Abstract
There remain significant obstacles in developing biologics to treat primary biliary cholangitis (PBC). Although a number of agents have been studied both in murine models and human patients, the results have been relatively disappointing. IL-22 is a member of the IL-10 family and has multiple theoretical reasons for predicting successful usage in PBC. We have taken advantage of an IL-22 expressing adeno-associated virus (AAV-IL-22) to address the potential role of IL-22 in not only protecting mice from autoimmune cholangitis, but also in treating animals with established portal inflammation. Using our established mouse model of 2-OA-OVA immunization, including α-galactosylceramide (α-GalCer) stimulation, we treated mice both before and after the onset of clinical disease with AAV-IL-22. Firstly, AAV-IL-22 treatment given prior to 2-OA-OVA and α-GalCer exposure, i.e. before the onset of disease, significantly reduces the portal inflammatory response, production of Th1 cytokines and appearance of liver fibrosis. It also reduced the liver lymphotropic chemokines CCL5, CCL19, CXCL9, and CXCL10. Secondly, and more importantly, therapeutic use of AAV-IL-22, administered after the onset of disease, achieved a greater hurdle and significantly improved portal pathology. Further the improvements in inflammation were negatively correlated with levels of CCL5 and CXCL10 and positively correlated with levels of IL-22. In conclusion, we submit that the clinical use of IL-22 has a potential role in modulating the inflammatory portal process in patients with PBC.
Keywords: IL-22, liver autoimmune disease, adeno-associated virus, chemokine, therapy
Introduction
IL-22 is a member of the IL-10 cytokine family, produced by a variety of immune cells, including T helper (Th) 17, Th22, γδ T, NKT, and innate lymphoid cells (ILCs) (1, 2). The IL-22 receptor (IL-22R) is a heterodimer that consists of IL-22R1 and the IL-10 receptor β subunit and is expressed by non-hematopoietic cells such as hepatocytes, keratinocytes, and epithelial cells of lung and intestine (3). IL-22 has a protective role in several experimental models of hepatic injury, including T cell-mediated hepatitis, liver ischemia-reperfusion injury, bacterial and parasitic infection, acute and chronic alcohol-induced liver damage and liver fibrosis (4–10). IL-22 has also been shown to modulate inflammation in other autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Sjögren's syndrome (SS), psoriasis, experimental autoimmune uveitis and experimental autoimmune myocarditis (11–18). However, IL-22, while protective before the onset of collagen induced arthritis, can exacerbate pathology if administered during the course of disease (19, 20). This dual role of IL-22 is also reflected in some murine models of psoriasis-like skin inflammation (15, 16).
Our laboratory has extensively studied a murine model of primary biliary cholangitis (PBC) induced following immunization with a mimotope of the inner lipoyl domain of the major mitochondrial autoantigen of PBC. This mimotope of PDC-E2 is coined 2-octynoic acid (2-OA). Mice immunized with 2-OA coupled to either BSA or OVA, and stimulated with α-galactosylceramide (α-GalCer) develop high titer anti-mitochondrial antibodies (AMAs) and autoimmune cholangitis with lymphocytic infiltrates, portal inflammation, granuloma formation, bile duct damage, and fibrosis (21, 22). In the study herein, we investigated whether IL-22 could modulate the natural history of autoimmune cholangitis by intravenously injecting mice either before or after the establishment of hepatic pathology with an IL-22 expressing recombinant adeno-associated virus (AAV-IL-22). Recombinant AAV is an appropriate vector for gene transfer in vivo based upon its replication defective nature and capability of infecting a broad range of cell types including non-dividing cells. It is retained in vivo as concatemers for long-term expression and elicits only a mild immune response compared with the older use of adenovirus (23). We report herein that the use of IL-22 not only prevents clinical autoimmune cholangitis but, more importantly, significantly reduces the portal inflammatory response even in mice with established clinical pathology. We submit that the mechanisms defined herein, namely the downregulation of specific chemokine pathways, suggest that IL-22 may be an attractive therapeutic venue for investigative study in humans with PBC.
Materials and Methods
Experimental mice
Female C57BL/6 mice aged 7-9 weeks were obtained from the National Laboratory Animal Center, Taiwan and mice maintained in the Animal Center of the College of Medicine, National Taiwan University. All experiments were performed following approval of The Institutional Animal Care and Use Committee (IACUC) of National Taiwan University College of Medicine and College of Public Health.
Preparation of AAV-IL-22
IL-22 cDNA was expressed and reversed from activated mouse T cells and cloned to adeno-associated virus vector (AAV-DJ). AAV-DJ is a recombinant AAV produced by a complex library of hybrid capsids from 8 different wild-type viruses (Cell Biolabs, San Diego, CA, USA). Earlier work has demonstrated that AAV-DJ vectors are not only superior to HBD-negative wild-type viruses (up to 100,000-fold superior to AAV-8 or AAV-9), but are also substantially better than AAV-2 (Cell Biolabs) (24). Briefly, the 540-bp cDNA containing murine IL-22 was inserted into a recombinant adeno-associated viral vector (pAAV-IRES-GFP). IL-22 inserted pAAV-IRES-GFP plasmid was co-transfected with pAAV-DJ and pHelper at a ratio of 1:1:1 into the adenovirus packaging AD293 cell line. Viruses were purified from infected cells 42–48 hours after infection by three freeze-thaw cycles followed by Hi-Trap Heparin column. Viral titers (transduction unit, TU) were measured by GFP expression in infected 293T cells using flow cytometry. Throughout these studies a mock AAV was used as a control; it did not contain a transgene in the expression cassette.
