, Soderlund DM. Differential state-dependent modification of rat Nav1.6 sodium channels expressed in human embryonic kidney (HEK293) cells by the pyrethroid insecticides tefluthrin and deltamethrin. Toxicology and Applied Pharmacology. 2011;257:377–387. doi: 10.1016/j.taap.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Soderlund DM. Functional expression of rat Nav1.6 voltage-gated sodium channels in HEK293 cells: modulation by the auxiliary β1 subunit. PLoS One. 2014;9:e85188. doi: 10.1371/journal.pone.0085188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCavera SJ, Soderlund DM. Differential binding of the pyrethroid insecticides S-bioallethrin, tefluthrin and deltamethrin to the open state of inactivation-deficient Nav1.6 sodium channels. Neurotoxicology. 2012;33:384–390. doi: 10.1016/j.neuro.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ. Developmentally-regulated sodium channel subunits are differentially sensitive to α-cyano containing pyrethroids. Toxicology and Applied Pharmacology. 2008;231:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovascular Research. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Patino GA, Isom LL. Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease. Neuroscience Letters. 2010;486:53–59. doi: 10.1016/j.neulet.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adeonviruses and the origin of HEK 293 cells. FASEB Journal. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. NeuroToxicology. 1998;19:823–832. [PubMed] [Google Scholar]
- Soderlund DM. Mode of action of pyrethrins and pyrethroids. In: Casida JE, Quistad GB, editors. Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses. Oxford University Press; New York: 1995. pp. 217–233. [Google Scholar]
- Soderlund DM. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pestic Biochem Physiol. 2010a;97:78–86. doi: 10.1016/j.pestbp.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Toxicology and mode of action of pyrethroid insecticides. In: Krieger RI, editor. Hayes’ Handbook of Pesticide Toxicology. Elsevier; New York: 2010b. pp. 1665–1686. [Google Scholar]
- Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol. 2012a;86:165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Pyrethroid action on sodium channels: isoform and species specificity. In: Knaak JB, Timchalk C, Tornero-Velez R, editors. Parameters for Pesticide QSAR and PBPK/PD Models for Human Risk Assessment. American Chemical Society; Washington, DC: 2012b. pp. 217–228. [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Sparks TC. Insecticide discovery: an evaluation and analysis. Pestic Biochem Physiol. 2013;107:8–17. doi: 10.1016/j.pestbp.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Narahashi T. Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the Type II pyrethroid deltamethrin. Journal of Pharmacology and Experimental Therapeutics. 1998;284:958–965. [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. 2009;30:81–89. doi: 10.1016/j.neuro.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Nav1.6 sodium channels. Toxicology and Applied Pharmacology. 2010;247:229–237. doi: 10.1016/j.taap.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Action of tefluthrin on rat Nav1.7 voltage-gated sodium channels expressed in Xenopus oocytes. Pestic Biochem Physiol. 2011a;101:21–26. doi: 10.1016/j.pestbp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Coexpression with auxiliary β subunits modulates the action of tefluthrin on rat Nav1.6 and Nav1.3 sodium channels. Pestic Biochem Physiol. 2011b;101:256–264. doi: 10.1016/j.pestbp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. Journal of Pharmacology and Experimental Therapeutics. 1994;270:595–603. [PubMed] [Google Scholar]
- Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. Journal of Pharmacological and Toxicological Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Whitaker WRJ, Clare JJ, Powell AJ, Chen YH, Faull RLM, Emson PC. Distribution of voltage-gated sodium channel α-subunit and β-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. Journal of Comparative Neurology. 2000;422:123–139. doi: 10.1002/(sici)1096-9861(20000619)422:1<123::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Whitaker WRJ, Faull RLM, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Molecular Brain Research. 2001;88:37–53. doi: 10.1016/s0169-328x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- Wu SN, Wu YH, Chen BS, Liu YC. Underlying mechanism of action of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology. 2009;2009:70–77. doi: 10.1016/j.tox.2009.01.009. [DOI] [PubMed] [Google Scholar]