Experimental protocol
Female mice, at 7-9 weeks of age, were intraperitoneally immunized with 2-OA-OVA in the presence of complete Freund's adjuvant (CFA, Sigma-Aldrich, St. Louis, MO, USA) and subsequently boosted at weeks 2, 4, 6 and 8 with 2-OA-OVA in incomplete Freund's adjuvant (IFA, Sigma-Aldrich). Two μg of α-galactosylceramide (Funakoshi, Tokyo, Japan) were injected with the first and second 2-OA-OVA immunizations. AAV-IL-22 was administered to mice at 3 days before (the preventive study) or at 3 weeks after (the therapeutic study) the first 2-OA-OVA immunization. Three weeks was chosen as the time period for the therapeutic phase of this study because at 3 weeks following initial immunization with 2-OA-OVA, mice exhibit florid portal inflammation (see below). Sera were obtained on all mice at 10 weeks post-immunization and levels of IL-22 were measured by ELISA. In addition in nested subgroups, mice were sacrificed at 5 weeks post immunization for hepatic chemokine assays; additional mice were sacrificed 10 weeks post-immunization for liver histopathology, definition of mononuclear cell phenotypes, cytokine profiles and titers of AMAs. All experiments were performed a minimum of 2–3 times with group sizes of 11–15 mice. Prior to this experimental protocol, two pilot studies were undertaken. Firstly, we assessed the basal level of expression of IL-22 as well as hepatic IL-22 by qRT-PCR in mice either immunized with 2-OA-OVA using our standard protocol or, for the purpose of controls, immunized with saline only. In addition, to verify the activity of AAV-IL-22, naive mice were injected with either AAV-IL-22, mock virus or normal saline and the levels of sera IL-22 and hepatic IL-22 mRNA quantitated at 10 weeks of age.
Determination of serum AMAs
Serum titers of IgM and IgG anti-PDC-E2 autoantibodies were measured by ELISA using our well standardized recombinant PDC-E2 (25). Briefly, purified mouse recombinant PDC-E2 at 10 μg/ml in carbonate buffer (pH 9.6) was coated onto ELISA plates at 4°C overnight. After blocking with 1% casein (Sigma-Aldrich), diluted sera was added for 2 hours at room temperature. In parallel one positive pool serum was diluted serially and added to each plate to constitute an internal standard. HRP-labelled anti-mouse IgG or IgM (Invitrogen, Camarillo, CA, USA) diluted 1/10000 in blocking buffer were added for detection of mouse antibodies. Optical density was read at 450 nm.
Liver mononuclear cell quantitation
Livers were perfused with PBS containing 0.2% BSA (PBS/0.2% BSA), passed through a 100μm nylon mesh, and re-suspended in PBS/0.2% BSA. The parenchymal cells were removed as pellets after centrifugation at 50 g for 5 minute and the non-parenchymal cells isolated using Histopaque-1077 (Sigma-Aldrich) (25) or 40% and 70% Percoll (GE HealthCare Biosciences, Quebec, Canada). After centrifugation, collected cells were washed with PBS/0.2% BSA and viability of cells was confirmed by trypan blue dye exclusion. Subsets of liver mononuclear cells were measured by flow cytometry. Before staining cells, with a previously defined optimal dilution of monoclonal antibodies (Abs), the cells were pre-incubated with anti-CD16/32 (clone 93) to block non-specific FcRγ binding. The following Abs were used in this study: anti-CD3, anti-CD4, anti-CD8a, anti-CD19, anti-NK1.1 (Biolegend, San Diego, CA, USA). For Foxp3 staining, after the cell surface staining, Foxp3 intracellular staining was performed using a commercially available kit from Biolegend. Stained cells were assessed on a FACSCalibur using FlowJo software (Tree Star, Inc., Ashland, OR, USA). Optimal concentrations of the mAbs were used throughout and all assays included positive and negative controls.
IL-22 levels and IL-22R1 expression
IL-22 expression in liver and IL-22R1 expression in liver mononuclear cells were detected by reverse transcription PCR. TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) was used to isolate RNA. cDNA was prepared using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) to reverse transcribe the isolated RNA. PCR were amplified for 35 cycles (1 min at 95°C, 1 min at 66°C, and 1 min at 72°C) and the products detected by 2% agarose ethidium bromide gel. H2O was used as a negative control, and LPS treated liver as a positive control. Serum levels of IL-22 were assayed by ELISA (R&D Systems, Minneapolis, MN, USA) and using positive and negative controls.
Determination of IFN-γ, TNF-α, collagen I, collagen III, and chemokines
IFN-γ, TNF-α, collagen I, collagen III, and chemokine expression in liver were detected by quantitative real time PCR on a 7500 Fast Real-Time PCR System (Applied Biosystems). β-actin serves as an internal control, and H2O serves as a negative control. Amplification reactions included oligonucleotide primers for each target gene, and for β-actin and as well as platinum Taq polymerase and SYBR Green DNA-binding dye. Fluorescence signals were analyzed during each of 40 cycles (denaturation 15 seconds at 95°C, annealing 15 seconds at 56°C, and extension 40 seconds at 72°C). Relative quantification was calculated using the comparative threshold cycle (CT) method. Primer sequences used in PCR are in Table 1.
Table 1.
List of primers for quantitative RT-PCR
| Gene Name | Sequence | |
|---|---|---|
| Mouse β-actin | Forward | 5' CACAGTGTTGTCTGGTGGTA 3' |
| Reverse | 5' GACTCATCGTACTCCTGCTT 3' | |
| CCL5 | Forward | 5' AGATCTCTGCAGCTGCCCTCA 3' |
| Reverse | 5' GGAGCACTTGCTGCTGGTGTAG 3' | |
| CXCL9 | Forward | 5' ATTGTGTCTCAGAGATGGTGCTAATG 3' |
| Reverse | 5' TGAAATCCCATGGTCTCGAAAG 3' | |
| CXCL10 | Forward | 5' GGATGGCTGTCCTAGCTCTG 3' |
| Reverse | 5' TGAGCTAGGGAGGACAAGGA 3' | |
| CXCL16 | Forward | 5' ACCCTTGTCTCTTGCGTTCTTCCT 3' |
| Reverse | 5' ATGTGATCCAAAGTACCCTGCGGT 3' | |
| CX3CL1 | Forward | 5' ACGAAATGCGAAATCATGTGC 3' |
| Reverse | 5' CTGTGTCGTCTCCAGGACAA 3' | |
| CCL19 | Forward | 5' CTGCCTCAGATTATCTGCCAT 3' |
| Reverse | 5' AGGTAGCGGAAGGCTTTCAC 3' | |
| CCL21 | Forward | 5' AAGGCAGTGATGGAGGGG 3' |
| Reverse | 5' CGGGGTAAGAACAGGATTG 3' | |
| IFN-γ | Forward | 5' GGCCATCAGCAACAACATAAGC 3' |
| Reverse | 5' TGGACCACTCGGATGAGCTCA 3' | |
| TNF-α | Forward | 5' CCCCAAAGGGATGAGAAGTTC 3' |
| Reverse | 5' TGAGGGTCTGGGCCATAGAA 3' | |
| IL-10 | Forward | GGTTGCCAAGCCTTATCGGA |
| Reverse | ACCTGCTCCACTGCCTTGCT | |
| Collagen-I | Forward | 5' ACGTCCTGGTGAAGTTGGTC 3' |
| Reverse | 5' CAGGGAAGCCTCTTTCTCCT 3' | |
| Collagen-III | Forward | 5' GTTCTAGAGGATGGCTGTACTAAACACA 3' |
| Reverse | 5' TTGCCTTGCGTGTTTGATATTC 3' | |
Histopathology
Portions of liver were excised and immediately fixed with 10% buffered formalin solution for 2 days at room temperature. Paraffin-embedded tissue sections were then cut into 4-μm slices for routine hematoxylin and eosin (H-E) and Masson's trichrome staining and observed by a blinded observer, Dr. C-E Loh.
Statistical analysis
Mann-Whitney U analysis was used to determine significant differences between groups. The Spearman test was used to evaluate correlations (Prism 5; Graph-Pad Software, La Jolla, CA, USA). Results are expressed as the mean ±standard error of the mean (SEM). Statistically significant differences were defined as P values of less than 0.05, less than 0.01, and less than 0.001.
Results
The kinetics of IL-22 expression
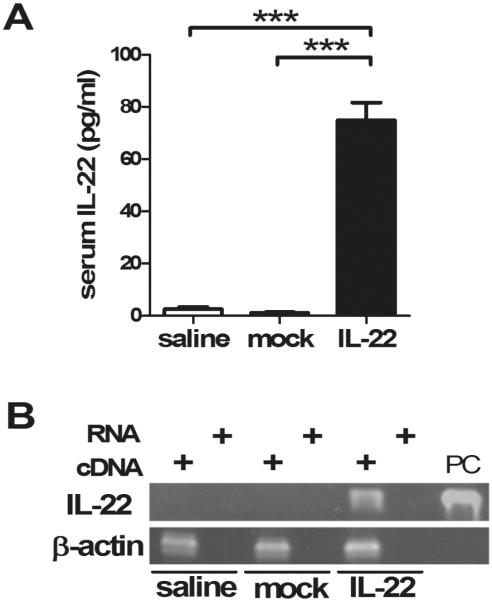
Firstly, we determined the basal expression level of IL-22 in the basic 2-OA-OVA immunized model and as expected there was no significant detection of either sera IL-22 or hepatic IL-22 by qRT-PCR in mice only exposed to the 2-OA-OVA protocol (Figure 1A, saline group). Thence, we examined mice for sera and hepatic IL-22 following treatment with a single intravenous injection of AAV-IL-22 or mock virus at a dose of 3×107 TU/mouse and then immunized with 2-OA-OVA. As shown in Figure 1A, IL-22 was readily detected in the serum of AAV-IL-22 injected mice but not in controls injected either with mock virus or normal saline. Similarly IL-22 mRNA expression was only detectable in the liver of mice injected with AAV-IL-22 (Figure 1B). Importantly, and as further controls, mice, at 10 weeks after injection, that were not immunized with 2-OA-OVA but injected with either AAV-IL-22 or the AAV-mock remained healthy without changes in body weight or abnormalities in liver histopathology (data not shown). Hence, our protocol, including the administration of AAV-IL-22 through a single intravenous injection was sufficient to achieve systemic transgenic expression but did not produce liver pathology. Further, we noted that there was production of AMA and intense portal inflammation at 3 weeks post immunization in the protocol used herein (Figure 2); hence the choice of this data point for the therapeutic trial.
Figure 1. IL-22 expression in mice.
Mice were injected with AAV-IL-22 (3×107TU/mouse), AAV mock (3×107TU/mouse) or normal saline three days before the first 2-OA-OVA immunization and sacrificed at week 10. (A) Serum IL-22 was measured by ELISA. n=15 mice per group. ***, p<0.001. (B) IL-22 expression in the liver was analyzed by PCR. RNA was converted to cDNA. PC, positive control.
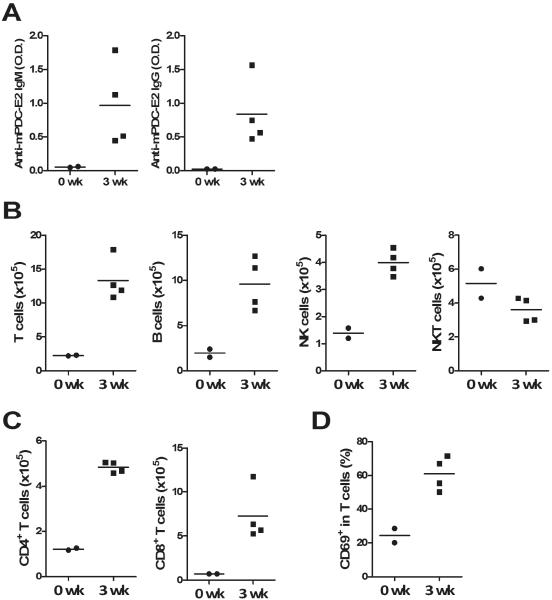
Figure 2. Portal inflammation was found at 3 weeks post immunization.
Mice were immunized with 2-OA-OVA and sacrificed at week 3. (A) Anti-PDC-E2 IgM and IgG were measured. O.D., optical density. (B) The numbers of T, B, NK and NKT cells in the liver were measured. (C) The numbers of CD4+ and CD8+ T cells in the liver were measured. (D) The frequency of activated T cells were detected. Each symbol represents an individual mouse and the horizontal lines represent the means.
Prophylactic use of IL-22 decreased autoimmune cholangitis and liver fibrosis
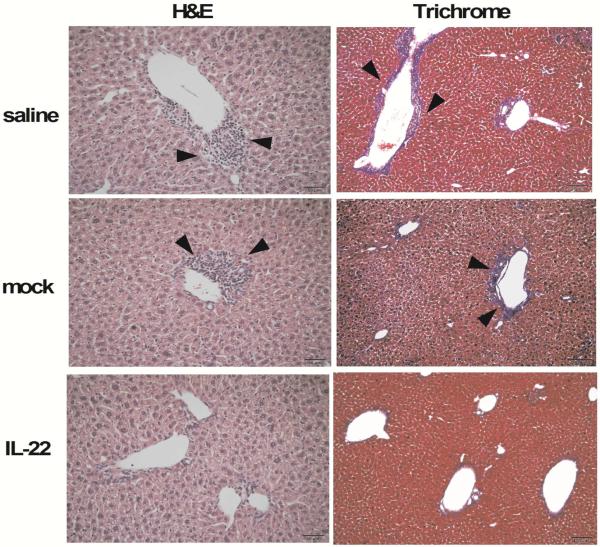
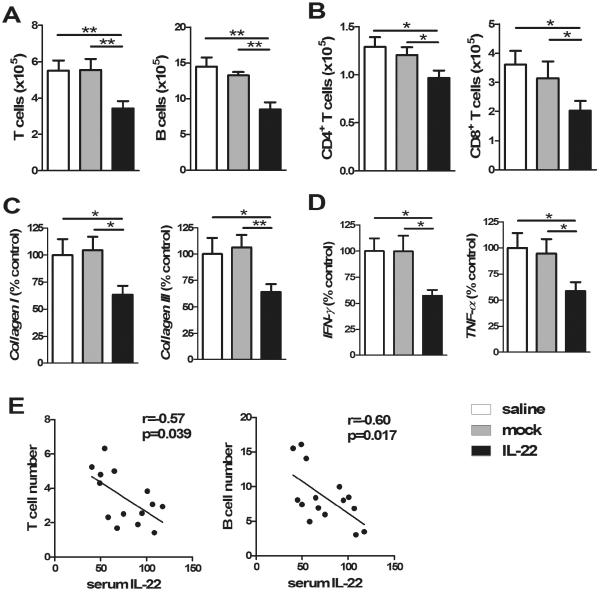
As expected control mice immunized with 2-OA-OVA as well as treated with AAV-mock and normal saline developed the expected degree of portal inflammation and fibrosis 10 weeks following immunization. In contrast, mice injected with AAV-IL-22 had a significant reduction in portal inflammation and fibrosis (Figure 3). These histologic observations are supported by quantitation of liver lymphocytic infiltrates in AAV-IL-22 treated 2-OA-OVA immunized mice compared to either the AAV mock or the normal saline control groups (Figure 4A and B). Consistent with this data, there was a lower expression of collagen I and III in mice treated with AAV-IL-22 compared to the AAV-mock group and normal saline controls (Figure 4C). Moreover, the Th1 cytokines, IFN-γ and TNF-α, were also significantly reduced in the liver of AAV-IL-22 treated mice than controls (Figure 4D). Interestingly there were no significant differences in the titers of anti-PDC-E2 antibodies amongst the three groups (data not shown). Of note, in AAV-IL-22 treated 2-OA-OVA immunized mice, the numbers of T and B cells in the liver had a significant negative correlation with the levels of serum IL-22 (r=−0.57, p<0.05 and r=−0.60, p<0.05, respectively) (Figure 4E). Taken together, intravenous administration of AAV-IL-22 before the initiation of PBC significantly reduced autoimmune cholangitis and liver fibrosis in 2-OA-OVA immunized mice.
Figure 3. Decreased portal inflammation and fibrosis in prophylactic IL-22 treated 2-OA-OVA immunized mice.
Mice were injected with AAV-IL-22 (3×107TU/mouse), AAV mock (3×107TU/mouse) or normal saline three days before the first 2-OA-OVA immunization and sacrificed at week 10. Representative stained liver sections of haematoxylin and eosin (H&E) (×200 magnification) and Masson's trichrome stain (×100 magnification). Arrowheads indicate cell infiltration in the liver (left panel) and fibrosis (right panel) in the liver.
Figure 4. Decreased lymphocytic infiltrates, cytokine levels, collagen levels in prophylactic IL-22 treated 2-OA-OVA immunized mice.
Mice were injected with AAV-IL-22 (3×107TU/mouse), AAV mock (3×107TU/mouse) or normal saline three days before the first 2-OA-OVA immunization and sacrificed at week 10. (A) The numbers of T and B cells in the liver were measured. (B) The numbers of CD4+ and CD8+ T cells in the liver were measured. (C) The expressions of collagen I and collagen III mRNA in the liver were detected by qRT-PCR. (D) The expressions of IFN-γ and TNF-α mRNA in the liver were detected by qRT-PCR. The results from normal saline treated 2-OA-OVA immunized mice (control) were set to a value of 100%. (E) The relationship between the serum level of IL-22 and liver T cells or B cells in the IL-22 treated 2-OA-OVA immunized mice. Each dot represents an individual mouse. n=15 mice per group.*, p<0.05; **, p<0.01.
Regulatory T cells and IL-10 in the liver of AAV-IL-22 treated 2-OA-OVA immunized mice
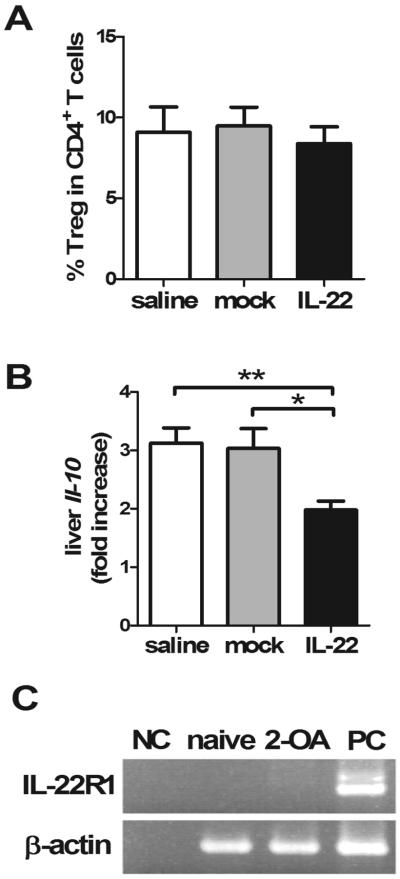
As IL-22 significantly decreased portal inflammation and liver fibrosis in 2-OA-OVA immunized mice, we hypothesized that IL-22 induced regulatory T cells and/or IL-10. As shown in Figure 5, there was no change in the frequency of regulatory T cells in the liver of AAV-IL-22 treated 2-OA-OVA immunized mice compared to controls (Figure 5A). In fact, liver expression of IL-10 was significantly decreased in AAV-IL-22 treated mice (Figure 5B), suggesting that the immunological effects of IL-22 in this model were not secondary to either regulatory T cells or IL-10. In addition, there was no expression of IL-22R in the liver mononuclear cells of naive or 2-OA-OVA immunized mice (Figure 5C).
Figure 5. There was no increase of regulatory T cells in IL-22 treated 2-OA-OVA immunized mice.
Mice were injected with AAV-IL-22 (3×107TU/mouse), AAV mock (3×107TU/mouse) or normal saline three days before the first 2-OA-OVA immunization and sacrificed at week 10. (A) The frequency of regulatory T cells (CD3+CD4+Foxp3+) in the liver was measured. Liver mononuclear cells were stained with anti-CD3, CD4 and Foxp3 Abs and assayed by a flow cytometer. (B) The expression of IL-10 in the liver was measured by qRT-PCR. This data represents the fold increase of data normalized to the naïve mice. n=15 mice per group. *, p<0.05; **, p<0.01. (C) Liver mononuclear cells from 2-OA-OVA immunized mice were collected and IL-22R expression was measured by RT-PCR. Naive, naïve mice; 2-OA, 2-OA-OVA immunized mice; NC, negative control; PC, positive control.
Expression of CCL5, CCL19, CXCL9, CXCL10 in liver
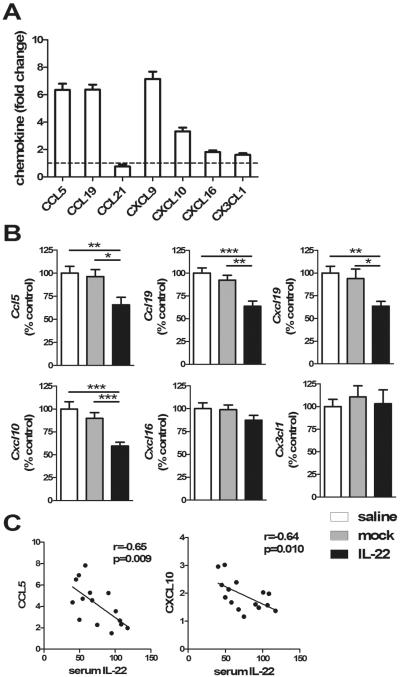
As noted in Figure 6A, compared to naïve mice, 2-OA-OVA immunized mice had a 3- to 8-fold increase in CCL5, CCL19, CXCL9, and CXCL10, 2-fold increase in CXCL16 and CX3CL1, and no increase in CCL21. There were no differences in these chemokines between normal saline and mock groups. In AAV-IL-22 treated 2-OA-OVA immunized mice, a 35–40 % decrease of CCL5, CCL19, CXCL9, and CXCL10 were observed compared to normal saline treated- and AAV-mock treated controls. However, there were no differences in the production of CXCL16 and CX3CL1 amongst the three groups (Figure 6B). Correlation analysis demonstrated that the chemokines CCL5 and CXCL10 in the liver were negatively correlated with serum levels of IL-22 in IL-22 treated 2-OA-OVA immunized mice (r=−0.65; p<0.01 and r= −0.64; p<0.05, respectively) (Figure 6C). These results suggest that increased expression of specific chemokines in 2-OA-OVA immunized mice stimulated recruitment of endogenous proinflammatory lymphocytes to the site of biliary inflammation; IL-22 suppressed the production of these chemokines.
Figure 6. Decrease of CCL5, CCL19, CXCL9, and CXCL10 in the liver of IL-22 treated 2-OA-OVA immunized mice.
(A) Mice were immunized with 2-OA-OVA and liver chemokine expression at 5 weeks post immunization was measured by qRT-PCR. This data represents the fold change of data normalized to the naïve mice. n=10 mice. (B) Mice were injected with AAV-IL-22 (3×107TU/mouse), AAV mock (3×107TU/mouse) or normal saline three days before the first 2-OA-OVA immunization and sacrificed at week 5. The expressions of chemokines in liver were measured by qRT-PCR. The results of each molecule from normal saline treated 2-OA-OVA immunized mice (control) were set to a value of 100%. (C) The relationship between the serum level of IL-22 and liver expression of CCL5 or CXCL10 in the IL-22 treated 2-OA-OVA immunized mice. Each dot represents an individual mouse. n=15 mice per group.*, p<0.05; **, p<0.01; ***, p<0.001.
Therapeutic use of AAV-IL-22 decreased autoimmune cholangitis and liver fibrosis
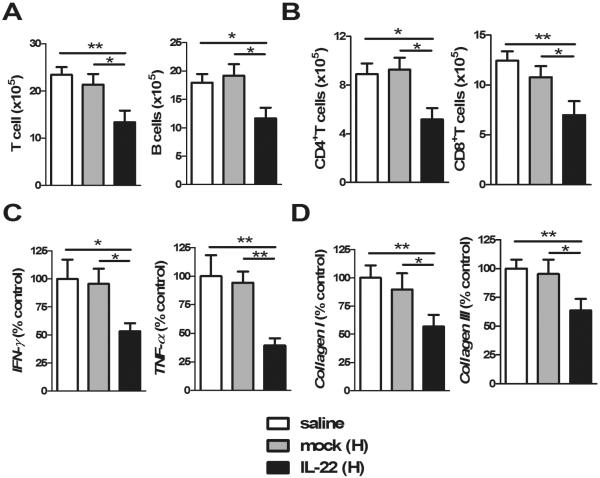
Importantly, based on the results of the preventive action of AAV-IL-22 in this model, we then studied whether IL-22 would attenuate autoimmune cholangitis in mice with established disease. Mice were treated with AAV-IL-22 or mock virus 3 weeks after 2-OA-OVA immunization and then studied at 10 weeks. At a dose of 3×107TU/mouse of AAV-IL-22, there were no changes in lymphocytic infiltrates, cytokine levels, collagen levels or anti-PDC-E2 antibodies (data not shown). However, when a dose of 9×107TU of AAV-IL-22 was used, there was a significant reduction in lymphocytic infiltrates, cytokine levels and collagen expression (Figure 7). However, there were no significant changes in the titers of anti-PDC-E2 antibodies in the mice independent of treatment.
Figure 7. High doses of AAV-IL-22 delivery at 3 weeks post 2-OA-OVA immunization reduced portal pathology.
Mice were injected with AAV-IL-22 (9×107TU/mouse), AAV mock (9×107TU/mouse) or normal saline at 3 weeks after the first 2-OA-OVA immunization and sacrificed at week 10. (A) The numbers of T and B cells in the liver were measured. (B) CD4+ and CD8+ T cells in the liver were measured. (C) The expressions of IFN-γ and TNF-α mRNA in the liver were detected by qRT-PCR. (D) The expressions of collagen I and collagen III mRNA in liver were detected by qRT-PCR. The results from normal saline treated 2-OA-OVA immunized mice (control) were set to a value of 100%. n=11–13 mice per group. *, p<0.05; **, p<0.01.
Discussion
Primary biliary cholangitis (PBC) is a progressive autoimmune liver disease characterized by immune-mediated destruction of intrahepatic small bile ducts, and production of anti-mitochondrial antibodies (26). Although there have been intensive efforts at understanding the genetic basis of PBC and major advances in dissecting underlying immunological defects, such data has not led to major new therapeutic agents (27–30). Characteristically, the disease is strongly associated with autoimmune phenomena such as the appearance of serum anti-mitochondrial autoantibodies and portal infiltrating T cells against the inner lipoyl domain in the E2 component of the pyruvate dehydrogenase complex (PDC-E2) (27, 31, 32). Although PBC is an autoimmune disease, there is not a clear benefit from immunosuppressive therapy, including corticosteroids, azathioprine, cyclosporine, and methotrexate (33). Treatment of patients with PBC is still not disease-specific and the standard of care involves therapy with the secondary bile acid ursodeoxycholic acid (UDCA) (34–36). We note, based upon the data herein, that the therapeutic improvements appear secondary to altering chemokine function and not due to several other T and B cell defects or autoantibody production which have been shown in patients with PBC (37–39).
In the study herein, we have demonstrated significant therapeutic potential of IL-22 in this model. We should emphasize that IL-22, by itself, did not appear to be important in the natural history of autoimmune cholangitis and liver fibrosis. However, when IL-22 in sera and liver achieved a pharmacologic increase, we demonstrated significant therapeutic benefits, whether administered before or after the onset of clinical disease. Importantly, this effect appears secondary to selective changes in lymphocytic homing and recruitment and not secondary to changes in regulatory T cell responses or levels of IL-10. These observations should be taken in the context that there are numerous mechanisms involved in the regulation of autoimmunity, including regulatory T cells and their interactions with forkhead box P3 (FoxP3)+ CD4+CD25+ cells, IL-10 secreting cells, and TGF-β-secreting cells. These latter effects appear secondary to changes in inflammatory cytokines, cell-cell contact, modulation of activation stage and alterations of antigen presenting cell function (40, 41). We submit that our results are secondary to chemokine expression and emphasize the potential of altering homing characteristics of lymphoid elements to modulate inflammatory diseases.
IL-22R1 expression is restricted to cells of epithelial origin, including hepatocytes and liver progenitor cells. Immune cells do not respond to IL-22 stimulation because of the lack of IL-22R1 expression (3). However, in some inflammatory autoimmune conditions, IL-22R1 expression can be detected on T cells and antigen presenting cells. In that case, IL-22 inhibits IFN-γ production of T cells directly or by IL-22 induced regulatory antigen presenting cells (17, 19). In our study there was no detectable IL-22R1 on the lymphoid infiltrates on the 2-OA-OVA immunized mice; this suggests that IL-22 is acting directly on lymphoid elements and emphasizes, again, the likelihood that its therapeutic effect is due to homing alterations.
Chemokines direct leukocyte trafficking and positioning within tissues, thus playing critical roles in regulating immune responses and inflammation. Chemokines CCL3, 4, 5, CXCL9, 10, 11, CXCL16, CX3CL1, and CCL19, 21 have been reported to play a role in liver inflammation (42). Lymphocytes that express CCR5 can be recruited to cross the portal endothelium, mediated in part by CCL3–5 (43). In addition, CXCL9–11 and CXCL16 recruit lymphocytes across the liver sinusoidal endothelium (44, 45). In addition, CXCL3 expression on biliary epithelial cells has been associated with recruitment of CX3CL1 expressing cells (46). In patients with PBC, increased expression of CXCL9, CXCL10, CXCL16, and CX3CL1 in the portal tracts have been reported (45, 47, 48). In 2-OA-OVA immunized mice, elevated expressions of CCL5, CCL19, CXCL9, CXCL10, CXCL16 and CX3CL1 in the liver were noted compared to naïve mice, suggesting that these chemokines are responsible for recruiting lymphocytes that bear their cognate receptors. Administration of IL-22 in vivo decreases hepatic expression of CCL5, CCL19, CXCL9, and CXCL10. In particular, CCL5 and CXCL10 were negatively correlated with levels of serum IL-22. These data suggest that IL-22 suppresses lymphocytic infiltration by decreasing the chemokines CCL5, CCL19, CXCL9, and CXCL10. Indeed, these findings are consistent with an earlier in vitro study in which IL-22 reduced CCL2 and CCL5 production of IFN-γ stimulated human hepatic stellate cells and CXCL9, CXCL10, CCL5 production of IFN-γ stimulated human hepatocytes (49).
There are multiple pathways in which IL-22 has shown involvement and indeed any studies involving therapeutic usage of IL-22 must take into account the observations that IL-22 may induce the anti-apoptotic proteins Bcl-2 and Bcl-XL, cyclin-dependent kinase 4 (CDK4), cyclin D1, c-myc, and p21 (4–6). In addition, IL-22 induces the senescence of hepatic stellate cells, which express both IL-10R2 and IL-22R1, thereby ameliorating liver fibrogenesis in carbon tetrachloride (CCl4)-induced liver fibrosis model (10). We should also emphasize that IL-22 may exacerbate chronic liver inflammation, at least in an HBV transgenic mouse model (49–51). Therefore, the use of IL-22 will depend very much on its mode of administration and the specific disease studied. However, the proof of principles emphasized herein emphasizes its potential usage in PBC, obviously in patients refractory to other therapies.
Highlights.
IL-22 engineered AAV vectors can be used to treat primary biliary cirrhosis
The use of such vectors not only prevents, but also treats mice with established biliary disease
The mechanism of this therapy is down-regulation of specific chemokines.
Such therapy has implications for directed immunotherapy which alters homing of autoreactive cells
Acknowledgements
We thank Yu-Ping Chiang (National Taiwan University Hospital) for providing technical advice.
Financial Support: This work was supported by grants from the Ministry of Science and Technology, Taiwan, MOST 104-2320-B-002-033-MY3 (YHC), National Health Research Institutes, Taiwan, NHRI-EX103-10027SC (YHC), and National Taiwan University, NTU-CDP-104R7880 (YHC) and in part by a grant from the National Institutes of Health, DK067003 (MEG).
Abbreviations
- 2-OA-OVA
2-octynoic acid conjugated ovalbumin
- AAV
adeno-associated virus
- AMAs
anti-mitochondrial antibodies
- MNCs
mononuclear cells
- PBC
primary biliary cholangitis
- TU
transduction unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 2.Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 5.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54:252–261. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz SM, Kohler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- 9.Chestovich PJ, Uchida Y, Chang W, Ajalat M, Lassman C, Sabat R, Busuttil RW, et al. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation. 2012;93:485–492. doi: 10.1097/TP.0b013e3182449136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin WZ, Chen LL, Pan HF, Leng RX, Zhai ZM, Wang C, Li RJ, et al. Expressions of IL-22 in circulating CD4+/CD8+ T cells and their correlation with disease activity in SLE patients. Clin Exp Med. 2011;11:245–250. doi: 10.1007/s10238-011-0134-9. [DOI] [PubMed] [Google Scholar]
- 12.Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, Nojima Y. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–1046. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 13.Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, Matthys P. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60:390–395. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- 14.Lavoie TN, Stewart CM, Berg KM, Li Y, Nguyen CQ. Expression of interleukin-22 in Sjogren's syndrome: significant correlation with disease parameters. Scand J Immunol. 2011;74:377–382. doi: 10.1111/j.1365-3083.2011.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, Fouser LA, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188:462–469. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- 17.Ke Y, Sun D, Jiang G, Kaplan HJ, Shao H. IL-22-induced regulatory CD11b+ APCs suppress experimental autoimmune uveitis. J Immunol. 2011;187:2130–2139. doi: 10.4049/jimmunol.1100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang H, Hanawa H, Liu H, Yoshida T, Hayashi M, Watanabe R, Abe S, et al. Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J Immunol. 2006;177:3635–3643. doi: 10.4049/jimmunol.177.6.3635. [DOI] [PubMed] [Google Scholar]
- 19.Justa S, Zhou X, Sarkar S. Endogenous IL-22 plays a dual role in arthritis: regulation of established arthritis via IFN-gamma responses. PLoS One. 2014;9:e93279. doi: 10.1371/journal.pone.0093279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar S, Zhou X, Justa S, Bommireddy SR. Interleukin-22 reduces the severity of collagen-induced arthritis in association with increased levels of interleukin-10. Arthritis Rheum. 2013;65:960–971. doi: 10.1002/art.37849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu SJ, Yang YH, Tsuneyama K, Leung PS, Illarionov P, Gershwin ME, Chuang YH. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. 2011;53:915–925. doi: 10.1002/hep.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CH, Chen YC, Yu YH, Tao MH, Leung PS, Ansari AA, Gershwin ME, et al. Innate immunity drives xenobiotic-induced murine autoimmune cholangitis. Clin Exp Immunol. 2014;177:373–380. doi: 10.1111/cei.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CH, Chen YC, Zhang W, Leung PS, Gershwin ME, Chuang YH. Innate immunity drives the initiation of a murine model of primary biliary cirrhosis. PLoS One. 2015;10:e0121320. doi: 10.1371/journal.pone.0121320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 27.Katsumi T, Tomita K, Leung PS, Yang GX, Gershwin ME, Ueno Y. Animal models of primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:142–153. doi: 10.1007/s12016-015-8482-y. [DOI] [PubMed] [Google Scholar]
- 28.Wang YH, Yang W, Yang JB, Jia YJ, Tang W, Gershwin ME, Ridgway WM, et al. Systems biologic analysis of T regulatory cells genetic pathways in murine primary biliary cirrhosis. J Autoimmun. 2015;59:26–37. doi: 10.1016/j.jaut.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb GJ, Hirschfield GM. Using GWAS to identify genetic predisposition in hepatic autoimmunity. J Autoimmun. 2015 doi: 10.1016/j.jaut.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Webb GJ, Siminovitch KA, Hirschfield GM. The immunogenetics of primary biliary cirrhosis: A comprehensive review. J Autoimmun. 2015 doi: 10.1016/j.jaut.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 32.He XS, Ansari AA, Ridgway WM, Coppel RL, Gershwin ME. New insights to the immunopathology and autoimmune responses in primary biliary cirrhosis. Cell Immunol. 2006;239:1–13. doi: 10.1016/j.cellimm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Silveira MG, Lindor KD. Treatment of primary biliary cirrhosis: therapy with choleretic and immunosuppressive agents. Clin Liver Dis. 2008;12:425–443. x–xi. doi: 10.1016/j.cld.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Imam MH, Talwalkar JA, Lindor KD. Clinical management of autoimmune biliary diseases. J Autoimmun. 2013;46:88–96. doi: 10.1016/j.jaut.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Floreani A, Franceschet I, Perini L, Cazzagon N, Gershwin ME, Bowlus CL. New therapies for primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:263–272. doi: 10.1007/s12016-014-8456-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Zhang W, Leung PS, Bowlus CL, Dhaliwal S, Coppel RL, Ansari AA, et al. Ongoing activation of autoantigen-specific B cells in primary biliary cirrhosis. Hepatology. 2014;60:1708–1716. doi: 10.1002/hep.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Sun Y, Zhang Z, Jia Y, Zou Z, Ding J, Li Y, et al. CXCR5+ CD4+ T follicular helper cells participate in the pathogenesis of primary biliary cirrhosis. Hepatology. 2015;61:627–638. doi: 10.1002/hep.27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Wang W, Tang L, He X, Yan X, Zhang X, Zhu Y, et al. Chemokine (C-X-C motif) ligand 13 promotes intrahepatic chemokine (C-X-C motif) receptor 5+ lymphocyte homing and aberrant B-cell immune responses in primary biliary cirrhosis. Hepatology. 2015;61:1998–2007. doi: 10.1002/hep.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 42.Oo YH, Shetty S, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. Dig Dis. 2010;28:31–44. doi: 10.1159/000282062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR 3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am J Pathol. 2005;167:887–899. doi: 10.1016/S0002-9440(10)62060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- 46.Isse K, Harada K, Zen Y, Kamihira T, Shimoda S, Harada M, Nakanuma Y. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41:506–516. doi: 10.1002/hep.20582. [DOI] [PubMed] [Google Scholar]
- 47.Chuang YH, Lian ZX, Cheng CM, Lan RY, Yang GX, Moritoki Y, Chiang BL, et al. Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J Autoimmun. 2005;25:126–132. doi: 10.1016/j.jaut.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Chemokine-chemokine receptor CCL2-CCR2 and CX3CL1-CX3CR1 axis may play a role in the aggravated inflammation in primary biliary cirrhosis. Dig Dis Sci. 2014;59:358–364. doi: 10.1007/s10620-013-2920-6. [DOI] [PubMed] [Google Scholar]
- 49.Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, Jin L, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology. 2014;59:1331–1342. doi: 10.1002/hep.26916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011;141:1897–1906. doi: 10.1053/j.gastro.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, Gershwin ME, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012;143:188–198. e187. doi: 10.1053/j.gastro.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]